Abstract
The mammalian proprotein convertases (PCs) are a family of secretory pathway enzymes that catalyze the endoproteolytic maturation of peptide hormones and many bioactive proteins. Two PCs, furin and PC6B, are broadly expressed and share very similar cleavage site specificities, suggesting that they may be functionally redundant. However, germline knockout studies show that they are not. Here we report the distinct subcellular localization of PC6B and identify the sorting information within its cytoplasmic domain (cd). We show that in neuroendocrine cells, PC6B is localized to a paranuclear, brefeldin A–dispersible, BaCl2-responsive post-Golgi network (TGN) compartment distinct from furin and TGN38. The 88-amino acid PC6B-cd contains sorting information sufficient to direct reporter proteins to the same compartment as full-length PC6B. Mutational analysis indicates that endocytosis is predominantly directed by a canonical tyrosine-based motif (Tyr1802GluLysLeu). Truncation and sufficiency studies reveal that two clusters of acidic amino acids (ACs) within the PC6B-cd contain differential sorting information. The membrane-proximal AC (AC1) directs TGN localization and interacts with the TGN sorting protein PACS-1. The membrane-distal AC (AC2) promotes a localization characteristic of the full-length PC6B-cd. Our results demonstrate that AC motifs can target proteins to distinct TGN/endosomal compartments and indicate that the AC-mediated localization of PC6B and furin contribute to their distinct roles in vivo.
INTRODUCTION
The identification of members of the mammalian family of proprotein convertases (PCs) has enhanced our understanding of how numerous proproteins and prohormones become proteolytically activated in the biosynthetic and endocytic pathways (Steiner et al., 1992; Seidah et al., 1994; Nakayama, 1997; Steiner, 1998). In addition, characterization of these enzymes has provided insight into areas as diverse as mechanisms of intracellular protein targeting, embryogenesis, and pathogen activation (Molloy et al., 1999). However, such studies have also raised several compelling questions concerning the relative roles of the PCs in vivo.
Structurally, the PCs share a similar domain organization, including an N-terminal signal peptide followed by a proregion necessary for enzyme transport and activation and a highly conserved catalytic domain characteristic of the subtilisin family of serine endoproteases (Seidah et al., 1994; Nakayama, 1997; Steiner, 1998; Molloy et al., 1999). Consistent with their shared structural similarity, all of the PCs have conserved enzymatic properties, including the cleavage of proproteins at sites containing multiple basic amino acids (e.g., Lys/Arg-Arg↓ or Arg-X-Lys/Arg-Arg↓).
The overall similarity of the in vivo processing sites in proproteins has raised the question of functional overlap between the PCs. Biochemical and cell biological analyses have provided some insight into this issue. The restricted tissue expression, acidic pH optima, and regulated pathway localization of the neuroendocrine-specific PC1/3 (hereafter termed PC3) and PC2 reflect their specialized function in the processing of prohormone substrates (Seidah et al., 1994; Nakayama, 1997; Steiner, 1998). Similarly, the unique expression of PC4 in the testes, together with impaired fertility in PC4-knockout mice (Mbikay et al., 1997), indicates a highly specialized function for this convertase. Several other PCs, however, have broader, overlapping expression patterns in a large number of tissues and cell types (Seidah et al., 1994; Steiner, 1998). These include the type I transmembrane proteins furin, LPC/PC7 (hereafter termed PC7), and PC5/6B (hereafter termed PC6B), all of which reportedly traffic in the constitutive secretory pathway.
Immunocytochemical studies show that furin, PC7, and PC6B each concentrate in Golgi-associated compartments (Molloy et al., 1994; De Bie et al., 1996; van de Loo et al., 1997). Although furin and PC7 both localize to the trans-Golgi network (TGN), recent studies showing their distinct cleavage site preferences suggest that they may process complementary sets of proproteins in vivo (van de Loo et al., 1997; J. Christian, personal communication). In contrast, furin and PC6B display overlapping substrate specificities. Both enzymes have been implicated in the proteolytic activation of BMP-4 (Cui et al., 1998) and are the only PCs potently targeted by the selective inhibitor α1-PDX (Jean et al., 1998). Thus, furin and PC6B appear to share several structural, biochemical, and cell biological characteristics that suggest that they could have overlapping roles in vivo. Their activities, however, are not fully redundant, because furin-knockout mice are embryonic lethal despite expressing PC6B (Roebroek et al., 1998).
The properties of furin, including its activation and intracellular trafficking, are well characterized (Molloy et al., 1999). Although furin is concentrated in the TGN, the enzyme cycles between this compartment and the cell surface through the endocytic pathway. The dynamic localization of furin within the TGN/endosomal system allows the protease to activate a large number of proproteins in multiple compartments. The cytoplasmic domain (cd) of furin is both necessary and sufficient to direct the TGN localization and recycling of the enzyme (Bosshart et al., 1994; Chapman and Munro, 1994; Molloy et al., 1994; Jones et al., 1995; Schäfer et al., 1995; Voorhees et al., 1995). A single cluster of acidic residues, which contains a pair of serines phosphorylated by casein kinase 2 (CK2) in vivo, is a key determinant for directing furin localization (Jones et al., 1995; Takahashi et al., 1995; Dittiéet al., 1997; Molloy et al., 1998; Wan et al., 1998). Furin is localized to the TGN by the interaction of the phosphorylated furin-cd with the connector protein PACS-1 (Wan et al., 1998).
Relative to furin, little is known regarding the intracellular trafficking of PC6B. When expressed in AtT-20 cells, PC6B has been shown to concentrate in a paranuclear compartment in which its staining pattern overlaps with that of TGN38 (De Bie et al., 1996). The shorter, lumenally restricted PC6A splice variant, however, is localized to regulated secretory granules (De Bie et al., 1996). This indicates that PC6B contains sorting information within its extended cysteine-rich lumenal, transmembrane, and/or cytoplasmic domains. The 88-amino acid PC6B-cd represents a candidate source for TGN/endosomal sorting information because it contains numerous sequences that resemble sorting motifs identified in other itinerant type I transmembrane proteins localized within TGN/endosomal compartments (Marks et al., 1997). These potential targeting signals include two tyrosine-based and one di-leucine–based clathrin-coated pit (CCP) recruitment motifs, as well as clusters of acidic residues. Similar acidic clusters (ACs) have been shown to direct several distinct sorting steps in the TGN/endocytic pathway, including localization to the TGN (furin, carboxypeptidase D, and varicellar zoster virus glycoprotein E; Schäfer et al., 1995; Voorhees et al., 1995; Alconada et al., 1996; Zhu et al., 1996; Eng et al., 1999), basolateral sorting (low-density lipoprotein [LDL] receptor and furin; Matter et al., 1994; Simmen et al., 1999), and delivery of lysosomal hydrolases (both the cation-dependent and cation-independent [CI] mannose-6-phosphate receptors [MPRs]; Chen et al., 1993; Mauxion et al., 1996; Boker et al., 1997; Schweizer et al., 1997).
As a first step toward determining the PC6B sorting itinerary, we report here a comparison of the localization of furin and PC6B in AtT-20 cells and an analysis of sorting motifs within the PC6B-cd. We show that, in contrast to furin, PC6B is localized to a paranuclear, post-Golgi compartment that is brefeldin A (BFA) dispersible, BaCl2 responsive, and in communication with early endosomes. Furthermore, the PC6B-cd is sufficient to direct sorting to the same compartments as the full-length protein. We also identified multiple active sorting signals, including a tyrosine-based internalization signal and two functional AC motifs. The membrane-proximal AC (AC1) interacts with the connector protein PACS-1 to direct TGN localization, whereas the second AC (AC2) promotes a distribution resembling that of the full-length PC6B-cd. These results identify unique targeting information in the PC6B-cd and further indicate that differential localization contributes to distinct roles for furin and PC6B in vivo.
MATERIALS AND METHODS
Recombinant DNA Constructs
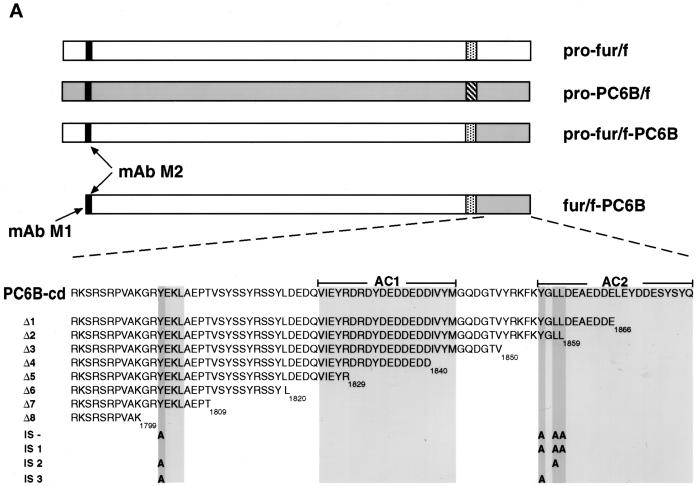
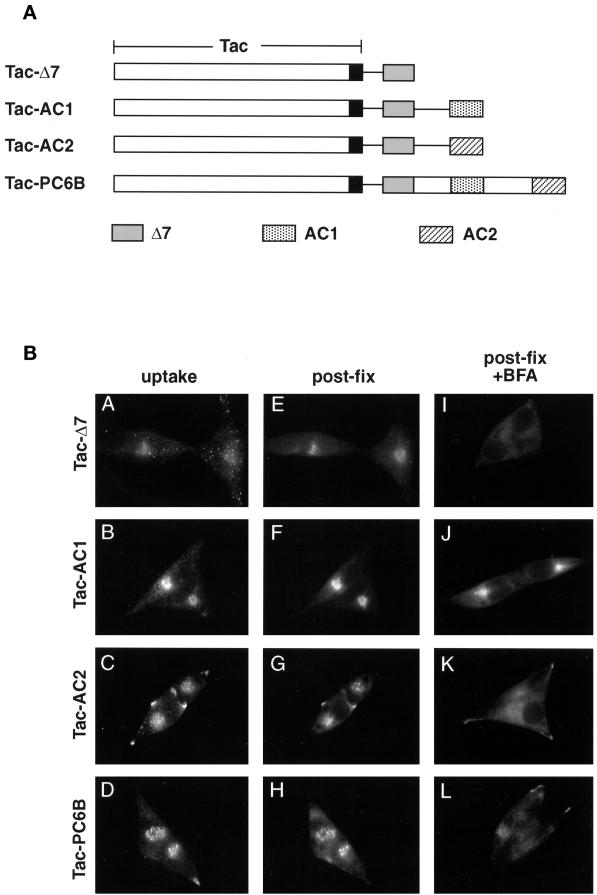
The DNA sequence of the rat PC6B-cd was obtained from intestine by reverse transcription PCR (Boehringer Mannheim, Indianapolis, IN) with two primers (5′-GGTGAATTCCTGCAGCTGCGGAAGTCTCGAAGCAGA [oligonucleotide {oligo} 1] and 5′-GGAGTCGACTTATTGGTAAGAGTAGCT [oligo 2]) containing sequences identical to the N and C termini of the mouse PC6B-cd (Nakagawa et al., 1993). Sequence analysis of the PCR product revealed a single amino acid difference compared with that reported for the mouse PC6B-cd (Thr1861 → Ala; Figure 1A). The PCR product was digested with PstI and SalI and subcloned into pGEM7Z.FBct cut with the same enzymes to replace the furin-cd with that of PC6B. The insert encoding furin–PC6B-cd was excised with BamHI and subcloned into pZVneofur/f cut with the same enzyme. This in-frame C-terminal “swap” generated a fur/f-PC6B chimera in which the furin-cd was replaced with that of PC6B. Point mutants and C-terminal truncations of the PC6B-cd were generated in the fur/f-PC6B chimera by either standard PCR or single-primer (Promega, Madison, WI) mutagenesis techniques. The mutagenic primers used for the PC6B-cd truncation constructs (stop codons are underlined) were oligo 3 (Δ1; 5′-GCGGGATCCTTATTACTCATCATCTTCTGCCTCATC), oligo 4 (Δ2; 5′-GCGGGATCCTTATTACAGCAGCCCGTACTTGAACTT), oligo 5 (Δ3; 5′-GCGGGATCCTTATTAAACAGTGCCATCTTGGCCCAT), oligo 6 (Δ4; 5′-GCGGGATCC TTATTAGTCATCCTCATCATCTTCATC), oligo 7 (Δ5; 5′-GCGGGATCCTTATTACCTGTACTCAATCACCTGGTC), oligo 8 (Δ6; 5′-GCGGGATCCTTATTAATAGCTGCTCCTGTAGGAGGA), oligo 9 (Δ7; 5′-GCGGGATCCTTATTAGGTGGGTTCTGCCAGCTTTTC), and oligo 10 (Δ8; 5′-GCGGGATCCTTATTACTTTGCCACAGGTC-TGCTTCG). Alanine substitutions (Ala codons are underlined) of potential PC6B-cd internalization motifs were created with the use of oligo 11 (L1858A; 5′-AAGTTCAAGTACGGGGCCCTGGATGAGGCAGAA), oligo 12 (Y1804A; 5′-GTGGCAAAGGGGCGC GCGGAAAAGCTGGCAGAA), oligo 13 (Y1856A; 5′-TACCGGAAGTTCAAGGCCGGGCTGCTGGATGAGGCA), or oligo 14 (YGLL/AGAA; 5′-TACCGGAAGTTCAAGGCCGGGGCCGCCGATG-AGGCAGAAGAT) in the appropriate combinations (Figure 1A).
Figure 1.
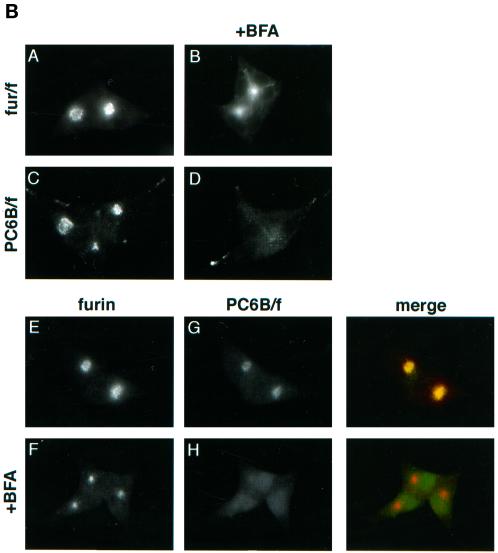
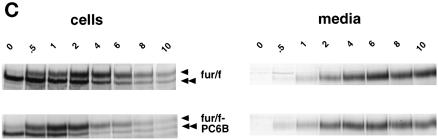
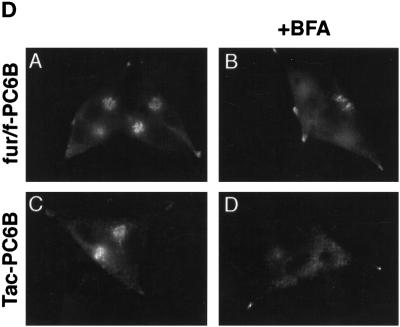
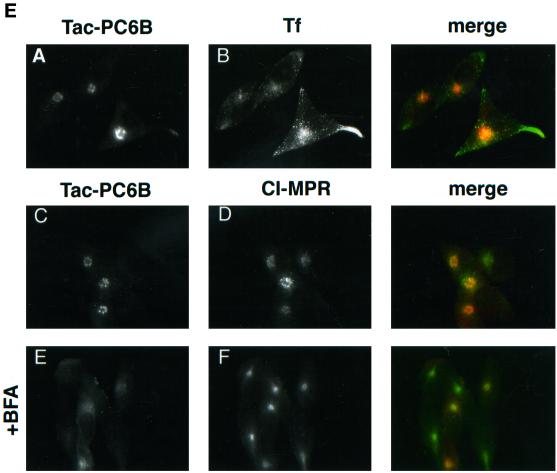
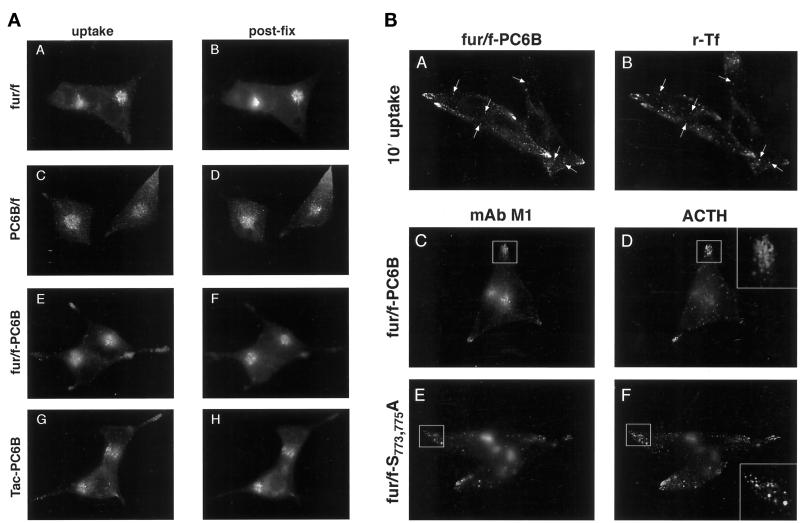
(A) Epitope-tagged furin and PC6B constructs. Furin (white) and PC6B (gray) are shown with transmembrane domains (stippled and striped, respectively) and the FLAG epitope (black). The differential reactivities of the FLAG-directed antibodies are also represented. mAb M1 recognizes only the N-terminal exposed FLAG sequence after excision of the proregion, whereas mAb M2 recognizes the epitope in both the pro and mature forms of the enzymes. The amino acid sequences of the PC6B-cd and its derivatives are shown. Membrane-proximal (AC1) and membrane-distal (AC2) acidic clusters as well as the potential tyrosine-based and di-leucine–like CCP recruitment motifs are highlighted in shaded boxes. Sites of Ala substitutions within these motifs are indicated with darker shading. (B) Comparison of intracellular localization between furin and PC6B. AtT-20 cells expressing either fur/f (A and B) or PC6B/f (C and D) were fixed either directly or after exposure to 10 μg/ml BFA for 20 min. The cells were stained after fixation with mAb M1. AtT-20 cells coexpressing non-epitope-tagged furin (E and F) and PC6B/f (G and H) were fixed either directly or after exposure to 10 μg/ml BFA for 20 min and then incubated with PA1-062 (anti-furin-cd) and mAb M1 (PC6B/f). The primary antibodies were distinguished with the use of fluorescently tagged species-specific secondary antisera conjugated to either FITC (PC6B/f) or TXR (furin). (C) Pulse-chase analysis of fur/f and fur/f-PC6B. Replicate plates of AtT-20 cells expressing either fur/f or fur/f-PC6B were pulse labeled with [35S]Met/Cys and harvested either directly (0) or after increasing chase times (0.5–10 h) in the absence of label. 35S-labeled proteins in both cells and media were immunoprecipitated and resolved by SDS-PAGE/fluorography. Migration positions of both nonsialylated (double arrowheads) and sialylated (based on neuraminidase sensitivity; single arrowheads) forms of the proteins are indicated. The diminution in signal in the chase samples is due primarily to shedding of the proteins into media (Molloy et al., 1994; Jean et al., 1998). (D) PC6B-cd is sufficient to direct protein localization. AtT-20 cells expressing either fur/f-PC6B (A and B) or Tac-PC6B (C and D) were fixed either directly or after exposure to 10 μg/ml BFA for 20 min. The fixed cells were stained with either mAb M1 (A and B) or anti-TAC (7G7) (C and D). (E) PC6B localization is distinct from CI-MPR and recycling endosomes. AtT-20 cells expressing Tac-PC6B were incubated with 50 μg/ml fluorescently labeled Tf (to load recycling endosomes; B) for 1 h, fixed, and incubated with anti-TAC (7G7) (A). AtT-20 cells expressing Tac-PC6B were fixed either directly (C and D) or after exposure to 10 μg/ml BFA for 20 min (E and F). The fixed cells were then stained with anti-TAC (7G7) (C and E) or anti-CI-MPR (D and F). The primary antibodies were distinguished with the use of fluorescently tagged species-specific secondary antisera conjugated to either FITC (CI-MPR) or TXR (Tac-PC6B).
Standard PCR was also used to amplify specific fragments of PC6B-cd from pZVneo-based plasmids for subcloning into pGEX-3X (Pharmacia, Piscataway, NJ) and expression of GST fusion proteins. For each construct, the 5′ primer was oligo 15 (5′-GTGGGATCCGGAAGTCTCGAAGCAGA), whereas oligos 2, 4, 16 (5′-CGCGTCGACTTACATATAGACGATGTCATC), and 17 (5′-CGGTGAGTCGACCACCTGGTCCTCGTCGAG) were used as the 3′ primers to amplify PC6B-cd, Δ2, Δ4, and Δ5, respectively. To amplify AC1, oligo 18 (5′-CAGCGGATCCTGATTGAGTACAGGGACCGA) and oligo 16 were used as primers, whereas to amplify AC2, oligo 19 (5′-AGCGGATCCAGTATGGGCTGCTGGATG) and oligo 2 were used. The PCR-amplified inserts were then subcloned into pGEX-3X. pET16b (Novagen, Madison, WI) expressing HtPACS-1fbr is described elsewhere (Wan et al., 1998).
The lumenal domain of T cell receptor α chain (Tac) cDNA was amplified by PCR of pBSSK(+)/Tac (provided by S. Milgram, University of North Carolina, Chapel Hill, NC) with oligo 20 (5′-GCCGGATCCGGTCCCAAGGGTCAGGAA) and oligo 21 (5′-GCGTCGACTTACTGCCAGGTTAACCCACTCAGGAGGAG) and then subcloned into pBSSK(+) cut with BamHI and SalI. The Δ7 fragment was amplified with oligo 22 (5′-GCGTCGACTTAGGTGGGTTCTGCTAGCCTTTTCGTAGCG) and oligo 23 (5′-GTGGGTTAACCTGGCAGCGGAAGTCTCGAAGCAGA). The fragment was cut with HpaI and SalI and subcloned into pBSSK(+) with the Tac lumenal domain to generate pBSSK(+)/Tac-Δ7. PCR was also used to amplify AC1 with oligo 18 and oligo 24 (5′-TCAGGCTAGCAGAACCCACCATTGAGTACAGGGACCGA) and to amplify AC2 with oligo 2 and oligo 25 (5′-TCAGGCTAGCAGAACCCACCTACGGGCTGCTGGATGAG). The fragments were subcloned into pBSSK(+)/Tac-Δ7 cut with NheI and SalI. Oligo 22 and oligo 2 were used to amplify rat PC6B-cd, and the product was subcloned into pBSSK(+)/Tac-Δ7 cut with HpaI and SalI to generate pBSSK(+)/Tac-PC6B.
Cell Culture and Vaccinia Virus Expression
AtT-20 cells (provided by B. Eipper, University of Connecticut Health Center) were cultured in complete DMEM containing 5% FCS. BSC-40 cells as well as the PACS-1 control (C12) and antisense (AS19) cell lines were maintained as described previously (Wan et al., 1998). Vaccinia recombinants expressing fur/f, furin, fur/fS773,775D, PC6B/f, and PC3/f have been described (Bresnahan et al., 1990; Molloy et al., 1994; Jones et al., 1995; Jean et al., 1998). PC6B-cd–containing expression vectors were generated by subcloning the DNA inserts into pZVneo (Hayflick et al., 1992), and recombinant viruses were generated as described (VanSlyke et al., 1995). Expression of each protein with the use of vaccinia recombinants was performed as described previously (Molloy et al., 1994).
For pulse-chase analysis, AtT-20 cells were grown to 80% confluence and then infected with recombinant viruses as described above. At 4 h after infection, the medium was removed and the cells were washed twice with Ca2+/Mg2+-free PBS and then incubated with methionine- and cysteine-free DMEM (ICN Biomedical, Costa Mesa, CA) supplemented with Express label (Met/Cys, 100 μCi/ml; New England Nuclear, Boston, MA) at 37°C for 30 min. Cells were then washed with PBS and harvested in mRIPA (50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 1% deoxycholate) or were refed with DMEM containing 10% serum and incubated for varying times before cell harvest. FLAG-tagged proteins were immunoprecipitated with the use of mAb M1 followed by isolation of the immune complexes with protein G–Sepharose. Digestion with neuraminidase (2 × 10−4 U; Boehr-inger Mannheim) was performed in 50 mM sodium citrate, pH 6.0, 0.1% SDS for 16 h at 37°C. Samples were resolved by SDS-PAGE (8% gels) and visualized by fluorography (Kodak [Rochester, NY] X-Omat film).
Immunofluorescence Microscopy and Antibodies
Immunofluorescence analyses were performed as described previously (Molloy et al., 1994) with the use of a Leica (Wetzlar, Germany) DMRB digital capture fluorescence microscope. Confocal images were acquired with a Bio-Rad (Richmond, CA) MRC 1024 ES laser scanning confocal imaging system. mAbs M1 (IgG2b) and M2 (IgG1) were from Kodak. Anti-TGN38 was provided by S. Milgram. Anti-adrenocorticotrophic hormone (ACTH) AS29 antibody was provided by S. Tooze (Imperial Cancer Research Fund, London, United Kingdom). Anti-Tac lumenal-domain antibody 2A3A1H (IgG1) was provided by F. Maxfield (Cornell University, New York, NY). Anti-Tac lumenal-domain antibody 7G7 (IgG2a) was obtained from the American Type Culture Collection (Rockville, MD). Anti-furin-cd PA1-062 antibody was from Affinity Bioreagents (Golden, CO). All FITC and Texas Red (TXR) secondary antibodies were from Fisher Scientific (Pittsburgh, PA). In double-label experiments, species- and subtype-specific secondary antisera were used to distinguish the primary antibodies. Fluorescently conjugated human transferrin (Tf) was from Molecular Probes (Eugene, OR).
Stimulated ACTH Release
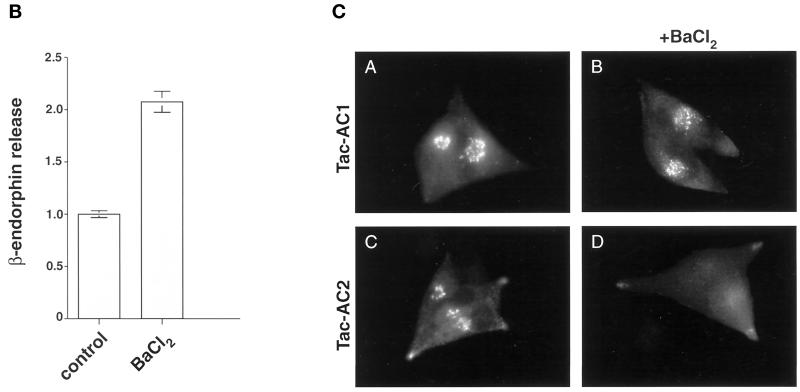
AtT-20 cells were rinsed three times with PBS and then incubated for 1 h at 37°C in 1 ml of Ca2+-free HBSS (125 mM NaCl, 4.75 mM KCl, 1.4 mM MgCl2, 10 mM glucose, 0.07% BSA, 25 mM HEPES, pH 7.35) in the absence or presence of 3 mM BaCl2. Released β-endorphin immunoreactive material was quantified by radioimmunoassay as described previously (Thorne et al., 1989).
Quantitative Internalization
mAb M1 was radioiodinated with the use of the chloramine T method and purified as described previously (Liu et al., 1997). For uptake assays, AtT-20 cells were infected with either wild-type vaccinia virus or vaccinia recombinants expressing FLAG-tagged constructs. At 4 h after infection, cells were detached by incubation with 10 mM EDTA in PBS. The suspended cells were then washed with DMEM containing 10 mM HEPES, pH 7.4, and incubated with 1 × 107 cpm/ml 125I-mAb M1 for 30 min on ice. Cells were washed twice with ice-cold medium and once with ice-cold complete medium. Cells were warmed to 37°C for increasing times to permit internalization of bound 125I-mAb M1. At each time point, replicate aliquots were removed and added to 500 μl of ice-cold DMEM/HEPES with or without 1 mg/ml protease K (Boehringer Mannheim). The suspensions were incubated for 30 min at 4°C and pelleted by centrifugation through a serum pad. Cell pellets were counted in a gamma counter. The activity of each sample was corrected by subtraction of activity values obtained for wild-type vaccinia virus–infected cells.
Bacterial Fusion Proteins and Binding Assay
GST fusion proteins were made from the PC6B-cd constructs cloned into the pGEX-3X expression vector as described above. The constructs were transformed into Escherichia coli BL21 lysS, and recombinant GST fusion proteins were purified with the use of glutathione-agarose beads. HtPACS-1fbr was made as described (Wan et al., 1998). For binding assays, 3 μg of Ht-PACS-1fbr was incubated for 45 min at room temperature with 5 μg of each GST protein in binding buffer (150 mM NaCl, 50 mM Tris, pH 7.5, 2 mM MgCl2, 2% NP-40). Protein complexes were isolated with the use of glutathione-agarose beads, resolved by SDS-PAGE, and visualized by Western blotting with the use of the anti-PACS-1fbr antiserum 678 (Wan et al., 1998) and chemiluminescent detection (New England Nuclear). Binding was quantified with the use of NIH Image software. Western blot analysis was performed as described (Molloy et al., 1994).
RESULTS
Differential Targeting of PC6B and Furin
To compare the localization of PC6B with that of furin, an epitope (FLAG) tag was introduced into the mouse full-length PC6B sequence (Figure 1A). The FLAG tag was inserted beyond the PC6B proregion cleavage site, which allows for specific detection of the proteolytically mature form of the enzyme (Molloy et al., 1994; Jean et al., 1998). Analysis by immunofluorescence showed furin and PC6B localized to distinct compartments in AtT-20 cells (Figure 1B).
Characteristic of its localization to the TGN (Molloy et al., 1994), furin concentrated in a paranuclear compartment that condensed upon treatment of cells with BFA before fixation. Although PC6B also showed a paranuclear concentration (consistent with a previous study by De Bie et al. [1996]), this staining was dispersed by BFA treatment, indicating non-TGN localization. In addition, PC6B displayed significant staining at the tips of AtT-20 cells, which was not observed for furin. This differential distribution of furin and PC6B was verified in double-labeling experiments (Figure 1B) in which the proteases were visualized independently with the use of antibodies directed against the cytoplasmic domain of nontagged furin (antiserum PA1-062) and the FLAG tag in PC6B/f (mAb M1). As seen in single-labeling experiments, both proteins were predominantly localized in the paranuclear region but the PC6B staining pattern was selectively dispersed by BFA treatment.
The PC6B Cytoplasmic Domain Mediates Sorting
The furin-cd contains sorting information that directs its trafficking within the TGN/endosomal system. Therefore, to determine the sorting capacity of the PC6B-cd, chimeric proteins were constructed consisting of the lumenal and transmembrane domains of either FLAG-tagged furin (fur/fR739t) or Tac and the PC6B-cd (fur/f-PC6B and Tac-PC6B, respectively; see Figures 1A and 5A). The Tac lumenal and transmembrane domains have been used previously to assess the sorting capacity of cytoplasmic sequences (Voorhees et al., 1995), whereas the use of fur/fR739t as an additional reporter provided a means to monitor the efficacy of transport of the proteins through early secretory pathway compartments. FLAG-directed antibody reactivity was used to monitor endoplasmic reticulum–localized propeptide cleavage (Molloy et al., 1994) as evidence of correct folding of the structurally similar lumenal domains (see legend to Figure 1A), and the presence of N-linked carbohydrate was used to monitor transport of the chimera through the Golgi complex.
Figure 5.
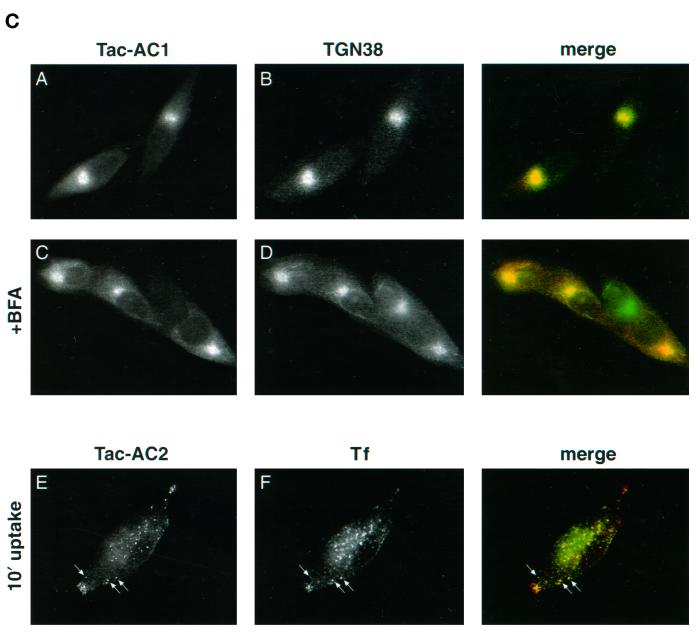
PC6B-cd ACs contain distinct sorting information. (A) Tac-PC6B-cd chimeras. The Tac lumenal (white) and transmembrane (black) domains, as well as the PC6B-cd Δ7 (gray), AC1 (stippled), and AC2 (striped) sequences are indicated. (B) AtT-20 cells expressing chimeric protein Tac-Δ7 (A, E, and I), Tac-AC1 (B, F, and J), Tac-AC2 (C, G, and K), or Tac-PC6B (D, H, and L) were incubated with anti-Tac mAb 2A3A1H (6 μg/ml) for 1 h (A–D), fixed, and costained with anti-Tac mAb 7G7 (E–H). Alternatively (I–L), cells were exposed to 10 μg/ml BFA for 20 min before fixation and staining with mAb 7G7. The primary mAbs were distinguished with the use of subtype-specific secondary antisera. (C) Colocalization of Tac/AC chimeras with subcellular markers. AtT-20 cells expressing Tac-AC1 were fixed either directly (A and B) or after a 20-min exposure to 10 μg/ml BFA (C and D). The fixed cells were then processed for double-label immunofluorescence with anti-Tac mAb 7G7 (A and C) and anti-TGN38 (B and D). The primary antibodies were distinguished with the use of species-specific secondary antisera conjugated to either FITC (TGN38) or TXR (TAC-AC1). AtT-20 cells expressing Tac-AC2 were incubated with anti-Tac mAb 2A3A1H (E) and fluorescently labeled Tf (F) for 10 min, fixed, and then processed for immunofluorescence. Internalized mAb 2A3A1H was visualized with the use of a TXR-conjugated secondary antibody. Arrows depict colocalized TAC-AC2 and Tf.
Metabolic labeling analyses showed that the PC6B-cd had no marked effect on the maturation and transit of reporter protein to late secretory pathway compartments (Figure 1C). Parallel plates of cells expressing either fur/f or fur/f-PC6B were pulse labeled with [35S]Met/Cys for 30 min and either harvested immediately or chased in the absence of label for various times. Immunoprecipitation of the cell lysates with mAb M1 (requiring a free N-terminal FLAG tag) showed that the pulse-labeled proteins underwent propeptide cleavage at the correct site (C terminal to Arg107) to generate the mature fur/f (98 kDa) and fur/f-PC6B (100 kDa) forms (Figure 1C, double arrowheads). During the initial chase periods (30 min), a larger, neuraminidase-sensitive molecular weight form (single arrowheads) appeared for both the fur/f (104 kDa) and fur/f-PC6B (106 kDa) constructs, demonstrating transport of the mature fur/f and fur/f-PC6B molecules to late secretory pathway compartments. Upon extended chase periods, both fur/f and fur/f-PC6B were released into the medium via a proteolytic shedding event (Figure 1C). Pulse-chase analysis of the full-length PC6B/f also showed proteolytic shedding with a time course indistinguishable from that of fur/f and the fur/f-PC6B chimera. The level of shed enzyme recovered in the medium (∼70%) indicated that neither construct underwent appreciable intracellular degradation.
Immunofluorescence analysis of the steady-state distribution of the Tac-PC6B (see Figure 5A) and fur/f-PC6B chimeras in AtT-20 cells showed a staining pattern matching that of full-length PC6B, including a concentration in the tips as well as in the paranuclear region (Figure 1D). As seen for the full-length PC6B, the paranuclear staining observed with the chimeric proteins was dispersed upon treatment with BFA, indicating non-TGN localization. Analysis with additional markers further indicated a distinct localization of PC6B. The paranuclear PC6B compartment appeared distinct from recycling endosomes because Tac-PC6B failed to colocalize with internalized fluorescently labeled Tf (loaded at high concentrations to detect the recycling compartment; Figure 1E). In addition, despite the similar staining patterns of Tac-PC6B and the CI-MPR, the two proteins appear to localize to distinct compartments because treatment of cells with BFA causes dispersal of Tac-PC6B but a clustering of the CI-MPR (Figure 1E). These observations demonstrate that the PC6B-cd contains sufficient sorting information to target reporter proteins to intracellular compartments indistinguishable from the full-length enzyme. Furthermore, this analysis indicates a distinct localization of PC6B compared with other TGN/endosomal markers.
Antibody (mAb M1) uptake analyses in AtT-20 cells (Figure 2A) further showed that fur/f, PC6B/f, and the chimeric proteins were all delivered to, and internalized from, the cell surface. However, in agreement with steady-state staining patterns, mAb M1 internalization indicated that the furin and PC6B-cds localized proteins to distinct compartments within the TGN/endosomal system. As described previously (Molloy et al., 1994; Jones et al., 1995), the native furin-cd directs efficient retrieval to the TGN, where the internalized mAb M1 and the postfixation mAb M2 staining patterns overlap. In contrast, PC6B and the PC6B-cd–containing chimeric proteins localized to both a paranuclear BFA-dispersible compartment and a population of punctate structures concentrated in the tips. These data demonstrate that the PC6B-cd can direct internalized proteins to compartments distinct from those of furin. Furthermore, the accessibility of the BFA-sensitive PC6B paranuclear compartment to endocytosed protein suggests that it represents post-TGN localization.
Figure 2.
(A) Comparison of sorting by PC6B and furin cds. AtT-20 cells expressing fur/f (A and B), PC6B/f (C and D), fur/f-PC6B (E and F), or Tac-PC6B (G and H) were incubated with mAb M1 (6 μg/ml) (A, C, and E) or anti-Tac (2A3A1H; 6 μg/ml) (G) for 1 h. The cells were then fixed, incubated with either mAb M2 (B, D, and F) or anti-Tac (7G7) (H), and processed for immunofluorescence. The primary mAbs were distinguished with the use of subtype-specific secondary antisera. (B) AtT-20 cells expressing fur/f-PC6B (A and B) were incubated with mAb M1 (6 μg/ml) and fluorescently labeled Tf (40 ng/ml) for 10 min and then fixed and processed for immunofluorescence. Internalized mAb M1 was visualized with the use of a TXR-conjugated secondary antiserum. Arrows depict colocalized fur/f-PC6B and Tf. AtT-20 cells expressing fur/f-PC6B (C and D) or fur/fS773,775A (E and F) were fixed and double labeled with mAb M1 (C and E) and anti-ACTH (AS-29) (D and F). The primary antibodies were distinguished with the use of fluorescently tagged species-specific secondary antisera conjugated to either FITC (ACTH) or TXR (fur/f-PC6B and fur/fS773,775A). Insets show enlarged tips marked by the boxes. Note the colocalization of ACTH and fur/fS773,775A (F, inset) but not fur/f-PC6B in MSGs (D, inset).
To assess the routing of internalized protein containing the PC6B-cd, cells expressing fur/f-PC6B or Tac-PC6B were incubated with both mAb M1 and fluorescently labeled Tf, which labels early endosomes at low concentrations. After short times of uptake, an overlap of the internalized Tf and mAb M1 was observed and seen to be concentrated in the tips (Figure 2B). If, after an initial 10 min of uptake of mAb M1, cells were chased for 50 min, substantial punctate staining remained in the tips. However, this tip-localized mAb M1 staining no longer colocalized with Tf. Double-labeling experiments (Figure 2B) with the use of mAb M1 and an antibody directed against ACTH, a granule marker, further showed that the vesicular PC6B-cd–directed tip staining of AtT-20s was not localized to mature secretory granules (MSGs). The absence of PC6B from MSGs contrasted with the colocalization of ACTH and a mislocalized, nonphosphorylatable form of furin (fur/fS773,775A) observed under the same conditions (Figure 2B) (Dittiéet al., 1997).
Together, these results demonstrate that the PC6B-cd 1) is sufficient to direct intracellular localization and cell surface internalization, and 2) localizes protein to late secretory pathway compartments with characteristics distinct from those of the TGN and MSGs and that are in communication with early endosomes. The latter observation indicates that the PC6B-cd directs a steady-state distribution different from that of the related endoprotease furin.
Truncation Analysis of the PC6B Cytoplasmic Domain
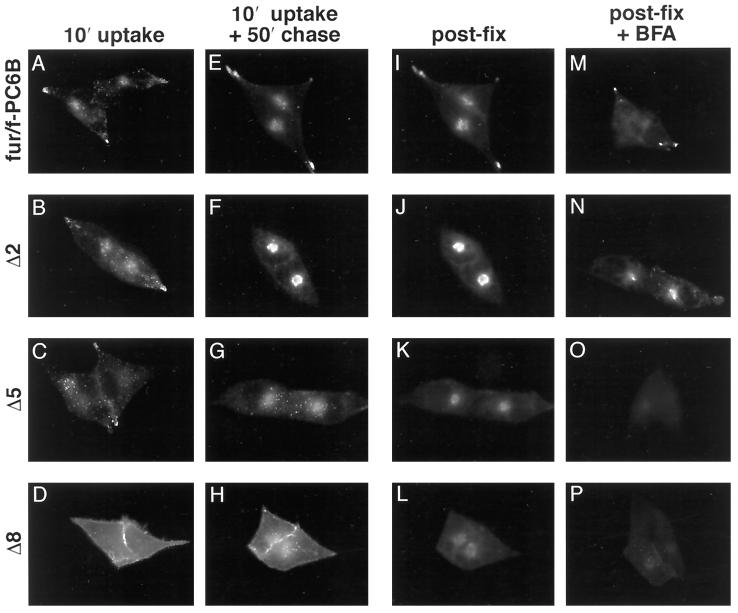
A series of incremental C-terminal truncations of fur/f-PC6B were constructed (Figure 1A) to begin to identify the PC6B-cd trafficking signals. The internalization and localization of each construct in AtT-20 cells were determined by mAb M1 uptake combined with postfixation mAb M2 staining either immediately after a short uptake period or after an additional chase (Figure 3). This protocol provided an assessment of the internalization of the constructs into early endosomes as well as their sorting to later compartments. The analysis revealed three distinct localization patterns represented by Δ1–Δ4, Δ5–Δ7, and Δ8, as described below. Deletion of the C-terminal 11 residues (Δ1; Leu1867–Gln1877) containing a cluster of acidic amino acids (AC2) resulted in a reduction in vesicular tip staining (after a 50-min chase) coupled with a concomitant increase in the paranuclear immunofluorescent signal. This redistribution was more pronounced upon removal of an additional 7 amino acids, including the remainder of the distal acidic cluster (Δ2; Figure 3). Further deletions of Ile1841–Leu1859 (Δ3 and Δ4) produced staining patterns indistinguishable from that of Δ2. Truncation of the next 10 residues (Δ5; Asp1830–Asp1840), including a second, membrane-proximal acidic cluster (AC1), resulted in a more dispersed, endosome-like punctate pattern after uptake and chase. Similar results were observed upon deletion of Val1810–Arg1829 (Δ6 and Δ7). Deletion of the 10 amino acids from Gly1800 to Thr1809 (Δ8), which contain a canonical tyrosine-based CCP recruitment motif (Tyr1802-Glu-Lys-Leu), blocked internalization of the chimera and led to its accumulation at the cell surface.
Figure 3.
Truncation analysis of the PC6B-cd by immunofluorescence. AtT-20 cells expressing either fur/f-PC6B or one of the PC6B-cd truncation constructs (Δ2, Δ5, or Δ8; see Figure 1A) were incubated with mAb M1 (6 μg/ml) for 10 min and fixed either directly (A–D) or after an additional 50-min chase (E–H). The fixed cells in panels E–H were then stained with mAb M2 (I–L). Alternatively (M–P), cells were treated with 10 μg/ml BFA for 20 min before fixation and staining with mAb M1. The primary mAbs were distinguished with the use of subtype-specific secondary antisera.
Treatment of cells expressing the various PC6B-cd truncation constructs with BFA revealed a further distinction in their localization patterns. In contrast to the results with the full-length PC6B-cd, the paranuclear concentration observed with Δ2 condensed with BFA treatment. This result indicates that removal of the distal 18 amino acids from the PC6B-cd leads to a redistribution of the chimeric protein to the TGN. Further truncation (e.g., Δ5), however, resulted in the loss of this TGN pattern. Together, these results indicate that there are at least three prominent sorting signals within the PC6B-cd: a membrane-proximal segment (Gly1800–Thr1809) containing a potential tyrosine-based endocytosis motif; a centrally located acidic cluster, AC1, promoting localization to the TGN; and a distal acidic cluster, AC2, which is required for the characteristic PC6B distribution (i.e., tips and a BFA-sensitive paranuclear compartment).
Identification of the PC6B-cd Internalization Motif
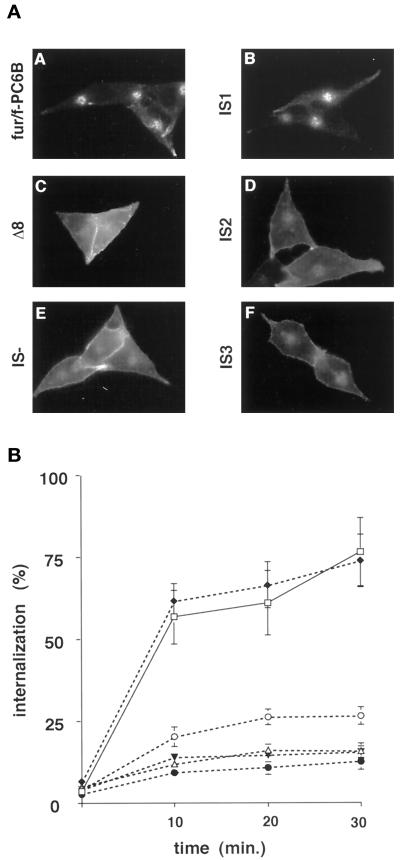
The pronounced accumulation of the Δ8 construct at the cell surface suggested that the tyrosine-based consensus internalization sequence (Tyr1802-Glu-Lys-Leu) directs endocytosis of PC6B. However, two other consensus internalization sequences are also present in the PC6B-cd (Figure 1A): a second tyrosine-based motif (Tyr1856-Gly-Leu-Leu1859) and a contiguous di-leucine motif (LeuLeu1859). To determine the relative contributions of these motifs to PC6B endocytosis, a series of point mutations was constructed. As expected, a fur/f-PC6B chimera in which all three potential internalization signals (IS) were disrupted by substitution with alanines (IS-) showed prominent cell surface staining with no discernible uptake of extracellularly applied mAb M1 (Figure 4A). Mutations in which combinations of two of the three signals were disrupted by alanine substitutions showed differential effects. The IS1 construct, with an intact membrane-proximal tyrosine-based motif (Tyr1802-Glu-Lys-Leu), displayed a staining pattern of internalized mAb M1 indistinguishable from that of fur/f-PC6B. In contrast, the IS2 and IS3 constructs, containing only intact C-terminal tyrosine or di-leucine motifs, respectively, showed significant cell surface staining with reduced internalization.
Figure 4.
Identification of PC6B internalization motifs. (A) AtT-20 cells expressing fur/f-PC6B, Δ8, or one of the fur/f-PC6B CCP mutants (IS-, IS1, IS2, or IS3; see Figure 1A) were incubated with mAb M1 (6 μg/ml) for 1 h and then fixed and processed for immunofluorescence microscopy. (B) AtT-20 cells expressing fur/f-PC6B, Δ8, IS-, IS1, IS2, or IS3 were chilled on ice and incubated with 125I-mAb M1. The washed cells were either harvested directly or warmed to 37°C, incubated for the indicated times, and then harvested. The internalized mAb M1 was measured by counting as described in MATERIALS AND METHODS. Assays were performed in triplicate. Error bars depict SD.
Quantitative uptake studies were performed to verify the differences in the relative effectiveness of the hydrophobic internalization motifs (Figure 4B). The endocytosis of each construct was determined by sequestration of 125I-labeled antibody as described in MATERIALS AND METHODS. The internalization data replicated the results of the immunofluorescence analysis, showing that IS1 alone was sufficient to support endocytosis at a level indistinguishable from that of the native PC6B-cd. In contrast, IS2 and IS3 supported only low levels of internalization. Thus, the point mutant analysis and the truncation studies (Figure 3) indicate that Tyr1802GluLysLeu is the principal internalization signal in the PC6B-cd.
PC6B ACs Direct Compartment-specific Localization
In addition to the internalization motifs, the truncation analysis shown in Figure 3 indicated the presence of at least two AC-containing sorting signals in the PC6B-cd, one between Leu1820 and Asp1840 (AC1) and a second between Leu1859 and the C terminus (AC2) (Figure 1A). To examine the sorting potential of the two ACs, they were analyzed independently. In the case of furin and the LDL receptor, the acidic motifs alone are not sufficient to support normal localization (Matter et al., 1994; Schäfer et al., 1995). Indeed, a CCP recruitment motif is also required to form a bipartite localization signal and allow efficient internalization from the cell surface (Matter et al., 1994; Jones et al., 1995; Schäfer et al., 1995). Our results with the IS mutations indicate that the localization of PC6B also requires an intact internalization motif. Therefore, each PC6B AC (Figure 1A) was fused to a Tac chimera, Tac-Δ7 (Figure 5A), consisting of residues Arg1790–Thr1809 of the PC6B-cd that include the PC6B-cd internalization signal (Tyr1802-Glu-Lys-Leu).
Analysis of the AC1 construct, Tac-AC1, by antibody uptake (mAb 2A3A1H; see MATERIALS AND METHODS) showed a concentrated paranuclear staining pattern that overlapped completely with postfixation (mAb 7G7) staining (Figure 5B). Upon treatment with BFA, the paranuclear staining of Tac-AC1 concentrated in a manner analogous to that seen with TGN-localized furin (compare with Figure 2). Double-label experiments (Figure 5C) further demonstrated that the Tac-AC1 chimera colocalized with the TGN marker TGN38 in both the presence and the absence of BFA, indicating that AC1 constitutes a functional TGN-targeting motif.
In contrast, antibody uptake by Tac-AC2 resulted in a pattern resembling that of PC6B/f and full-length PC6B-cd chimeras, with pronounced labeling of punctate structures in tips and a less concentrated paranuclear component overlapping the postfixation staining (Figure 5B). As seen for constructs containing the full-length PC6B-cd (Figure 1D), BFA treatment caused the paranuclear component of the Tac-AC2 staining pattern to disperse, indicating non-TGN localization. Furthermore, the peripheral vesicular staining observed for Tac-AC2 with antibody uptake overlapped with internalized Tf (Figure 5C), indicating transport through early endosomes. Thus, unlike AC1, the AC2 region confers a distribution similar to that of the full-length PC6B-cd, including both endosomal and BFA-dispersible paranuclear compartments.
PC6B AC1 Binds to the TGN Sorting Protein PACS-1
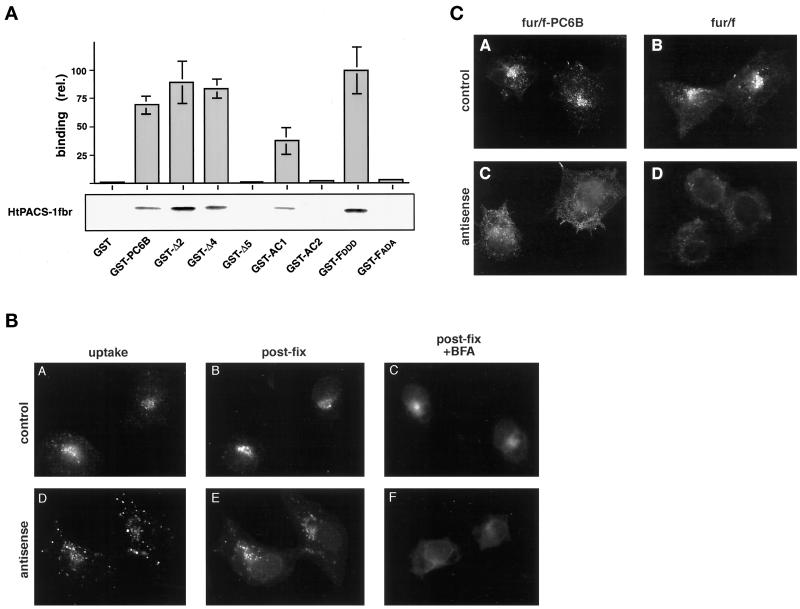
A protein–protein binding assay was used to test the ability of the PC6B-cd to interact with PACS-1 (Figure 6A). A His-tagged construct composed of the 141-amino acid fragment of PACS-1, including the furin-cd binding region, HtPACS-1fbr, was incubated with GST or GST fusion proteins containing the entire PC6B-cd, the Δ2, Δ4, or Δ5 truncated cd (Figure 1A), or the individual AC1 and AC2 regions. As controls, two GST furin-cd constructs, GST-FDDD, a positive control, and GST-FADA, a negative control, were also tested (Wan et al., 1998). In analyses of test constructs, binding of HtPACS-1fbr to the entire PC6B-cd was readily detected. Analysis of the PC6B-cd truncations showed that constructs containing the AC1 sequence (Δ2cd and Δ4cd) bound HtPACS-1fbr, whereas Δ5cd, a PC6B-cd truncation lacking the AC1 sequence, did not. Furthermore, and consistent with the localization of Tac-AC1 to the TGN, the GST construct containing only the 19-residue AC1 bound HtPACS-1fbr. In contrast, the analogous AC2 construct did not support binding in these assays. This differential PACS-1 binding by AC motifs is similar to that reported previously for the CI-MPR (Wan et al., 1998).
Figure 6.
Role of PACS-1 in PC6B AC1 targeting. (A) In vitro binding of PC6B-cd and the PACS-1fbr. HtPACS-1fbr was incubated with either GST or GST fusion proteins containing either furin-cd constructs (GST-FDDD, a positive control, and GST-FADA, a negative control) or PC6B-cd sequences including the full-length PC6B-cd, the Δ2cd, Δ4cd, and Δ5cd truncation mutants, or AC1 or AC2. Bound HtPACS-1fbr was separated with glutathione beads, resolved by SDS-PAGE, and visualized by Western blotting with the anti-PACS-1fbr antiserum 678. Binding was quantified and expressed relative to that observed with GST-FDDD (21% of input HtPACS-1fbr). Data represent averages from three independent experiments. Error bars represent SD. (B) PACS-1 is required for AC1-directed TGN localization. Parallel cultures of PACS-1 control (A–C) and PACS-1 antisense (D–F) cells expressing Tac/AC1 were incubated with anti-Tac mAb 2A3A1H (6 μg/ml) for 1 h, fixed, and costained with anti-Tac mAb 7G7. Cells in panels C and F were incubated with 10 μg/ml BFA for 20 min before fixation and staining with anti-Tac mAb 7G7. The primary mAbs were distinguished with the use of subtype-specific secondary antisera. (C) Parallel cultures of PACS-1 control (A and B) and PACS-1 antisense (C and D) cells expressing fur/f-PC6B (A and C) or fur/f (B and D) were incubated with mAb M1 (6 μg/ml) for 1 h, fixed, and processed for immunofluorescence microscopy.
PACS-1 Is Required for AC1-directed TGN Localization
The results of the binding assays suggested that the ability of AC1 to function as a TGN localization signal is dependent on its interaction with PACS-1. To examine this possibility, Tac-AC1 was expressed in either PACS-1 antisense or control (empty vector) cells (see MATERIALS AND METHODS). The antisense cells were used previously to establish the requirement of PACS-1 for the localization of furin (Wan et al., 1998). Antibody uptake in control cells showed that Tac-AC1 cycles to the cell surface and is retrieved to the TGN, where the staining pattern overlaps with that of postfixation staining. In contrast, in PACS-1 antisense cells, antibody uptake failed to concentrate in the paranuclear region and resulted in a dispersed punctate staining pattern. Furthermore, the postfixation staining pattern was distended from the paranuclear region and, unlike Tac-AC1 in control cells, dispersed after treatment with BFA. Together, these results demonstrate that AC1-directed sorting to the TGN requires PACS-1 and further suggest that AC1 functions in a manner analogous to the phosphorylated form of the furin-cd AC.
The interaction of AC1 with PACS-1 suggested that localization of PC6B was dependent, in part, on PACS-1. To examine this possibility, the localization of fur/f-PC6B and fur/f was examined in parallel plates of PACS-1 control and antisense cells (Figure 6C). Antibody uptake studies showed that in control cells, fur/f-PC6B was localized to a paranuclear compartment, whereas in PACS-1 antisense cells, this construct was mislocalized and exhibited a diffuse staining pattern. Similarly, and in agreement with previous studies (Molloy et al., 1998; Wan et al., 1998), TGN localization of fur/f required expression of PACS-1. These results suggest that steady-state localization of PC6B requires cycling through the TGN or some other PACS-1–dependent step.
The PC6B Compartment Is Mobilized by Stimulated Secretion
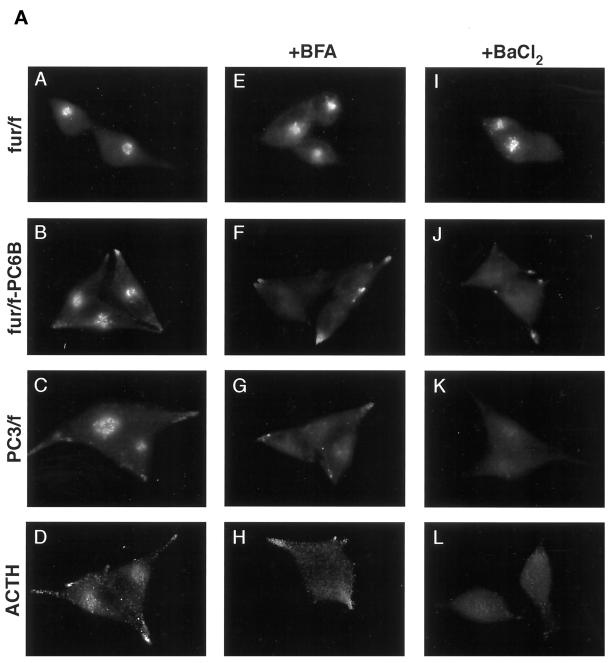
To help define the paranuclear, BFA-dispersible compartment in which PC6B localizes at steady state, the localization of several markers of the late secretory pathway was compared in AtT-20 cells (Figure 7A). Both ACTH and the epitope (FLAG)-tagged form of the neuroendocrine-specific endoprotease PC3 (PC3/f; visualized with mAb M1) show staining patterns resembling that of PC6B at steady state, including both paranuclear and tip staining. This distribution is consistent with the presence of ACTH and PC3 in multiple compartments of the regulated secretory pathway, i.e., immature secretory granules and MSGs. Interestingly, like PC6B, the paranuclear staining for both of these regulated pathway markers dispersed upon treatment with BFA. This similarity prompted an analysis of protein localization after treatment with BaCl2 to stimulate secretory granule exocytosis. When AtT-20 cells were treated with BaCl2, the paranuclear pools of ACTH, PC3/f, and fur/f-PC6B were depleted. This BaCl2 effect correlated with the enhanced release of β-endorphin immunoreactivity into the culture medium (Figure 7B). However, in agreement with a lack of PC6B in MSGs (Figure 2B), the tip-localized fur/f-PC6B was unaffected by the secretagogue. Furthermore, this effect was specific for the BFA-dispersible markers, because the paranuclear staining for TGN-localized furin was unaffected by the secretagogue. Together, these data indicate that the paranuclear component of the PC6B staining pattern shares some characteristics with those of known regulated secretory pathway markers.
Figure 7.
(A) Furin and PC6B cds impart differential sensitivity to stimulated release. AtT-20 cells expressing fur/f (A, E, and I), fur/f-PC6B (B, F, and J), or PC3/f (C, G, and K) were fixed either directly or after exposure to either 10 μg/ml BFA for 20 min (E–H) or 3 mM BaCl2 for 1 h (I–L). The cells were then labeled after fixation with mAb M1 or antibody against ACTH (D, H, and L). (B) Stimulated release of ACTH. AtT-20 cells were incubated for 1 h with either control media or 3 mM BaCl2, and released β-endorphin immunoreactivity was quantified by radioimmunoassay (see MATERIALS AND METHODS). Data represent the average values of three independent experiments. Error bars represent SD. (C) PC6B AC2 is sufficient for targeting to the BaCl2-responsive compartment. AtT-20 cells expressing Tac-AC1 (A and B) or Tac-AC2 (C and D) were fixed either before (A and C) or after (B and D) exposure to 3 mM BaCl2, stained with anti-Tac mAb 7G7, and processed for immunofluorescence.
PC6B AC2 Imparts Sensitivity to the Secretagogue
To examine the ability of the PC6B acidic clusters to replicate the BaCl2 sensitivity imparted by the full-length PC6B-cd, the Tac-AC1 and Tac-AC2 constructs were analyzed by immunofluorescence microscopy as described above. Consistent with its similarity to furin and localization to the TGN, the steady-state distribution of Tac-AC1 was unaffected by BaCl2 treatment (Figure 7C). In contrast, the paranuclear staining observed for Tac-AC2 was depleted. This indicates that AC2, in conjunction with the IS1 CCP recruitment motif (Tyr1802GluLysLeu), can replicate several characteristics of PC6B trafficking, including localization to a BFA-dispersible, BaCl2-responsive paranuclear compartment that is in communication with the endocytic pathway.
DISCUSSION
These studies show that the mammalian PCs furin and PC6B have distinct distributions within the TGN/endosomal pathway. Although furin localizes to the TGN, PC6B accumulates in a BFA-dispersible and BaCl2-responsive paranuclear compartment and in the tips of AtT-20 cells (Figures 1B and 7A). Furthermore, we demonstrated that the PC6B-cd contains targeting information that directs endocytosis and promotes localization of reporter proteins to compartments characteristic of full-length PC6B (Figures 1 and 2). A combination of truncation, point mutation, and chimeric constructs further showed that the trafficking information contained within the PC6B-cd is distributed between multiple sorting motifs. These include a tyrosine-based motif for internalization (Tyr1802-Glu-Lys-Leu) as well as two clusters of acidic residues, or ACs, each of which imparts a distinct intracellular localization (Figures 3–5). The function of the membrane-proximal PC6B AC, AC1, is dependent on a previously identified sorting protein, PACS-1, which directs the TGN localization of multiple AC-containing proteins (including the related endoprotease furin [Wan et al., 1998]). In contrast, the distal AC, AC2, which does not efficiently bind PACS-1, promotes a localization similar to that of PC6B itself.
Intracellular Localization May Determine Distinct Roles for PCs In Vivo
Furin and PC6B have several common characteristics in addition to their structural similarity. Both are delivered to the plasma membrane and recycled via Tf-containing early endosomes (Figure 2) (Jones et al., 1995; Molloy et al., 1998), and both share enzymatic features such as overlapping cleavage site specificities and sensitivity to the selective inhibitor α1-PDX (Jean et al., 1998). Furthermore, both furin and PC6B have been implicated in the processing of common substrates, including neurotrophins and BMP-4 (Seidah et al., 1996; Cui et al., 1998). Despite these similarities, our data show that, at steady state, PC6B and furin are concentrated in different parts of the TGN/endosomal system. Such differential localization may facilitate the activation of substrates within distinct cellular processing compartments in vivo and also may help explain the inability of embryonic PC6B to compensate for the absence of furin in knockout mice (Roebroek et al., 1998). This would contrast with furin and PC7, which colocalize within the TGN but have distinct substrate specificities (van de Loo et al., 1997; Jean et al., 1998; J. Christian, personal communication). Thus, a combination of differential cleavage site specificity and subcellular localization may contribute to the requirement for multiple PCs within TGN/endosomal compartments. Indeed, whereas some furin (or PC6B) substrates are reported to be cleaved in the TGN, others are apparently cleaved in post-TGN compartments within the biosynthetic pathway (Sariola et al., 1995).
Communication between Multiple Post-TGN Processing Compartments
Although the accumulation of PC6B in a paranuclear compartment is consistent with a previous description of Golgi-like staining (De Bie et al., 1996), our data showing dispersal by BFA (Figures 1B and 7A) suggest that this pattern is not indicative of TGN localization. Similarly, and also in agreement with De Bie et al. (1996), the lack of overlap between PC6B and a marker for MSGs indicates that the enzyme does not concentrate in late compartments of the regulated secretory pathway (Figures 2B and 7A). However, the redistribution of PC6B upon treatment of cells with secretagogue (Figure 7A) suggests that the paranuclear pool of PC6B is in communication with the regulated pathway. One possibility is that, like furin and the CI-MPR, PC6B enters early compartments of the regulated pathway only to be removed during the granule maturation process. Although MPR and furin are likely to be retrieved ultimately to endosomes and the TGN, respectively, PC6B may be transported to a distinct endosomal compartment. Upon stimulation of secretion, the enhanced membrane flux may shift the equilibrium of the PC6B retrieval pathway, leading to a depletion of the paranuclear pool (as seen for the bona fide regulated pathway proteins ACTH and PC3; Figure 7A). This is in contrast to what is seen with furin, which still appears to be effectively localized to the TGN (Figure 7A).
This link with regulated secretion suggests several similarities between PC6B and another secretory pathway protein, peptidylglycine α-amidating monooxygenase (PAM), which catalyzes essential modifications in the biosynthesis of neuroendocrine peptides (Milgram et al., 1997). Like PC6A and PC6B, this bifunctional protein is expressed in multiple isoforms, including one (PAM1) with transmembrane and cytosolic domains. As described here for PC6B, the PAM1-cd contains trafficking information that directs retrieval from the cell surface and concentration in a perinuclear region showing partial overlap with TGN markers. In addition, the truncated, soluble isoforms of both PAM and PC6 (PC6A) are localized to MSGs. Furthermore, the PAM1-cd is thought to direct its retrieval from the maturing granule. Although the exact roles of the transmembrane isoforms of PC6 and PAM have not been elucidated, they could represent key components of a post-TGN endosomal protein-processing system. The observation that PC6B and furin maintain their distinct distributions in epithelial cells (our unpublished data) indicates that any such specialized role for PC6B may not be limited to neuroendocrine cell types.
Protein Sorting by Bipartite AC Motifs
The steady-state localization of furin to the TGN is dependent on phosphorylation of a pair of serine residues that form CK2 phosphorylation sites within its single AC (Jones et al., 1995; Takahashi et al., 1995). Phosphorylation of the AC regulates the binding of PACS-1 to both the furin-cd and the varicellar zoster virus glycoprotein E–cd (Wan et al., 1998). Although the PC6B AC1 also functions as a PACS-1–dependent TGN localization signal (Figures 5–7), its binding is not phosphorylation dependent (Figure 6). This result is consistent with our recent demonstraction that PACS-1 binds to the nonphosphorylatable AC in the HIV-1 Nef (Piguet et al., 2000) and indicates that PACS-1 can participate in the sorting of additional proteins with nonphosphorylatable ACs. Although it is not yet known how the interaction between PC6B-cd and PACS-1 may be regulated in vivo, these results indicate that it is independent of AC1 modification. The observation that PACS-1 is a phosphoprotein that can be recruited to membranes (Wan et al., 1998) suggests that its phosphorylation state and/or subcellular localization could modulate binding.
Although the PC6B AC1 can function as a TGN localization signal, the distal AC (AC2) appears to mediate a different sorting step, producing an accumulation in the BFA-dispersed paranuclear compartment and tips of AtT-20 cells. The distinct localization pattern obtained for AC2-directed sorting suggests that this AC may interact with a different member of the PACS family or perhaps an unrelated binding partner. Regardless of its effector, the ability of AC2 to localize a reporter protein to the same compartments as the full-length PC6B-cd (compare Figures 2 and 5) suggests that it is the dominant signal for directing steady-state localization of PC6B. Although our data indicate that AC2 is the dominant sorting signal, the mislocalization of fur/f-PC6B in the PACS-1 antisense cells (Figure 6C) indicates a role for AC1 in the steady-state localization of PC6B. It is possible that PC6B cycles through the TGN in an AC1-dependent manner to maintain its steady-state localization. Furthermore, it seems likely that under some conditions AC1 may play a more prominent role, perhaps redistributing PC6B to the TGN in response to particular stimuli. This potential for dynamic interplay between multiple AC motifs could also arise for additional proteins routed through the TGN/endosomal system, such as CI-MPR.
The importance of an intact internalization signal for PC6B-cd–directed sorting (Figure 4) is consistent with previous observations showing that, although AC motifs can be dominant localization signals, they generally function in the context of additional sorting information (Molloy et al., 1999). Indeed, the idea of bipartite localization signals has arisen in part from studies of AC-directed furin and LDL receptor trafficking (Matter et al., 1994; Jones et al., 1995; Schäfer et al., 1995; Simmen et al., 1999). Although the phosphorylated furin AC is a TGN localization signal, it requires the presence of an independent internalization motif for efficient targeting (Matter et al., 1994; Jones et al., 1995; Schäfer et al., 1995; Simmen et al., 1999). Similarly, clusters of acidic residues with nearby tyrosine- and phenylalanine-based motifs regulate the basolateral sorting of LDL receptor and furin in polarized epithelial cells (Matter et al., 1994; Simmen et al., 1999). One explanation for these observations is that the CCP recruitment motifs are essential for directing the proteins into vesicles that bud from the cell surface and/or the TGN. Once in endosomal compartments, however, the AC motifs act to localize the protein within the TGN/endosomal system. Although the point mutation analysis shown here indicates that the Tyr1802GluLysLeu motif is the most efficient internalization signal in the PC6B-cd, it does not rule out an important role for the Tyr1856GlyLeuLeu sequence in modulating the action of one or both of the ACs or in a separate routing step.
Together, the results discussed here highlight several interesting aspects of protein sorting by cytosolic targeting motifs, including 1) the importance of ACs in regulating multiple steps in the TGN/endocytic trafficking pathway, 2) the ability of structurally similar motifs to produce unique patterns of localization (e.g., the phosphorylatable furin AC and PC6B AC1 versus PC6B AC2), and 3) the relationship between differential interaction with cytosolic binding proteins and AC function. In addition, the differential localization observed for furin and PC6B suggests that these enzymes have unique properties and may activate proproteins in distinct subcellular compartments. The continued study of the proprotein convertases will help establish the importance of localization within the TGN/endosomal system in determining their functions in vivo and will provide insight into the mechanics and regulation of protein sorting.
ACKNOWLEDGMENTS
The authors thank J. Christian and members of the Thomas laboratory for reading of the manuscript and helpful comments. We thank B. Eipper, B. Hoflack, F. Maxfield, S. Milgram, and S. Tooze for reagents. Y.X. is the recipient of a Tartar Trust fellowship. This work was supported by National Institutes of Health grants DK37274 and DK44629.
Abbreviations used:
- AC
acidic cluster
- ACTH
adrenocorticotrophic hormone
- BFA
brefeldin A
- CCP
clathrin-coated pit
- cd
cytoplasmic domain
- CI-MPR
cation-independent mannose-6-phosphate receptor
- CK2
casein kinase 2
- IS
internalization signal
- LDL
low-density lipoprotein
- MSG
mature secretory granule
- oligo
oligonucleotide
- PAM
peptidylglycine α-amidating monooxygenase
- PC
proprotein convertase
- Tac
T cell receptor α chain
- Tf
transferrin
- TGN
trans-Golgi network
- TXR
Texas Red
REFERENCES
- Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- Boker C, von Figura K, Hille-Rehfeld A. The carboxy-terminal peptides of 46 kDa and 300 kDa mannose 6-phosphate receptors share partial sequence homology and contain information for sorting in the early endosomal pathway. J Cell Sci. 1997;110:1023–1032. doi: 10.1242/jcs.110.8.1023. [DOI] [PubMed] [Google Scholar]
- Bosshart H, Humphrey J, Deignan E, Davidson J, Drazba J, Yuan L, Oorschot V, Peters PJ, Bonifacino JS. The cytoplasmic domain mediates localization of furin to the trans-Golgi network en route to the endosomal/lysosomal system. J Cell Biol. 1994;126:1157–1172. doi: 10.1083/jcb.126.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnahan PA, Leduc R, Thomas L, Thorner J, Gibson HL, Brake AJ, Barr PJ, Thomas G. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves Pro-β-NGF in vivo. J Cell Biol. 1990;111:2851–2859. doi: 10.1083/jcb.111.6.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RE, Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO J. 1994;13:2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Remmler J, Delaney JC, Messner DJ, Lobel P. Mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor: a consensus casein kinase II site followed by 2 leucines near the carboxyl terminus is important for intracellular targeting of lysosomal enzymes. J Biol Chem. 1993;268:22338–22346. [PubMed] [Google Scholar]
- Cui Y, Jean F, Thomas G, Christian JL. BMP-4 is proteolytically activated by furin and/or PC6 during vertebrate embryonic development. EMBO J. 1998;17:4735–4743. doi: 10.1093/emboj/17.16.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bie I, Marcinkiewicz M, Malide D, Lazure C, Nakayama K, Bendayan M, Seidah NG. The isoforms of proprotein convertase PC5 are sorted to different subcellular compartments. J Cell Biol. 1996;135:1261–1275. doi: 10.1083/jcb.135.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittié AS, Thomas L, Thomas G, Tooze SA. Interaction of furin in immature secretory granules from neuroendocrine cells with the AP-1 adaptor complex is modulated by casein kinase II phosphorylation. EMBO J. 1997;16:4859–4870. doi: 10.1093/emboj/16.16.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng FJ, Varlamov O, Fricker LD. Sequences within the cytoplasmic domain of Gp180/carboxypeptidase D mediate localization to the trans-Golgi network. Mol Biol Cell. 1999;10:35–46. doi: 10.1091/mbc.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick JS, Wolfgang WJ, Forte MA, Thomas G. A unique Kex2-like endoprotease from Drosophila melanogaster is expressed in the central nervous system during early embryogenesis. J Neurosci. 1992;12:705–717. doi: 10.1523/JNEUROSCI.12-03-00705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean F, Stella K, Thomas L, Liu G, Xiang Y, Reason AJ, Thomas G. α1-Antitrypsin Portland, a bioengineered serpin highly selective for furin: application as an antipathogenic agent. Proc Natl Acad Sci USA. 1998;95:7293–7298. doi: 10.1073/pnas.95.13.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BG, Thomas L, Molloy SS, Thulin CD, Fry MD, Walsh KA, Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Thomas L, Warren RA, Enns CA, Cunningham CC, Hartwig JH, Thomas G. Cytoskeletal protein ABP-280 directs the intracellular trafficking of furin and modulates proprotein processing in the endocytic pathway. J Cell Biol. 1997;139:1719–1733. doi: 10.1083/jcb.139.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Ohno H, Kirchhausen T, Bonifacino JS. Protein sorting by tyrosine-based signals. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- Matter K, Yamamoto EM, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauxion F, Le Borgne R, Munier-Lehmann H, Hoflack B. A casein kinase II phosphorylation site in the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor determines the high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2171–2178. doi: 10.1074/jbc.271.4.2171. [DOI] [PubMed] [Google Scholar]
- Mbikay M, et al. Impaired fertility in mice deficient for the testicular germ-cell protease PC4. Proc Natl Acad Sci USA. 1997;94:6842–6846. doi: 10.1073/pnas.94.13.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram SL, Kho ST, Martin GV, Mains RE, Eipper BA. Localization of integral membrane peptidylglycine alpha-amidating monooxygenase in neuroendocrine cells. J Cell Sci. 1997;110:695–706. doi: 10.1242/jcs.110.6.695. [DOI] [PubMed] [Google Scholar]
- Molloy SS, Anderson E, Jean F, Thomas G. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- Molloy SS, Thomas L, Kamibayashi C, Mumby MC, Thomas G. Regulation of endosome sorting by a specific PP2A isoform. J Cell Biol. 1998;142:1399–1411. doi: 10.1083/jcb.142.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy SS, Thomas L, VanSlyke JK, Stenberg PE, Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 1994;13:18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Murakami K, Nakayama K. Identification of an isoform with an extremely large Cys-rich region of PC6, a Kex2-like processing endoprotease. FEBS Lett. 1993;327:165–171. doi: 10.1016/0014-5793(93)80163-o. [DOI] [PubMed] [Google Scholar]
- Nakayama K. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J. 1997;327:625–635. doi: 10.1042/bj3270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet V, Wan L, Borel C, Mangasarian A, Demaurex N, Thomas G, Trono D. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat Cell Biol. 2000;2:163–167. doi: 10.1038/35004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroek AJ, Umans L, Pauli IG, Robertson EJ, van Leuven F, van de Ven WJ, Constam DB. Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase furin. Development. 1998;125:4863–4876. doi: 10.1242/dev.125.24.4863. [DOI] [PubMed] [Google Scholar]
- Sariola M, Saraste J, Kuismanen E. Communication of post-Golgi elements with early endocytic pathway: regulation of endoproteolytic cleavage of Semliki Forest virus p62 precursor. J Cell Sci. 1995;108:2465–2475. doi: 10.1242/jcs.108.6.2465. [DOI] [PubMed] [Google Scholar]
- Schäfer W, Stroh A, Berghofer S, Seiler J, Vey M, Kruse ML, Kern HF, Klenk HD, Garten W. Two independent targeting signals in the cytoplasmic domain determine trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A, Kornfeld S, Rohrer J. Proper sorting of the cation-dependent mannose 6-phosphate receptor in endosomes depends on a pair of aromatic amino acids in its cytoplasmic tail. Proc Natl Acad Sci USA. 1997;94:14471–14476. doi: 10.1073/pnas.94.26.14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Pareek S, Savaria D, Hamelin J, Goulet B, Laliberte J, Lazure C, Chretien M, Murphy RA. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem J. 1996;314:951–960. doi: 10.1042/bj3140951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Chretien M, Day R. The family of subtilisin/kexin like pro-protein and pro-hormone convertases: divergent or shared functions. Biochimie. 1994;76:197–209. doi: 10.1016/0300-9084(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Simmen T, Nobile M, Bonifacino JS, Hunziker W. Cycling of furin occurs via the basolateral surface of MDCK cells and requires a phenylalanine-isoleucine motif together with an acidic amino acid cluster. Mol Cell Biol. 1999;19:3136–3144. doi: 10.1128/mcb.19.4.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- Steiner DF, Smeekens SP, Ohagi S, Chan SJ. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992;267:23435–23438. [PubMed] [Google Scholar]
- Takahashi S, Nakagawa T, Banno T, Watanabe T, Murakami K, Nakayama K. Localization of furin to the trans-Golgi network and recycling from the cell surface involves Ser and Tyr residues within the cytoplasmic domain. J Biol Chem. 1995;270:28397–28401. doi: 10.1074/jbc.270.47.28397. [DOI] [PubMed] [Google Scholar]
- Thorne BA, Caton LW, Thomas G. Expression of mouse proopiomelanocortin in an insulinoma cell line: requirements for β-endorphin processing. J Biol Chem. 1989;264:3545–3552. [PubMed] [Google Scholar]
- van de Loo JW, Creemers JW, Bright NA, Young BD, Roebroek AJ, Van de Ven WJ. Biosynthesis, distinct post-translational modifications, and functional characterization of lymphoma proprotein convertase. J Biol Chem. 1997;272:27116–27123. doi: 10.1074/jbc.272.43.27116. [DOI] [PubMed] [Google Scholar]
- VanSlyke JK, Thomas L, Thomas G. Use of vaccinia virus vectors to study neuropeptide processing. In: Smith AI, editor. Peptidases and Neuropeptide Processing. Vol. 23. San Diego, CA: Academic Press; 1995. pp. 45–64. [Google Scholar]
- Voorhees P, Deignan E, van Donselaar E, Humphrey J, Marks MS, Peters PJ, Bonifacino JS. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Molloy SS, Thomas L, Liu G, Xiang Y, Rybak SL, Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Hao Y, Gershon MD, Ambron RT, Gershon AA. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]