Abstract
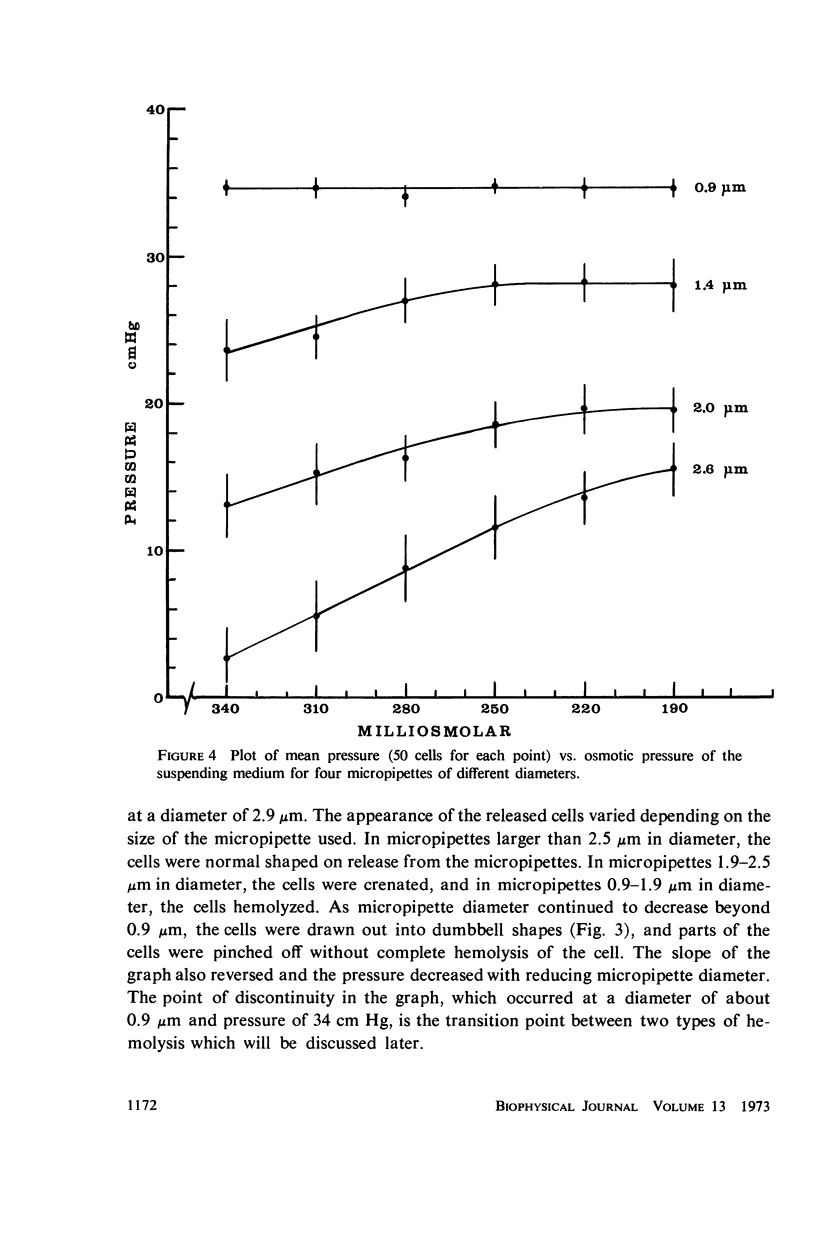
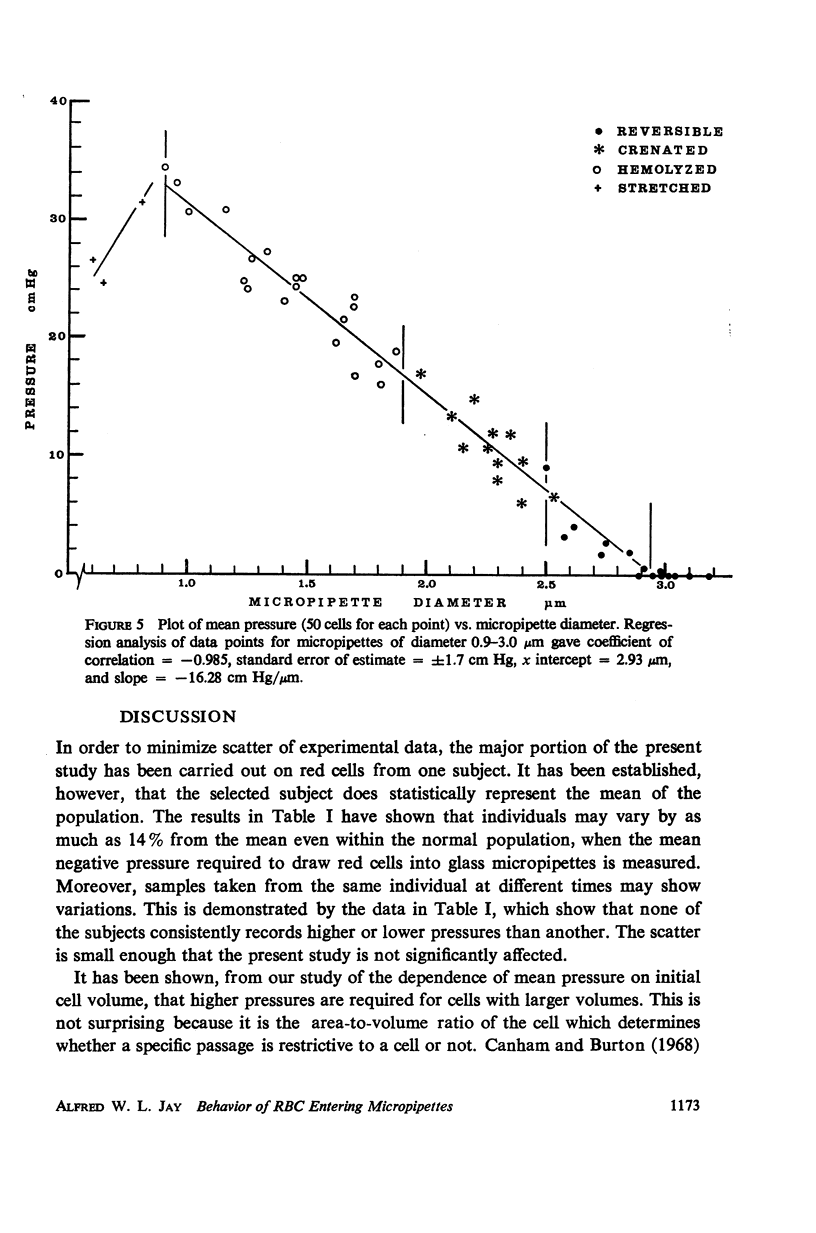
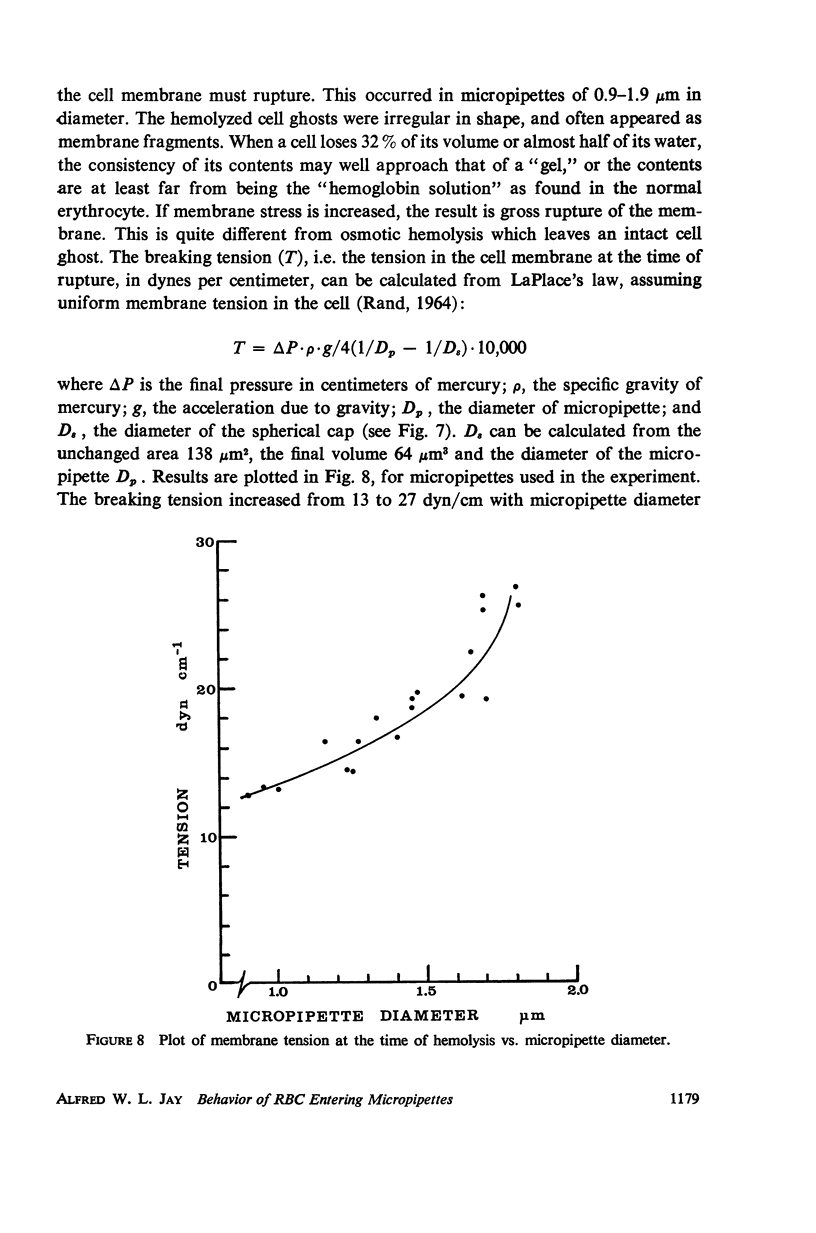
Single human red blood cells suspended in buffered Ringer's solution were rapidly drawn, at recorded pressures, into glass micropipettes of diameter 0.6-3.2 μm. Cells could enter micropipettes of diameter ≥ 2.9 μm with minimal pressure. In micropipettes of 0.9-2.9 μm, the pressure required increased linearly with decreasing diameter. For diameters 2.5-2.9 μm, pressures ranged up to 7 cm Hg, and the cells returned to normal biconcave shape on release. For diameters 1.9-2.5 μm, the required pressures ranged from 7 to 17 cm Hg. The released cells were crenated. In micropipettes 0.9-1.9 μm, the pressures required ranged from 17 to 34 cm Hg. The cells hemolyzed on entry. As diameter decreased from 0.9 to 0.6 μm, cells were drawn into dumbbell shapes and parts of the cells were pinched off without complete hemolysis of the cell. Using an accepted value of 138 μm2 for the mean cell area, the mean volume of the human red cell was calculated to be 94 μm3. Under mechanical stress, about 12% of this volume is rapidly exchangeable with the external medium. The cell volume may further decrease by 20% which is not reversible.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J. S., Jay A. W., Saari J. T. Effects of cytochalasin B on osmotic fragility and deformability of human erythrocytes. Can J Physiol Pharmacol. 1972 Jul;50(7):684–688. doi: 10.1139/y72-099. [DOI] [PubMed] [Google Scholar]
- Canham P. B., Burton A. C. Distribution of size and shape in populations of normal human red cells. Circ Res. 1968 Mar;22(3):405–422. doi: 10.1161/01.res.22.3.405. [DOI] [PubMed] [Google Scholar]
- Chien S., Luse S. A., Bryant C. A. Hemolysis during filtration through micropores: a scanning electron microscopic and hemorheologic correlation. Microvasc Res. 1971 Apr;3(2):183–203. doi: 10.1016/0026-2862(71)90022-7. [DOI] [PubMed] [Google Scholar]
- Jay A. W., Rowlands S., Skibo L. The resistance to blood flow in the capillaries. Can J Physiol Pharmacol. 1972 Oct;50(10):1007–1013. doi: 10.1139/y72-145. [DOI] [PubMed] [Google Scholar]
- LEFEVRE P. G. THE OSMOTICALLY FUNCTIONAL WATER CONTENT OF THE HUMAN ERYTHROCYTE. J Gen Physiol. 1964 Jan;47:585–603. doi: 10.1085/jgp.47.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGANELLI C. V., SOLOMON A. K. The rate of exchange of tritiated water across the human red cell membrane. J Gen Physiol. 1957 Nov 20;41(2):259–277. doi: 10.1085/jgp.41.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND R. P., BURTON A. C. Area and volume changes in hemolysis of single erythrocytes. J Cell Comp Physiol. 1963 Jun;61:245–253. doi: 10.1002/jcp.1030610306. [DOI] [PubMed] [Google Scholar]
- RAND R. P., BURTON A. C. MECHANICAL PROPERTIES OF THE RED CELL MEMBRANE. I. MEMBRANE STIFFNESS AND INTRACELLULAR PRESSURE. Biophys J. 1964 Mar;4:115–135. doi: 10.1016/s0006-3495(64)86773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND R. P. MECHANICAL PROPERTIES OF THE RED CELL MEMBRANE. II. VISCOELASTIC BREAKDOWN OF THE MEMBRANE. Biophys J. 1964 Jul;4:303–316. doi: 10.1016/s0006-3495(64)86784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogausch H. Decreased erythrocyte deformability in cholestatic jaundice. Acta Haematol. 1971;46(4):211–220. doi: 10.1159/000208577. [DOI] [PubMed] [Google Scholar]
- SAVITZ D., SIDEL V. W., SOLOMON A. K. OSMOTIC PROPERTIES OF HUMAN RED CELLS. J Gen Physiol. 1964 Sep;48:79–94. doi: 10.1085/jgp.48.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIDEL V. W., SOLOMON A. K. Entrance of water into human red cells under an osmotic pressure gradient. J Gen Physiol. 1957 Nov 20;41(2):243–257. doi: 10.1085/jgp.41.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRS J. A. THE FACILITATED UPTAKE OF NITRIC OXIDE BY HAEMOGLOBIN IN ERYTHROCYTES. Biochim Biophys Acta. 1964 Jul 15;90:108–116. doi: 10.1016/0304-4165(64)90123-0. [DOI] [PubMed] [Google Scholar]
- Seeman P., Kwant W. O., Sauks T., Argent W. Membrane expansion of intact erythrocytes by anesthetics. Biochim Biophys Acta. 1969;183(3):490–498. doi: 10.1016/0005-2736(69)90163-1. [DOI] [PubMed] [Google Scholar]
- Seeman P. Transient holes in the erythrocyte membrane during hypotonic hemolysis and stable holes in the membrane after lysis by saponin and lysolecithin. J Cell Biol. 1967 Jan;32(1):55–70. doi: 10.1083/jcb.32.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESTERMAN M. P., PIERCE L. E., JENSEN W. N. ERYTHROCYTE LIPIDS: A COMPARISON OF NORMAL YOUNG AND NORMAL OLD POPULATIONS. J Lab Clin Med. 1963 Sep;62:394–400. [PubMed] [Google Scholar]
- Weed R. I., LaCelle P. L., Merrill E. W. Metabolic dependence of red cell deformability. J Clin Invest. 1969 May;48(5):795–809. doi: 10.1172/JCI106038. [DOI] [PMC free article] [PubMed] [Google Scholar]




