Abstract
Chronic engagement of the B cell receptor by soluble autoantigen leads to reduced B cell survival. Using the Ig and hen egg lysozyme double transgenic mouse model, we demonstrate that the survival of soluble autoantigen-engaged B cells is further reduced in mice lacking CD4 T cells or deficient in CD40. Mixed bone marrow chimera experiments reveal that, under homeostatic conditions, the CD40L–CD40 pathway can augment autoreactive B cell survival in a non-cell-autonomous manner. Naive CD4 T cells are shown to constitutively express CD40L mRNA and protein, although cell surface CD40L abundance is low because of engagement with CD40 on other cells. These observations indicate that the CD40L–CD40 pathway can augment the survival of autoantigen-engaged B cells in the absence of T cell activation. We propose that constitutive CD40L expression by naive CD4 T cells influences the composition of the B cell repertoire and may also affect the homeostasis of other cell types such as regulatory T cells in lymphoid organs.
Keywords: homeostasis
Autoreactive B cells that arise in the bone marrow are kept in check by a series of self-tolerance mechanisms. Cells with B cell receptors (BCRs) that bind multivalent autoantigens in the bone marrow undergo receptor editing or deletion. B cells that are specific for autoantigens of lower valency are able to enter the periphery, but chronic exposure to autoantigen induces IgM down-modulation and reduced coupling of the BCR to downstream pathways, a state known as B cell anergy (1). A study in humans has shown that many autoreactive B cells reach the periphery but fail to enter the long-lived mature B cell pool (2).
In the Ig/hen egg lysozyme (HEL) double transgenic (Tg) mouse model, the HEL autoantigen-binding B cells have a short (≈1 week) half-life compared with the 4-week half-life of Ig Tg B cells in the absence of HEL (3, 4). The basis for this difference in lifespan is not fully defined, but anergic B cells were shown to have elevated expression of the proapoptotic molecule BIM and were more dependent on the prosurvival factor BAFF (5, 6). A further reduction in autoantigen-binding B cell survival was observed when the Ig/HEL transgenes were crossed onto a recombinase-activating gene 1 (RAG1)-deficient background (7). RAG1-deficient mice were not found to have reduced BAFF production (5), leaving the basis for this effect unclear.
Here we establish that naive CD4 T cells augment HEL autoantigen-binding B cell survival. Examination of CD40 as a candidate receptor downstream of CD4 T cells revealed that autoantigen-binding B cells have reduced survival in the absence of CD40 expression by hematopoietic cells. Mixed bone marrow chimera analysis revealed that the CD40L–CD40 pathway can rescue autoantigen-binding B cells through a non-cell-autonomous mechanism. Moreover, we show that CD40L is constitutively expressed by naive CD4 T cells and that surface levels are down-modulated by interactions with CD40-expressing cells. These findings indicate that, in addition to the well established role of the CD40L–CD40 pathway in immune activation, the pathway functions during immune homeostasis to influence the properties of autoantigen-engaged B cells and, most likely, other cell types.
Results
T Cells Augment Autoantigen-Binding B Cell Survival.
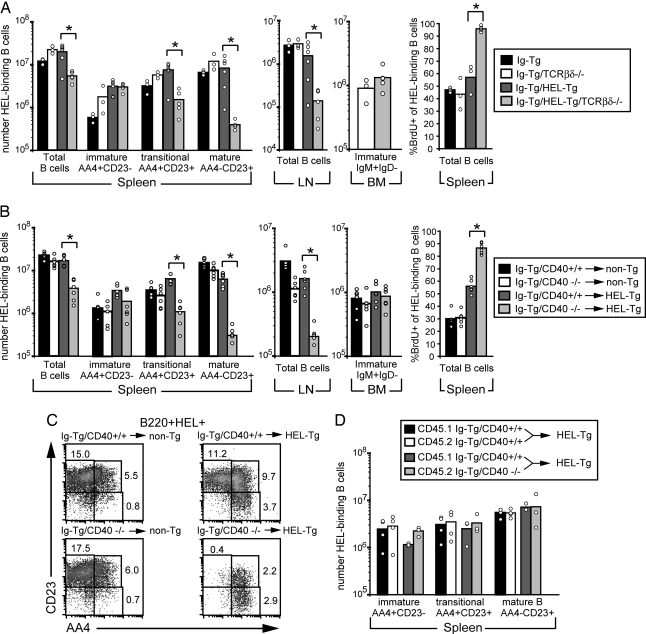
To determine whether the reduced survival of HEL autoantigen-binding B cells developing on a RAG1−/− background was due to the absence of T cells, the Ig/HEL transgenes were crossed to a T cell antigen receptor (TCR)-βδ-deficient background. On a WT background, the numbers of B cells in the spleens of Ig and Ig/HEL Tg mice were similar, and they were reduced by ≈2-fold in lymph nodes of Ig/HEL double Tg mice, in agreement with previous results (3, 4). However, in mice lacking T cells, autoreactive B cell numbers were decreased ≈5-fold in the spleen and 10-fold in lymph nodes (Fig. 1A). The remaining B cells were predominantly immature, based on CD23 and AA4 staining (8). Moreover, BrdU incorporation studies (Fig. 1A Right) showed that the majority (≈90%) of Ig/HEL Tg B cells in T cell-deficient mice became BrdU-labeled in 5 days, indicating that the cells had a reduced lifespan.
Fig. 1.
Reduced survival of HEL autoantigen-binding B cells in T cell-deficient mice and in mice lacking CD40. (A) Adult mice of the indicated types were given water containing BrdU for 5 days and analyzed. Spleen and lymph node (LN) cells were stained for CD19, HEL, AA4, and CD23 to distinguish immature (AA4+CD23−), transitional (T2- and T3-inclusive and AA4+CD23+), and mature (AA4−CD23+) HEL-binding B cells (8). Bone marrow (BM) cells were stained for B220, IgM, and IgD, and immature B cells were identified as B220+IgM+IgD−. Spleen cells were also stained for B220, HEL binding, and BrdU. Data show three to six mice per group from two separate experiments. (B) Bone marrow from CD40+/+ Ig Tg or CD40−/− Ig Tg mice was used to reconstitute lethally irradiated non-Tg and HEL Tg recipient mice. After 6–8 weeks, mice were given water containing BrdU for 5 days and then analyzed as in A. Data show six mice per group from one set of bone marrow chimeras and are representative of two experiments. (C) Examples of AA4 and CD23 profiles of splenic B cells of mice in B. Cells were stained for B220, HEL binding, CD23, and AA4 and pregated on size, B220, and HEL binding (B220+HEL+). (D) Bone marrow from CD45.1-positive CD40+/+ Ig Tg mice and CD45.2-positive CD40+/+ Ig Tg or CD45.2-positive CD40−/− Ig Tg mice was mixed at a 1:1 ratio and used to reconstitute lethally irradiated HEL Tg recipient mice. After 6–8 weeks, spleen cells were stained for CD45.1, CD19, AA4, CD23, and HEL-binding. Data show three to four mice per group from one set of bone marrow chimeras and are representative of two experiments. ∗, P < 0.002 by Student’s t test.
CD40 in Hematopoietic Cells Is Required for Autoreactive B Cell Survival.
A candidate T cell-derived B cell survival factor was CD40L. To investigate whether autoreactive Ig/HEL Tg B cell survival was influenced by CD40 signaling, we bred Ig Tg mice with CD40-deficient mice and used bone marrow from the resulting mice to reconstitute lethally irradiated non-Tg or HEL Tg recipient mice. Autoreactive B cell numbers in Ig/HEL Tg bone marrow chimeras were decreased ≈5-fold in the spleen and 10-fold in lymph nodes in the absence of CD40 on hematopoietic cells (Fig. 1B), decreases similar to those seen in T cell-deficient mice (Fig. 1A). Again, the remaining autoantigen-binding B cells were predominantly immature, based on CD23 and AA4 staining (Fig. 1C). In the absence of HEL autoantigen, Ig Tg B cells in the CD40−/− and WT bone marrow chimeras showed similarly low levels of BrdU incorporation after 1 week of labeling (Fig. 1B). In contrast, the majority (≈90%) of B cells in the CD40−/− Ig/HEL Tg chimeras became BrdU-labeled, compared with 50% of B cells in the WT Ig/HEL Tg chimeras (Fig. 1B), indicating that in mice lacking CD40 on hematopoietic cells the B cells were turning over more rapidly. To determine whether the CD40 requirement was B cell-intrinsic, HEL Tg or control mice were reconstituted with equal mixtures of CD40−/− and WT Ig Tg bone marrow. When using this approach, CD40-deficient and WT HEL autoantigen-binding B cells developed side by side in the same animals. In contrast to the above findings, the number of CD40-deficient autoantigen-engaged B cells at each stage of splenic B cell maturation was not reduced compared with the numbers of WT autoantigen-engaged B cells in control chimeras (Fig. 1D). Taken together, these observations indicate that CD40 is required within hematopoietic cells but that it can function extrinsically from autoantigen-binding B cells to promote their survival.
Requirement for CD40L.
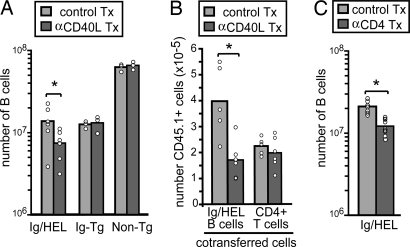
To test whether the CD40 requirement for autoantigen-binding B cell survival reflected a requirement for CD40L, Ig/HEL Tg mice were treated with a CD40L-blocking antibody (clone MR1) (Fig. 2A). Splenic Ig/HEL Tg B cells were transferred into the mice at the time of treatment to allow a cohort of cells to be tracked in the absence of further contribution by bone marrow-derived cells (Fig. 2B). In Ig/HEL Tg mice treated with CD40L-blocking antibody for 1 week, there was a 2-fold reduction in autoreactive B cells numbers. A similar 2-fold decrease was seen in the number of transferred autoreactive B cells in the CD40L-blocked mice, although transferred T cell numbers remained unaffected. Non-Tg and Ig Tg mice lacking HEL autoantigen were also treated with CD40L-blocking antibody, but there was no effect on B cell numbers (Fig. 2A). These findings indicate that autoantigen-binding B cell survival was augmented by CD40L engagement of CD40. To determine whether CD4 T cells provided the CD40L signal, we compared the effects of CD40L blocking with those of CD4 T cell depletion. Similar to the effect of CD40L blocking, CD4 T cell depletion by day 9 of treatment with the GK1.5 anti-CD4 antibody led to a 2-fold decrease in autoreactive B cell numbers in the spleen (Fig. 2C).
Fig. 2.
CD40L blocking and depletion of CD4 T cells lead to similar reductions in autoantigen-binding B cell numbers. (A) Non-Tg, Ig Tg, and Ig/HEL Tg mice were treated with 250 μg of anti-mouse CD40L (clone MR1) or control IgG on days 0, 2, and 5. After 7 days, HEL binding (in Ig Tg and Ig/HEL Tg mice) or total (in non-Tg mice) B cells in spleen and lymph nodes were enumerated. Ig/HEL Tg data show six mice from two experiments; non-Tg and Ig Tg data show three mice from one experiment. (B) CD45.1-positive Ig/HEL Tg cells were transferred into CD45.2-positive Ig/HEL Tg recipients 1 h after treatment of recipient mice with anti-CD40L or control antibody. Mice were treated and analyzed as in A by using anti-CD45.1 to identify the transferred cells. Data show five to six mice from two experiments. (C) Depletion of CD4+ T cells leads to decreased survival of autoreactive B cells. Ig/HEL Tg mice were treated with 1 mg of anti-mouse CD4 (clone GK1.5) (d0, d4) to deplete CD4 T cells. After 9 days, HEL-binding B cells were enumerated as in A and B. Data show nine mice from three separate experiments. Tx, antibody-treated. ∗, P < 0.05 by Student’s t test.
Constitutive CD40L Expression by Naive T Cells and Modulation by CD40-Expressing Cells.
CD40L is typically considered to be a T cell activation molecule. To determine whether CD40L was expressed in the absence of T cell activation, we examined transcript abundance in whole spleen and in purified T cells. Real-time PCR analysis of whole spleen from unimmunized WT mice revealed notable CD40L expression, and this signal was absent in TCR-β−/−δ−/− animals (Fig. 3A). Analysis of purified cells revealed that CD40L mRNA expression was highly enriched in total CD4 T cells compared with whole spleen and CD62LhiCD44loCD25− naive CD4 T cells showed the highest expression (Fig. 3B). CD8 T cells and CD25+ CD4 T cells also expressed CD40L, although at lower abundance than in naive CD4 T cells.
Fig. 3.
CD40L is constitutively expressed by naive CD4 T cells. (A) CD40L transcript abundance in spleen tissue harvested from WT (n = 5), CD40−/− (n = 5), or TCR-β−/−δ−/− (n = 3) mice. CD40L and hypoxanthine phosphoribosyltransferase (HPRT) transcripts were quantitated by real-time PCR. (B) CD40L transcript abundance in T cell subsets sorted from WT splenocytes by using the following criteria: total CD4+, total CD8+, naive CD4+ (CD4+CD25−CD62LhiCD44lo), naive CD8+ (CD8+ CD62Lhi), and CD4+CD25+. Data show results from two separate experiments. Fresh and PMA and ionomycin (P/I)-treated CD4+ T cells from spleens were prepared as in Materials and Methods. (C and D) Flow cytometric analysis of CD40L expression by CD4 and CD8 T cells. Spleen and lymph node (LN) cells from WT, CD40−/−, and CD40L−/− mice were stained for CD4, CD8, CD62L, CD25, and CD40L. Data are representative of more than five experiments. SA-PE, streptavidin phycoerythrin. (E and F) Flow cytometric analysis of CD40L (E) and CD69 (F) expression on in vitro incubated WT CD4 cells. Cells in the absence or presence of puromycin (puro) were incubated on ice (fresh) or at 37°C without (rest) or with P/I for 2 or 4 h. Cells were harvested and stained for CD4, CD40L, l-selectin, and CD69. The background staining of CD40L−/− cells is included for comparison.
By flow cytometric analysis, CD40L expression was low to undetectable on the surface of freshly isolated WT spleen or lymph node T cells (Fig. 3C). However, in vitro studies have shown that CD40-mediated ligation of CD40L can cause down-modulation of the ligand from the cell surface (9, 10), and we therefore examined CD40L expression on T cells from CD40 deficient mice. We detected strong CD40L expression on the surface of CD4 T cells from spleen and lymph nodes of CD40-deficient donors (Fig. 3C). Consistent with the mRNA expression analysis, the highest levels of surface CD40L were detected on naive (CD62Lhi) CD4 T cells with lower expression on regulatory (CD25+) and memory (CD62Llo) CD4 T cells (Fig. 3D) and little to no detectable expression on CD8 T cells (Fig. 3C). After activation by phorbol 12-myristate 13-acetate (PMA) and ionomycin treatment, CD40L transcript and surface expression could be further up-regulated (Fig. 3 B and E). CD40L mRNA levels were the same in WT and CD40−/− spleens and T cells, indicating that the detectable CD40L surface expression in cells from CD40−/− mice was not due to increases in transcript abundance (Fig. 3A and data not shown).
To further test whether naive CD4 T cells in WT mice contain preformed CD40L protein, lymph node cells were incubated in vitro at low density to avoid cell-to-cell contact and CD40–CD40L engagement. CD40L surface expression was observed on L-selectin-high, CD69-negative CD4 T cells within 2 h of in vitro incubation (Fig. 3E) at levels approaching those of CD4 T cells from CD40-deficient mice. This surface expression of CD40L was not prevented by inclusion of the protein synthesis inhibitor puromycin from the start of in vitro incubation (Fig. 3E). By contrast, puromycin inhibited the additional induction of surface CD40L expression achieved by exposure to PMA and ionomycin (Fig. 3E). Up-regulation of the activation antigen, CD69, only occurred in the presence of PMA and ionomycin, and this induction was also blocked by puromycin (Fig. 3F), providing a further control showing that new protein synthesis was being inhibited. CD8 T cells failed to show CD40L expression after in vitro incubation, consistent with the lack of CD40L mRNA in these cells and the lack of surface expression in CD40−/− animals (Fig. 3 D and E). These results provide further evidence that naive CD4 T cells in WT mice contain preformed CD40L protein.
B Cells and Non-B Cells Down-Modulate CD40L Expression by Naive T Cells.
To examine which CD40-expressing cells contribute to the down-modulation of CD40L from the surface of naive CD4 T cells, we determined the impact of B cell deficiency. CD40L could be detected at low levels on spleen, but not lymph node, T cells from B cell-deficient μMT mice (Fig. 4A). These observations indicate that B cells contribute but that other cell types make a greater contribution to CD40L down-modulation. Consistent with CD40L down-modulation depending on contact between CD4 T cells and CD40-expressing cells, CD40L was detectable on the surface of WT naive CD4 T cells circulating in the blood (Fig. 4A). The detection of CD40L on naive CD4 T cells in blood provides further evidence that WT cells constitutively produce CD40L protein. As an additional approach to examine whether an encounter with CD40-expressing cells was responsible for down-modulating CD40L in vivo, splenocyte adoptive transfer experiments were performed. In CD40−/− mice that received transfers of total splenocytes or purified WT B cells, there was marked CD40L down-modulation on recipient CD4+ T cells 2.5 days later (Fig. 4B). An intermediate level of CD40L modulation was observed as soon as 3 h after transfer of WT splenocytes, whereas no down-modulation occurred after transfer of CD40-deficient cells (Fig. 4C). These observations support the conclusion that naive T cells in WT mice down-modulate surface CD40L secondary to the engagement of CD40 on CD40-expressing cells.
Fig. 4.
CD40 on B cells, as well as non-B cells, causes the down-modulation of CD40L on naive T cells. (A) CD40L expression in WT, CD40−/−, and μMT spleen, lymph nodes (LN), and blood, with the corresponding CD40L−/− cell populations used as staining controls. CD40L expression on CD4+CD62Lhi cells is shown, and the data are representative of five mice of each type from four experiments. (B) CD40L expression on CD4+CD62Lhi T cells in CD40−/− hosts 2.5 days after transfer of 5 × 106 CD40+/+ B cells compared with T cells from WT or CD40−/− mice not receiving transferred cells. (C) CD40L expression on CD4+CD62Lhi T cells in CD40−/− hosts 3 h or 2.5 days after transfer of WT or CD40−/− splenocytes containing 107 B cells. Data in B and C are representative of three experiments. SA-PE, streptavidin phycoerythrin; SA-APC, streptavidin allophycocyanin.
Discussion
The above findings establish that, under conditions of immune homeostasis, the CD40L–CD40 pathway, in a non-cell-autonomous manner, augments the survival of autoantigen-engaged peripheral B cells. Under resting conditions, naive CD4 T cells are demonstrated to express CD40L, whereas CD25+ CD4 T cells express lower amounts, and CD8 T cells show minimal expression of this membrane-bound cytokine. Exposure to CD40 expressed by B cells and non-B cells down-modulates CD40L from the T cell surface. These findings indicate that, in addition to the well established roles of the CD40L–CD40 pathway in immune activation, the pathway functions during immune homeostasis, augmenting the survival of B cells that have chronically engaged BCRs. We speculate that this pathway helps to retain polyreactive specificities in the B cell repertoire whose usefulness in responses to foreign pathogens outweighs the risks associated with their weak autoreactivity (1). In addition to the effects on B cells, we postulate that CD40L from naive CD4 T cells contributes to the maintenance of regulatory T cells, because both CD40- and CD40L-deficient animals display a reduction in CD25+ and FoxP3+ regulatory T cell numbers (refs. 11 and 12; R.L., L.M.K., and J.G.C., unpublished work).
The finding that naive CD4+ T cells express CD40L mRNA was unexpected, because CD40L was generally thought to be restricted to activated T cells (13, 14). A small number of studies had noted the presence of CD40L mRNA in freshly isolated T cells, although the significance of this expression was not further assessed (15, 16). In keeping with previous studies (9, 10, 12–15), CD40L on the surface of T cells freshly isolated from lymphoid tissues was low to undetectable. However, our analysis of cells from CD40-deficient hosts, cells incubated for brief periods in the absence of CD40 engagement, and cells in WT blood revealed readily detectable surface CD40L. In vitro studies have shown that CD40L is down-modulated after encountering CD40-expressing cells (9, 17). We suggest that this is an ongoing process in vivo, with CD40L reaching the surface of naive CD4 T cells but then being rapidly down-modulated after engagement of CD40 on other cells. Although our studies here indicate that naive T cells express sufficient amounts of CD40L to influence autoantigen-engaged B cell survival, it is important to note that exposure to activating stimuli can further induce CD4 T cell CD40L mRNA and protein expression by at least 10-fold. Different extents of CD40 engagement by CD40L or crosslinking antibodies can lead to different outcomes in the cell (18, 19). Thus, rather than working in an on/off manner, CD40L may function in a graded manner, allowing CD4 T cells to induce distinct effects in target cells depending on the level of CD40L expression. In this regard it is notable that several other TNF family members that were initially identified through their immune activating functions, such as TNF and lymphotoxin, have subsequently been found to have important homeostatic roles (20, 21).
Although we initially anticipated that the survival-promoting role of CD40 would be intrinsic to the autoantigen-binding B cells, we found an important extrinsic role for this pathway. Within the hematopoietic compartment, CD40 may signal in dendritic cells (DCs), macrophages, or, possibly, other B cells to lead to the production of factors that augment autoreactive B cell survival. Non-cell-autonomous effects of CD40 signaling have previously been suggested to influence the survival of plasma cells and to contribute to anti-pneumococcal polysaccharide antibody responses (22, 23). Although they show the existence of an extrinsic pathway, our present studies do not exclude the possibility that the CD40L–CD40 pathway can also act in a cell-intrinsic manner to promote autoantigen-binding B cell survival. Short-term CD40L neutralization and short-term CD4 T cell ablation both led to a 2-fold reduction in autoantigen-binding B cell numbers, indicating that there is a constitutive requirement for CD40L to maintain these cells. The less severe effect of the antibody treatments compared with genetic CD40 or T cell deficiency may be due to incomplete blocking or T cell ablation achieved by the antibody treatments. Alternatively, the extrinsic effect of CD40L–CD40 signaling may operate over longer time periods than those affected by the 1-week antibody treatments. Transcript levels of the B cell survival factor BAFF within the spleen were not diminished in the absence of T cells (5) or in the absence of CD40 (data not shown), indicating that the extrinsic CD40L–CD40 pathway regulates other factors that augment autoreactive B cell survival.
Mice deficient in Bruton’s tyrosine kinase (Btk) have reduced numbers of B cells and, analogously to our observations with autoantigen-engaged B cells, the B cell deficiency is more severe in mice that also lack T cells or in animals also lacking CD40 (24–27). The Btk studies (24–27) did not establish the source of CD40L that augmented the survival of the cells, but from our experiments it can be postulated that naive T cells are involved. Because basal BCR signaling is important for B cell survival and because Btk contributes to BCR signaling, it seems possible that Btk-deficient cells become dependent on the naive T cell-derived CD40L that we identify here as being able to augment HEL autoantigen-binding B cell survival.
Naive T cells are known to undergo frequent encounters with DCs, and we propose that there is a continual low-level engagement of DC CD40 by naive T cell CD40L and that, in turn, this is the principal interaction responsible for down-modulating CD40L on naive T cells. Engagement of CD40 on DCs may contribute to the CD40-dependent maintenance of regulatory T cells (11, 12) and may also account for the naive CD4 T cell dependence of some CD8 memory cells (28). CD40 potently synergizes with Toll-like receptor (TLR) ligands to promote DC maturation (29), and we speculate that this synergy occurs as soon as TLR-stimulated DCs arrive in the lymphoid organ T zone. There has been less evidence for frequent encounters between naive T and B cells. However, the down-modulation of CD40L on the majority of naive CD4 T cells in CD40-deficient animals after the transfer of 5 × 106 purified B cells suggests that this small number of B cells comes into contact with many T cells within hours of transfer. Our findings do not exclude the possibility that CD40 is secreted and able to modulate CD40L at a distance. However, although a splice variant of CD40 has been described that lacks the transmembrane domain, this form was not secreted (30). Moreover, it is notable that T cells from the blood of WT animals have detectable CD40L, favoring the view that CD40L down-modulation occurs through cell-to-cell contact within lymphoid tissues, rather than by the actions of soluble CD40. In addition to effects on autoantigen-engaged cells, B cells that have bound foreign antigen may benefit from exposure to naive T cell CD40L before their encounter with rare antigen-specific T cells (31).
In conclusion, we propose that, in the normal B cell repertoire, a subset of polyreactive B cells receives sufficient chronic BCR stimulation to become dependent on CD40L–CD40 pathway signals for survival. This may be one of a variety of mechanisms that counterbalance the need to maintain self-tolerance with the need for a diverse B cell repertoire to respond to pathogens (1). The CD40L–CD40 pathway has frequently been implicated in the development of autoimmunity, including lupus and other autoantibody-mediated diseases, and the inhibition of CD40L was found to have therapeutic benefit in mouse disease models and in human lupus patients (32). It is notable that, in mouse and human lupus, elevations in CD40L have sometimes been observed on bulk T cell populations or as a soluble form in circulation (33–38). Such elevations in CD40L might lead to retention of increasingly autoreactive clones in the B cell repertoire. In this context, a recent study (39) provided evidence that the naive B cell repertoires of lupus patients contain increased frequencies of autoreactive B cells. It has been suggested that anti-CD40L therapy may have therapeutic benefit by inhibiting the initial cognate CD40L–CD40 interaction during antigen-specific T and B cell encounter or by disrupting CD40L–CD40 interactions between germinal center T cells and centrocytes (40). Our studies here suggest a further stage wherein CD40L blockade may have therapeutic benefit, by reducing the survival of autoreactive B cells, helping to purge the cells from the repertoire before even any encounter with autoreactive T cells.
Materials and Methods
Reagents.
Anti-mouse CD40L (clone MR1) and polyclonal hamster IgG control for in vivo blockade were from Pharmingen and BioExpress, Inc. (West Lebanon, NH). Anti-mouse CD4 (clone GK1.5) for in vivo depletion was from BioExpress or Richard Locksley (University of California, San Francisco). HEL, BrdU, PMA, and ionomycin were from Sigma-Aldrich.
Animals.
C57BL/6 (B6) Ig/HEL Tg mice were generated by crossing HEL-specific MD4 Ig Tg mice to ML5 soluble HEL Tg mice or by using MD4 Ig Tg bone marrow to repopulate lethally irradiated (1,100 rad) HEL Tg mice (5). B6 CD40−/− mice were from The Jackson Laboratory, and some were crossed with MD4 Ig Tg mice. Radiation chimeras were made to generate Ig/HEL Tg/CD40−/− mice. Ig/HEL Tg/TCR-β−/−δ−/− mice were generated by crossing Ig/HEL Tg mice to B6 TCR-β−/−δ−/− mice from The Jackson Laboratory. B6 CD40L−/− and μMT mice were from The Jackson Laboratory. Mixed bone marrow chimeras were produced by reconstituting HEL Tg mice with 50% CD45.2 Ig Tg/CD40+/+ or Ig Tg/CD40−/− bone marrow and 50% CD45.1 Ig Tg bone marrow. All mice were maintained in a specific pathogen-free barrier facility. In experiments wherein purified B cells were transferred, CD45.1 spleen cells were negatively depleted by AutoMACS (Miltenyi Biotec, Auburn, CA) by using biotinylated antibodies against CD43, CD11c, and Ter119, followed by streptavidin microbeads. Enriched cells were 97–98% CD19+.
Flow Cytometry.
Single-cell suspensions from splenic, mesenteric, and brachial lymph nodes or from blood (RBC lysed) were incubated with various antibodies for four-color flow cytometry on a Becton Dickinson FACSCalibur. Monoclonal antibodies to BrdU, CD45.1, CD19, B220, CD23, CD40, CD62L, CD44, CD25, IgD, and streptavidin phycoerythrin were from BD Pharmingen. Anti-IgM FITC was from Jackson ImmunoResearch. Anti-lysozyme was from Rockland Immunochemicals. Antibodies to CD4 and CD8 were from Caltag (Burlingame, CA). AA4 allophycocyanin (APC) and anti-mouse CD40L biotin (clone MR1) were from eBioscience (San Diego). Hamster IgG biotin isotype control was from Biolegend (San Diego). Streptavidin APC was from Molecular Probes. HEL-specific monoclonal antibody HyHEL9 conjugated to PECy5.5 was from custom conjugations performed by Caltag. All sorted cells were >98% pure.
Real-Time PCR Analysis.
For purified T cells, B6 spleens were harvested and T cells were enriched before sorting by negative selection on an AutoMACS separator to deplete CD19+, CD11c+, and Ter119+ cells. Remaining cells were stained for CD4, CD8, CD62L, CD25, CD44, and propidium iodide to exclude dead cells. Cells were sorted on a Becton Dickinson FACSAria. To generate T cell-enriched preparations, spleen cells were incubated with biotinylated CD19, CD8, Gr-1, Mac-1, Ter119, CD11c, CD25, NK1.1, and I-Ab (Pharmingen) followed by streptavidin microbeads (Miltenyi Biotec) and run on an AutoMACS separator. Cell pellets were snap-frozen in liquid nitrogen, and RNA was prepared by using an RNeasy kit (Qiagen, Valencia, CA). Equivalent amounts of cDNA were used in quantitative PCR on an ABI 7300 sequence detection instrument (Applied Biosystems) by using primer sets with SYBR Green (Bio-Rad). Primer pairs were as follows (forward, F; reverse, R): hypoxanthine phosphoribosyltransferase (HPRT) F, AGGTTGCAAGCTTGCTGGT, and HPRT R, TGAAGTACTCATTATAGTCAAGGGCA; CD40 ligand F, GTGAGGAGATGAGAAGGCAA, and CD40 ligand R, CACTGTAGAACGGATGCTGC; and CD40 F, CTGCCCAGTCGGCTTCTTCTC, and CD40 R, ATGGTGATGAGGATGCCCATC.
Cell Culture.
CD4+ T cells from WT and CD40−/− spleens were enriched by negative selection on an AutoMACS separator by using biotinylated antibodies against CD19, CD11c, and Ter119, followed by streptavidin microbeads. “Fresh” cells were kept on ice, and remaining cells were plated on 12-well plates at 3.5 × 106 cells per milliliter in RPMI medium 1640 plus 10% FCS at 37°C and rested or were stimulated for 2 h with 10 ng/ml PMA and 1 μg/ml ionomycin and then snap-frozen for mRNA quantitation. To test for sensitivity to puromycin, freshly isolated lymph node or spleen cells were premixed or not with 100 μM puromycin while on ice, and then 0.5 ml were plated at 5 × 105 cells per milliliter on 24-well plates. After a 20-min incubation at 37°C, PMA and ionomycin were added to some wells as described above.
Acknowledgments
We thank Olivia Lam for excellent assistance with the mouse colonies, Chris Allen for helpful discussions, and Susan Schwab for assistance with cell sorting and for comments on the manuscript. J.G.C. is an Investigator of the Howard Hughes Medical Institute. This work was supported by National Institutes of Health Grant AI-40098.
Abbreviations
- BCR
B cell receptor
- HEL
hen egg lysozyme
- Tg
transgenic
- TCR
T cell antigen receptor
- PMA
phorbol 12-myristate 13-acetate
- DC
dendritic cell.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Goodnow C. C., Sprent J., Fazekas de St Groth B., Vinuesa C. G. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 2.Wardemann H., Yurasov S., Schaefer A., Young J. W., Meffre E., Nussenzweig M. C. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 3.Fulcher D. A., Basten A. J. Exp. Med. 1994;179:125–134. doi: 10.1084/jem.179.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cyster J. G., Goodnow C. C. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 5.Lesley R., Xu Y., Kalled S. L., Hess D. M., Schwab S. R., Shu H. B., Cyster J. G. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 6.Thien M., Phan T. G., Gardam S., Amesbury M., Basten A., Mackay F., Brink R. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt K. N., Cyster J. G. J. Immunol. 1999;162:284–291. [PubMed] [Google Scholar]
- 8.Allman D., Lindsley R. C., DeMuth W., Rudd K., Shinton S. A., Hardy R. R. J. Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 9.Yellin M. J., Sippel K., Inghirami G., Covey L. R., Lee J. J., Sinning J., Clark E. A., Chess L., Lederman S. J. Immunol. 1994;152:598–608. [PubMed] [Google Scholar]
- 10.Ludewig B., Henn V., Schroder J. M., Graf D., Kroczek R. A. Eur. J. Immunol. 1996;26:3137–3143. doi: 10.1002/eji.1830261246. [DOI] [PubMed] [Google Scholar]
- 11.Kumanogoh A., Wang X., Lee I., Watanabe C., Kamanaka M., Shi W., Yoshida K., Sato T., Habu S., Itoh M., et al. J. Immunol. 2001;166:353–360. doi: 10.4049/jimmunol.166.1.353. [DOI] [PubMed] [Google Scholar]
- 12.Guiducci C., Valzasina B., Dislich H., Colombo M. P. Eur. J. Immunol. 2005;35:557–567. doi: 10.1002/eji.200425810. [DOI] [PubMed] [Google Scholar]
- 13.Roy M., Waldschmidt T., Aruffo A., Ledbetter J. A., Noelle R. J. J. Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- 14.Ford G. S., Barnhart B., Shone S., Covey L. R. J. Immunol. 1999;162:4037–4044. [PubMed] [Google Scholar]
- 15.Lee B. O., Haynes L., Eaton S. M., Swain S. L., Randall T. D. J. Exp. Med. 2002;196:693–704. doi: 10.1084/jem.20020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armant M., Armitage R., Boiani N., Delespesse G., Sarfati M. Eur. J. Immunol. 1996;26:1430–1434. doi: 10.1002/eji.1830260705. [DOI] [PubMed] [Google Scholar]
- 17.van Kooten C., Gaillard C., Galizzi J. P., Hermann P., Fossiez F., Banchereau J., Blanchard D. Eur. J. Immunol. 1994;24:787–792. doi: 10.1002/eji.1830240402. [DOI] [PubMed] [Google Scholar]
- 18.Randall T. D., Heath A. W., Santos-Argumedo L., Howard M. C., Weissman I. L., Lund F. E. Immunity. 1998;8:733–742. doi: 10.1016/s1074-7613(00)80578-6. [DOI] [PubMed] [Google Scholar]
- 19.McCloskey N., Pound J. D., Holder M. J., Williams J. M., Roberts L. M., Lord J. M., Gordon J. Eur. J. Immunol. 1999;29:3236–3244. doi: 10.1002/(SICI)1521-4141(199910)29:10<3236::AID-IMMU3236>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Locksley R. M., Killeen N., Lenardo M. J. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 21.Gommerman J. L., Browning J. L. Nat. Rev. Immunol. 2003;3:642–655. doi: 10.1038/nri1151. [DOI] [PubMed] [Google Scholar]
- 22.Garcia de Vinuesa C., MacLennan I. C., Holman M., Klaus G. G. Eur. J. Immunol. 1999;29:3216–3224. doi: 10.1002/(SICI)1521-4141(199910)29:10<3216::AID-IMMU3216>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 23.Jeurissen A., Billiau A. D., Moens L., Shengqiao L., Landuyt W., Wuyts G., Boon L., Waer M., Ceuppens J. L., Bossuyt X. J. Immunol. 2006;176:529–536. doi: 10.4049/jimmunol.176.1.529. [DOI] [PubMed] [Google Scholar]
- 24.Wortis H. H., Burkly L., Hughes D., Roschelle S., Waneck G. J. Exp. Med. 1982;155:903–913. doi: 10.1084/jem.155.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sprent J., Bruce J. J. Exp. Med. 1984;160:335–340. doi: 10.1084/jem.160.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan W. N., Nilsson A., Mizoguchi E., Castigli E., Forsell J., Bhan A. K., Geha R., Sideras P., Alt F. W. Int. Immunol. 1997;9:395–405. doi: 10.1093/intimm/9.3.395. [DOI] [PubMed] [Google Scholar]
- 27.Oka Y., Rolink A. G., Andersson J., Kamanaka M., Uchida J., Yasui T., Kishimoto T., Kikutani H., Melchers F. Int. Immunol. 1996;8:1675–1685. doi: 10.1093/intimm/8.11.1675. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton S. E., Wolkers M. C., Schoenberger S. P., Jameson S. C. Nat. Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 29.Napolitani G., Rinaldi A., Bertoni F., Sallusto F., Lanzavecchia A. Nat. Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tone M., Tone Y., Fairchild P. J., Wykes M., Waldmann H. Proc. Natl. Acad. Sci. USA. 2001;98:1751–1756. doi: 10.1073/pnas.98.4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Pinto D., Moreno J. Eur. J. Immunol. 2005;35:1097–1105. doi: 10.1002/eji.200425732. [DOI] [PubMed] [Google Scholar]
- 32.Toubi E., Shoenfeld Y. Autoimmunity. 2004;37:457–464. doi: 10.1080/08916930400002386. [DOI] [PubMed] [Google Scholar]
- 33.Yellin M. J., D’Agati V., Parkinson G., Han A. S., Szema A., Baum D., Estes D., Szabolcs M., Chess L. Arthritis Rheum. 1997;40:124–134. doi: 10.1002/art.1780400117. [DOI] [PubMed] [Google Scholar]
- 34.Koshy M., Berger D., Crow M. K. J. Clin. Invest. 1996;98:826–837. doi: 10.1172/JCI118855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai-Mehta A., Lu L., Ramsey-Goldman R., Datta S. K. J. Clin. Invest. 1996;97:2063–2073. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato K., Santana-Sahagun E., Rassenti L. Z., Weisman M. H., Tamura N., Kobayashi S., Hashimoto H., Kipps T. J. J. Clin. Invest. 1999;104:947–955. doi: 10.1172/JCI7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vakkalanka R. K., Woo C., Kirou K. A., Koshy M., Berger D., Crow M. K. Arthritis Rheum. 1999;42:871–881. doi: 10.1002/1529-0131(199905)42:5<871::AID-ANR5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 38.Lettesjo H., Burd G. P., Mageed R. A. J. Immunol. 2000;165:4095–4104. doi: 10.4049/jimmunol.165.7.4095. [DOI] [PubMed] [Google Scholar]
- 39.Yurasov S., Wardemann H., Hammersen J., Tsuiji M., Meffre E., Pascual V., Nussenzweig M. C. J. Exp. Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grammer A. C., Lipsky P. E. Arthritis Res. Ther. 2003;5(Suppl. 4):S22–S27. doi: 10.1186/ar1009. [DOI] [PMC free article] [PubMed] [Google Scholar]