Abstract
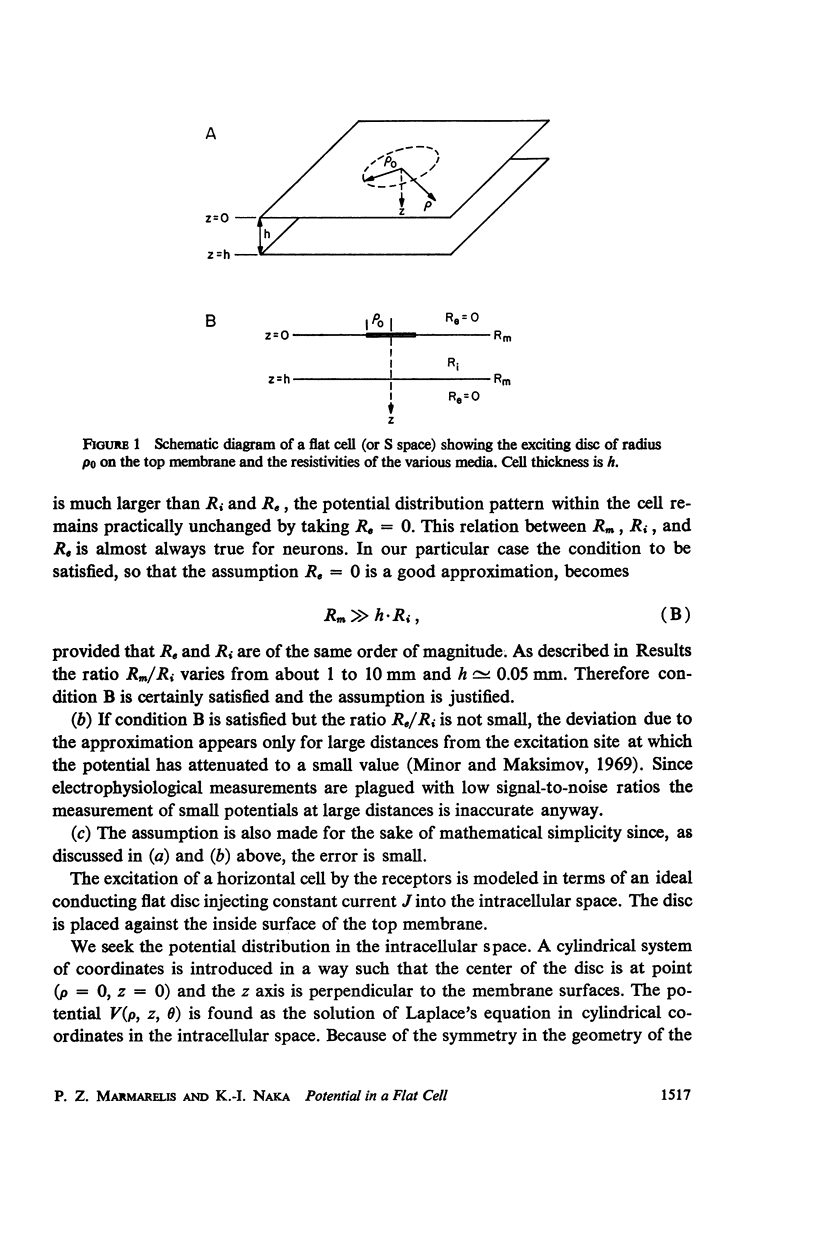
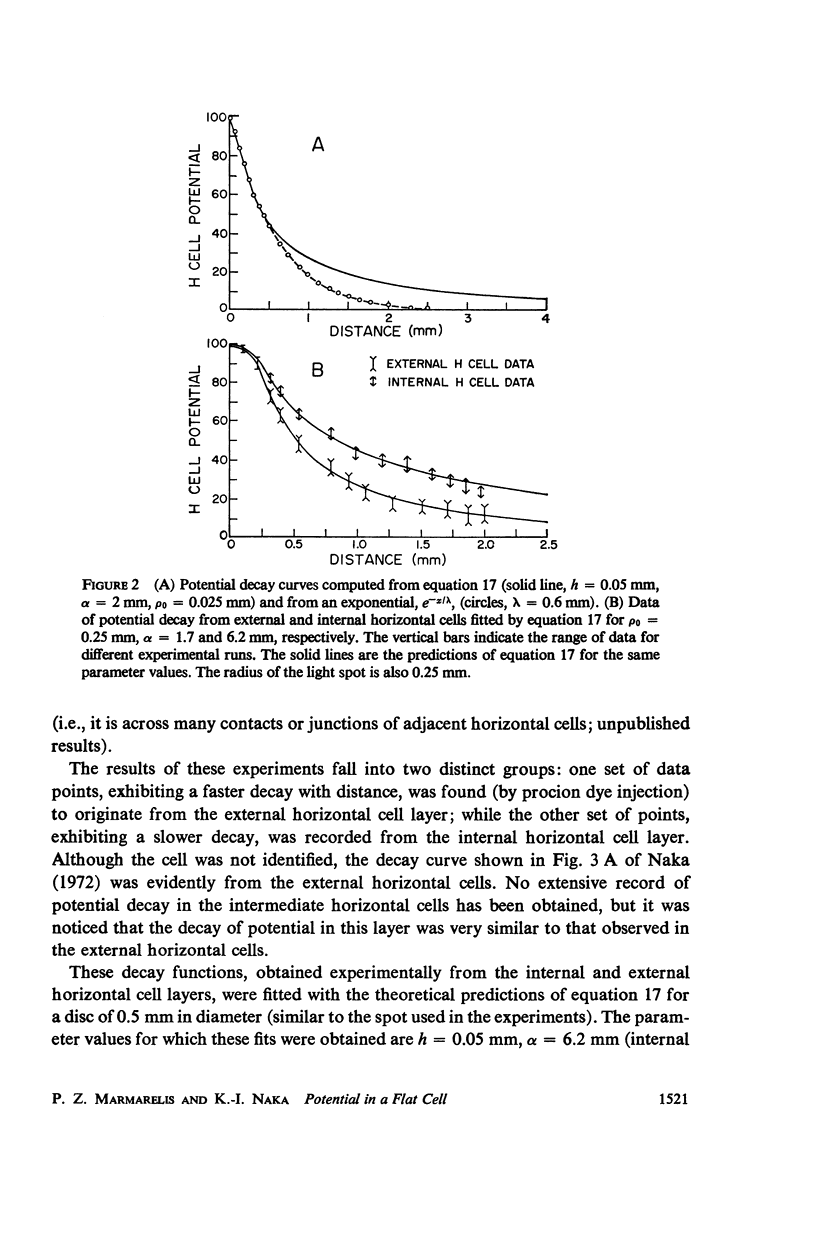
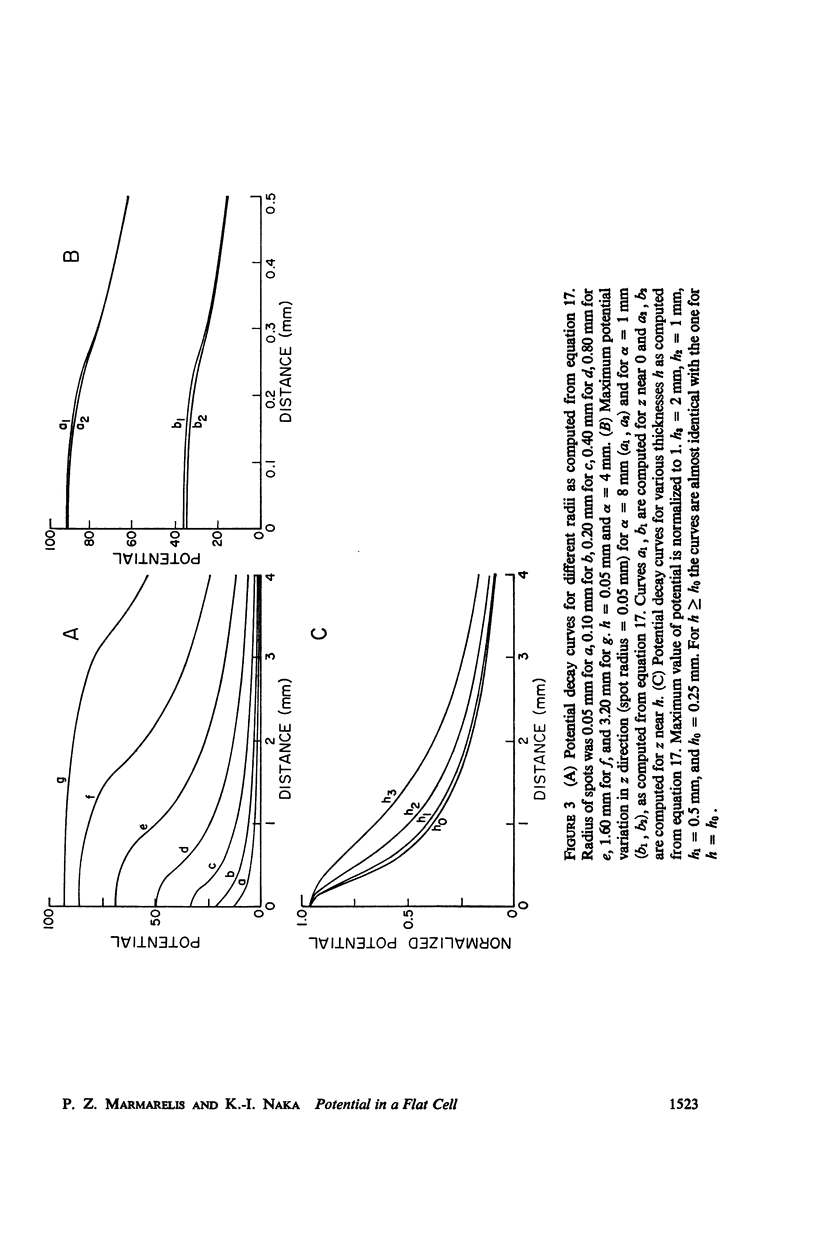
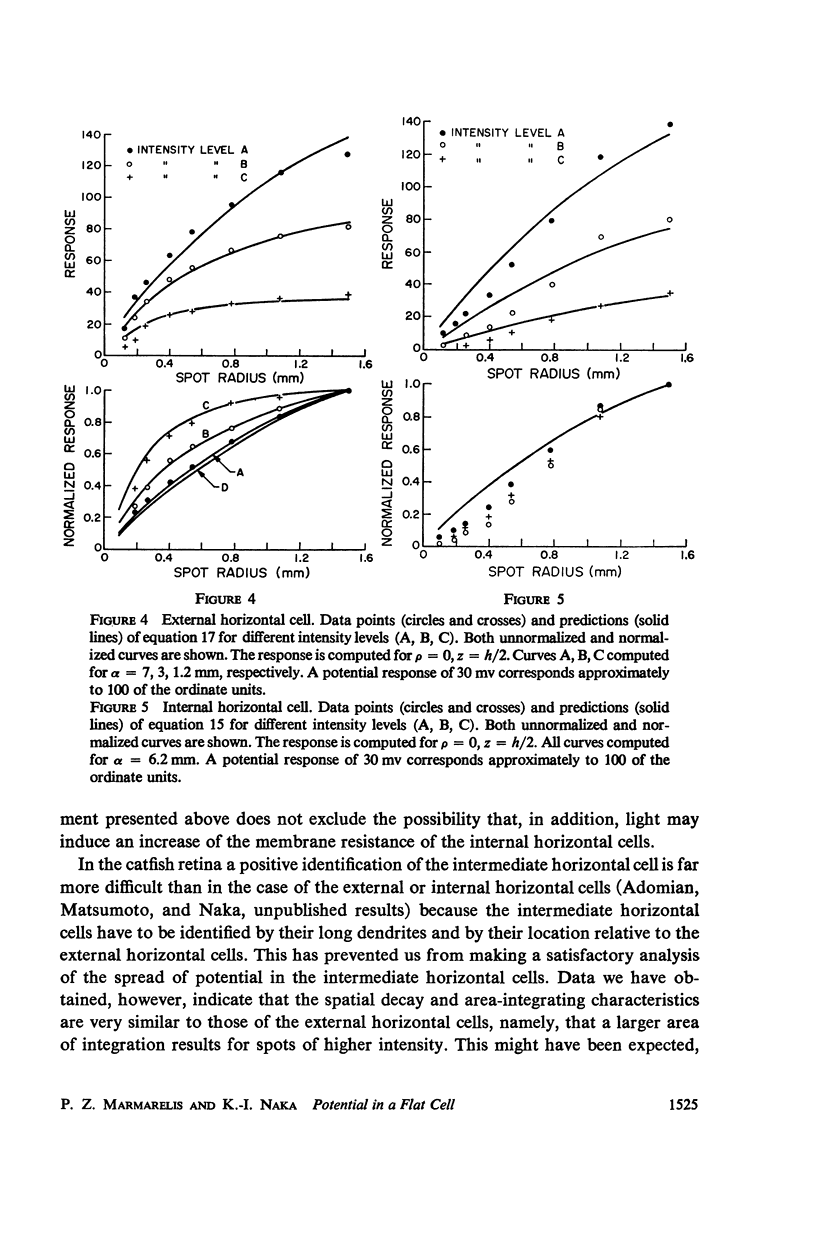
An analytical solution is obtained for the three-dimensional spatial distribution of potential inside a flat cell, such as the layer of horizontal cells, as a function of its geometry and resistivity characteristics. It was found that, within a very large range of parameter values, the potential is given by [Formula: see text] where r = ρ/ρ0, z̄ = z/ρ0, ρ = (Ri/Rm)·ρ0, δ = h/ρ0; K is a constant; J is the assumed synaptic current; ρ, z are cylindrical coordinates; ρ0 is the radius of the synaptic area of excitation; h is the cell thickness; and Ri, Rm are the intracellular and membrane resistivities, respectively. Formula A closely fits data for the spatial decay of potential which were obtained from the catfish internal and external horizontal cells. It predicts a decay which is exponential down to about 40% of the maximum potential but is much slower than exponential below that level, a characteristic also exhibited by the data. Such a feature in the decay mode allows signal integration over the large retinal areas which have been observed experimentally both at the horizontal and ganglion cell stages. The behavior of the potential distribution as a function of the flat cell parameters is investigated, and it is found that for the range of the horizontal cell thicknesses (10-50 μ) the decay rate depends solely on the ratio Rm/Ri. Data obtained from both types of horizontal cells by varying the diameter of the stimulating spot and for three widely different intensity levels were closely fitted by equation A. In the case of the external horizontal cell, the fit for different intensities was obtained by varying the ratio Rm/Ri; in the case of the internal horizontal cell it was found necessary, in order to fit the data for different intensities, to vary the assumed synaptic current J.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daw N. W. Colour-coded ganglion cells in the goldfish retina: extension of their receptive fields by means of new stimuli. J Physiol. 1968 Aug;197(3):567–592. doi: 10.1113/jphysiol.1968.sp008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. S-potentials in the skate retina. Intracellular recordings during light and dark adaptation. J Gen Physiol. 1971 Aug;58(2):163–189. doi: 10.1085/jgp.58.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE E. P. Resistance values in a syncytium. Aust J Exp Biol Med Sci. 1961 Jun;39:267–274. doi: 10.1038/icb.1961.27. [DOI] [PubMed] [Google Scholar]
- GOURAS P. Graded potentials of bream retina. J Physiol. 1960 Jul;152:487–505. doi: 10.1113/jphysiol.1960.sp006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor A. V., Maksimov V. V. Passivnye élektricheskie svoistva modeli ploskoi kletki. Biofizika. 1969 Mar-Apr;14(2):328–335. [PubMed] [Google Scholar]
- Naka K. I., Nye P. W. Receptive-field organization of the catfish retina: are at least two lateral mechanisms involved? J Neurophysiol. 1970 Sep;33(5):625–642. doi: 10.1152/jn.1970.33.5.625. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Nye P. W. Role of horizontal cells in organization of the catfish retinal receptive field. J Neurophysiol. 1971 Sep;34(5):785–801. doi: 10.1152/jn.1971.34.5.785. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from luminosity units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):587–599. doi: 10.1113/jphysiol.1966.sp008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. The generation and spread of S-potentials in fish (Cyprinidae). J Physiol. 1967 Sep;192(2):437–461. doi: 10.1113/jphysiol.1967.sp008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi K., Sutija V. Lateral spread of light-induced potentials along different cell layers in the teleost retina. Vision Res. 1969 Aug;9(8):881–893. doi: 10.1016/0042-6989(69)90095-9. [DOI] [PubMed] [Google Scholar]
- Norton A. L., Spekreijse H., Wolbarsht M. L., Wagner H. G. Receptive field organization of the S-potential. Science. 1968 May 31;160(3831):1021–1022. doi: 10.1126/science.160.3831.1021. [DOI] [PubMed] [Google Scholar]
- OIKAWA T., OGAWA T., MOTOKAWA K. Origin of so-called cone action potential. J Neurophysiol. 1959 Jan;22(1):102–111. doi: 10.1152/jn.1959.22.1.102. [DOI] [PubMed] [Google Scholar]
- Shiba H., Kanno Y. Further study of the two-dimensional cable theory: an electric model for a flat thin association of cells with a directional intercellular communication. Biophysik. 1971;7(4):295–301. doi: 10.1007/BF01190241. [DOI] [PubMed] [Google Scholar]
- TOMITA T., TOSAKA T., WATANABE K., SATO Y. The fish EIRG in response to different types of illumination. Jpn J Physiol. 1958 Mar 30;8(1):41–50. doi: 10.2170/jjphysiol.8.41. [DOI] [PubMed] [Google Scholar]
- Tanaka I., Sasaki Y. On the electrotonic spread in cardiac muscle of the mouse. J Gen Physiol. 1966 Jul;49(6):1089–1110. doi: 10.1085/jgp.0491089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda J., Nosaki H., Tomita T. Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Res. 1969 Apr;9(4):453–463. doi: 10.1016/0042-6989(69)90134-5. [DOI] [PubMed] [Google Scholar]
- WATANABE K., TOSAKA T. Functional organization of the cyprinid fish retina as revealed by discriminative responses to spectral illumination. Jpn J Physiol. 1959 Mar 25;9(1):84–93. doi: 10.2170/jjphysiol.9.84. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Dowling J. E. Synaptic relationships in the plexiform layers of carp retina. Z Zellforsch Mikrosk Anat. 1969;100(1):60–82. doi: 10.1007/BF00343821. [DOI] [PubMed] [Google Scholar]
- Yamada E., Ishikawa T. The fine structure of the horizontal cells in some vertebrate retinae. Cold Spring Harb Symp Quant Biol. 1965;30:383–392. doi: 10.1101/sqb.1965.030.01.038. [DOI] [PubMed] [Google Scholar]


