Abstract
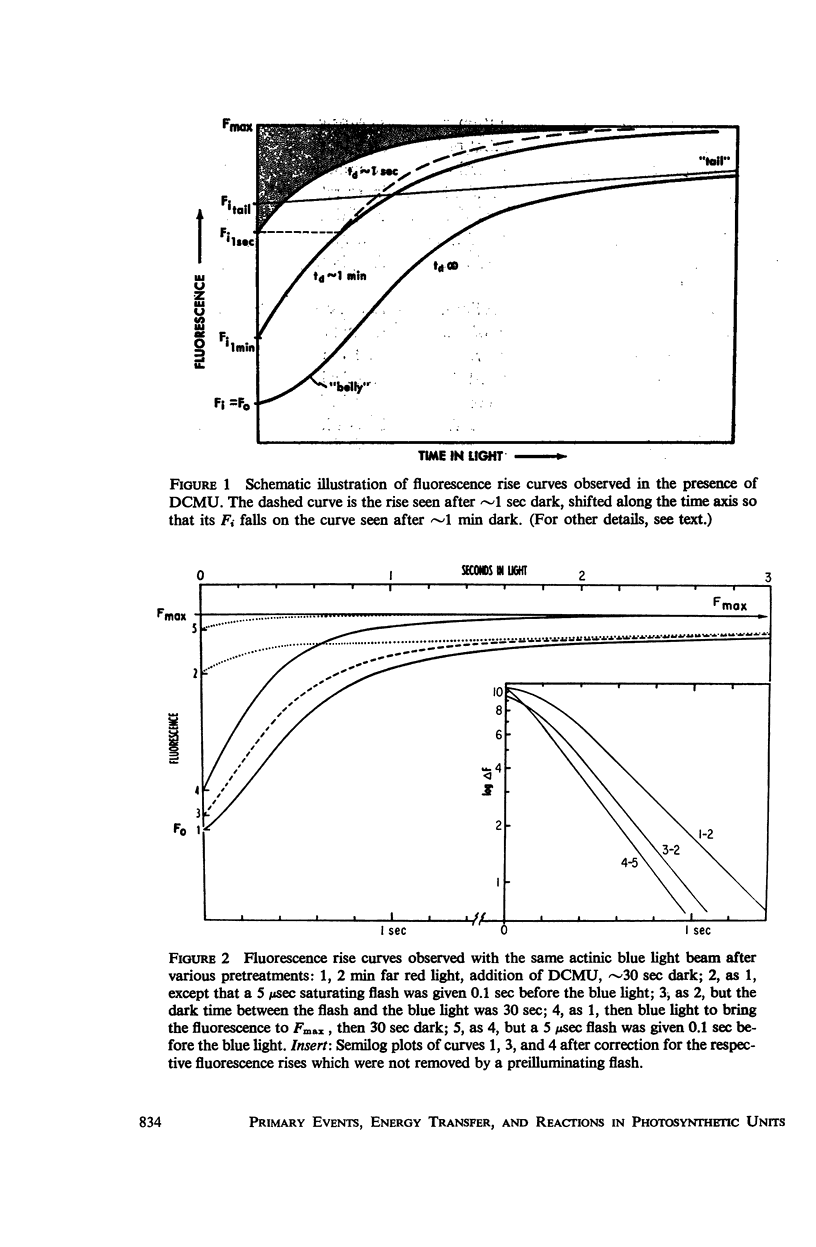
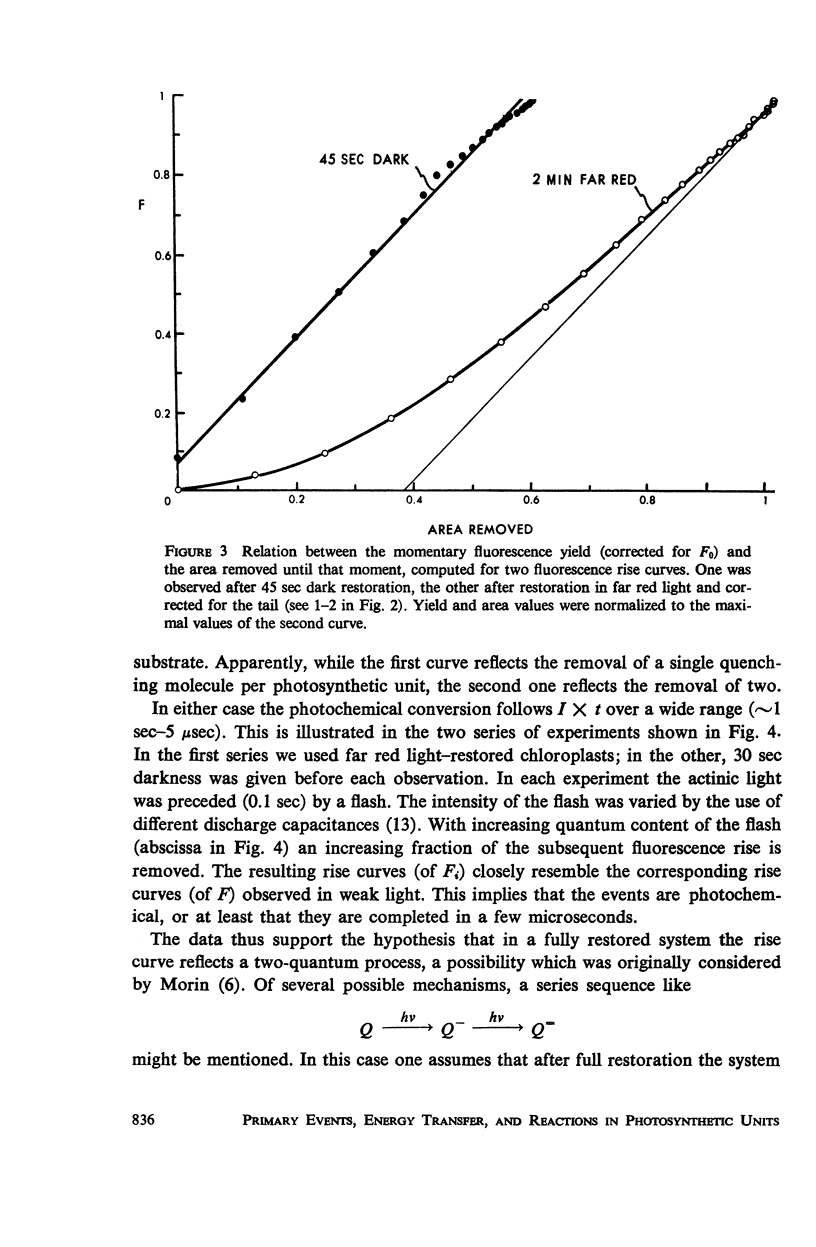
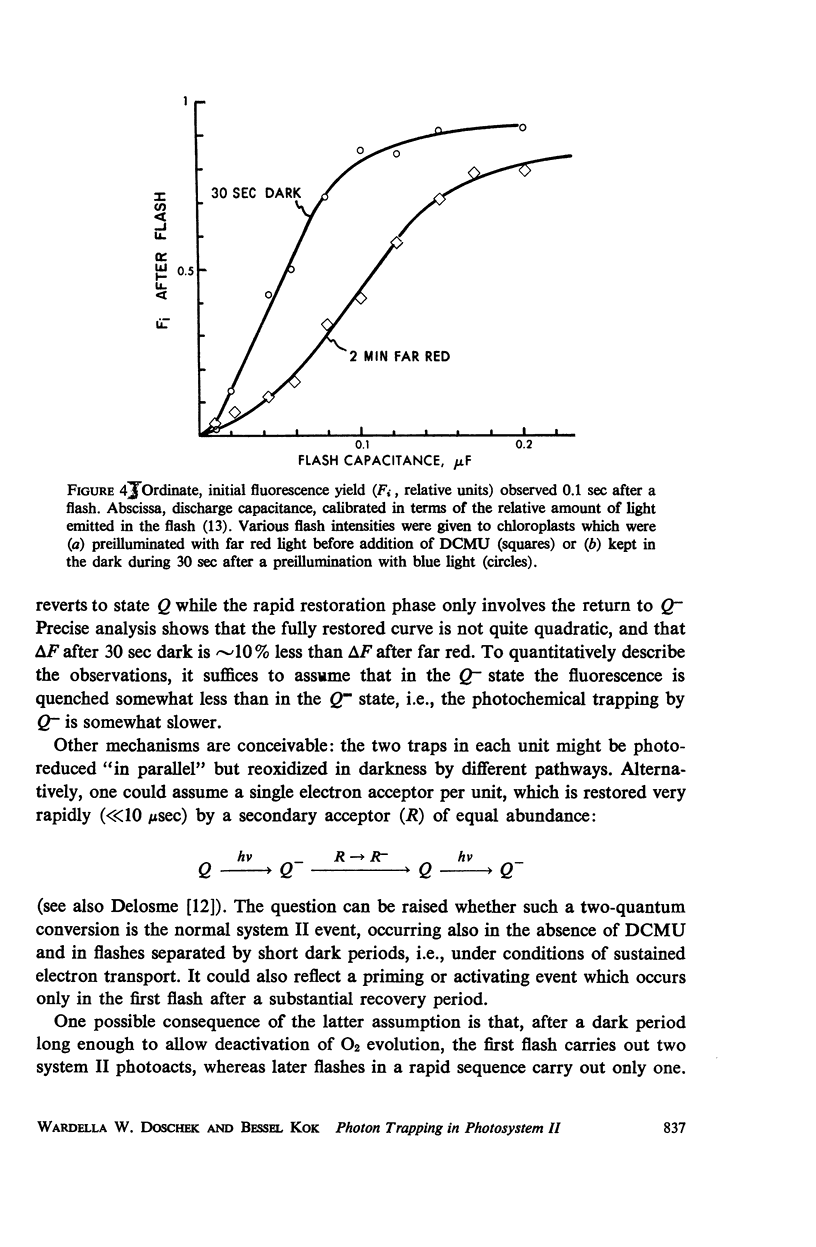
Using isolated chloroplasts in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), an analysis was made of the rise of the fluorescence yield effected by weak light. Depending on the pretreatment, the time-course of the rapid photochemical part of the rise varied between nearly first-order and quadratic kinetics, i.e., reflected either a one-quantum or a two-quantum conversion. We consider the occurrence of two photoreductants per system II unit, which are reoxidized in different dark reactions. The data further showed that the “first-order process” is also inhomogeneous.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennoun P. Réoxydation du quencher de fluorescence "Q" en présence de 3-(3,4-dichlorophényl)-1,1-diméthylurée. Biochim Biophys Acta. 1970 Sep 1;216(2):357–363. doi: 10.1016/0005-2728(70)90227-6. [DOI] [PubMed] [Google Scholar]
- Delosme R. Etude de l'induction de fluorescence des algues vertes et des chloroplastes au début d'une illumination intense. Biochim Biophys Acta. 1967 Jul 5;143(1):108–128. doi: 10.1016/0005-2728(67)90115-6. [DOI] [PubMed] [Google Scholar]
- Forbush B., Kok B. Reaction between primary and secondary electron acceptors of photosystem II of photosynthesis. Biochim Biophys Acta. 1968 Aug 20;162(2):243–253. doi: 10.1016/0005-2728(68)90106-0. [DOI] [PubMed] [Google Scholar]
- Heath R. L. Kinetics studies on the fluorescence quencher in isolated chloroplasts. Biophys J. 1970 Dec;10(12):1173–1188. doi: 10.1016/S0006-3495(70)86363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot P. Cinétiques des réactions liées a l'émission d'oxygène photosynthétique. Biochim Biophys Acta. 1965 May 25;102(1):116–134. [PubMed] [Google Scholar]
- KAUTSKY H., APPEL W., AMANN H. [Chlorophyll fluorescence and carbon assimilation. Part XIII. The fluorescence and the photochemistry of plants]. Biochem Z. 1960;332:277–292. [PubMed] [Google Scholar]
- Kok B., Forbush B., McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem Photobiol. 1970 Jun;11(6):457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Schwartz M. N-tetramethyl-rho-phenylenediamine as a catalyst of photophosphorylation. Biochim Biophys Acta. 1966 Feb 7;112(2):204–212. doi: 10.1016/0926-6585(66)90321-9. [DOI] [PubMed] [Google Scholar]
- Witt H. T., Döring G., Rumberg B., Schmidt-Mende P., Siggel U., Stiehl H. H. Electron transport in photosynthesis. Brookhaven Symp Biol. 1966;19:161–168. [PubMed] [Google Scholar]


