Abstract
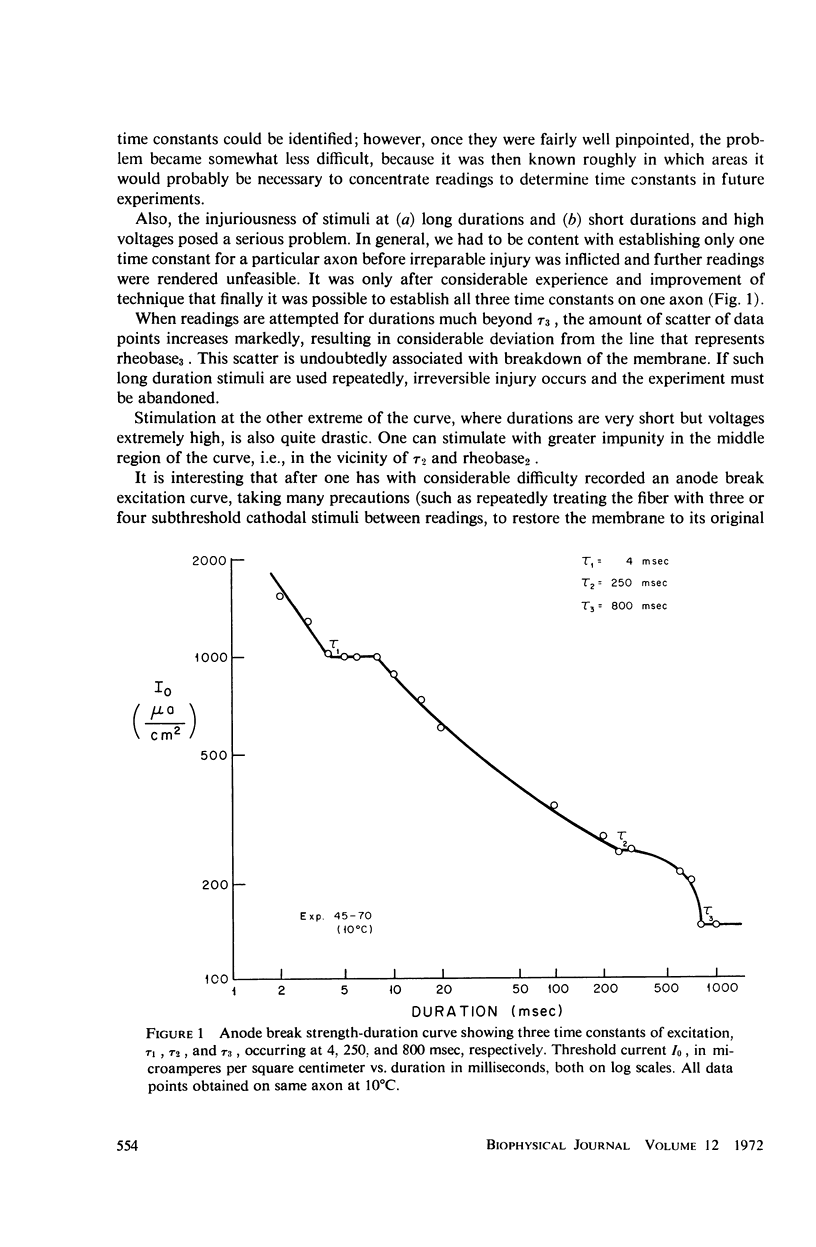
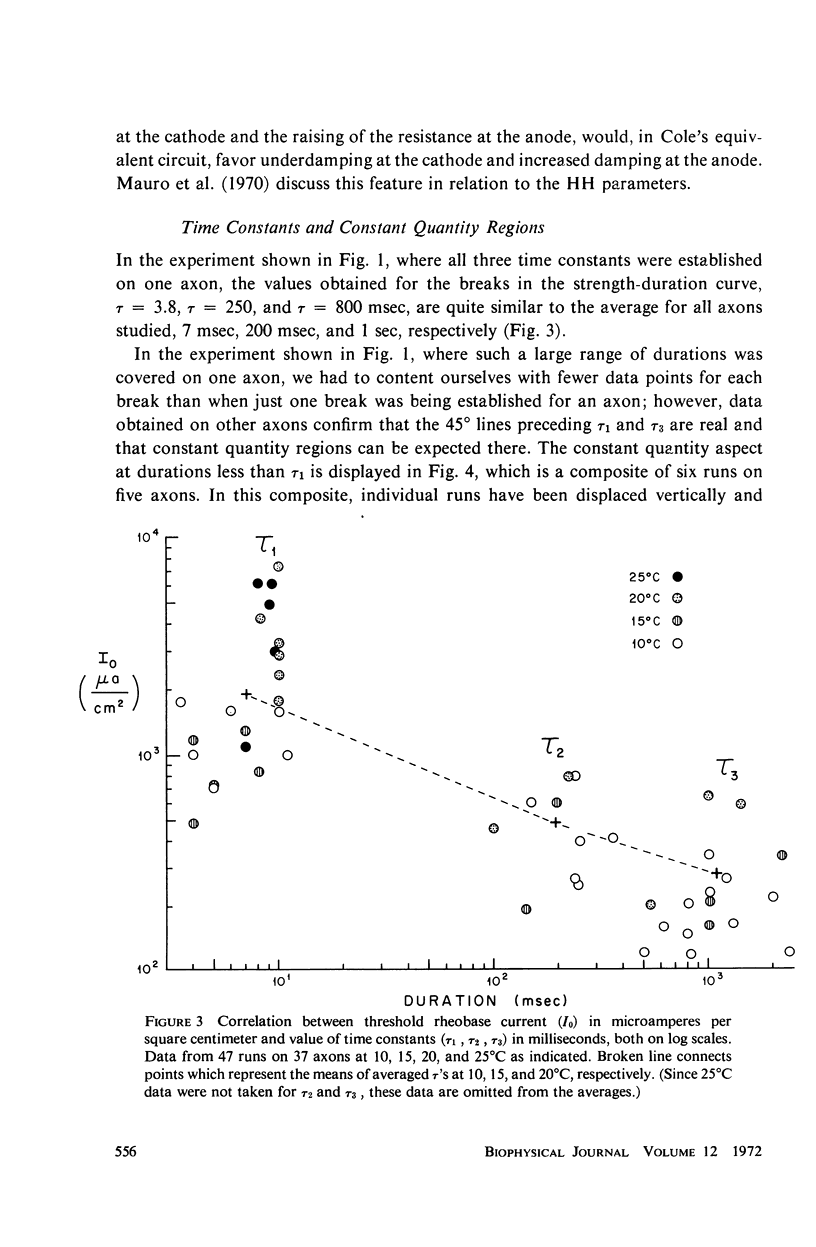
Strength-duration curves for space-clamped squid axons, using square wave anode breaks as stimuli, established the existence of four distinct regions. For the average experimental axon the intersection of the first two regions, τ1, occurs at about 7 msec. This agrees with computations based on the Hodgkin-Huxley (HH) equations and corresponds to the accommodation time constant found previously for a linearly rising ramp, as given by the HH equations and as found experimentally. The second break in the curve, τ2, at about 200 msec, and the third break, τ3, at 1 sec, are far beyond the range of the HH equations and may be the counterpart in the excitability of the long time constants, which have been apparent from a number of other types of experiments. The regions of the curve before 1 msec and beyond 2 or 3 sec are quite variable and may represent breakdown. Rheobase increases in both experimental and computed axons when temperature is raised. In both experimental and computed axons τ1 descreases slightly when the temperature is raised from 10 to 15°C. At 20 and 25°C, τ1 of the experimental axon increases markedly.
Full text
PDF

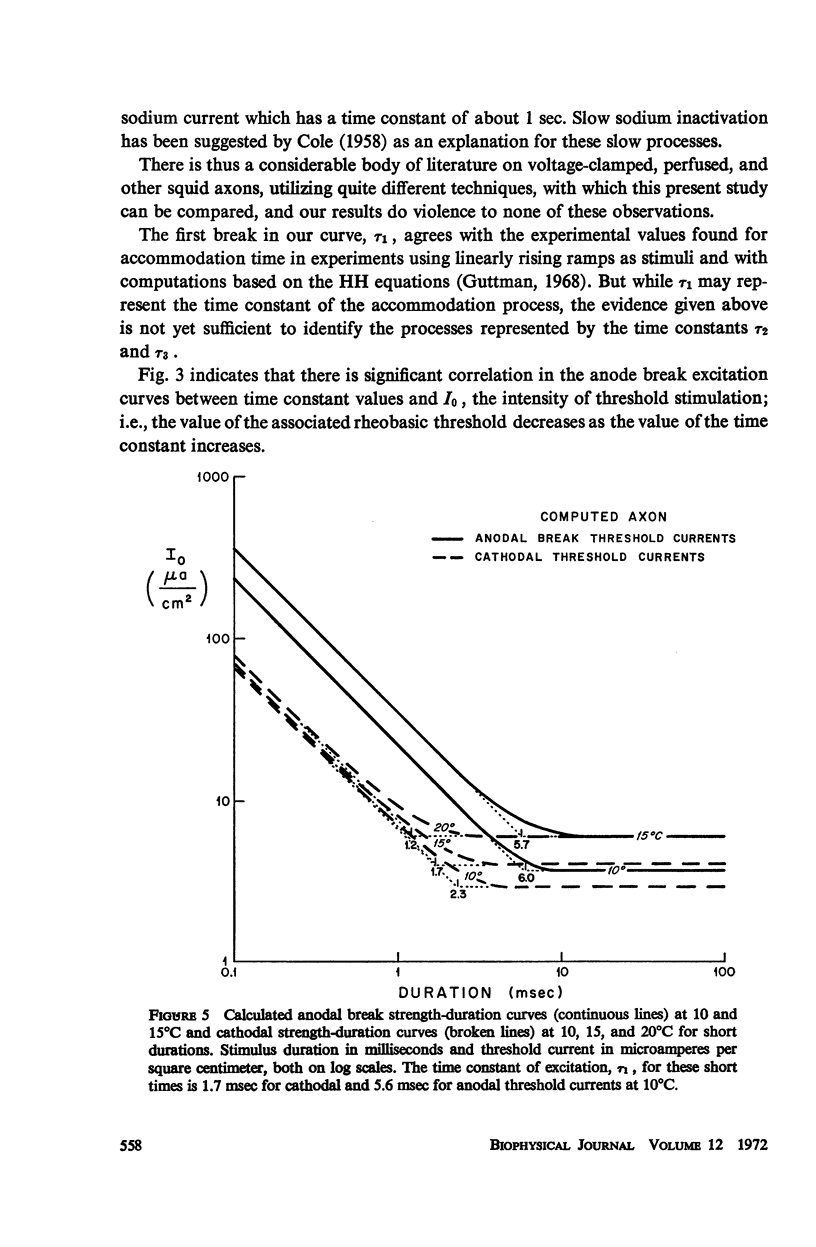

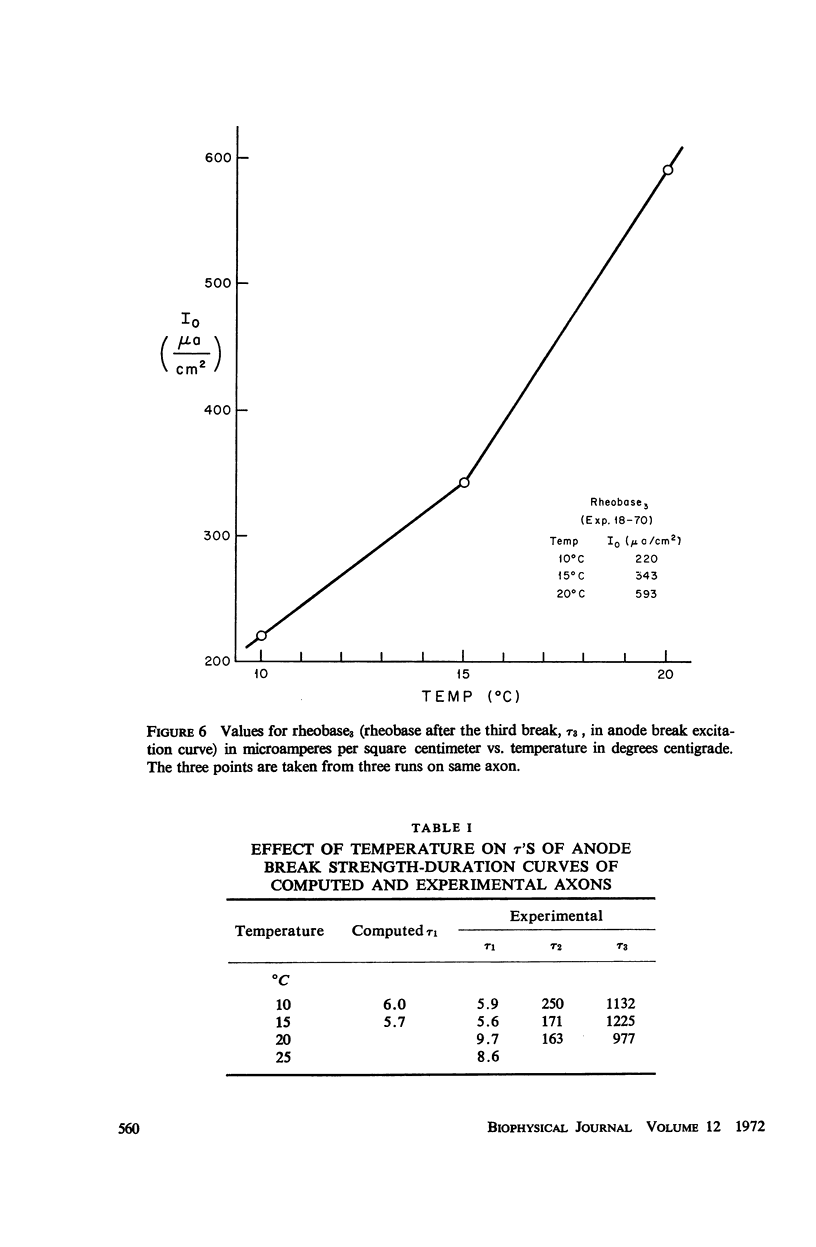



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Palti Y. The effects of external potassium and long duration voltage conditioning on the amplitude of sodium currents in the giant axon of the squid, Loligo pealei. J Gen Physiol. 1969 Nov;54(5):589–606. doi: 10.1085/jgp.54.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Slow changes in membrane permeability and long-lasting action potentials in axons perfused with fluoride solutions. J Physiol. 1970 Dec;211(3):707–728. doi: 10.1113/jphysiol.1970.sp009300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K. S. RECTIFICATION AND INDUCTANCE IN THE SQUID GIANT AXON. J Gen Physiol. 1941 Sep 20;25(1):29–51. doi: 10.1085/jgp.25.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman R., Barnhill R. Oscillation and repetitive firing in squid axons. Comparison of experiments with computations. J Gen Physiol. 1970 Jan;55(1):104–118. doi: 10.1085/jgp.55.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFEVRE P. G. Excitation characteristics of the squid giant axon: a test of excitation theory in a case of rapid accommodation. J Gen Physiol. 1950 Sep;34(1):19–36. doi: 10.1085/jgp.34.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecar H., Nossal R. Theory of threshold fluctuations in nerves. I. Relationships between electrical noise and fluctuations in axon firing. Biophys J. 1971 Dec;11(12):1048–1067. doi: 10.1016/S0006-3495(71)86277-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas K. The analysis of complex excitable tissues by their response to electric currents of short duration. J Physiol. 1907 Mar 27;35(4):310–331. doi: 10.1113/jphysiol.1907.sp001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A., Conti F., Dodge F., Schor R. Subthreshold behavior and phenomenological impedance of the squid giant axon. J Gen Physiol. 1970 Apr;55(4):497–523. doi: 10.1085/jgp.55.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J. S., Gorman A. L. Photoreceptor potentials of opposite polarity in the eye of the scallop, Pecten irradians. J Gen Physiol. 1970 Sep;56(3):376–391. doi: 10.1085/jgp.56.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]




