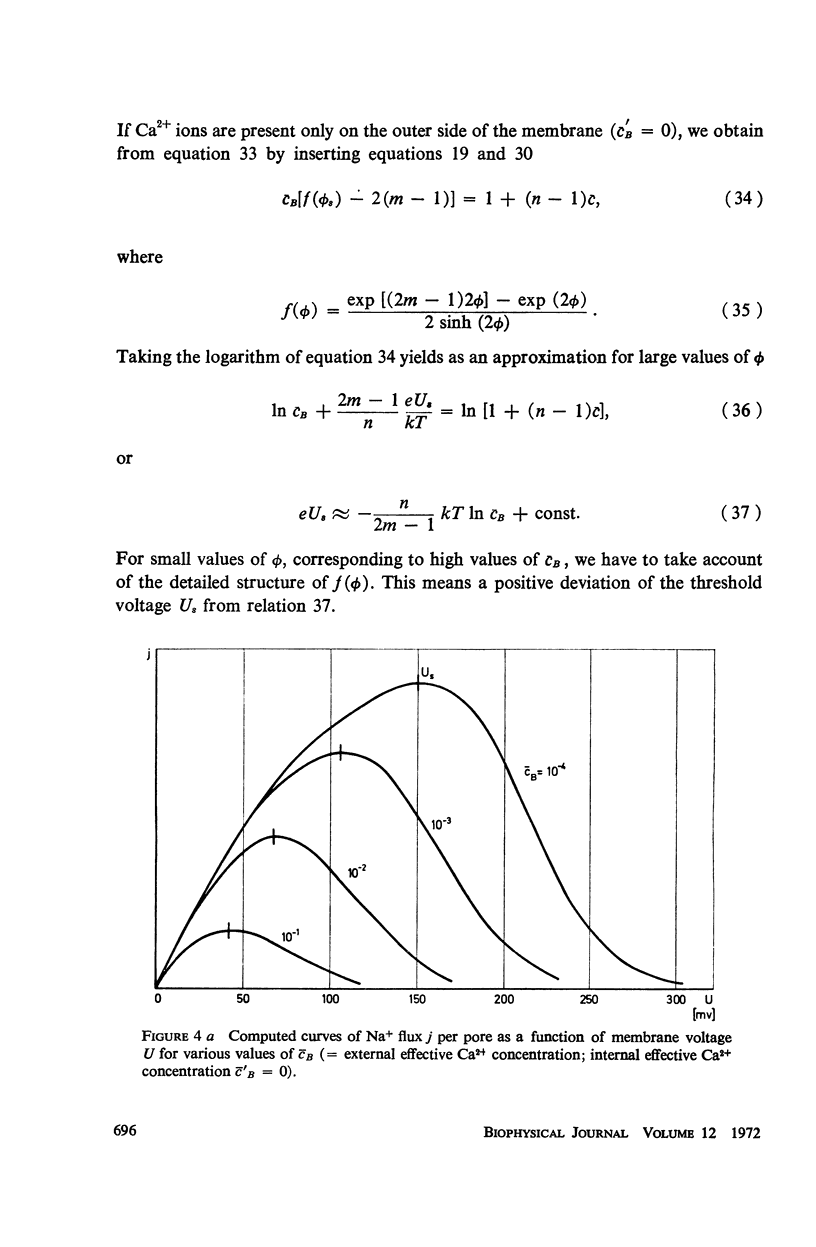
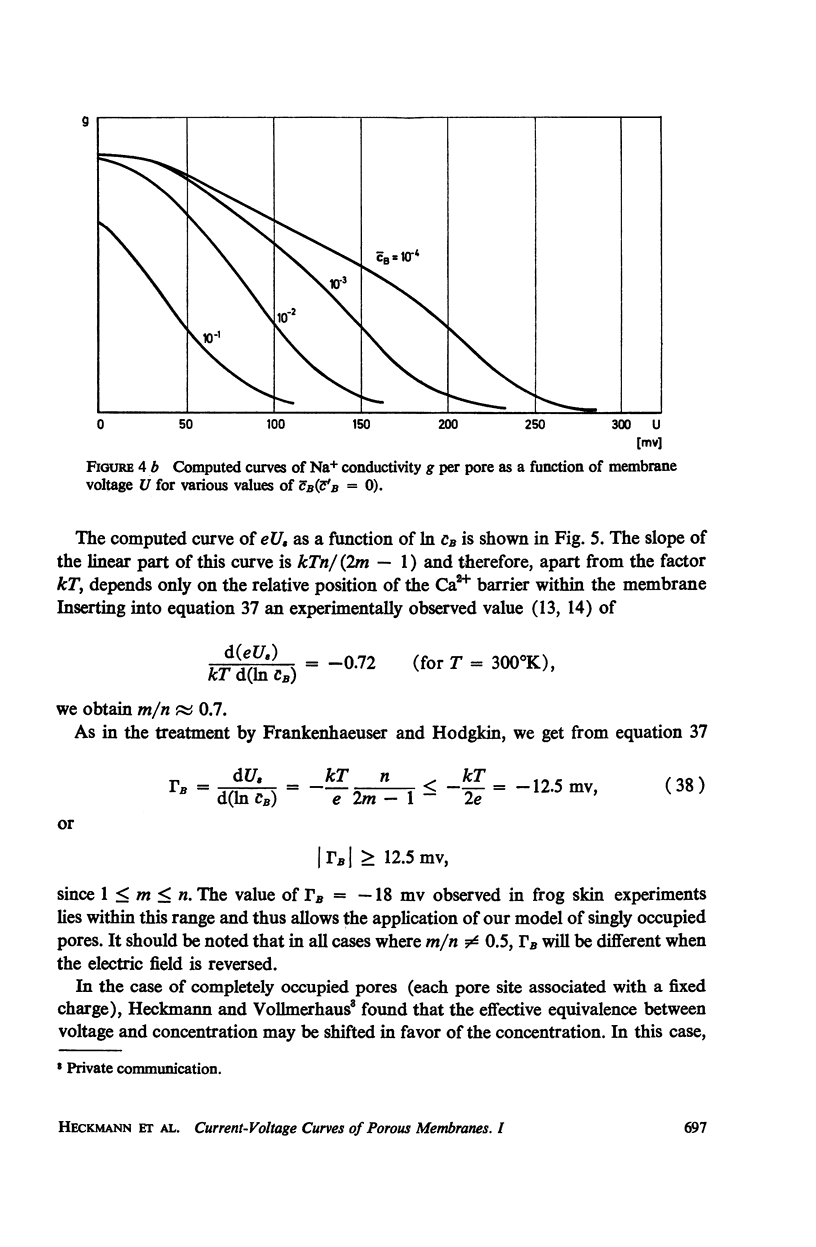
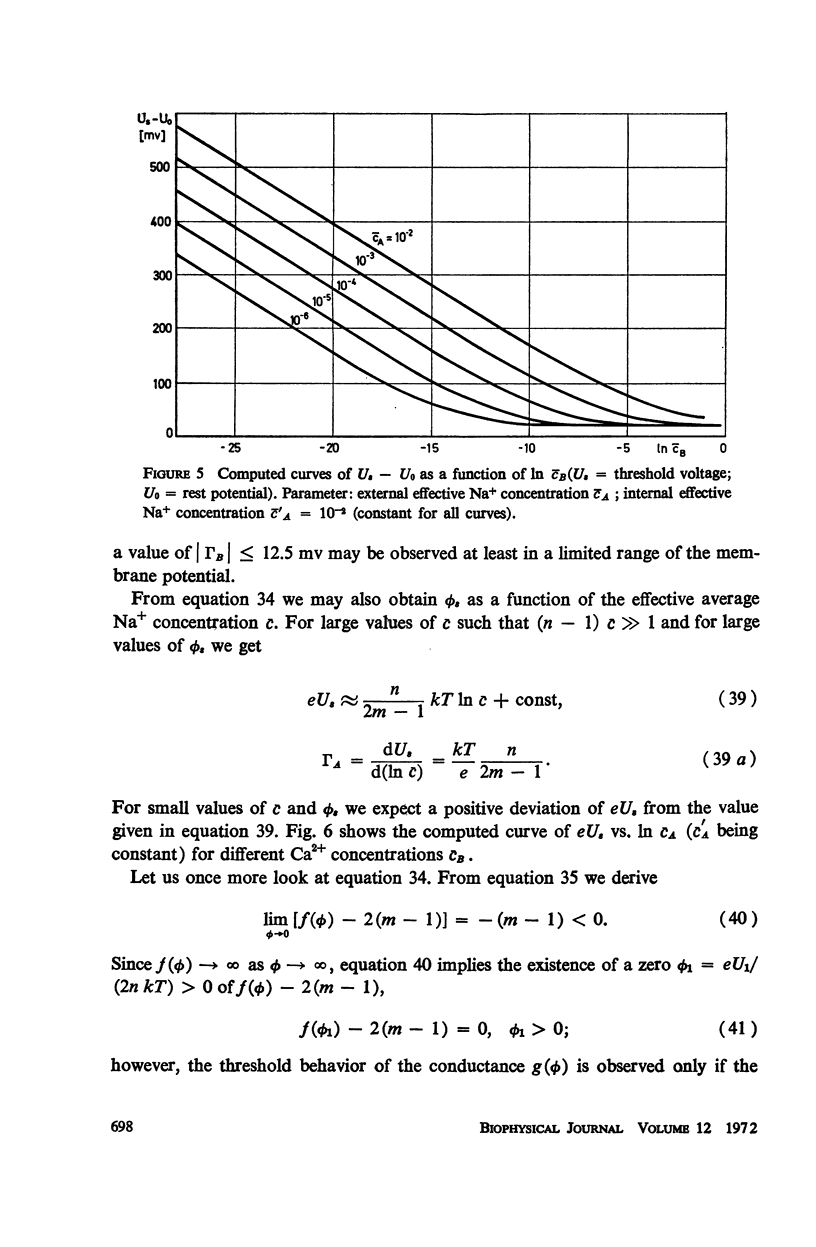
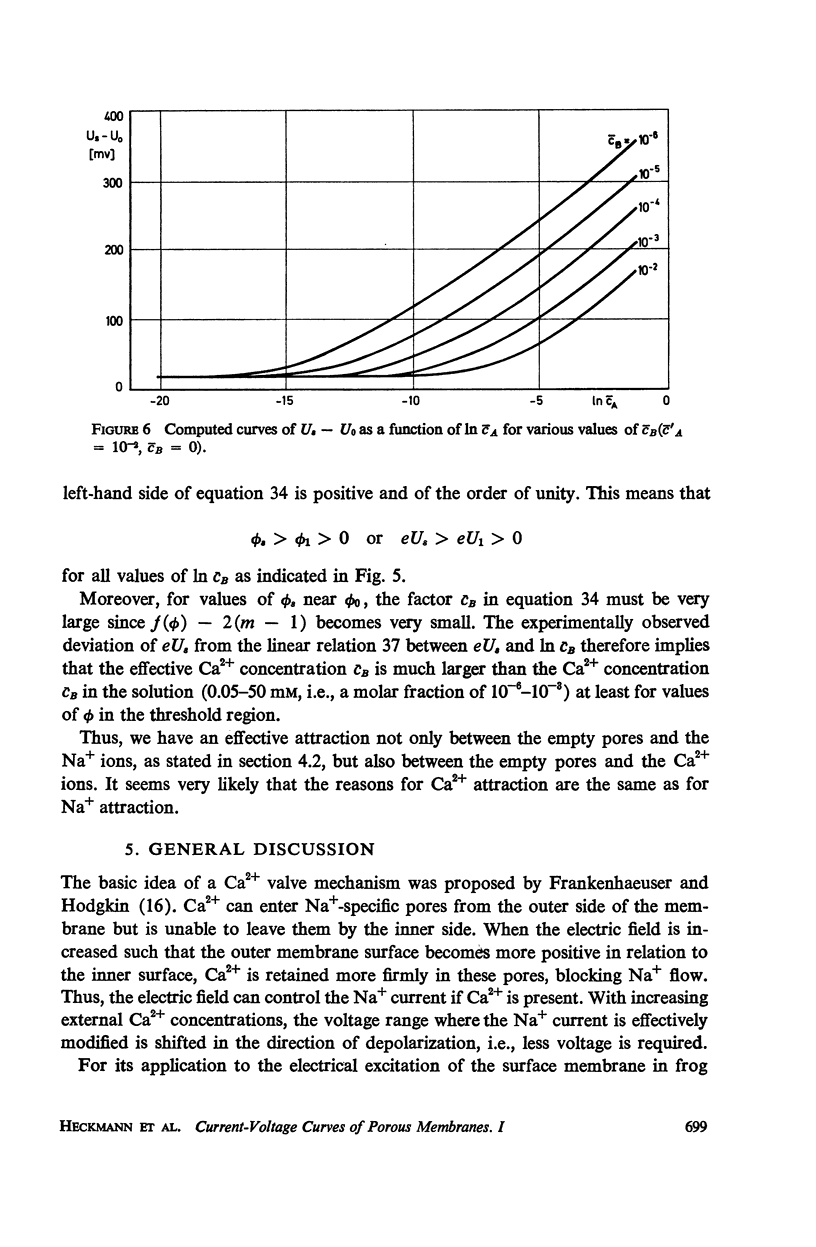
Abstract
We propose a physical model for voltage-dependent conductance changes of excitable cell membranes. It is based on competition of uni- and bivalent ions for chains of stable sites extending through the membrane. These one-dimensional pathways (pores) have different profiles of chemical potential for the two ionic species so that bivalent ions can block the passage of univalent ions at large membrane potentials. We treat the special case that each pore is either empty or, because of electrostatic repulsion, contains no more than one uni- or bivalent ion at a time. A system of linear differential equations describes the time-dependent probabilities of the various possible pore states. The states are limited by transition rate constants involving the profile of the chemical potential, the membrane voltage, the ionic concentrations in the adjacent baths, and electrostatic interactions between the ions. The steady-state solutions (Kirchhoff-Hill theorem) yield expressions for the relationship between the small signal conductance of univalent ions and the concentration of these ions in the external bathing medium (a saturation curve) and for the ionic currents and the steady-state current-voltage curve (N-shaped). From the latter curve we compute the shift of theshold potential caused by concentration changes of the external bathing medium. The model yields a number of predictions which can be tested experimentally.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman H. M., Macey R. I. The N-shaped current-potential characteristic in frog skin. 3. Ionic dependence. Biophys J. 1969 Feb;9(2):151–162. doi: 10.1016/s0006-3495(69)86376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman H. M., Macey R. I. The N-shaped current-potential characteristic in frog skin. I. Time development during step voltage clamp. Biophys J. 1969 Feb;9(2):127–139. doi: 10.1016/S0006-3495(69)86374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman H. M., Macey R. I. The N-shaped current-potential characteristic in frog skin. II. Kinetic behavior during ramp voltage clamp. Biophys J. 1969 Feb;9(2):140–150. doi: 10.1016/S0006-3495(69)86375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN D. E. GATE CONTROL OF ION FLUX IN AXONS. J Gen Physiol. 1965 May;48:SUPPL–SUPPL:77. doi: 10.1085/jgp.48.5.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYT R. C. THE SQUID GIANT AXON. MATHEMATICAL MODELS. Biophys J. 1963 Sep;3:399–431. doi: 10.1016/s0006-3495(63)86829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Studies in irreversible thermodynamics. IV. Diagrammatic representation of steady state fluxes for unimolecular systems. J Theor Biol. 1966 Apr;10(3):442–459. doi: 10.1016/0022-5193(66)90137-8. [DOI] [PubMed] [Google Scholar]
- Lindemann B. Sodium- and calcium-dependence of threshold potential in frog skin excitation. Biochim Biophys Acta. 1968 Nov 5;163(3):424–426. doi: 10.1016/0005-2736(68)90130-2. [DOI] [PubMed] [Google Scholar]
- MULLINS L. J. An analysis of pore size in excitable membranes. J Gen Physiol. 1960 May;43:105–117. doi: 10.1085/jgp.43.5.105. [DOI] [PMC free article] [PubMed] [Google Scholar]


