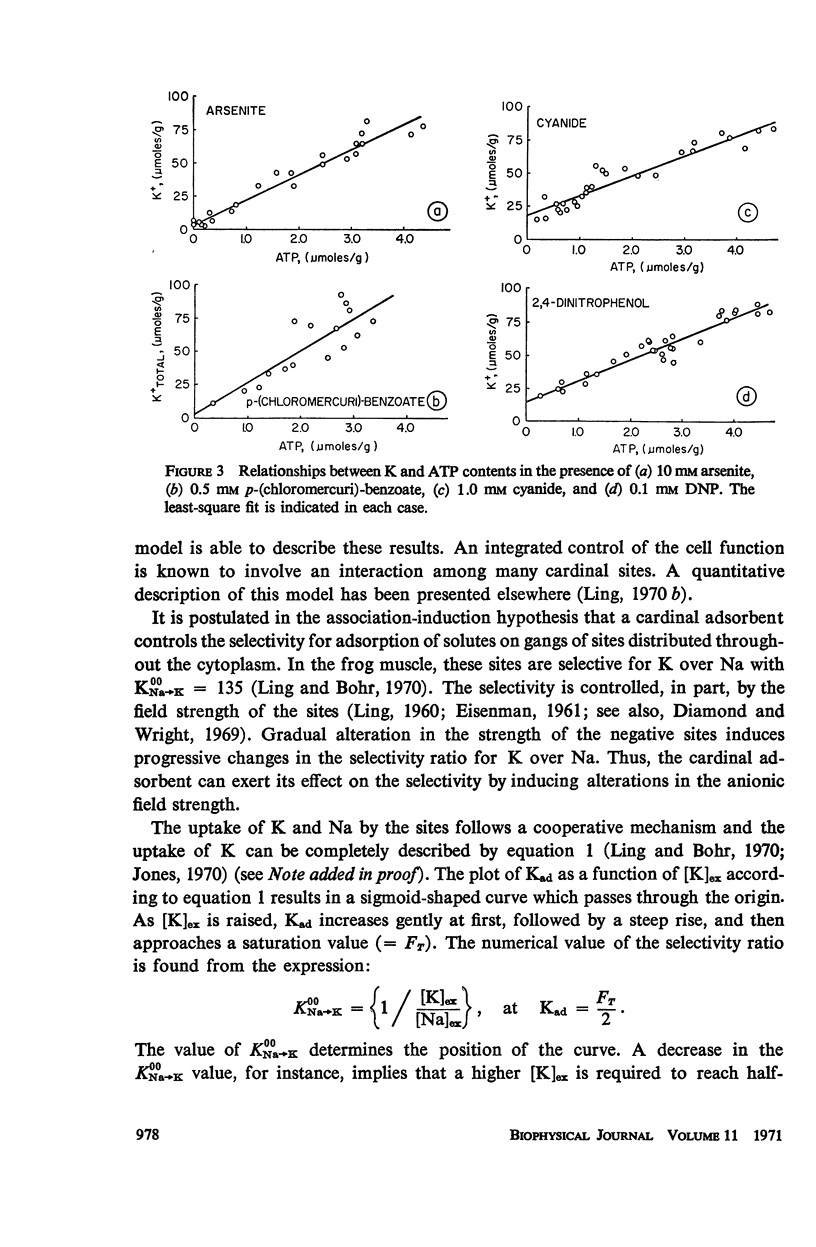
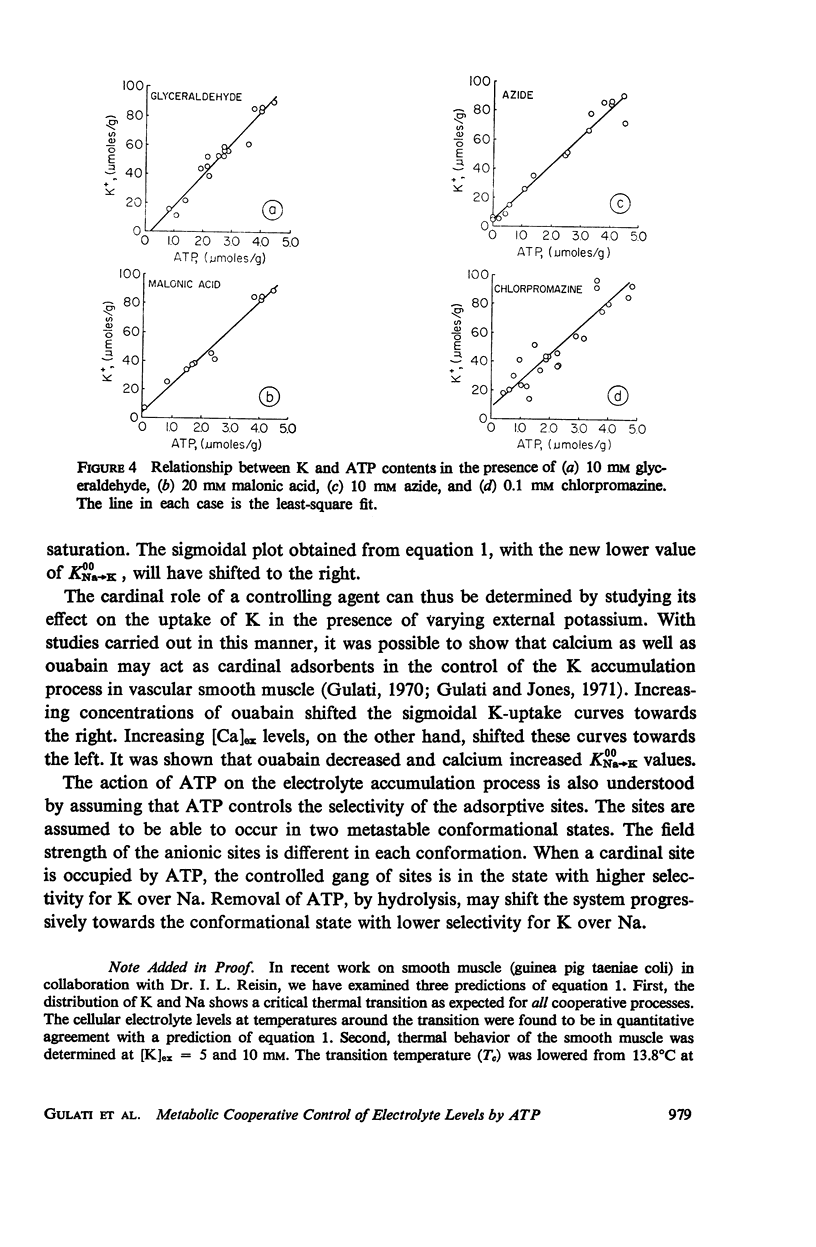
Abstract
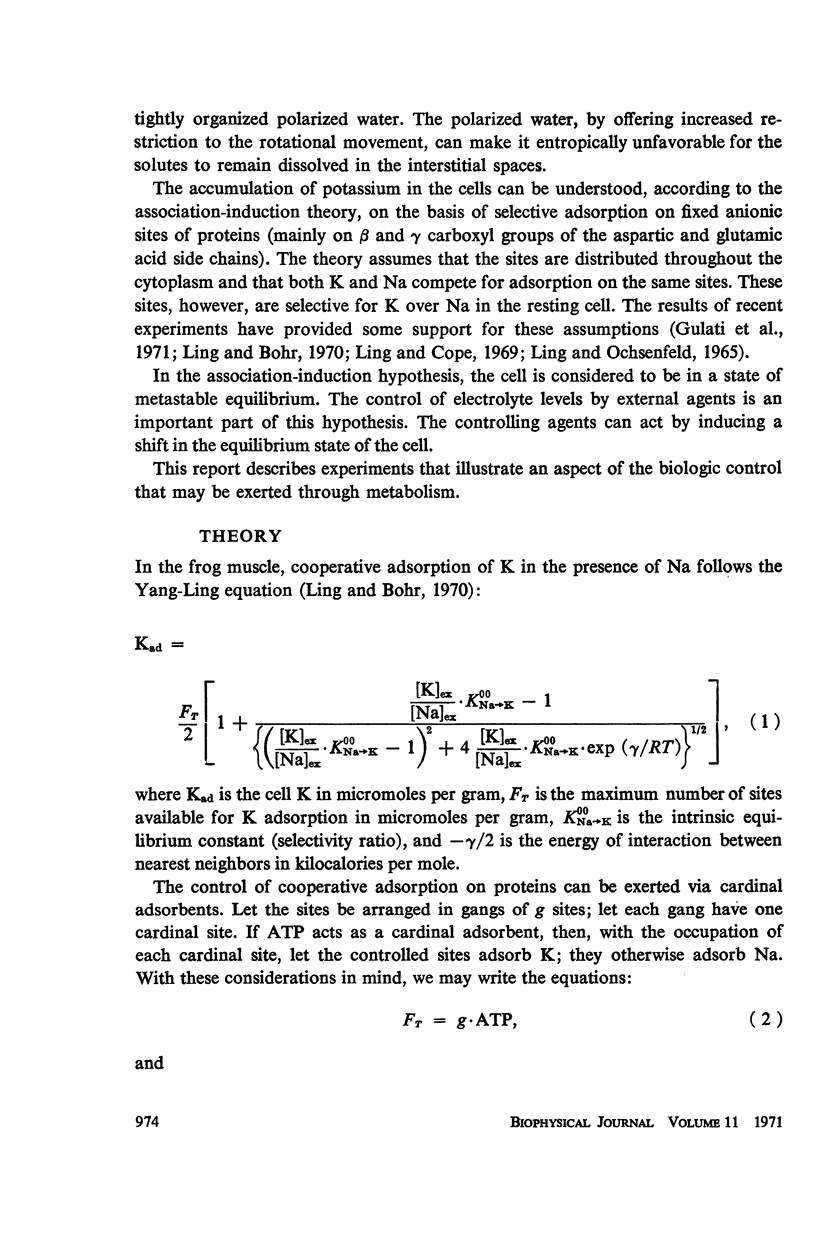
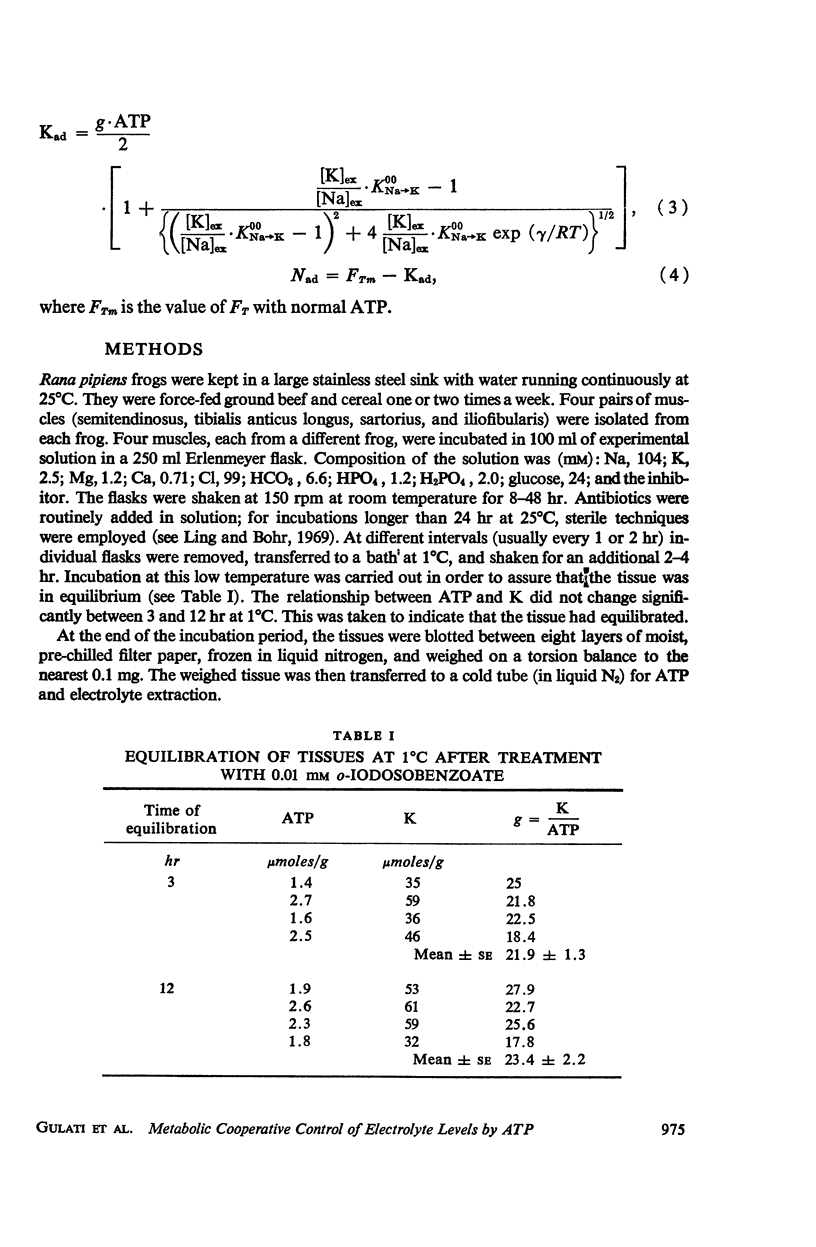
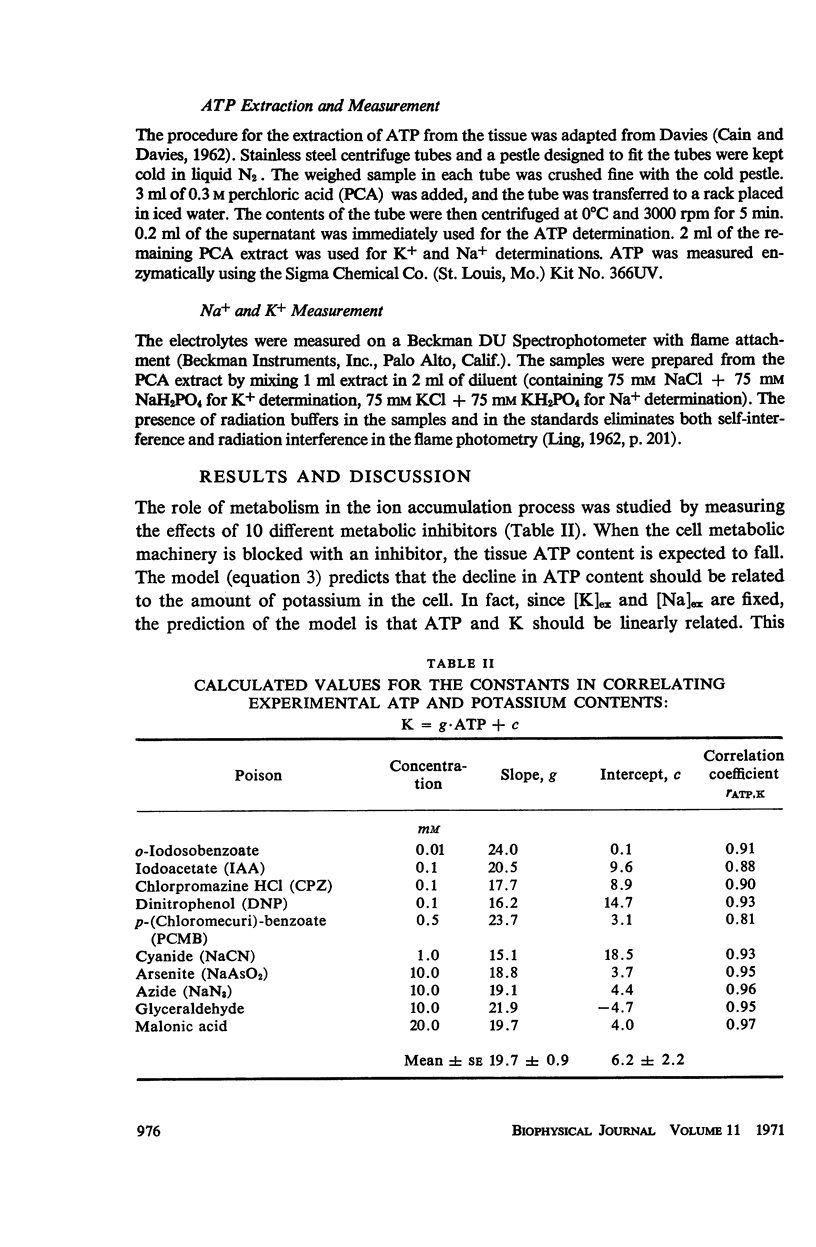
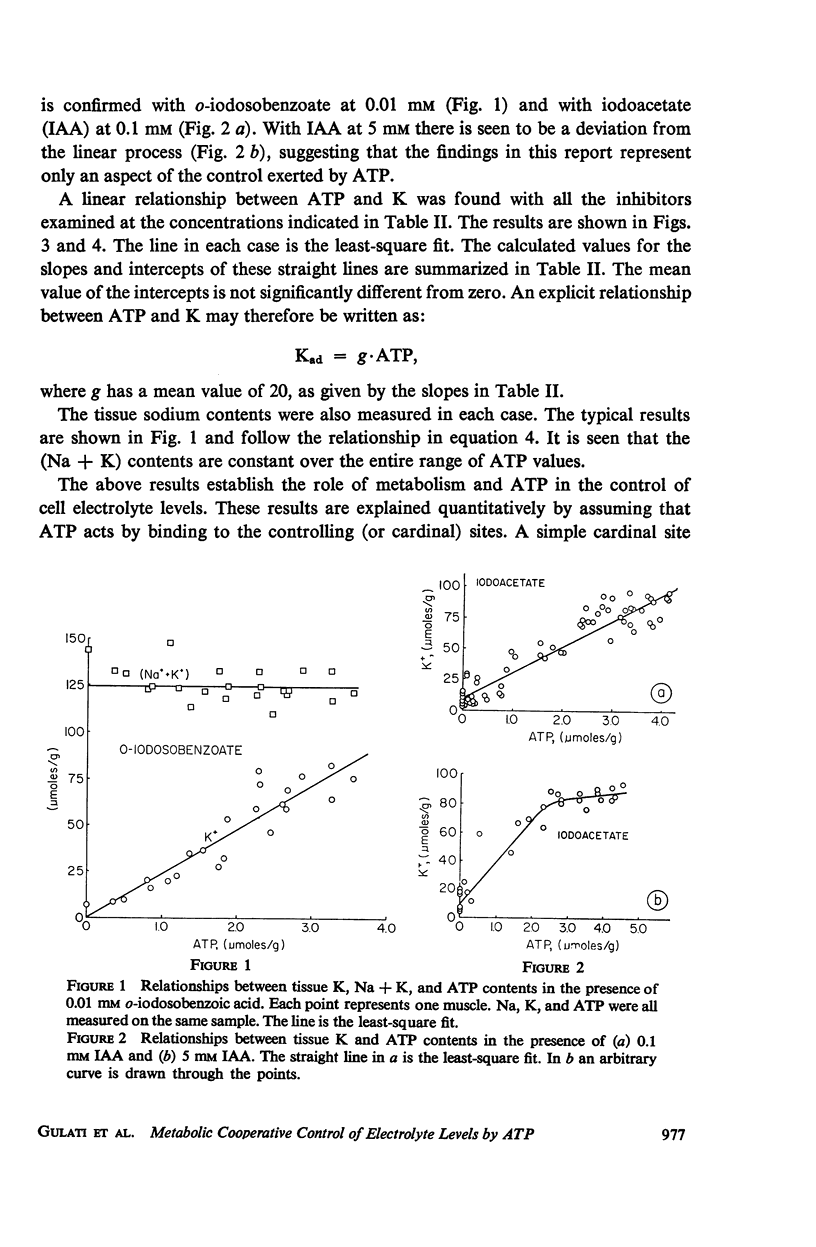
This study examines the effects of metabolic inhibitors on the content of cellular K, Na, and adenosine triphosphate (ATP). ATP and K are seen to fall in the inhibited tissues. The ATP content is correlated with the K content. The role of ATP is examined according to a recent biophysical approach. It is suggested that ATP may control the electrolyte levels by inducing conformational changes in the cytoplasmic proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cope F. W. Spin-echo nuclear magnetic resonance evidence for complexing of sodium ions in muscle, brain, and kidney. Biophys J. 1970 Sep;10(9):843–858. doi: 10.1016/S0006-3495(70)86339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler J. L., Fritz O. G., Jr, Swift T. J. Direct evidence from nuclear magnetic resonance studies for bound sodium in forg skeletal muscle. Biophys J. 1970 Mar;10(3):260–268. doi: 10.1016/s0006-3495(70)86298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Jones A. W. Cooperative control of potassium accumulation by ouabain in vascular smooth muscle. Science. 1971 Jun 25;172(3990):1348–1350. doi: 10.1126/science.172.3990.1348. [DOI] [PubMed] [Google Scholar]
- Hazlewood C. F., Nichols B. L., Chamberlain N. F. Evidence for the existence of a minimum of two phases of ordered water in skeletal muscle. Nature. 1969 May 24;222(5195):747–750. doi: 10.1038/222747a0. [DOI] [PubMed] [Google Scholar]
- Hinke J. A. Solvent water for electrolytes in the muscle fiber of the giant barnacle. J Gen Physiol. 1970 Oct;56(4):521–541. doi: 10.1085/jgp.56.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. W., Karreman G. Potassium accumulation and permeation in the canine carotid artery. Biophys J. 1969 Jul;9(7):910–924. doi: 10.1016/S0006-3495(69)86426-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G. N., Bohr G. Studies on ion distribution in living cells. II. Cooperative interaction between intracellular potassium and sodium ions. Biophys J. 1970 Jun;10(6):519–538. doi: 10.1016/S0006-3495(70)86317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G. N., Cope F. W. Potassium ion: is the bulk of intracellular K+ adsorbed? Science. 1969 Mar 21;163(3873):1335–1336. doi: 10.1126/science.163.3873.1335. [DOI] [PubMed] [Google Scholar]
- Ling G. N. Diphosphoglycerate and inosine hexaphosphate control of oxygen binding by hemoglobin: a theoretical interpretation of experimental data. Proc Natl Acad Sci U S A. 1970 Sep;67(1):296–301. doi: 10.1073/pnas.67.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G. N., Ochsenfeld M. M. Studies on the ionic permeability of muscle cells and their models. Biophys J. 1965 Nov;5(6):777–807. doi: 10.1016/S0006-3495(65)86752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G. N. The physical state of water in living cells and its physiological significance. Int J Neurosci. 1970 Dec;1(2):129–152. doi: 10.3109/00207457009147626. [DOI] [PubMed] [Google Scholar]



