Abstract
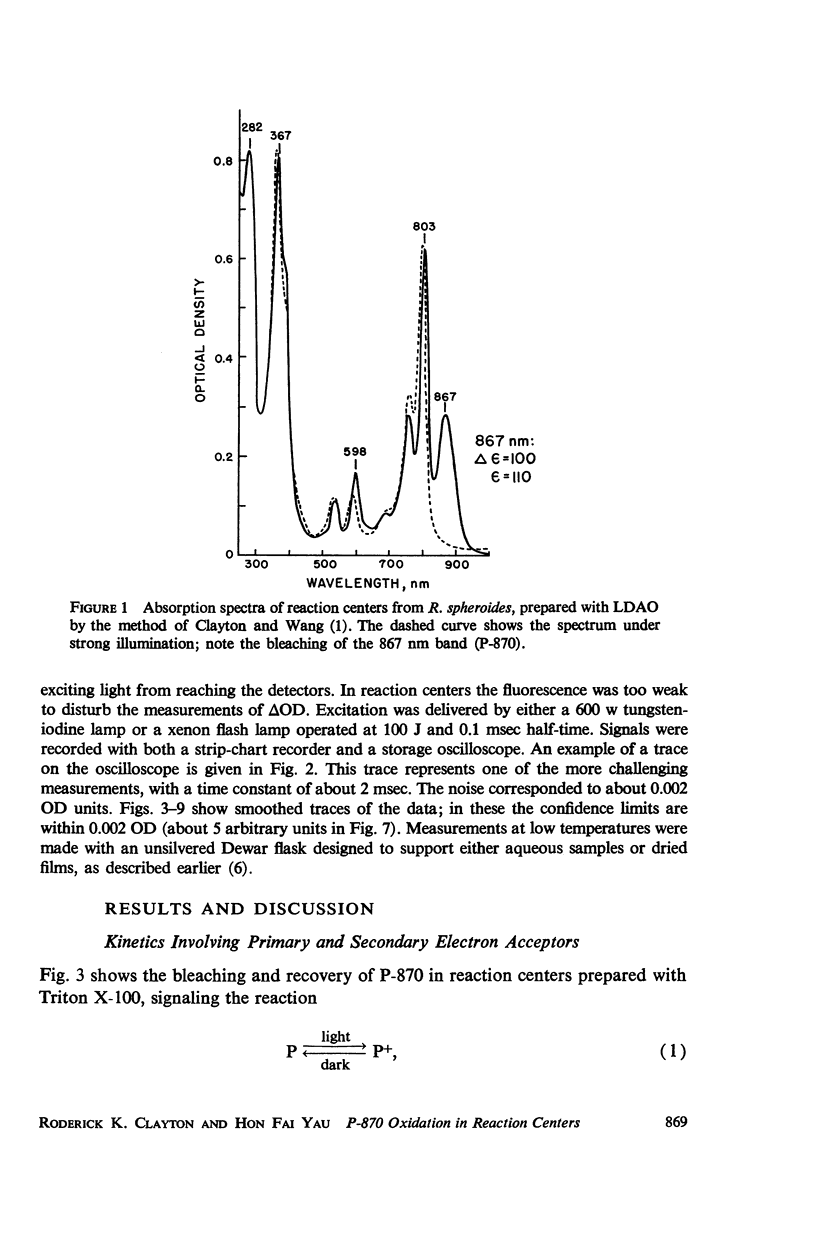
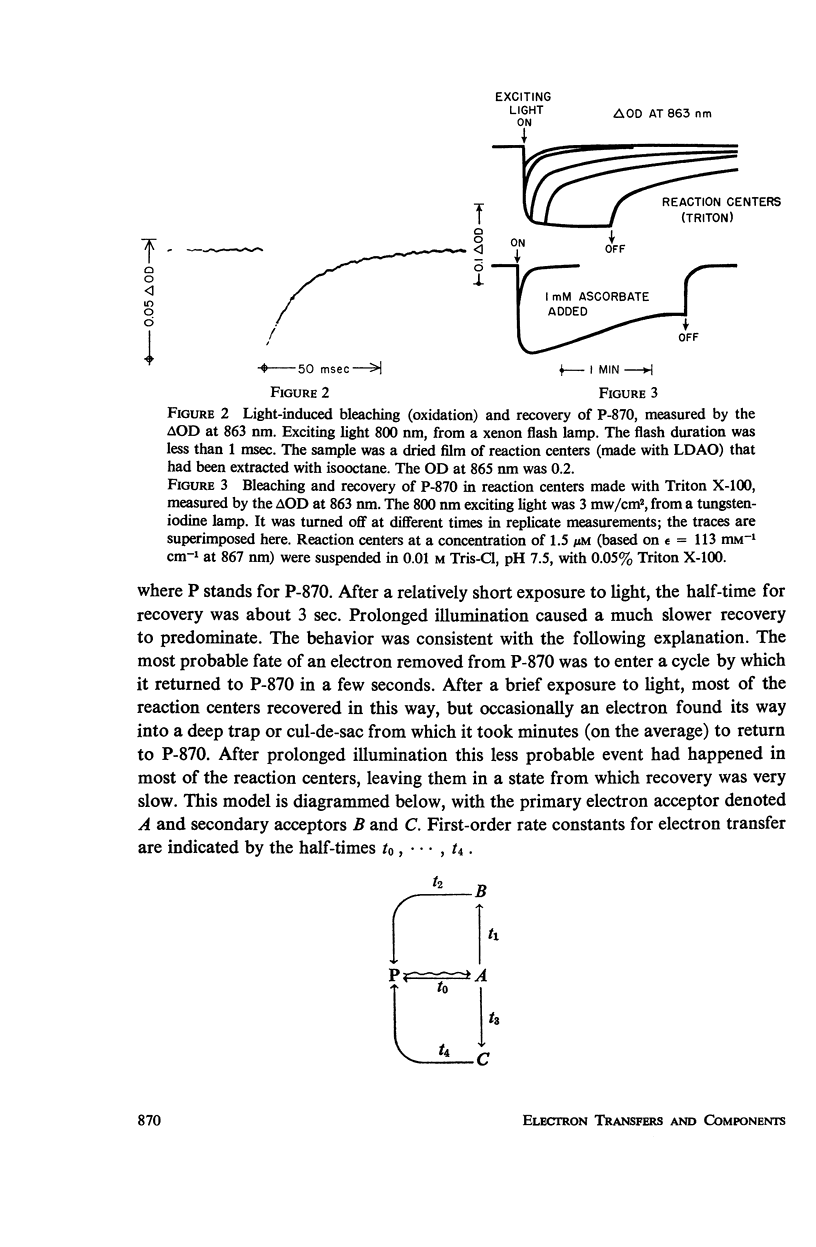
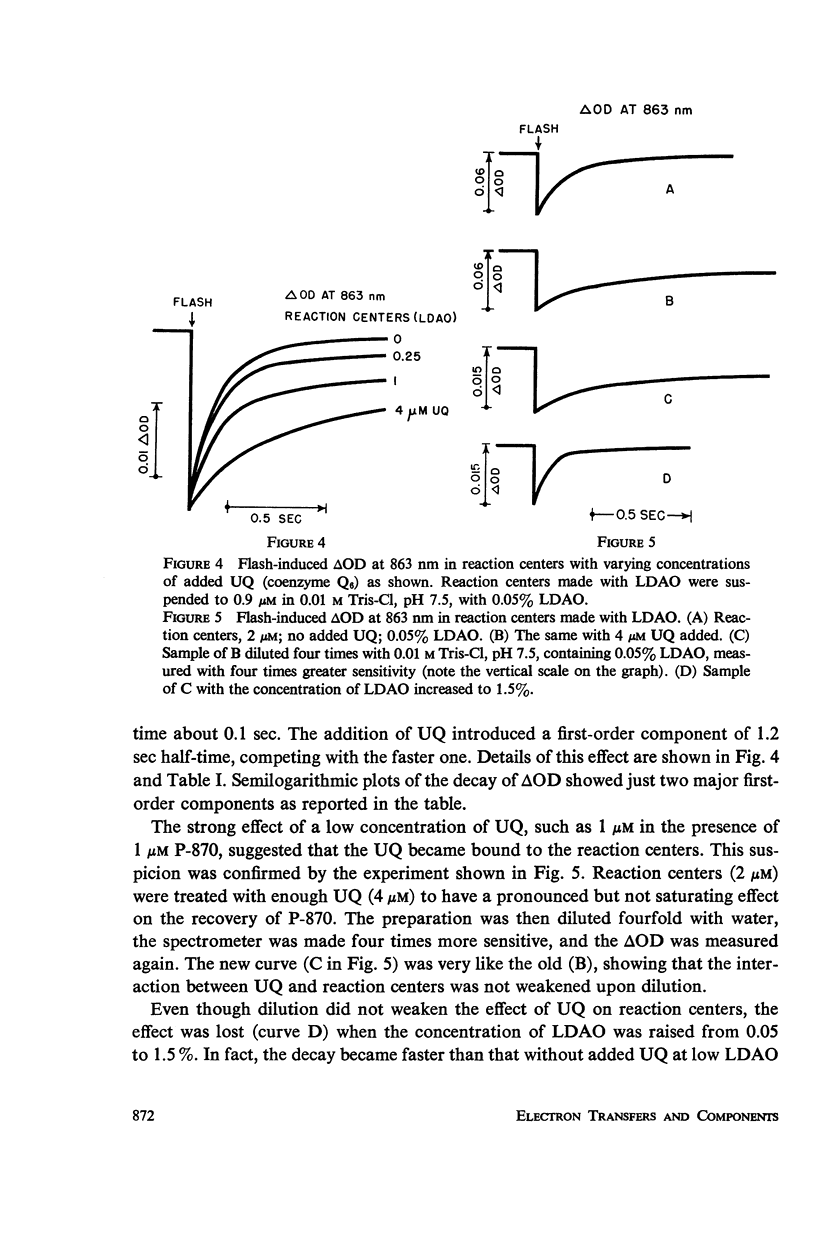
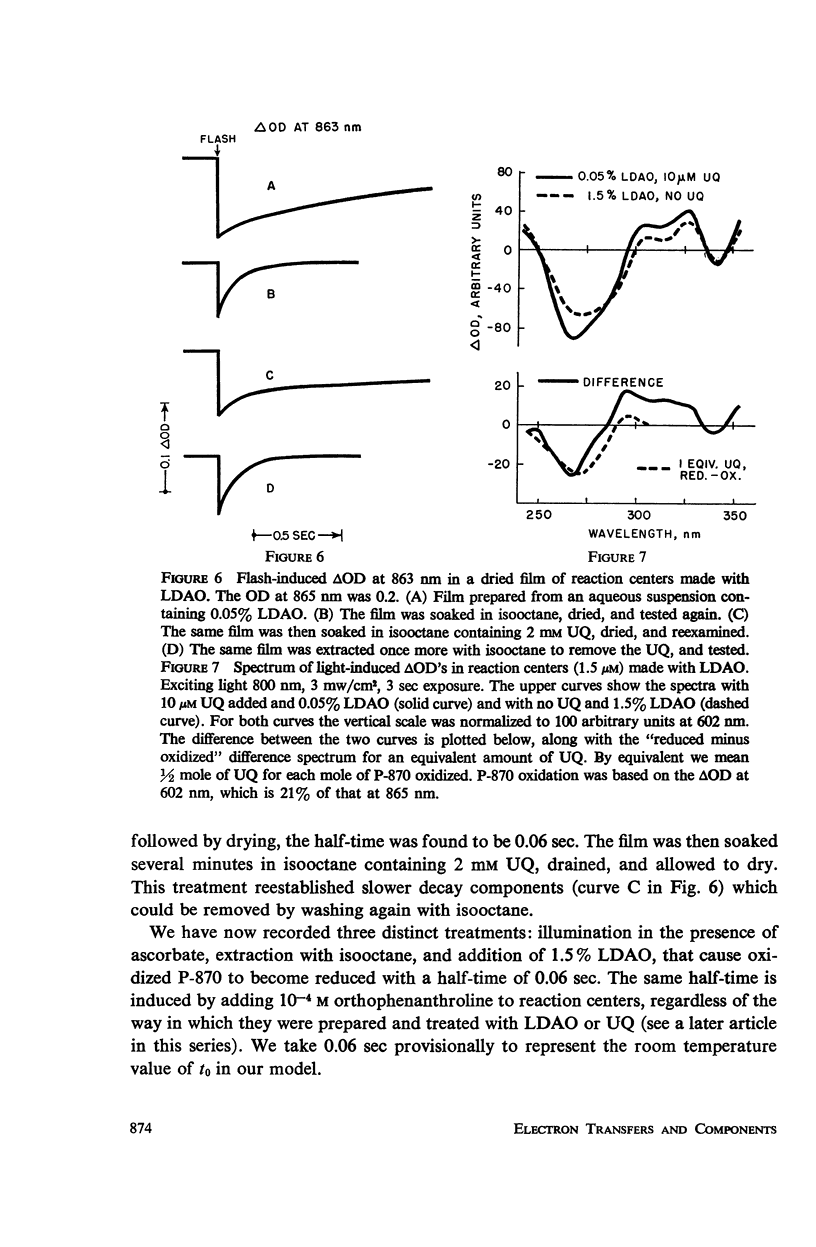
Photosynthetic reaction centers from Rhodopseudomonas spheroides were prepared with the detergent lauryl dimethylamine oxide (LDAO). In contrast to reaction centers made with Triton X-100, these contained no cytochromes and little or no ubiquinone (UQ). The reduction of P-870, after its photochemical oxidation, was studied in these materials with the following results. In reaction centers made with Triton X-100, slow kinetic components (seconds to minutes) could be attributed to secondary electron acceptors or traps. In reaction centers made with LDAO the kinetics were predominantly fast (half-times, 100 msec or less); slower components could be introduced by adding UQ. Added UQ appeared to become bound to reaction centers made with LDAO, but the binding might have meant only that both components were trapped within detergent micelles. Ferricyanide could retard the reduction of oxidized P-870, apparently by capturing electrons from the reducing side of the photochemical system. Under conditions in which the participation of secondary electron acceptors seemed to have been eliminated, the recovery of P-870 was mainly by a first-order process with a half-time of about 60 msec at room temperature and 20-30 msec at about -80°C and below. The transition with decreasing temperature suggested the presence of a mixed population, exhibiting both the 60 and 20 msec components, but variations in the absorption spectra with temperature did not suggest the presence of a mixed population. Absorption difference spectra in the ultraviolet were compatible with the idea that UQ added to reaction centers became reduced in the light.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W., Clayton R. K. THE FIRST STEP IN PHOTOSYNTHESIS: EVIDENCE FOR ITS ELECTRONIC NATURE. Proc Natl Acad Sci U S A. 1960 Jun;46(6):769–776. doi: 10.1073/pnas.46.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton J. R., Clayton R. K., Reed D. W. An identification of the radical giving rise to the light-induced electron spin resonance signal in photosynthetic bacteria. Photochem Photobiol. 1969 Mar;9(3):209–218. doi: 10.1111/j.1751-1097.1969.tb07285.x. [DOI] [PubMed] [Google Scholar]
- Clayton R. K., Straley S. C. An optical absorption change that could be due to reduction of the primary photochemical electron acceptor in photosynthetic reaction centers. Biochem Biophys Res Commun. 1970;39(6):1114–1119. doi: 10.1016/0006-291x(70)90674-1. [DOI] [PubMed] [Google Scholar]
- Feher G. Some chemical and physical properties of a bacterial reaction center particle and its primary photochemical reactants. Photochem Photobiol. 1971 Sep;14(3):373–387. doi: 10.1111/j.1751-1097.1971.tb06180.x. [DOI] [PubMed] [Google Scholar]
- GOEDHEER J. C. Spectral and redox properties of bacteriochlorophyll in its natural state. Biochim Biophys Acta. 1960 Mar 11;38:389–399. doi: 10.1016/0006-3002(60)91274-9. [DOI] [PubMed] [Google Scholar]
- McElroy J. D., Feher G., Mauzerall D. C. On the nature of the free radical formed during the primary process of bacterial photosynthesis. Biochim Biophys Acta. 1969 Jan 14;172(1):180–183. doi: 10.1016/0005-2728(69)90105-4. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Clayton R. K. The reducing potential of the bacterial photosynthetic reaction center. Photochem Photobiol. 1969 Apr;9(4):395–399. doi: 10.1111/j.1751-1097.1969.tb07305.x. [DOI] [PubMed] [Google Scholar]
- Reed D. W., Clayton R. K. Isolation of a reaction center fraction from Rhodopseudomonas spheroides. Biochem Biophys Res Commun. 1968 Mar 12;30(5):471–475. doi: 10.1016/0006-291x(68)90075-2. [DOI] [PubMed] [Google Scholar]


