Abstract
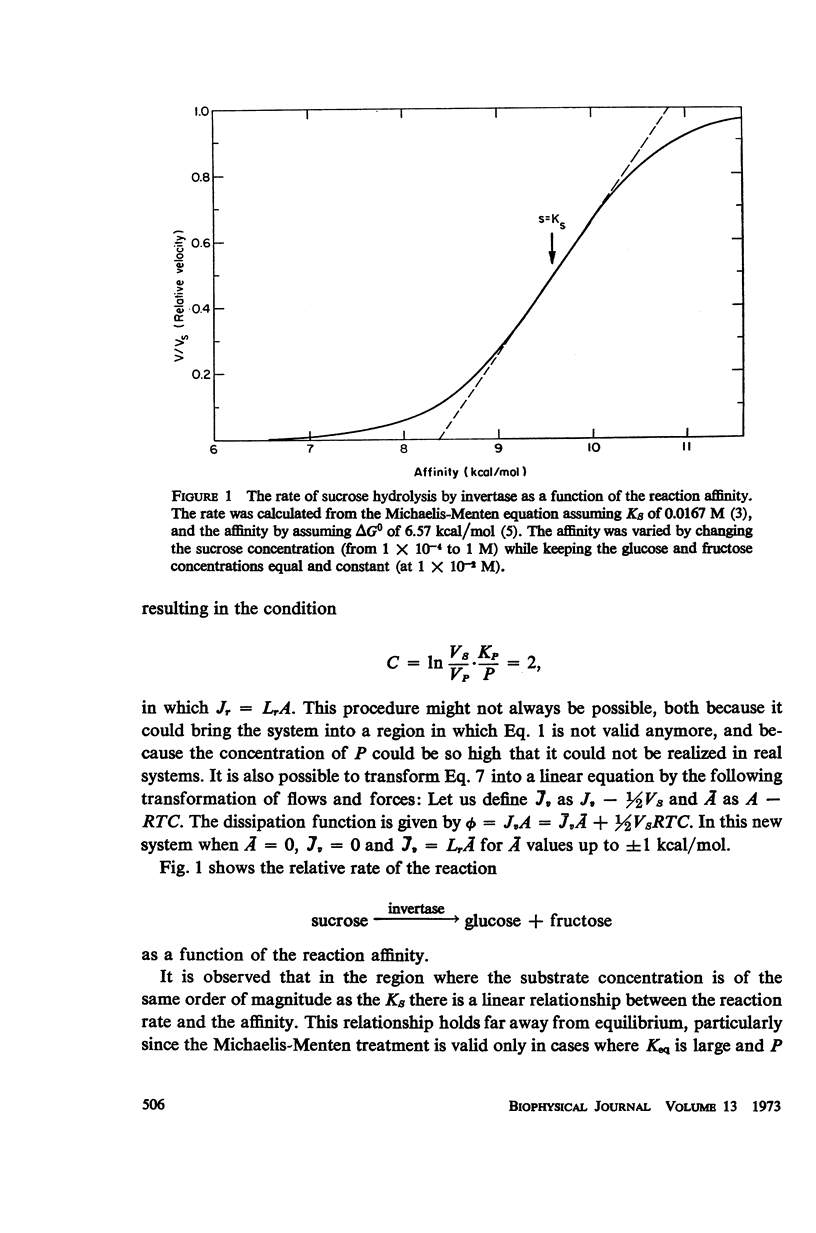
The rate of enzyme-catalyzed reactions is a proportional function of the reaction affinity over a range of more than 2 kcal/mol, (i.e., 25-fold substrate concentration change). For kinetically irreversible reactions, proportionality is obeyed when the substrate concentration is of the same order of magnitude as the Km of the reaction. Linearity can be obtained by proper choice of product concentration or alternatively by a linear transformation which allows the description of the system by slightly different parameters. For kinetically reversible reactions, the linear range could be obtained and extended to both sides of equilibrium provided the concentration of the substrate is fixed at a proper value and the affinity is varied by the product concentration. In oxidative phosphorylation, a coupled system of enzymatic reactions, proportional regions are found for both oxidation and phosphorylation. These findings justify the use of linear phenomenological equations in bioenergetics.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal R., Caplan S. R., Kedem O. The coupling of an enzymatic reaction to transmembrane flow of electric current in a synthetic "active transport" system. Biophys J. 2008 Dec 31;7(6):735–757. doi: 10.1016/S0006-3495(67)86620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson J. E., Noltmann E. A. The effect of pH and temperature on the kinetic parameters of phosphoglucose isomerase. Participation of histidine and lysine in a proposed dual function mechanism. J Biol Chem. 1968 Apr 10;243(7):1401–1414. [PubMed] [Google Scholar]


