Abstract
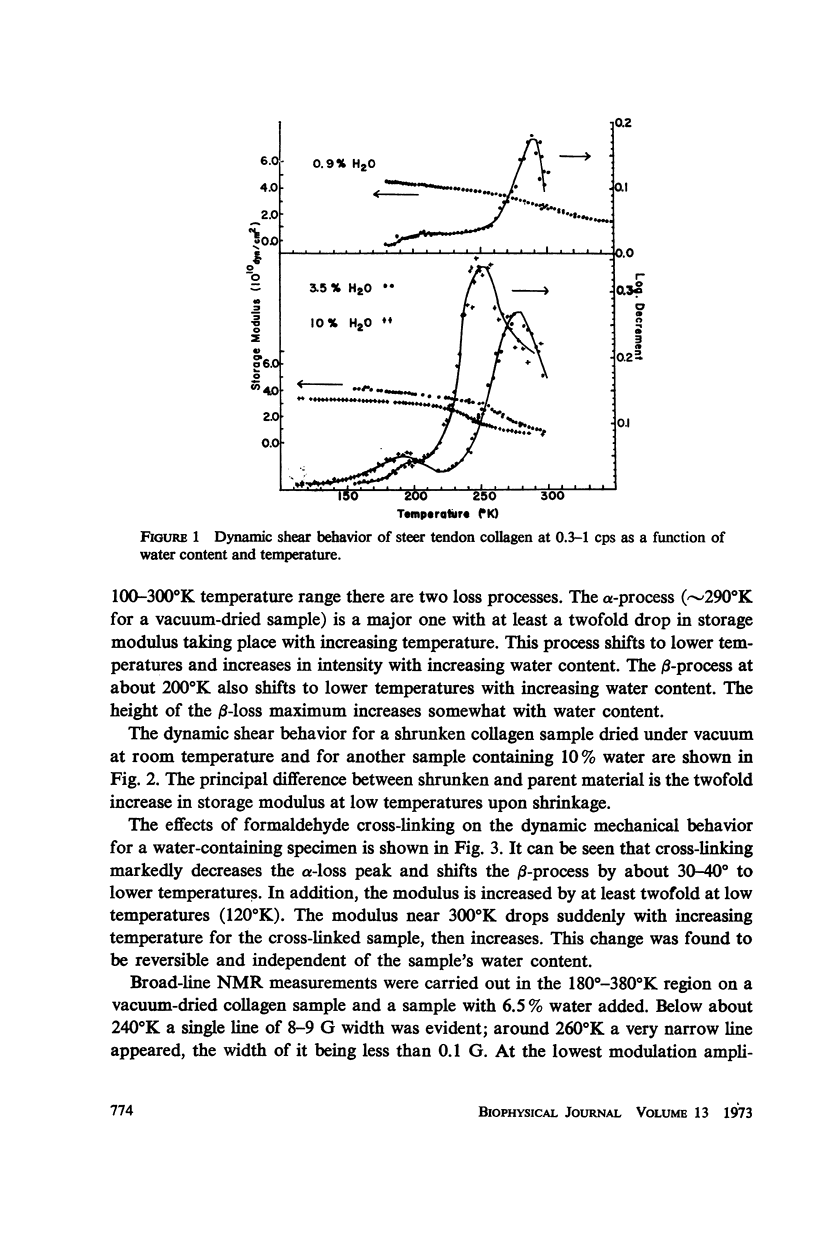
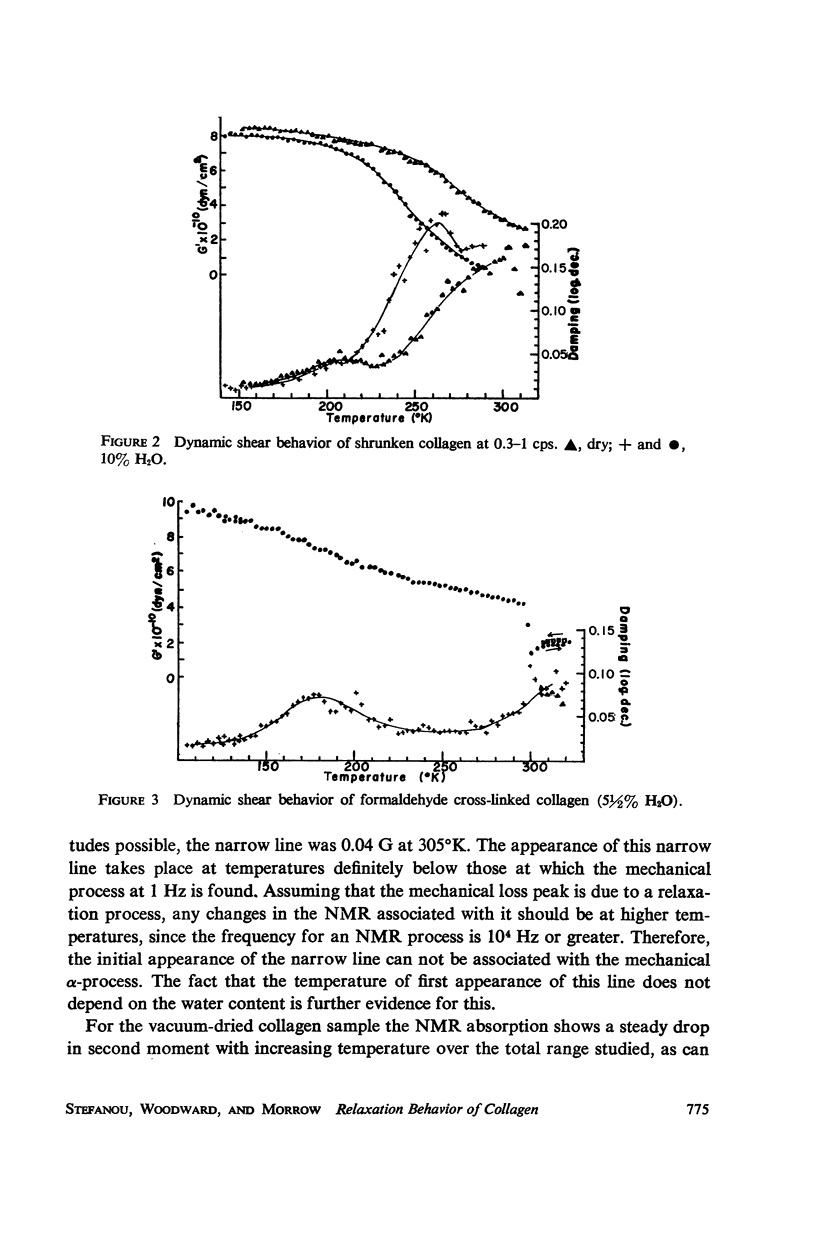
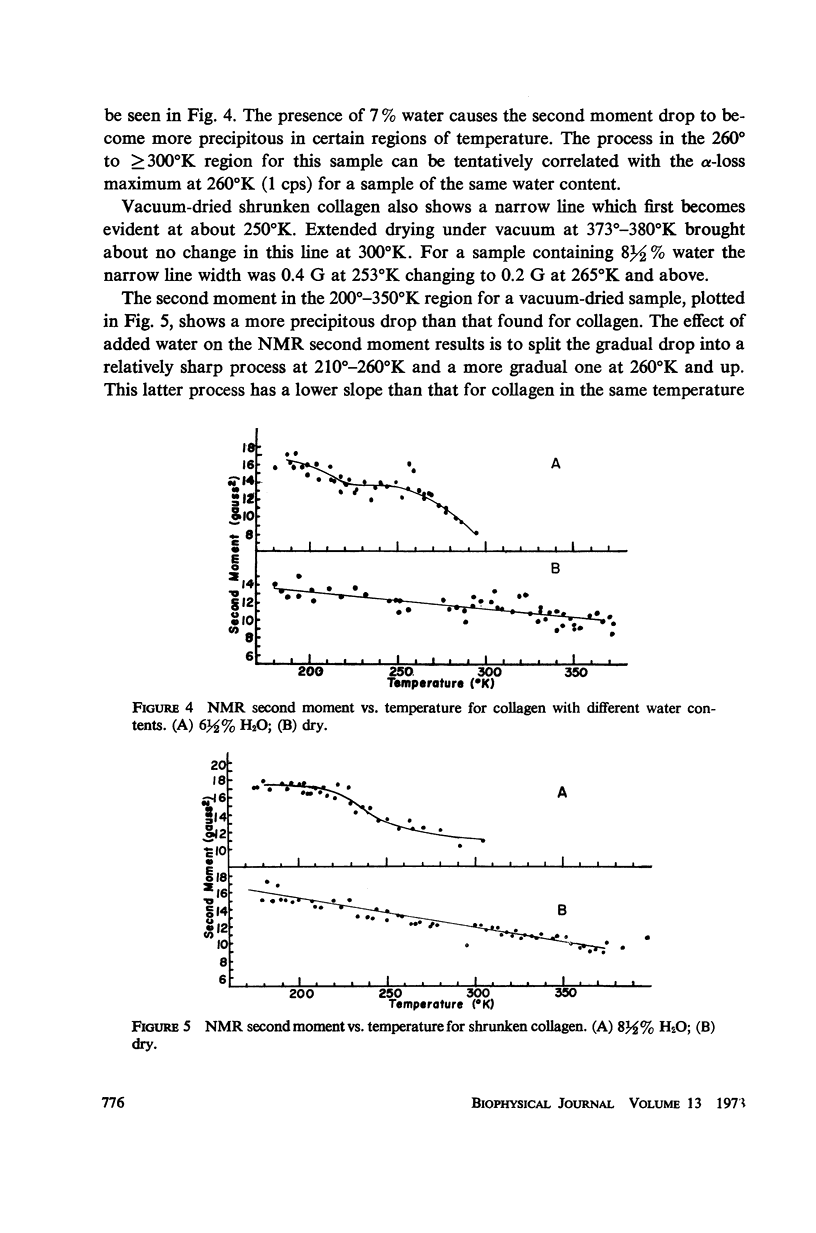
The dynamic mechanical properties of purified collagen from bovine tendon were studied using a torsion pendulum in the temperature range of 120°-360°K at 0.3-1 cps. In the temperature range studied, two loss peaks were observed: a β-peak at about 200°K, and an α-peak approximately five times larger at about 280°K. The temperature of the α-transition is shown to be dependent on water content, decreasing with increasing amount of water and shifting to lower temperatures. Broad-line proton magnetic resonance results were also obtained on similar samples. A narrow nuclear magnetic resonance (NMR) line appears at about 250°C. The effects of shrinkage to form gelatin and of cross-linking on the relaxation behavior of collagen were also studied. The motions taking place in collagen over the 120°-360°K range are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chien J. C., Chang E. P. Dynamic mechanical and rheo-optical studies of collagen and gelatin. Biopolymers. 1972;11(10):2015–2031. doi: 10.1002/bip.1972.360111003. [DOI] [PubMed] [Google Scholar]
- De Santis P., Giglio E., Liquori A. M., Ripamonti A. Van der Waals interaction and the stability of helical polypeptide chains. Nature. 1965 May 1;206(983):456–458. doi: 10.1038/206456a0. [DOI] [PubMed] [Google Scholar]
- Dehl R. E., Hoeve C. A. Broad-line NMR study of H2O and D2O in collagen fibers. J Chem Phys. 1969 Apr 15;50(8):3245–3251. doi: 10.1063/1.1671547. [DOI] [PubMed] [Google Scholar]
- Lim J. J., Shamos M. H. An investigation of the bound water in tendon by dielectric measurement. Biophys J. 1971 Aug;11(8):648–663. doi: 10.1016/S0006-3495(71)86244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


