Abstract
RNA interference (RNAi) provides a powerful experimental tool for sequence-specific gene silencing, allowing efficient analysis of gene function in a multitude of cell types. However, application of RNAi in primary mammalian neurons has been limited by low-transfection efficiency and considerable toxicity of conventional transfection methods. In this study, we evaluated a peptide-mediated and a polymer/lipid-based cellular delivery method for siRNA into rat primary neurons and compared the results with a commonly used liposomal transfection reagent. Stearylated octaarginine (Stearyl-R8) was used as polypeptide and artificial virus-like particles (AVPs) were used as a combined liposomal-polymeric vector, since both reagents have been previously shown to successfully transfect DNA into cell lines. Stearyl-R8 and AVPs both promoted siRNA transfection into primary hippocampal neurons via the endosomal pathway. SiRNA-mediated gene silencing could be effectively induced in primary neuron cultures. In comparison with the commonly used cationic liposome transfection agent, both novel reagents were less detrimental to cell metabolic activity. We conclude that these novel transfection methods yield performances comparable to cationic liposome-mediated transfection for siRNA, while being less cytotoxic in primary neurons. Stearyl-R8 and AVPs may therefore represent novel and more cost-efficient alternatives to conventional siRNA-transfection reagents.
Keywords: RNAi, transfection, cytotoxicity, polypeptide, virus-like particle
INTRODUCTION
RNA interference induced by introduction of siRNA has become an important research tool for targeted gene ablation in neuronal cells. However, functional studies using RNA interference depend not only on successful gene silencing, but also on unimpaired viability of the culture by the transfection reagents themselves.
A large number of commercial transfection reagents are available for the introduction of nucleic acids into cell lines. The most frequently applied method uses cationic lipid-based reagents (Maurer et al. 1999; Dalby et al. 2004). Cationic lipid-based transfection reagents efficiently deliver siRNA into cell lines without exerting major cytotoxic side effects. On the other hand, such compounds are toxic for many primary mammalian cells, e.g., primary endothelial, dendritic, or smooth muscle cells, and primary neurons (Davidson et al. 2004; Kiefer et al. 2004). Nevertheless, cationic lipid-based transfection reagents have been used for the induction of RNA interference in primary neurons when long-term survival of the culture represented a less critical issue (Krichevsky and Kosik 2002; Lingor et al. 2004).
Recently, various innovative reagents have been successfully used to transfect DNA into cell lines. Lipophilic polypeptides, such as stearylated octaarginine (Stearyl-R8), are able to encapsulate and shuttle nucleic acids through cell membranes without the need to covalently attach the nucleic acid to the arginine-containing carrier peptide. For DNA transfection, Stearyl-R8 has proven to be a superior peptide-based transfection reagent that is at least as effective as conventional lipid-based reagents (Futaki et al.2001a). The stearylation on the N terminus of the peptides increases the efficiency of transfection by facilitating the absorption of the complex to the membranes through its hydrophobic moiety.
Consequently, new lipid-based transfection methods have been developed that use polymeric vectors combined with liposomal artificial virus-like envelopes (so-called artificial virus-like particles, AVPs). Here, nucleic acids are condensed with a potent transfection reagent (polyethyleneimine or protamine sulfate) and complexed with a liposomal envelope (artificial viral envelope, AVE) that mimics the lipid composition of natural enveloped viruses. Endosomal escape is facilitated because of additional endosomolytic properties. The use of AVEs has been shown to yield significantly improved transfection rates for DNA, for example, into primary endothelial cells (Muller et al. 2001; Nahde et al. 2001).
In our present study, both novel peptide-based (Stearyl-R8) and lipid-based (AVP) delivery methods were applied for the transfection of siRNAs into rat primary neurons and compared with a widely used liposomal transfection reagent (Lipofectamine). We evaluated the uptake mechanism and intracellular localization of siRNA, verified RNAi induction, and assessed the impairment of cell metabolic activity as a measure for cytotoxic effects.
RESULTS
Intracellular localization of transfected siRNA
The intracellular localization of siRNA after transfection is crucial for its successful function as an inductor of the RNA interference process. DNA transfection approaches depend on efficient delivery of the nucleic acid to the nucleus. For induction of RNAi, cytoplasmatic delivery of siRNA is required, because the assembly of the siRNA-containing RISC complex occurs in the cytoplasm (Zeng and Cullen 2002; Sontheimer 2005). In this study, we used Cy3-labeled siRNA in order to visualize transfection kinetics and intracellular localization of siRNA. Fluorescent siRNA was transfected into primary neurons either in conjugation with the conventional transfection reagent Lipofectamine, the novel siRNA transfection reagents Stearyl-octaarginine (Stearyl-R8), or as artificial virus-like particles (AVPs). “Naked” siRNA applied without any transfection reagent served as control.
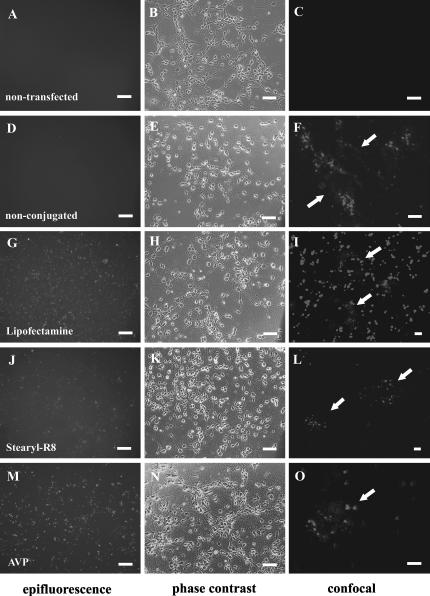
Except for “naked” siRNA (Fig. 1D,E), all tested transfection agents initially resulted in the formation of extracellular siRNA-containing complexes, which eventually adhered to the cell membrane and could be visualized by conventional epifluorescence. The most prominent extracellular liposome/siRNA aggregates were observed after application of Lipofectamine-conjugated siRNA and persisted over several days in culture (Fig. 1G,H). Extracellular aggregate formation was less prominent with Stearyl-R8- and AVP-conjugated siRNA (Fig. 1J,K,M,N). Confocal images taken 72 h after transfection show prominent granular localization of Cy3 fluorescence next to diffuse cytoplasmatic staining (Figs. 1I,L,O). As we have previously shown (Lingor et al. 2004), application of nonconjugated “naked” siRNA to primary neurons also induced a modest granular uptake of siRNA (Fig. 1F).
FIGURE 1.
SiRNA transfection of primary hippocampal neuron cultures. Epifluorescence (left), corresponding phase contrast (middle) overview images and confocal microscopy (right) detail images of primary neuron cultures 24 h after transfection. Nontransfected cells are shown in A–C. SiRNA was applied nonconjugated (D–F) or conjugated with Lipofectamine (G–I), Stearyl-R8 (J–L), and AVP (M–O). Epifluorescence images demonstrate the distribution of siRNA conjugates in the culture with variable aggregate formation. Confocal images show the intracellular distribution of labeled siRNA within granular structures 72 h after transfection in greater detail. Individual cells in confocal images are labeled with arrows. Bars in epifluorescence and phase contrast images, 50 μm; bars in confocal images, 10 μm.
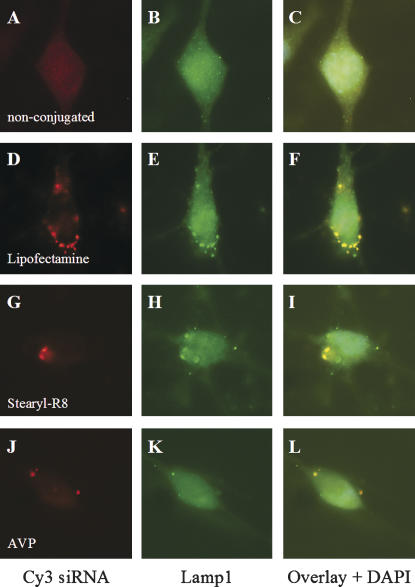
Double-labeling with the lysosomal marker Lamp1 demonstrated that the granular structures containing the complexes of siRNA and transfection reagent were present in the lysosomal compartment (Fig. 2). The final intracellular distribution pattern was not markedly different between the three transfection reagents tested. Notably, the nucleus remained spared of the Cy3 signal in all transfection paradigms, as visualized by confocal images and immunostaining. All three tested transfection reagents thus mediated endocytic uptake of siRNA, which finally resulted in cytoplasmic localization of the nucleic acid.
FIGURE 2.
Intracellular localization of transfected Cy3-labeled siRNA. Transfection with Lipofectamine (D–F), Stearyl-R8 (G–I), and AVP (J–L) results in a lysosomal localization of intracellular Cy3-labeled siRNA, as demonstrated by colocalization with the lysosomal marker Lamp1 24 h after transfection. Nonconjugated siRNA (A–C) shows a less-intense fluorescence pattern, indicating low-transfection efficiency.
To examine the kinetics of siRNA uptake, we performed time-lapse microscopy. Once siRNA/transfection reagent complexes adhere to the cell membrane, they can divide into multiple siRNA-containing particles, which then diminish in size and eventually decrease in fluorescence. Meanwhile, an increase of cytoplasmatic Cy3 fluorescence is observed, where RNAi could occur. Figure 3 shows this process for Stearyl-R8-transfected neurons. Similar uptake kinetics were observed using AVP-transfected siRNA (data not shown).
FIGURE 3.
Neuronal uptake of Cy3-labeled siRNA transfected with Stearyl-R8 shown by time-lapse microscopy (8 h). (Left) Start of live imaging; (right) end of live imaging in phase contrast; (middle) sequential images of live microscopy taken in intervals of 60 min. Arrows indicate the same neuron throughout the time-lapse imaging. SiRNA-containing, Cy3-positive vesicles in the culture medium adhere to the cell membrane. Cellular Cy3 fluorescence representing transfected siRNA increases over time in the cell cytosol, sparing the nucleus.
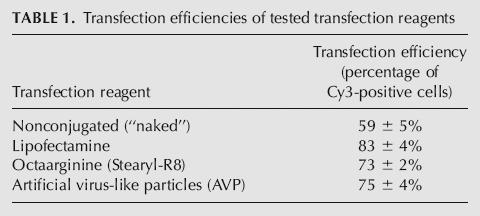
The transfection efficiency was determined by quantification of the percentage of Cy3-positive cells relative to all cells in culture (Table 1). The percentage of transfected cells was the lowest for nonconjugated “naked” siRNA (59 ± 5%), while Lipofectamine showed the highest transfection efficiency (83 ± 4%). Novel transfection reagents displayed slightly lower transfection efficiencies compared with Lipofectamine (Stearyl-R8 73 ± 2%, AVP 75 ± 4%).
TABLE 1.
Transfection efficiencies of tested transfection reagents
Induction of RNA interference by transfected siRNA
In order to demonstrate that conjugated siRNA remains functional, the induction of RNA interference was tested for an exogenously transfected reporter protein (EGFP) and the endogenous structure protein MAP2B.
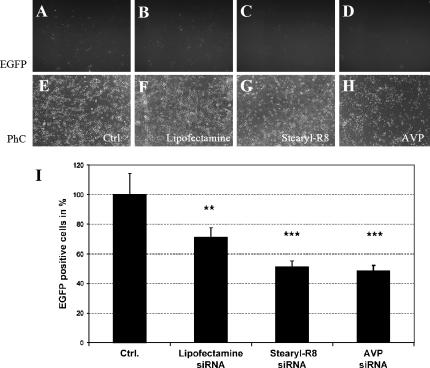
Hippocampal neurons were transfected with an EGFP-expressing DNA plasmid by nucleofection, and EGFP expression was allowed to continue for 3 d in vitro (Fig. 4A). Anti-EGFP siRNA was then applied to the primary neuron cultures in conjugation with Lipofectamine (Fig. 4B), Stearyl-R8 (Fig. 4C, Stearyl-R8:siRNA ratio of 2:1), or AVPs (Fig. 4D).
FIGURE 4.
RNAi induction in primary neuronal cultures. Lipofectamine (B), Stearyl-R8 (C), and AVP (D) effectively induce siRNA-mediated gene silencing in primary neurons previously transfected with an EGFP-expressing plasmid. EGFP-fluorescence (A–D), phase contrast (E–H) microscopy, and quantification of EGFP-positive cells (I) are shown.
Silencing of EGFP expression was quantified by counting EGFP-positive neurons 48 h after siRNA application. Lipofectamine, Stearyl-R8- and AVP- effectively induced siRNA-mediated gene silencing of the EGFP reporter gene. Using the same amount of siRNA, Stearyl-R8- and AVP-mediated siRNA delivery silenced EGFP expression more efficiently than Lipofectamine (Fig. 4I). Transfection of “naked” siRNA has previously been shown to have only minimal effects on EGFP expression (Lingor et al. 2004).
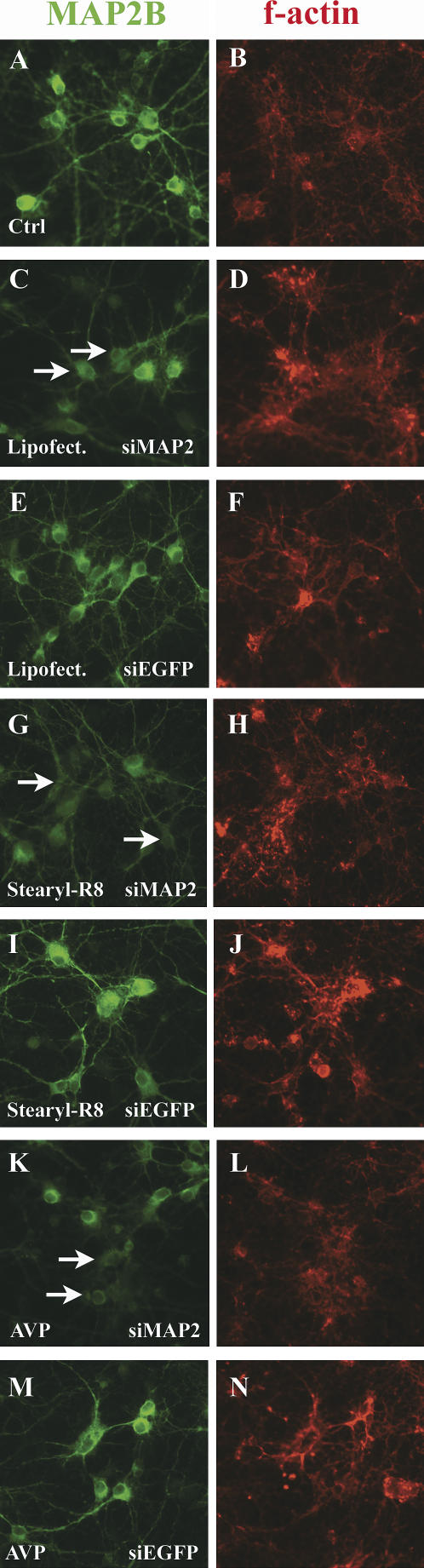
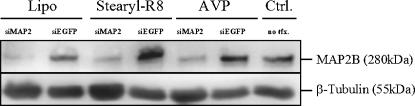
In order to verify the specificity of siRNA-induced gene silencing and to exclude unspecific silencing effects limited to the exogenously transfected EGFP, we targeted the endogenous MAP2 gene. After plating, neurons were transfected with anti-MAP2 siRNA on DIV2 and the down-regulation was observed on DIV 9 by immunocytochemistry. Anti-EGFP siRNA served as control. All tested transfection reagents induced a down-regulation of MAP2 as visualized by decreased immunofluorescence for MAP2B, a splicing variant, which is highly expressed throughout neuronal development (Fig. 5A–N). The silencing activity in the total culture was additionally verified by Western blots of protein lysates obtained from cultures on DIV 9 (Fig. 6).
FIGURE 5.
Silencing of endogenous MAP2B in hippocampal cultures. Nontransfected control cultures show a homogeneous MAP2B staining (A). Counterstaining with phalloidin (indicative for f-actin) served as control for a second, nonregulated structure protein (B). Cultures treated with siRNA against MAP2 show an increased number of neurons, which show a weak immunoreactivity for MAP2B (C,G,K). The arrows mark exemplary neurons with most prominent down-regulation. Cultures treated with anti-EGFP siRNA show an unchanged immunoreactivity for MAP2B (E,I,M). Phalloidin counterstain indicates unaltered levels of the nontargeted structure protein f-actin (D,F,H,J,L,N).
FIGURE 6.
Western blots demonstrating specific down-regulation of the endogenous MAP2B protein. Anti-MAP2 siRNA transfected with Lipofectamine, Stearyl-R8, or with AVPs reduces MAP2B expression to similar levels. Control transfections using anti-EGFP siRNA do not influence MAP2B expression. All lanes were loaded with the same amount of total protein as indicated by the β-Tubulin detection.
Cytotoxicity of transfection reagents for primary neuron cultures
Next to successful induction of RNA interference, the lowest possible cytotoxic effects in primary neuron cultures were our second major criterion in the evaluation of novel siRNA transfection reagents. To evaluate cytotoxic effects in culture, we measured cell metabolic activity as a marker for functional integrity and survival of primary neurons after application of conjugated siRNA. In contrast to frequently used nuclear stains (e.g., Hoechst), quantification of mitochondrial metabolic activity has been shown to detect functional changes at an earlier stage, where nuclear morphology is yet unchanged (Vistica et al. 1991).
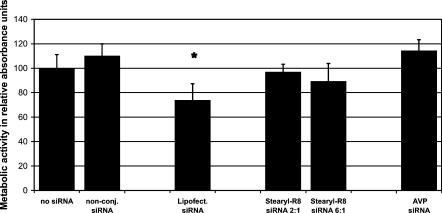
Lipofectamine considerably impaired cell viability, reducing the metabolic activity to 73%, compared with nontransfected controls of our primary neuron culture paradigm (Fig. 7, third column) 24 h after transfection. SiRNA transfection using Lipofectamine additionally started inducing morphologic changes in neurites already after 12 h in culture (data not shown), which is in accordance with previous reports of cationic lipid toxicity (Lappalainen et al. 1994).
FIGURE 7.
Toxicity of siRNA transfection reagents. Metabolic activity assessed by WST1 assay indicating functional integrity of the neuronal culture after application of siRNA without transfection reagents, and siRNA complexed with Lipofectamine, Stearyl-R8, or AVP 24 h after transfection. Results are given as relative absorption units compared with nontransfected control cultures.
All tested Stearyl-R8:siRNA ratios induced no significant impairment of metabolic activity, ranging from 89% to 96% compared with the control culture (Fig. 7, fourth and fifth column). We noted a tendency to increased toxicity with higher Stearyl-R8:siRNA ratios, which, however, remained insignificant as compared with controls. AVP-mediated siRNA transfection did not show any significant impairment of metabolic activity in our culture paradigm (Fig. 7, last column).
DISCUSSION
Numerous innovative reagents for nucleic acid transfection have been developed in the past two decades. Strategies focused on peptide-mediated and polymer/lipid-based cellular DNA delivery. Here, we have applied these strategies to transfect siRNA into primary neurons and compared the efficiency to an established cationic lipid-based transfection reagent, Lipofectamine. Next to a successful delivery of siRNA to the primary cells, a main objective of our study was to determine the cytotoxic effects of the novel transfection reagents.
Futaki and coworkers have tested several oligoarginine compounds composed of four, eight, 12, or 16 arginine residues with and without stearylation for DNA transfection (Futaki et al.2001a,b). Stearylated octaarginine has been shown to be the most efficient compound to transfect plasmid DNA into COS-7 cells, and was therefore selected for our siRNA transfection approach. We used Stearyl-R8 in a similar cation:anion charge ratio as described for the DNA transfection approach. Since the total amount of nucleic acids has to be lower for siRNA transfection approaches in order to retain specificity of gene silencing and avoid the induction of an interferon response (Sledz et al. 2003), the amount of octaarginine used was equally lower as compared with DNA transfection methods. In the case of AVPs, the same holds true. The reduced concentration required of both novel transfection reagents most likely explains the low cytotoxic side effects seen in our primary culture (Lingor et al. 2004). Rapid degradation of arginyl peptides by intracellular trypsin-like proteases could additionally account for the low cytotoxicity of the tested polypeptide (Futaki et al.2001b).
Branched histidine and lysine polymers have been used in a similar manner for siRNA transfection approaches, demonstrating that the ability to successfully transfect DNA does not per se qualify for effective siRNA transfection (Leng et al. 2005). Other approaches used siRNA chemically coupled to the membrane permeant peptides penetratin1 or transportan via a disulfide bond and have equally shown to mediate efficient transfection of primary neurons (Davidson et al. 2004; Muratovska and Eccles 2004). However, siRNAs have to be synthesized with a 5′ thiol on the sense strand, and the coupling procedure has to be performed before application, rendering this approach more elaborate and cost expensive.
Lipoplexes from anionic lipids have been previously shown to successfully transfect plasmid DNA into cell lines and primary neurons (Nahde et al. 2001; da Cruz et al. 2004; Patil et al. 2004). We now show that these so-called artificial virus-like particles are equally effective in transduction of small double-stranded RNA molecules for induction of RNA interference. Delivery systems using anionic liposomes seem to be less cytotoxic in vitro than cationic gene delivery systems (Welz et al. 2000), which we equally observed in our siRNA transfection experiments. This can be partly due to a more physiologic composition of the anionic lipids, which limits unspecific interactions with the cell surface as it is seen with cationic transfection agents (Muller et al. 2001; Nahde et al. 2001). Stearylated octaarginine, however, which is a highly basic compound, was not toxic in our experiments, which may be due to its intracellular degradation.
As shown by time-lapse- and confocal microscopy, Stearyl-R8 and AVPs formed complexes with fluorescent siRNA and resulted in formation of granule-like intracellular inclusions, which could be attributed to the lysosomal compartment. Similarly to Lipofectamine, Stearyl-R8- and AVP- thus use the endosomal uptake machinery in order to shuttle siRNA into the cytoplasm—a mechanism that has been described for most chemical transfection methods (Luo and Saltzman 2000). In the cytoplasm, less intense Cy3 fluorescence was found, indicating that Cy3-labeled siRNA had been released from lysosomal compartments. The lysosomal release has been shown to be a crucial property of nucleic acid transfection reagents, as RNA degradation occurs in the cell cytoplasm (Zeng and Cullen 2002). We did not observe any nuclear translocalization of siRNA. Unlike for DNA transfection, this is not required for efficient RNAi induction. Using EGFP as reporter protein, we confirmed that siRNA transfected with Stearyl-R8 or AVPs effectively induces gene silencing in primary neurons. The silencing efficiency did not significantly differ between the three transfection methods evaluated. RNAi-induced silencing was not limited to exogenously transfected EGFP, but equally down-regulated the endogenous neuronal structure protein MAP2B, while not affecting levels of two other structure proteins f-actin or β-tubulin.
In summary, we present two additional alternatives for the transfection of siRNA into primary mammalian neurons that could be particularly useful when only little impairment of cell viability after transfection is desired. The peptide-mediated transfection with stearylated octaarginine as well as the polymer/lipid-based cellular delivery with artificial virus-like particles both are easy to handle, as only pipet mixing of siRNA and the respective transfection reagent is necessary, and no further chemical modifications of the siRNA have to be performed. Both reagents represent cost-efficient alternatives to cationic lipid-based transfection reagents, because low amounts of transfection reagent are required.
These novel methods for siRNA delivery thus foster the use of RNAi as an experimental biological tool and in putative therapeutic approaches for targeted inhibition of genes involved in the pathogenesis of human disease.
MATERIALS AND METHODS
Primary hippocampal neuron culture
Briefly, pregnant Wistar rats were sacrificed at embryonic day 18 by CO2 intoxication. Both hippocampi were dissected and tissue pieces were trypsinated and dissociated using a fire-polished Pasteur pipette. Cells were seeded on poly-L-ornithine/laminin (both Sigma-Aldrich)-coated glass coverslips at a density of 50,000/cm2. Cultures were maintained in 24-well plates containing 600 μL of medium at 37°C and 5% CO2 in a humidified atmosphere. Culture medium was based on Neurobasal (NB; GIBCO) containing B27 supplement, transferrin, glutamine (all Sigma-Aldrich), and PSN antibiotic mixture (GIBCO). Medium changes were not performed.
siRNA preparation
Anti-EGFP siRNA for uptake studies was chemically synthesized by Biospring and Cy3 conjugated at the 5′ end. Oligonucleotides were freshly annealed before use in annealing buffer containing 100 mM potassium acetate, 30 mM Hepes–KOH (pH 7.4), and 2 mM magnesium acetate (all Fluka). Nonlabeled anti-EGFP siRNA for silencing experiments was generated using the Silencer SiRNA Construction Kit (Ambion). The siRNA yield was quantified by spectrophotometry for each individual siRNA preparation. The following sequence was used for both siRNA preparations: anti-EGFP-sense 5′-GACGUAAACGGCCACAAGUUC-3′, anti-EGFP-antisense 5′-ACUUGUGGCCGUUUACGUCGC-3′. Anti-MAP2 siRNA was synthesized by QIAGEN according to a published sequence containing anti-MAP2-sense 5′-CGAGAGGAAAGACGAAGGAUU-3′ and anti-MAP2-antisense 5′-UCCUUCGUCUUUCCUCUCGUG-3′ (Krichevsky and Kosik 2002). Lyophilized siRNA was reconstituted following the manufacturer's instructions.
Preparation of AVEs
For the preparation of AVEs, 1,2-dioleoyl-sn-glycero-3-(phospho-L-serine) (DOPS), 1,2 dioleoyl-sn-Glycero-3-Phosphoethanolamine (DOPE) (both from Avanti Polar Lipids, Inc.) and Cholesterol (Calbiochem) were used. Lipids were used as stock solutions in Chloroform and kept for storage at −20°C.
For the preparation of AVE-DOPE (composition DOPS:DOPE:Chol 1:1:1) lipids were dissolved in chloroform and dried into a thin film in a rotating 100-mL glass vessel warmed by a water bath at 40°C under gentle vacuum. Residual chloroform was removed by vacuum desiccation for 1 h. The lipid film was hydrated by the addition of 1 mL of 40°C warm sterile Tris buffer (10 mM at pH 7.4), resulting in a total lipid concentration of 10 mM. The multilamellar liposome suspension was sonicated in a ultrasonic bath for 1 h. Final preparation of the small unilamellar liposomes was done by extrusion through polycarbonate membrane filters with a pore size of 50 nm in a Liposofast (Avestin). Liposome size and size distribution were checked by photon correlation spectroscopy (Zetasizer Nano ZS, Malvern Instruments). Liposomes were sterile filtered (220 nm).
siRNA application
For transfection and targeting of EGFP-expression, siRNA was complexed with Lipofectamine 2000 (Invitrogen), Stearyl-R8, or PEI and AVEs in order to obtain AVP. All transfections were performed on day 3 in vitro (DIV 3), unless otherwise stated. Cell cultures were fixed and processed for immunocytochemistry on DIV 4 for endosomal stainings and on DIV 9 for MAP2B staining.
Lipofectamine 2000
According to the manufacturer's instructions, per well of a 24-well culture plate, 1 μL of Lipofectamine 2000 was diluted in 50 μL of Neurobasal medium without supplements at room temperature and 5 min later was combined with 10 pmol of siRNA in 50 μL of Neurobasal medium without supplements. Incubation was continued for 20 min at room temperature, and the mixture was applied to culture wells, resulting in a final siRNA concentration of 16 nM.
Stearyl-R8
Transfections were performed according to an adapted protocol from DNA transfection experiments (Futaki et al.2001a). Stearyl-R8 was chemically synthesized by SynPep. Transfection was carried out using different cation:anion charge ratios of a Stearyl-R8:siRNA mixture (data not shown). A Stearyl-R8:siRNA ratio of 2:1 yielded best results and was chosen for all further experiments. Per well of a 24-well culture plate, 1.5 μL of Stearyl-R8 was diluted in 50 μL of Neurobasal medium without supplements and combined with 10 pmol of siRNA in 50 μL of Neurobasal medium without supplements after 5 min incubation at room temperature. Incubation was continued for 20 min at room temperature and the mixture was applied to the culture wells, resulting in a final siRNA concentration of 16 nM.
AVP
Transfection protocols were adapted from DNA transfection experiments (Nahde et al. 2001). A total of 0.3 μg of low molecular weight, branched polyethyleneimine (PEI; Lupasol G100, BASF) was added in aqueous solution (adjusted to pH 7.4 and sterile filtered) to 10 pmol of siRNA, resulting in a ratio of polyethyleneimine nitrogen:siRNA of 21:1. Lower ratios resulted in reduced transduction, while higher ratios had only marginal impact in experiments with plasmid DNA (data not shown). Complexation was performed by pipetting up and down and a further incubation for 15 min. Next, an AVE-suspension containing 0.66 nmol of lipid was added to 10 pmol of siRNA, precomplexed with PEI, and gently mixed by pipetting up and down in an eppendorf tube. The formulation was continued for 20 min at room temperature, yielding AVPs ready for transfection. For siRNA application, experiments without cationic lipids, 10 pmol of “naked” siRNA per well was added directly to the cell suspension after preparation. Cells were plated as described, resulting in a final siRNA concentration of 16 nM.
For cell viability experiments without siRNA, equal amounts of transfection reagents were used as mentioned above.
DNA transfection and RNAi
Freshly dissociated hippocampal neurons were DNA transfected by electroporation using the Amaxa Nucleofector (Amaxa biosystems). A total of 2.0 × 106 cells were resuspended in nucleofection buffer, and 3 μg of DNA plasmid (pU1.EGFP) were added according to the manufacturer's instructions. Electroporation was performed using program O-05 (Gresch et al. 2004) and cells were cultured as described above. Transfection of anti-EGFP siRNA with transfection reagents was done on DIV 3. EGFP-positive neurons were counted in five random ocular fields 48 h after siRNA transfection using a Zeiss-Axioplan fluorescence microscope (Zeiss).
Immunocytochemistry
Cultures were fixed with formaldehyde (4%, 10 min), unspecific binding was blocked with normal goat serum (NGS; 10% in PBS, 10 min), and the primary antibody (anti-Lamp1, 1:250, kind gift of Dr. M. Sereda, Department of Neurogenetics, Max Planck Institut of Experimental Medicine, Göttingen) was applied in blocking solution overnight. Secondary Cy2-coupled anti-rat antibody (1:300; Jackson Labs) was applied for 45 min at 37°C, and cultures were nuclear stained with DAPI (1:1000, 10 min; Sigma-Aldrich) and mounted in Moviol (Hoechst) on glass slides. A Leica confocal laser-scanning system with a Krypton/Argon laser equipped with a Leica inverted microscope and a 40X objective was used (Leica).
For detection of endogenous MAP2B, hippocampal neuron cultures were processed as detailed above. Here, the primary anti-MAP2B (1:400, BD Biosciences) and the secondary Cy2-coupled anti-mouse antibody (1:300; Jackson Labs) were used. Additionally, rhodamine-labeled phalloidin (1:400, Molecular Probes) was applied together with the secondary anti-mouse antibody to visualize f-actin.
Western blotting
Hippocampal neuron lysates were prepared after 7 d of siRNA treatment using a lysis buffer containing 10 mM HEPES (pH 7.2), 142 mM KCl, 5 mM MgCl2, 1 mM EGTA, and 1% IGEPAL plus protease inhibitors (“Complete tablets,” Roche). Protein quantification was determined using the bicinchoninic acid assay (Pierce). Then, 20 μg of protein from each sample was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were blocked for 1 h at room temperature in a blocking solution containing 5% BSA, 0.1% Tween-20, and Tris-buffered saline (50 mM Tris-Hcl at pH 7.6, 150 mM NaCl). After blocking, the membranes were incubated overnight at 4°C with the mouse anti-MAP2B (1:1000, BD Biosciences) and mouse anti-βTubulin (1:2000, Sigma) antibodies diluted in blocking solution. The membranes were washed three times with 0.1% Tween-20 in Tris-buffered saline (15 min each) and incubated with a horseradish peroxidase-conjugated goat anti-mouse IgG antibody diluted in blocking solution (1:2000, Sigma). The membranes were washed with Tris-buffered saline as described before and then developed for analysis using a chemiluminesence method. Images were recorded and analyzed with the Quantity One Image Analysis Software (Bio-Rad).
Assessment of metabolic activity
Overall metabolic activity in culture was determined using the WST1 assay (Roche) according to the manufacturer's instructions. Briefly, WST1 reagent was added 1:10 to the culture medium and incubated for 2 h at 37°C. Absorbance was measured at 450 against 620-nm reference wavelength using an ELISA reader (Tecan).
Statistics
Data obtained in toxicity assays and cell counts are given as means ± SD. Intergroup differences were considered significant at *p < 0.05, **p < 0.01, and ***p < 0.001 according to one-way ANOVA.
ACKNOWLEDGMENTS
We thank Dr. M. Sereda, Department of Neurogenetics, Max Planck Institut of Experimental Medicine, Göttingen, for kindly providing the Lamp1 antibody; Dr. Ch. Stadelmann, Institute of Neuropathology, University of Göttingen, for kindly providing the secondary Cy2-coupled anti-rat antibody; Alexandra Marten and Ulrike Schöll for excellent technical assistance; Ajit Singh Dhaunchak and Asparuh Iliev for help with confocal imaging; and Katrin Meuer for help with time-lapse microscopy. Funded by Deutsche Forschungsgemeinschaft through the DFG-Research Center for Molecular Physiology of the Brain.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2252206.
REFERENCES
- da Cruz M.T., Simoes S., de Lima M.C. Improving lipoplex-mediated gene transfer into C6 glioma cells and primary neurons. Exp. Neurol. 2004;187:65–75. doi: 10.1016/j.expneurol.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Dalby B., Cates S., Harris A., Ohki E.C., Tilkins M.L., Price P.J., Ciccarone V.C. Advanced transfection with Lipofectamine 2000 reagent: Primary neurons, siRNA, and high-throughput applications. Methods. 2004;33:95–103. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Davidson T.J., Harel S., Arboleda V.A., Prunell G.F., Shelanski M.L., Greene L.A., Troy C.M. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J. Neurosci. 2004;24:10040–10046. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futaki S., Ohashi W., Suzuki T., Niwa M., Tanaka S., Ueda K., Harashima H., Sugiura Y. Stearylated arginine-rich peptides: A new class of transfection systems. Bioconjug. Chem. 2001a;12:1005–1011. doi: 10.1021/bc015508l. [DOI] [PubMed] [Google Scholar]
- Futaki S., Suzuki T., Ohashi W., Yagami T., Tanaka S., Ueda K., Sugiura Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001b;276:5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- Gresch O., Engel F.B., Nesic D., Tran T.T., England H.M., Hickman E.S., Korner I., Gan L., Chen S., Castro-Obregon S., et al. New non-viral method for gene transfer into primary cells. Methods. 2004;33:151–163. doi: 10.1016/j.ymeth.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Kiefer K., Clement J., Garidel P., Peschka-Suss R. Transfection efficiency and cytotoxicity of nonviral gene transfer reagents in human smooth muscle and endothelial cells. Pharm. Res. 2004;21:1009–1017. doi: 10.1023/b:pham.0000029291.62615.ec. [DOI] [PubMed] [Google Scholar]
- Krichevsky A.M., Kosik K.S. RNAi functions in cultured mammalian neurons. Proc. Natl. Acad. Sci. 2002;99:11926–11929. doi: 10.1073/pnas.182272699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen K., Jaaskelainen I., Syrjanen K., Urtti A., Syrjanen S. Comparison of cell proliferation and toxicity assays using two cationic liposomes. Pharm. Res. 1994;11:1127–1131. doi: 10.1023/a:1018932714745. [DOI] [PubMed] [Google Scholar]
- Leng Q., Scaria P., Zhu J., Ambulos N., Campbell P., Mixson A.J. Highly branched HK peptides are effective carriers of siRNA. J. Gene Med. 2005;7:977–986. doi: 10.1002/jgm.748. [DOI] [PubMed] [Google Scholar]
- Lingor P., Michel U., Scholl U., Bahr M., Kugler S. Transfection of “naked” siRNA results in endosomal uptake and metabolic impairment in cultured neurons. Biochem. Biophys. Res. Commun. 2004;315:1126–1133. doi: 10.1016/j.bbrc.2004.01.170. [DOI] [PubMed] [Google Scholar]
- Luo D., Saltzman W.M. Synthetic DNA delivery systems. Nat. Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- Maurer N., Mori A., Palmer L., Monck M.A., Mok K.W., Mui B., Akhong Q.F., Cullis P.R. Lipid-based systems for the intracellular delivery of genetic drugs. Mol. Membr. Biol. 1999;16:129–140. doi: 10.1080/096876899294869. [DOI] [PubMed] [Google Scholar]
- Muller K., Nahde T., Fahr A., Muller R., Brusselbach S. Highly efficient transduction of endothelial cells by targeted artificial virus-like particles. Cancer Gene Ther. 2001;8:107–117. doi: 10.1038/sj.cgt.7700280. [DOI] [PubMed] [Google Scholar]
- Muratovska A., Eccles M.R. Conjugate for efficient delivery of short interfering RNA (siRNA) into mammalian cells. FEBS Lett. 2004;558:63–68. doi: 10.1016/S0014-5793(03)01505-9. [DOI] [PubMed] [Google Scholar]
- Nahde T., Muller K., Fahr A., Muller R., Brusselbach S. Combined transductional and transcriptional targeting of melanoma cells by artificial virus-like particles. J. Gene Med. 2001;3:353–361. doi: 10.1002/jgm.203. [DOI] [PubMed] [Google Scholar]
- Patil S.D., Rhodes D.G., Burgess D.J. Anionic liposomal delivery system for DNA transfection. AAPS J. 2004;6 doi: 10.1208/aapsj060429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledz C.A., Holko M., de Veer M.J., Silverman R.H., Williams B.R. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 2003;5:834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- Sontheimer E.J. Assembly and function of RNA silencing complexes. Nat. Rev. Mol. Cell Biol. 2005;6:127–138. doi: 10.1038/nrm1568. [DOI] [PubMed] [Google Scholar]
- Vistica D.T., Skehan P., Scudiero D., Monks A., Pittman A., Boyd M.R. Tetrazolium-based assays for cellular viability: A critical examination of selected parameters affecting formazan production. Cancer Res. 1991;51:2515–2520. [PubMed] [Google Scholar]
- Welz C., Neuhuber W., Schreier H., Metzler M., Repp R., Rascher W., Fahr A. Nuclear transport of oligonucleotides in HepG2-cells mediated by protamine sulfate and negatively charged liposomes. Pharm. Res. 2000;17:1206–1211. doi: 10.1023/a:1026410612600. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Cullen B.R. RNA interference in human cells is restricted to the cytoplasm. RNA. 2002;8:855–860. doi: 10.1017/s1355838202020071. [DOI] [PMC free article] [PubMed] [Google Scholar]