Abstract
Objective
The objective of this study was to determine if two-dimensional ultrasound adds diagnostic information to that provided by the examination of three-dimensional/four-dimensional (3D/4D) volume datasets alone.
Material and methods
Ninety-nine fetuses were examined by 3D/4D volume ultrasonography. Volume datasets were evaluated by a blinded independent examiner who, after establishing an initial diagnostic impression by 3D/4D ultrasonography, performed a 2D ultrasound examination. The frequency of agreement and diagnostic accuracy of each modality to detect congenital anomalies was calculated and compared.
Results
Fifty-four normal and 45 fetuses with 82 anomalies diagnosed by 2D ultrasonography were examined. Agreement between 3D/4D and 2D ultrasonography occurred for 90.4% of the findings (123/136; intraclass correlation coefficient: 0.834, 95% CI: 0.774-0.879). Six anomalies were missed by 3D/4D when compared to 2D ultrasonography [(ventricular septal defect (n=2), interrupted inferior vena cava with azygous continuation (n=1), tetralogy of Fallot (n=1), horseshoe kidney (n=1), and cystic adenomatoid malformation (n=1)). There were two discordant diagnoses: transposition of the great arteries diagnosed as a double outlet right ventricle, and pulmonary atresia misinterpreted as tricuspid atresia by 3D/4D ultrasonography. One case of occult spinal dysraphism was suspected by 3D but not confirmed by 2D ultrasonography. When compared to diagnoses performed after delivery (n=106), the sensitivity and specificity of 3D/4D [92.2% (47/51) and 76.4% (42/55)] and 2D ultrasonography [96.1% (49/51) and 72.7% (40/55)] were not significantly different (p=0.233).
Conclusion
Information provided by 2D ultrasonography is consistent, in the majority of cases, with information provided by the examination of 3D/4D volume datasets alone.
Keywords: Three-dimensional, four-dimensional, 3D, 4D, sonographic tomography, prenatal diagnosis, accuracy, congenital anomalies, fetus, STIC
Introduction
Three-dimensional (3D) ultrasonography has been increasingly used for examination of the human fetus. This technology allows examiners to move from a 3D mental reconstruction of two-dimensional (2D) images to actual 3D visualization of anatomical structures.1 Thus, sonologists are no longer constrained by limitations of static 2D images to establish a diagnosis, but can, instead, interact with volume datasets to examine anatomical structures of interest in planes of section other than the original acquisition planes.2–5 Other potential benefits of 3D ultrasonography include: (1) the ability to review volume data interactively after the patient has left the examination room;3,5 (2) the availability of a variety of rendering methods that allows visualization of different characteristics of the same structure (e.g., the same volume dataset of the fetal back can reveal the external aspect of a meningomyelocele when rendered using the surface mode or, alternatively, the underlying bones when the volume dataset is rendered using the maximum intensity mode6); (3) the possibility of rotating the volume dataset and examine anatomical structures from different perspectives;7 (4) improved accuracy for volume measurements, including volume measurements of irregular objects;8–13 (5) the possibility to standardize ultrasound examinations;3,14,15 (6) the ability to transmit data over networks for consultation in tertiary care centers;3,16–18 and (7) the potential to use offline software programs as an interactive educational tool.5,19 More recently, motion information has been incorporated into 3D volume datasets [four-dimensional (4D) ultrasonography], and the fetal heart can now be examined using either spatiotemporal image correlation (STIC)20,21 or 2D matrix array technology.22–25
Traditionally, 3D/4D ultrasonography has been used as an adjunctive imaging modality to 2D ultrasonography. Thus, the current paradigm consists of performing 3D/4D ultrasonography as part of a target scan, after an initial diagnostic impression has been established by 2D ultrasonography. Benacerraf et al.14,15 proposed that 3D/4D ultrasonography could be used as the primary imaging modality to perform a complete fetal anatomical survey, and coined the term “sonographic tomography” to describe this novel approach to obstetrical ultrasound. Potential advantages of sonographic tomography over conventional 2D ultrasonography include: (1) less dependency on the operator; (2) decreased scanning time; and (3) the possibility of standardizing the entire process of performing an examination.15 However, whether examiners can rely exclusively on the evaluation of 3D/4D volume datasets to diagnose congenital anomalies remains to be determined. This study was conducted to: (1) determine if 2D ultrasound adds diagnostic information to what is provided by the examination of 3D/4D volume datasets alone; and (2) compare the diagnostic accuracy of 3D/4D and 2D ultrasonographic examinations for the diagnosis of congenital anomalies confirmed after birth.
Material and Methods
Ninety-nine fetuses (91 singletons and 4 pairs of twins) were prospectively examined by two sonographers, each with more than one year of experience in 3D ultrasonography. Sonographers were instructed to acquire representative 3D/4D volume datasets of the fetal anatomy, including the head, face, chest, abdomen, limbs and spine. Volume acquisitions were performed using both transverse and longitudinal sweeps through the maternal abdomen. The goal of the examination was to document fetal anatomical structures and congenital anomalies as thoroughly as possible. Decisions regarding scanning technique and number of volume datasets to be acquired in each study were left to the discretion of the sonographer performing the examination. In addition, no time limit to conclude the scan was pre-determined. The fetal heart was examined using 4D ultrasonography with STIC. All 3D/4D examinations were performed with Voluson 730 equipment (Voluson 730 Expert, GE Healthcare, Milwaukee, WI). Patients were enrolled in research protocols approved by the Institutional Review Boards of both Wayne State University, Detroit, Michigan, and the National Institute of Child Health and Human Development, Bethesda, Maryland. All patients gave written informed consent before participating in the study.
After acquisition of the volume datasets by the sonographers, an independent examiner blinded to the indication for the examinations was allowed to enter the room. Three-dimensional volume datasets were explored, using either multiplanar or rendering techniques, by the independent examiner, who established an initial diagnostic impression by 3D/4D ultrasonography alone. Immediately after examination of the 3D/4D volume datasets, the same examiner performed a 2D ultrasound examination and established a final ultrasonographic diagnosis.
Findings by 3D/4D ultrasonography were compared to those of 2D ultrasonography. The frequency of agreement between the two modalities was calculated and the inter-method reliability was tested by the intraclass correlation coefficient. In case of discordance between 3D/4D and 2D ultrasonographic findings, volume datasets were re-evaluated to determine if the information provided by 2D ultrasonography could have been present in the 3D volume dataset, but overlooked by the examiner during the prospective evaluation.
In 79 cases, complete post-natal follow-up was available and, thus, it was possible to compare the diagnoses established by 3D/4D and 2D ultrasonography against those established by examination of the newborn or autopsy. Sensitivity and specificity, as well as the degree of agreement (kappa index of agreement) for the diagnosis of congenital anomalies by each modality were calculated and compared using the McNemar-Bowker’s test. Statistical analysis was performed with SPSS version 12.0 (SPSS Inc., Chicago, IL), Microsoft Excel 2003 for Windows (Microsoft Corporation, Redmond, WA) and NCSS PASS 2004 (NCSS, Kaysville, UT).
Results
Of the 99 fetuses examined, 54 were considered normal and 45 fetuses had 82 congenital anomalies diagnosed by 2D ultrasonography (Table I). The mean gestational age (± SD) at the time of examination was 24.4 ± 6.5 weeks. Complete agreement between 2D and 3D/4D ultrasonography was observed for 90.4% of the findings (123/136; intraclass correlation coefficient: 0.834, 95% CI: 0.774-0.879). Anomalies missed by 3D/4D when compared to 2D ultrasonography were: ventricular septal defects (VSD; n=2), interrupted inferior vena cava with azygous continuation (n=1; Figure 1), tetralogy of Fallot (n=1; Figure 2), horseshoe kidney (n=1), and cystic adenomatoid malformation (n=1; Table II). One of the cases of VSD suspected by 2D, but not by 3D/4D ultrasonography, occurred at 12 4/7 weeks of gestation in a fetus with cystic hygroma and hydrops. In three cases, a VSD suspected by 3D/4D could not be confirmed by 2D ultrasonography (Table II, cases 19, 37 and 45). The fourth case occurred in a fetus with a lemon-shaped head for which coronal views of the spine obtained by 3D/4D ultrasonography suggested splaying of the lateral pedicles in the lumbar region, leading to the hypothesis of occult spinal dysraphism. The fetus had a small occipital encephalocele, which was missed by both 3D/4D and 2D ultrasonography.
Table I.
Abnormalities identified by two-dimensional (2D) ultrasound in 45 fetuses with congenital anomalies.
| Findings | N |
|---|---|
| Central Nervous System | |
| Absent cavum septum pellucidum | 1 |
| Agenesis of the corpus callosum | 2 |
| Anencephaly | 2 |
| Arachnoid cyst | 1 |
| Dandy-Walker variant | 1 |
| Encephalocele | 1 |
| Ventriculomegaly | 1 |
| Spina bifida | 3 |
| Cardiac | |
| Absent pulmonary valve syndrome | 1 |
| Aortic stenosis | 1 |
| Atrioventricular canal | 2 |
| Coarctation of the aorta | 1 |
| Constricted ductus arteriosus | 1 |
| Double outlet right ventricle | 2 |
| Hydrops | 1 |
| Hypoplastic left ventricle | 2 |
| Hypoplastic right ventricle | 2 |
| Interrupted inferior vena cava with azygous continuation | 2 |
| Pulmonary atresia | 1 |
| Situs ambiguous | 1 |
| Situs inversus | 1 |
| Tetralogy of Fallot | 3 |
| Transposition of the great arteries | 2 |
| Tricuspid regurgitation | 1 |
| Ventricular size disproportion | 1 |
| Ventricular septal defect | 15 |
| Anomalies of twin pregnancies | |
| Acardiac twin | 1 |
| Anomalies of the placenta and membranes | |
| Amniotic bands | 1 |
| Gastrointestinal anomalies and abdominal wall defects | |
| Ascites | 1 |
| Gastroschisis | 4 |
| Omphalocele | 1 |
| Musculoskeletal anomalies | |
| Bowed humerus, radius, ulna | 1 |
| Digit amputations | 1 |
| Rockerbottom feet | 1 |
| Scoliosis | 1 |
| Short ulna/bowed radius | 1 |
| Splayed ribs | 1 |
| Neck and chest anomalies | |
| Congenital cystic adenomatoid malformation of the lungs | 3 |
| Cystic hygroma | 1 |
| Diaphragmatic hernia | 2 |
| Renal anomalies | |
| Echogenic cystic right kidney | 1 |
| Echogenic left kidney | 1 |
| Horseshoe kidney | 1 |
| Megacystis | 1 |
| Multicystic kidney | 2 |
| Urethral atresia | 1 |
| Facial anomalies | |
| Lingual cyst | 1 |
| Micrognathia | 2 |
| Total | 82 |
Figure 1.
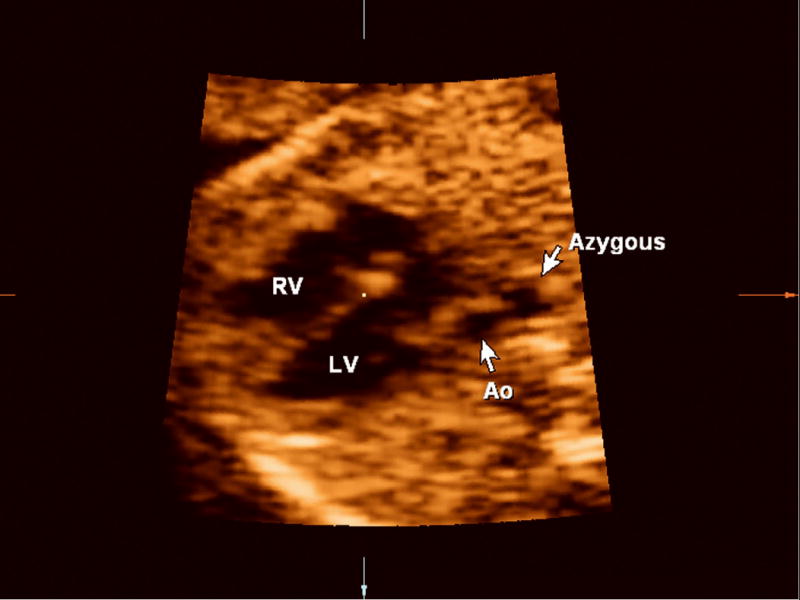
Retrospective evaluation of the volume dataset shows a dilated azygous vein to the right of the aorta (Ao; “double vessel sign”), demonstrating that the anomaly was actually present in the volume dataset but was overlooked prospectively by the examiner.
Figure 2.
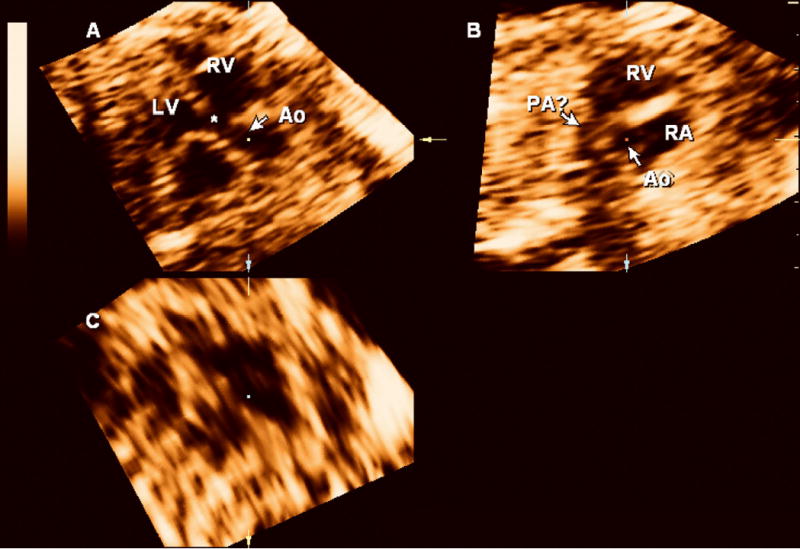
3D multiplanar display of the fetal heart. On panel A, the aorta (Ao) overrides the ventricular septum (*). A short axis view of the right ventricle (RV) is shown in panel B and the pulmonary artery (PA) is poorly visualized. The findings are suggestive of a tetralogy of Fallot and were overlooked during the prospective examination of the volume dataset. Panel C: coronal view. LV: left ventricle; RA: right atrium.
Table II.
Discordant diagnoses by three-dimensional/four-dimensional (3D/4D) and two-dimensional (2D) ultrasonography (n=12).
| Case # | GA (weeks) | Prospective 3D/4D findings | 2D findings | Retrospective 3D/4D findings | Comments | Postnatal findings |
|---|---|---|---|---|---|---|
| 3 | 32 1/7 | Hypoplastic RV
VSD Tricuspid atresia |
Hypoplastic RV
VSD Pulmonary atresia |
Hypoplastic RV | Poor quality volume datasets: excessive shadowing and fetal movement | Hypoplastic RV
Normal IVS Pulmonary atresia |
| 7 | 12 4/7 | Cystic hygroma
Hydrops |
Cystic Hygroma
Hydrops VSD |
Cystic hygroma
hydrops |
No 3D/4D volumes of the heart | Cystic hygroma
Hydrops |
| 14 | 23 2/7 | Omphalocele
VSD |
Omphalocele
VSD Interrupted IVC with azygous continuation |
Omphalocele
VSD Interrupted IVC with azygous continuation |
Interrupted IVC with azygous continuation overlooked in the prospective evaluation of the 4D volume datasets (Figure 1) | Omphalocele
Interrupted IVC with azygous continuation |
| 18 | 25 1/7 | Normal | CCAM | Normal | Poor quality volume datasets: shadowing. | Spontaneous resolution |
| 19 | 20 3/7 | VSD | Normal | VSD | VSD suspected by 3D/4D US with color and power Doppler. Revised 2D images confirmed initial suspicion | Lost to follow-up |
| 37 | 22 6/7 | VSD | Normal | Normal | VSD suspected in only one of 4 volume datasets | Lost to follow-up |
| 38 | 22 6/7 | Normal | Tetralogy of Fallot | VSD + overriding aorta | Pulmonary artery poorly seen (Figure 2) | Lost to follow-up |
| 45 | 34 2/7 | HLHS
Aortic stenosis Coarctation of Aorta VSD |
HLHS
Aortic stenosis Coarctation of Aorta |
HLHS
Aortic stenosis Coarctation of Aorta |
— | HLHS
Aortic stenosis Coarctation of Aorta |
| 75 | 26 0/7 | Spina bifida
Scoliosis Splayed ribs Normal kidneys |
Spina bifida
Scoliosis Splayed ribs Horseshoe kidneys |
Spina bifida
Scoliosis Splayed ribs Horseshoe kidney |
Horseshoe kidneys were overlooked in the first prospective evaluation of the 4D volume datasets | Spina bifida
Scoliosis Splayed ribs Horseshoe kidneys |
| 78 | 20 3/7 | DORV (Figure 3)
VSD |
TGA
VSD |
TGA (Figure 4)
VSD |
Poor quality volume datasets: motion | TGA
VSD |
| 97 | 19 4/7 | Normal | VSD | Normal | Normal | |
| 98 | 20 2/7 | Lemon shaped BPD
Spina bifida occulta? |
Lemon shaped BPD
Normal spine |
Lemon shaped BPD
Normal spine |
Encephalocele present in the neonate could not be retrospectively identified in the volume datasets | Microcephaly
Hemivertebra Small encephalocele |
BPD: biparietal diameter; CCAM: congenital cystic adenomatoid malformation; DORV: double outlet right ventricle; HLHS: hypoplastic left heart syndrome; IVC: inferior vena cava; IVS: interventricular septum; RV: right ventricle; TGA: transposition of the great arteries; VSD: ventricular septal defect.
In two cases of cardiac anomalies, there was discordance between specific findings observed by 3D/4D when compared to 2D ultrasonography. The first was a case of pulmonary atresia with an intact ventricular septum, which was interpreted by 3D/4D ultrasonography as tricuspid atresia (Table II, case 3). The second was a case of transposition of the great arteries, which was interpreted by 3D/4D ultrasonography as a double outlet right ventricle (Table II, case 78, Figures 3 and 4). In the case of pulmonary atresia, poor quality of the volume datasets due to excessive shadowing from ribs and fetal movement prevented adequate visualization of the right outflow tract. The case of transposition of the great arteries, which was misinterpreted as a double outlet right ventricle, illustrates a potential pitfall of 3D/4D ultrasonography to evaluate the relationships between the outflow tracts and the ventricular chambers. This particular volume dataset was acquired using transverse sweeps through the fetal thorax during a period of excessive fetal movement and the alignment between the aorta and the interventricular septum was artificially displaced, giving the impression of overriding (Figure 3). Retrospective examination of another volume dataset of the same fetus acquired using sagittal sweeps through the fetal thorax ruled out the overriding aorta and revealed the correct diagnosis of transposition of the great arteries (Figure 4).
Figure 3.
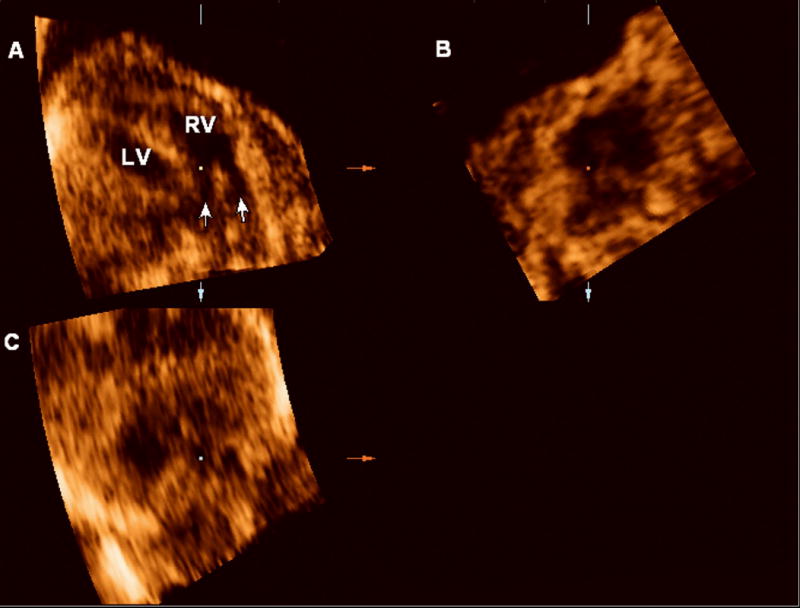
4D multiplanar display of the fetal heart. Volume acquired with a transverse sweep through the fetal chest during excessive fetal motion. Panel A: Two vessels (arrows) apparently connect to the right ventricle (RV), suggesting a double outlet right ventricle. Panel B: sagittal view. Panel C: coronal view.
Figure 4.
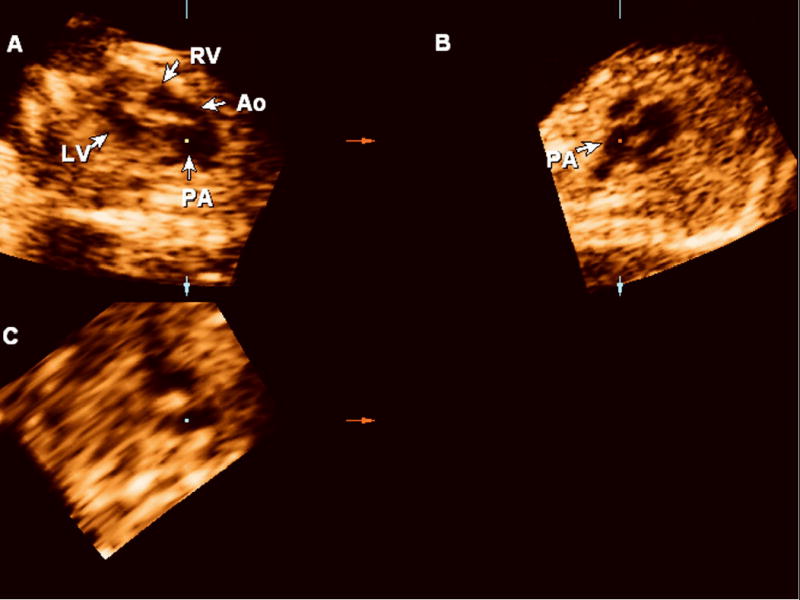
Retrospective review of the volume dataset acquired using sagittal sweeps through the fetal chest reveals two vessels leaving the ventricles in parallel and the correct diagnosis of transposition of the great arteries (Panel A). Panel B: sagittal view. Panel C: coronal view. Ao: aorta; LV: left ventricle; PA: pulmonary artery.
Complete follow-up was available for 79 fetuses. The sensitivity and specificity for 3D/4D and 2D ultrasonography to detect congenital anomalies are displayed in Table III. No significant difference in diagnostic conformity to neonatal outcomes was observed when the two methods were compared (kappa = 0.821; McNemar-Bowker’s test: 3.00, p=0.223). Four anomalies detected after birth were missed by 3D/4D ultrasonography (hemivertebra, small occipital encephalocele, horseshoe kidney, and a VSD) and two were missed by 2D ultrasonography (hemivertebra and a small occipital encephalocele). There were 13 false-positive diagnoses by 3D/4D ultrasonography [micrognathia (n=2), tricuspid regurgitation (n=1), spina bifida occulta (n=1), and VSDs (n=9)] and 15 false-positive diagnoses by 2D ultrasonography [micrognathia (n=2), tricuspid regurgitation (n=1), ventricular size disproportion (n=1), VSDs (n=10), and a congenital cystic adenomatoid malformation (n=1)].
Table III.
Diagnostic accuracy of three-dimensional (3D)/four-dimensional (4D) and two-dimensional (2D) ultrasonography for the detection of congenital anomalies
| Diagnostic modality | Sensitivity | Specificity |
|---|---|---|
| 3D/4D | 47/51
92.2% (80.3%-97.5%) |
42/55
76.4% (62.7%-86.3%) |
| 2D | 49/51
96.1% (85.4%-99.3%) |
40/55
72.7% (58.8%-83.4%) |
Calculations based on the outcome of 79 fetuses for which postnatal follow-up was available. Calculations were based on comparisons of individual findings by 3D/4D or 2D ultrasonography and postnatal outcome (n=106). Values are expressed as percentage (95% confidence interval).
Kappa index of agreement: 0.821; McNemar-Bowker’s test: 3.00, p=0.223.
Discussion
Several studies have compared 3D to 2D ultrasonography for the diagnosis of congenital anomalies, yielding conflicting results (Table IV).5,26–32 While some have reported that 3D ultrasonography would be advantageous for the diagnosis of congenital anomalies, others have suggested that this method does not provide significant additional information over what is provided by 2D ultrasonography. A potential bias of these studies is that the examiner was aware of the indications for, and the results of, the 2D ultrasound examination at the time that the 3D volume datasets were examined. Therefore, only the complementary role of 3D over 2D ultrasonography could be evaluated.
Table IV.
Prenatal diagnosis of congenital anomalies with three-dimensional (3D) and two-dimensional (2D) ultrasound (US).
|
3DUS |
2DUS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | GA (weeks) | n | Population | Outcome measures | n | % | n | % | P |
| Merz et al.5 | 1995 | 16–38 | 204 | Patients with a fetal malformation detected by conventional 2DUS | 3D advantageous to demonstrate fetal structures
3D provided similar information 3D provided less information* |
127
73 4 |
62.3%
35.8% 2.0% |
|||
| Merz et al.26 | 1995 | 16–38 | 458 | 242 normal fetuses and 216 with congenital anomalies diagnosed by 2DUS | Diagnostic gain over 2DUS | 139 | 64.2% | |||
| Platt et al.27 | 1998 | 6 to 35 | 161 | Obstetric and gynecologic patients attending a clinic. Clinically suspected anomalies or findings (n=32) | 3D provided additional information or changed diagnosis
3D provided similar information |
3
29 |
9%
91% |
|||
| Baba et al.28 | 1999 | 13 to 35 | 19 | 36 abnormalities detected in 19 pregnancies complicated by congenital malformations. All exams with 4DUS | Anomaly visualized by 4DUS only‡
Anomaly visualized by 2DUS only† Anomaly visualized by both 2D and 4DUS Additional information provided by 4DUS |
2
16 9 9 |
6%
44% 25% 25% |
|||
| Dyson et al.29 | 2000 | 12 to 38 | 63 | 103 anomalies examined by 2D and 3DUS. Patients selected for the study on the basis that 3DUS might provide useful information. Review of 3DUS data done remote from the time of 2DUS examination | 3D provided additional information
3D provided similar information 3D disadvantageous 3D provided similar information Anomalies detected exclusively by 3D |
53
46 4 355 42 |
51.5%
44.7% 3.9% 35.1% 4.2% |
|||
| Scharf et al.30 | 2001 | 7 to 41 | 433 | Mixed high and low risk population; 40 fetuses with congenital anomalies | Visualization rate of congenital anomalies | 28 | 68.3% | 39 | 97.5% | <0.05† |
| Xu et al.31 | 2002 | 16 to 42 | 216 | High-risk pregnancies; 41 fetuses with confirmed congenital anomalies | Definitive diagnosis of a congenital anomaly | 38 | 92.7% | 32 | 78.0% | <0.05† |
| Merz et al.32 | 2005 | 11 to 35 | 3472 | Pregnancies at high-risk for anomalies; 1012 congenital anomalies detected in 906 pregnancies | 3D advantageous to demonstrate anomalies | 615 | 60.8% | |||
4 fetuses with heart defects; lack of information attributed to motion artifacts.
In this study, anomalies detectable by 2DUS only represented abnormalities of the internal organs.
The two anomalies detectable by 4DUS only were facial dysmorphism and clubfoot.
GA: gestational age; 4DUS: four-dimensional ultrasound.
Reproduced with permission from Gonçalves et al. JUM 2005;24:1599–1624.
In this study, we deliberately inverted the order that 3D/4D examinations are normally performed in clinical practice to ask the question of whether the examination of volume datasets alone could provide reliable diagnostic information when compared to 2D ultrasonography or neonatal outcome. A single examiner, who was blinded to study indications, performed all studies. In 90% of the cases, there was complete agreement between the findings of 3D/4D and 2D ultrasonography. In 50% of discordant cases (6/12), volume datasets were either of poor diagnostic quality (n=4) or the anomaly, although present in the volume dataset, was overlooked by the examiner during prospective examination. An unintended, but important, observation of this study was that motion artifacts interfered with the evaluation of anatomical relationships between the great vessels and the cardiac chambers in one case, leading to the erroneous suspicion of a double outlet right ventricle (Figure 3) when the correct diagnosis was transposition of the great arteries (Figure 4). It is possible that a static volume acquisition of the fetal chest, to evaluate the heart, could have attenuated the effect of fetal movements observed in this case.
When diagnoses established by 3D/4D and 2D ultrasonography were compared to postnatal diagnoses, there was no statistical difference in the detection rates of the two diagnostic modalities, despite the potential bias of this study favoring 2D ultrasonography. A hemivertebra and a small occipital encephalocele were missed, in the same fetus, by both 3D/4D and 2D ultrasonography. Two-dimensional ultrasonography, in contrast, correctly diagnosed a horseshoe kidney and a VSD, which were detected after delivery. Most false-positive diagnoses, either by 3D/4D or 2D ultrasonography, were VSDs not identified after birth. However, between 32.7% and 46.1% of VSDs diagnosed during the prenatal period underwent spontaneous closure in utero.33,34
Knowledge of whether or not the examination of 3D volume datasets can provide accurate diagnostic information is important if this technology is to be used as the primary modality to establish a diagnosis. This may be the case in remote consultation settings (telemedicine)16,17,35, or in situations where the volume dataset is the primary source of information available to the sonologist (e.g., sonographic tomography).14,15
Regarding the potential application of 3D/4D ultrasonography in telemedicine, Nelson et al.16 investigated the possibility of performing virtual 3D ultrasound examinations at remote clinical sites. One hundred patients underwent 2D and 3D ultrasonography at the University of California in San Diego and the volume datasets were sent over computer networks or magnetic media for review at Thomas Jefferson University in Philadelphia, PA (remote site). Differences between measurements obtained by 2D and 3D ultrasound methods were, in general, less than 5% for individual measurements. 3D performed better than 2D for the visualization of first trimester fetal structures and organs (e.g., stomach, kidneys, cord insertion, lateral ventricles, and extremities) and the visualization rate was equivalent during the second trimester. A cystic adenomatoid malformation was missed by 3D ultrasonography in the second trimester and there was one false-positive diagnosis of a cleft lip. Limitations of the study included individual variability in the interpretation between physicians and unavailability of gating techniques to examine the fetal heart by 3D/4D ultrasound. In the present study, a single examiner analyzed the volume datasets and performed the 2D ultrasound examination, and volume datasets of the fetal heart were acquired with STIC technology. The use of STIC technology for telemedicine applications has been previously described by Viñals et al.,17 who have successfully examined volume datasets of the fetal heart transmitted from remote locations over the internet in order to confirm or exclude cardiac malformations.
Our observations complement those of Benacerraf et al.,14,15 who have proposed that 3D ultrasonography (sonographic tomography) could be used in lieu of 2D ultrasound to perform fetal anatomical surveys, and that the examination could be performed in a manner similar to contemporary CT and MR cross-sectional imaging. Structural anatomical surveys would be performed by the examination of 3D volume datasets alone, in approximately half of the time required to perform a 2D ultrasound examination. Besides the gain in time, this approach would also allow the physician interpreting the scan to review the entire volume dataset as though he or she was scanning the patient directly, and to evaluate for anomalies that the sonographer might not have specifically recognized or recorded.15
We conclude that the evaluation of fetal anatomy and diagnosis of congenital anomalies is possible by the examination of 3D/4D volume datasets alone. Discordance between 3D/4D and 2D diagnoses generally occurred for volume datasets of poor diagnostic quality and, in two cases, anomalies that were initially overlooked by the examiner could be retrospectively identified by review of the volume datasets. The design employed in this study could be used to validate sonographic tomography in diagnostic units considering a more extensive application of this technology in clinical practice.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
References
- 1.Benacerraf BR. Three-dimensional fetal sonography: use and misuse. J Ultrasound Med. 2002;21:1063–1067. doi: 10.7863/jum.2002.21.10.1063. [DOI] [PubMed] [Google Scholar]
- 2.Hamper UM, Trapanotto V, Sheth S, DeJong MR, Caskey CI. Three-dimensional US: preliminary clinical experience. Radiology. 1994;191:397–401. doi: 10.1148/radiology.191.2.8153312. [DOI] [PubMed] [Google Scholar]
- 3.Pretorius DH, Nelson TR. Three-dimensional ultrasound. Ultrasound Obstet Gynecol. 1995;5:219–221. doi: 10.1046/j.1469-0705.1995.05040219.x. [DOI] [PubMed] [Google Scholar]
- 4.Jurkovic D, Geipel A, Gruboeck K, et al. Three-dimensional ultrasound for the assessment of uterine anatomy and detection of congenital anomalies: a comparison with hysterosalpingography and two-dimensional sonography. Ultrasound Obstet Gynecol. 1995;5:233–237. doi: 10.1046/j.1469-0705.1995.05040233.x. [DOI] [PubMed] [Google Scholar]
- 5.Merz E, Bahlmann F, Weber G. Volume scanning in the evaluation of fetal malformations: a new dimension in prenatal diagnosis. Ultrasound Obstet Gynecol. 1995;5:222–227. doi: 10.1046/j.1469-0705.1995.05040222.x. [DOI] [PubMed] [Google Scholar]
- 6.Riccabona M, Pretorius DH, Nelson TR, Johnson D, Budorick NE. Three-dimensional ultrasound: display modalities in obstetrics. J Clin Ultrasound. 1997;25:157–167. doi: 10.1002/(sici)1097-0096(199705)25:4<157::aid-jcu2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Sohn C, Grotepass J, Menge KH, Ameling W. [Clinical application of 3-dimensional ultrasound display. Initial results] Dtsch Med Wochenschr. 1989;114:534–537. doi: 10.1055/s-2008-1066630. [DOI] [PubMed] [Google Scholar]
- 8.Brinkley JF, McCallum WD, Muramatsu SK, Liu DY. Fetal weight estimation from ultrasonic three-dimensional head and trunk reconstructions: evaluation in vitro. Am J Obstet Gynecol. 1982;144:715–721. doi: 10.1016/0002-9378(82)90443-4. [DOI] [PubMed] [Google Scholar]
- 9.Favre R, Nisand G, Bettahar K, Grange G, Nisand I. Measurement of limb circumferences with three-dimensional ultrasound for fetal weight estimation. Ultrasound Obstet Gynecol. 1993;3:176–179. doi: 10.1046/j.1469-0705.1993.03030176.x. [DOI] [PubMed] [Google Scholar]
- 10.Steiner H, Gregg AR, Bogner G, et al. First trimester three-dimensional ultrasound volumetry of the gestational sac. Arch Gynecol Obstet. 1994;255:165–170. doi: 10.1007/BF02335080. [DOI] [PubMed] [Google Scholar]
- 11.Hughes SW, D’Arcy TJ, Maxwell DJ, et al. Volume estimation from multiplanar 2D ultrasound images using a remote electromagnetic position and orientation sensor. Ultrasound Med Biol. 1996;22:561–572. doi: 10.1016/0301-5629(96)00022-1. [DOI] [PubMed] [Google Scholar]
- 12.Gilja OH, Hausken T, Berstad A, Odegaard S. Measurements of organ volume by ultrasonography. Proc Inst Mech Eng [H ] 1999;213:247–259. doi: 10.1243/0954411991534951. [DOI] [PubMed] [Google Scholar]
- 13.Pretorius DH, Borok NN, Coffler MS, Nelson TR. Three-dimensional ultrasound in obstetrics and gynecology. Radiol Clin North Am. 2001;39:499–521. doi: 10.1016/s0033-8389(05)70294-3. [DOI] [PubMed] [Google Scholar]
- 14.Benacerraf BR, Shipp TD, Bromley B. How sonographic tomography will change the face of obstetric sonography: a pilot study. J Ultrasound Med. 2005;24:371–378. doi: 10.7863/jum.2005.24.3.371. [DOI] [PubMed] [Google Scholar]
- 15.Benacerraf BR, Shipp TD, Bromley B. Three-dimensional US of the Fetus: Volume Imaging. Radiology 2006; [DOI] [PubMed]
- 16.Nelson TR, Pretorius DH, Lev-Toaff A, et al. Feasibility of performing a virtual patient examination using three-dimensional ultrasonographic data acquired at remote locations. J Ultrasound Med. 2001;20:941–952. doi: 10.7863/jum.2001.20.9.941. [DOI] [PubMed] [Google Scholar]
- 17.Vinals F, Mandujano L, Vargas G, Giuliano A. Prenatal diagnosis of congenital heart disease using four-dimensional spatio-temporal image correlation (STIC) telemedicine via an Internet link: a pilot study. Ultrasound Obstet Gynecol. 2005;25:25–31. doi: 10.1002/uog.1796. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DD, Pretorius DH, Riccabona M, Budorick NE, Nelson TR. Three-dimensional ultrasound of the fetal spine. Obstet Gynecol. 1997;89:434–438. doi: 10.1016/S0029-7844(96)00498-X. [DOI] [PubMed] [Google Scholar]
- 19.Kossoff G. Three-dimensional ultrasound--technology push or market pull? Ultrasound Obstet Gynecol. 1995;5:217–218. doi: 10.1046/j.1469-0705.1995.05040217.x. [DOI] [PubMed] [Google Scholar]
- 20.Goncalves LF, Romero R, Espinoza J, et al. Four-dimensional ultrasonography of the fetal heart using color Doppler spatiotemporal image correlation. J Ultrasound Med. 2004;23:473–481. doi: 10.7863/jum.2004.23.4.473. [DOI] [PubMed] [Google Scholar]
- 21.Goncalves LF, Lee W, Chaiworapongsa T, et al. Four-dimensional ultrasonography of the fetal heart with spatiotemporal image correlation. Am J Obstet Gynecol. 2003;189:1792–1802. doi: 10.1016/s0002-9378(03)00913-x. [DOI] [PubMed] [Google Scholar]
- 22.Deng J, Sullivan ID, Yates R, et al. Real-time three-dimensional fetal echocardiography--optimal imaging windows. Ultrasound Med Biol. 2002;28:1099–1105. doi: 10.1016/s0301-5629(02)00560-4. [DOI] [PubMed] [Google Scholar]
- 23.Scharf A, Geka F, Steinborn A, et al. 3D real-time imaging of the fetal heart. Fetal Diagn Ther. 2000;15:267–274. doi: 10.1159/000021020. [DOI] [PubMed] [Google Scholar]
- 24.Sklansky MS, Nelson T, Strachan M, Pretorius D. Real-time three-dimensional fetal echocardiography: initial feasibility study. J Ultrasound Med. 1999;18:745–752. doi: 10.7863/jum.1999.18.11.745. [DOI] [PubMed] [Google Scholar]
- 25.Maulik D, Nanda NC, Singh V, et al. Live three-dimensional echocardiography of the human fetus. Echocardiography. 2003;20:715–721. doi: 10.1111/j.0742-2822.2003.03166.x. [DOI] [PubMed] [Google Scholar]
- 26.Merz E, Bahlmann F, Weber G, Macchiella D. Three-dimensional ultrasonography in prenatal diagnosis. J Perinat Med. 1995;23:213–222. doi: 10.1515/jpme.1995.23.3.213. [DOI] [PubMed] [Google Scholar]
- 27.Platt LD, Santulli T, Jr, Carlson DE, Greene N, Walla CA. Three-dimensional ultrasonography in obstetrics and gynecology: preliminary experience. Am J Obstet Gynecol. 1998;178:1199–1206. doi: 10.1016/s0002-9378(98)70323-0. [DOI] [PubMed] [Google Scholar]
- 28.Baba K, Okai T, Kozuma S, Taketani Y. Fetal abnormalities: evaluation with real-time-processible three-dimensional US--preliminary report. Radiology. 1999;211:441–446. doi: 10.1148/radiology.211.2.r99mr02441. [DOI] [PubMed] [Google Scholar]
- 29.Dyson RL, Pretorius DH, Budorick NE, et al. Three-dimensional ultrasound in the evaluation of fetal anomalies. Ultrasound Obstet Gynecol. 2000;16:321–328. doi: 10.1046/j.1469-0705.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- 30.Scharf A, Ghazwiny MF, Steinborn A, Baier P, Sohn C. Evaluation of two-dimensional versus three-dimensional ultrasound in obstetric diagnostics: a prospective study. Fetal Diagn Ther. 2001;16:333–341. doi: 10.1159/000053937. [DOI] [PubMed] [Google Scholar]
- 31.Xu HX, Zhang QP, Lu MD, Xiao XT. Comparison of two-dimensional and three-dimensional sonography in evaluating fetal malformations. J Clin Ultrasound. 2002;30:515–525. doi: 10.1002/jcu.10109. [DOI] [PubMed] [Google Scholar]
- 32.Merz E, Welter C. 2D and 3D Ultrasound in the evaluation of normal and abnormal fetal anatomy in the second and third trimesters in a level III center. Ultraschall Med. 2005;26:9–16. doi: 10.1055/s-2004-813947. [DOI] [PubMed] [Google Scholar]
- 33.Paladini D, Palmieri S, Lamberti A, et al. Characterization and natural history of ventricular septal defects in the fetus. Ultrasound Obstet Gynecol. 2000;16:118–122. doi: 10.1046/j.1469-0705.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 34.Axt-Fliedner R, Schwarze A, Smrcek J, et al. Isolated ventricular septal defects detected by color Doppler imaging: evolution during fetal and first year of postnatal life. Ultrasound Obstet Gynecol. 2006;27:266–273. doi: 10.1002/uog.2716. [DOI] [PubMed] [Google Scholar]
- 35.Michailidis GD, Simpson JM, Karidas C, Economides DL. Detailed three-dimensional fetal echocardiography facilitated by an Internet link. Ultrasound Obstet Gynecol. 2001;18:325–328. doi: 10.1046/j.0960-7692.2001.00520.x. [DOI] [PubMed] [Google Scholar]


