Abstract
Cancer vaccines targeting ‘self’ antigens that are expressed at consistently high levels by tumor cells are potentially useful in immunotherapy, but immunological tolerance may block their function. Here, we describe a novel, naked DNA vaccine encoding an alphavirus replicon (self-replicating mRNA) and the self/tumor antigen tyrosinase-related protein-1. Unlike conventional DNA vaccines, this vaccine can break tolerance and provide immunity to melanoma. The vaccine mediates production of double-stranded RNA, as evidenced by the autophosphorylation of protein kinase R. Double-stranded RNA is critical to vaccine function because both the immunogenicity and the anti-tumor activity of the vaccine are blocked in mice deficient for the RNase L enzyme, a key component of the 2′,5′-linked oligoadenylate synthetase antiviral pathway involved in double-stranded RNA recognition. This study shows for the first time that alphaviral replicon-encoding DNA vaccines activate innate immune pathways known to drive antiviral immune responses, and points the way to strategies for improving the efficacy of immunization with naked DNA.
Vaccine vectors based on recombinant viruses have been used for many years, but the delivery of target antigens can be accompanied by unwanted side effects. First, preexisting antibodies can neutralize the recombinant virus before it is able to deliver its payload. Second, structural proteins from the virus can dominate T- and B-cell–mediated immune responses, diverting immunity away from the target immunogen1,2. Hence, there is a critical need to develop vaccine vectors that are not only highly immunogenic, but also antigenically simple.
The simplest of all recombinant vectors, naked plasmid DNA vaccines, have successfully been used in animal models to induce immune responses to many pathogens and model antigens. DNA vaccines are easy to produce, inexpensive and safe3, but for some applications insufficiently immunogenic. A variety of attempts have been made to improve DNA vaccines4, including the production of pro-apoptotic proteins5. Despite these advances, the poor immunogenicity of plasmid DNA remains apparent when attempting to elicit immunity to weak immunogens, such as non-mutated ‘self’ tumor-associated antigens that are recognized by anti-tumor T cells.
One promising new strategy to improve naked DNA vaccines is to express the target antigen under the control of an alphaviral replicase6,7 with the premise of using the ability of alphavirus to produce large amounts of viral mRNA (refs. 8,9). In alphavirus-derived DNA and RNA vaccines, the encoded alphaviral replicase-enzyme complex amplifies self-replicating RNA (replicon). In model systems, replicon containing nucleic acid vaccines display therapeutic efficacy at doses several logs lower than those required by conventional DNA vaccines10–12. In the current study, we examined whether a replicase-based DNA vaccine encoding a non-mutated self-antigen could be used to break tolerance and prevent B16 melanoma, a goal not previously accomplished with conventional DNA vaccines13. Subsequently, we explored the mechanism behind the superior efficacy of replicase-based nucleic acid vaccines compared with conventional DNA vaccines. We found no correlation between immunogenicity and antigen production of a DNA vaccine. While apoptotic death induced by replicase-based DNA may prevent prolonged production of the target antigen, adaptive immunity is nevertheless still efficiently activated. We show for the first time that innate antiviral pathways implicated in the molecular mechanisms of innate antiviral immunity (double-stranded (ds) RNA recognition and interferon action14) form at least one mechanism underlying the superior efficacy of replicase-based DNA vaccines. Activating such pathways enables a DNA vaccine to break tolerance to self-antigens.
A conventional TRP-1 plasmid is only immunogenic in Tyrp1−/−mice
Previous reports showed poor immunogenicity of conventional plasmids encoding non-mutated melanocyte/melanoma antigens13,15,16. Using β-galactosidase (β-gal) as the model antigen, we demonstrated significant enhancement of the immunogenicity and efficacy of RNA and DNA vaccines by placing antigen expression under the control of an alphavirus replicase enzyme12,17. We attempted to engineer a replicase-based DNA vaccine that breaks immune tolerance and elicits immunity against a non-mutated, tumor-associated self-antigen. The target antigen, gp75 (tyrosinase-related protein-1 (TRP-1), encoded by the gene Tyrp1), is the mouse homologue of a human tumor rejection antigen expressed at high levels in melanoma cells18. The mouse TRP-1 constructs were compared with DNA plasmids encoding the cross-reactive human protein (Figs. 1a and b). A conventional DNA vaccine encoding human TRP-1 (‘non-self’) was previously shown to elicit a protective immune response to B16 melanoma in mice, unlike DNA plasmids encoding mouse TRP-1 (‘self’; ref. 13 and K.R. Irvine, pers. comm.). In the pSin vectors (Fig. 1a), human or mouse TYRP1 were cloned downstream of the replicase gene12. In the conventional plasmids, pCMV-mTRP-1 and pCMV-hTRP-1, antigen expression is controlled by a cytomegalovirus (CMV) promoter (Fig. 1b). The functionality of all plasmids was confirmed by immunoblot analysis of transfected BHK-21 cells (Fig. 1c). pCMV-mTRP-1 induced a significant antibody response (Fig. 1d) and tumor protection (data not shown) in Tyrp1−/− mice, but not in heterozygous littermates, confirming that the poor immunogenicity of the conventional mouse TRP-1 DNA vaccine is due to immunological tolerance when this antigen is constitutively expressed by the host.
Fig. 1.
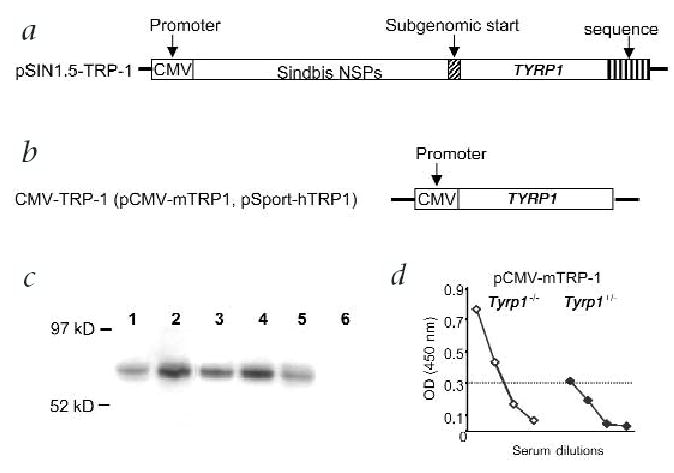
Design of plasmids used for this study. a, The pSin1.5 vector12 containing the genes for the mouse or human TRP-1 antigen. b, Two conventional plasmids (pcDNA3 and pSport), encoding mouse and human TRP-1, respectively. In case of the pSin vector, self-replicating RNA (replicon) is transcribed under control of a CMV promoter. This replicon encodes the replicase gene and the tumor-associated antigen. After translation, more RNA copies are produced in the transfected cell through the action of the replicase–enzyme complex8,26 (non-structural proteins, NSP). c, Antigen expression was confirmed in vitro by transfection of BHK-21 cells and western blot using sera from mice immunized with vaccinia-mTRP-1. Lane 1, B16.F10 melanoma; lane 2, CMV-mTRP-1; lane 3, pCMV-hTRP-1; lane 4, pSin-mTRP-1; lane 5, pSin-hTRP-1; lane 6, pCMV-EGFP. d, pCMV-mTRP-1 is a functional vaccine in Tyrp1−/−, but is unable to immunize (that is, break tolerance in) wild-type (Figs. 2 and 3a) or Tyrp1+/− mice. Serum (diluted 1:5, 1:25, 1:125, 1:625) was analyzed by ELISA after 5 immunizations. Shown is the average of 5 mice after subtraction of background values from naïve mice. P value at 1:125 by Wilcoxon rank sum test is 0.051.
Replicase-based DNA vaccines break self-tolerance without increased antigen production
To determine whether replicase-based DNA plasmid can break tolerance in wild-type mice, we measured serum antibody titers in immunized mice (Fig. 2). Immunization with pCMV-mTRP-1 only marginally increased the titer of antibody against mouse TRP-1 above background levels, as previously published13. In contrast, the pSin-mTRP-1 plasmid induced high antibody titers. We then analyzed the level of antigen produced by the two types of DNA vaccines. Alphaviral expression vectors were originally developed with the goal of increasing antigen production to enhance immunogenicity. To test this hypothesis, plasmids were delivered into the skin and the homogenized target tissue was evaluated for antigen expression. No significant differences in the amount of antigen were found between skin areas intradermally injected with the various plasmids (data not shown). Replicase-based DNA (pSin-β-gal) delivered by gene gun also did not trigger higher antigen production than the conventional plasmid (Fig. 2), although gene-gun immunization triggered higher antigen production than intradermal injection. This is consistent with previously published in vitro results12, thus confirming that replicase-based vectors owe their increased immunogenicity to mechanisms other than simple overproduction of antigen.
Fig. 2.
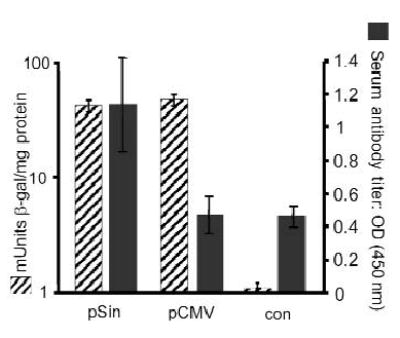
Antigen expression does not correlate with immunogenicity. After delivery by gene gun, we observed no difference in the expression of a reporter gene (β-gal) encoded on either a conventional (pCMV) or replicase-based (pSin) plasmid (hatched bars). Skin biopsies of 3 mice each were harvested 24 h after immunization with a single shot (theoretical dose = 1 μg plasmid). Results are shown as mUnits β-gal with s.e.m. (left scale). Similarly, no significant differences in antigen levels were detected after intradermal injection of the plasmids (10 μg each; data not shown). Average serum antibody titers (n = 4) after gene gun immunization with DNA plasmids encoding mouse TRP-1 (right scale) or a control plasmid (con = pSin-β-gal) are shown as filled bars with standard error. Mice were bled 1 week after the last immunization and antibody titers were measured by ELISA. Sera were diluted 1:125 and measured individually. The experiment was repeated 4 times with sera from independently immunized mice yielding comparable results. Results shown are from a representative experiment.
Replicase-based DNA vaccine protects from melanoma
Immunization with a conventional DNA plasmid encoding human TRP-1 delivered by gene gun has previously been shown to protect mice from B16 melanoma13. B16 constitutively expresses non-mutated mouse TRP-1 (Fig. 1c; ref. 19). To test the efficacy of replicase-based TRP-1 plasmids, gene-gun–immunized mice were challenged subcutaneously with B16 melanoma. Both plasmids encoding the cross-reactive human TRP-1 antigen (pCMV-hTRP-1 and pSin-hTRP-1) provided strong tumor protection (Fig. 3a). Of the vectors encoding mouse TRP-1, the conventional DNA vaccine (pCMV-mTRP-1) was ineffective, whereas the replicase-based vector (pSin-mTRP-1) induced the highest level of tumor protection of all vectors. Thus, a replicase-based DNA plasmid encoding a non-mutated, tumor-associated self-antigen (TRP-1) broke tolerance and protected against tumor, whereas the same antigen encoded by a conventional DNA plasmid was ineffective. This implies that replicase-based nucleic acid vaccines trigger different effector mechanisms than conventional DNA vaccines.
Fig. 3.
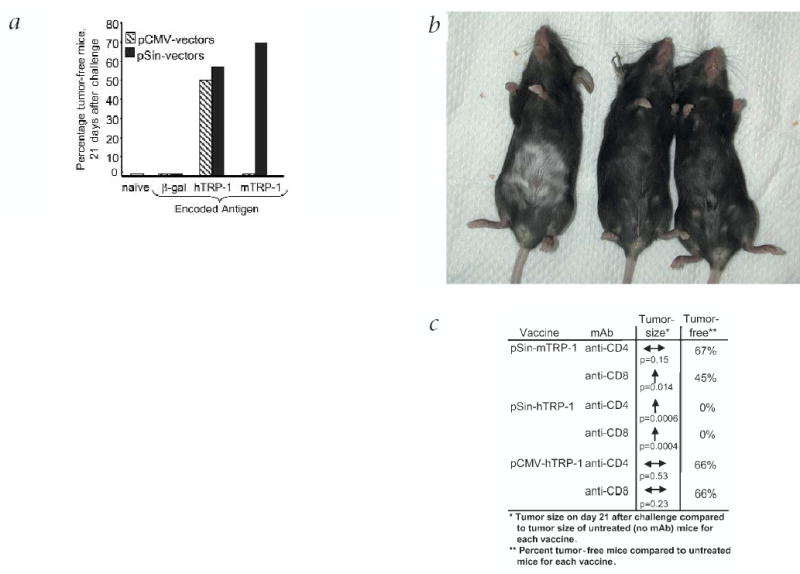
Tumor prevention by immunization with various plasmids encoding TRP-1. a, Mice were immunized 5 times at weekly intervals by gene gun and challenged subcutaneously with 1 × 105 B16.F10 melanoma cells. Shown is the compiled percentage of tumor-free mice 21 d after challenge. Tumors were measured for 3 weeks after challenge in a blinded fashion. Total number of mice per group was between 10 (β-gal plasmids and pCMV-mTRP-1) and 50 (all others). Mice immunized with pSin-mTRP-1 were also protected after challenge with a tumor inoculum of 5 × 105 B16 cells (data not shown). b, Vitiligo is only induced by immunization with a conventional vector encoding human TRP-1, but not with a replicase-based vector encoding either human or mouse TRP-1. Shown is one representative mouse for each vaccine, 10 days after 5 weekly immunizations. Depigmentation begins at the site of immunization with the gene gun (shaved abdomen). c, Impact of the depletion of CD4+ or CD8+ cells at the effector phase (pre-challenge). Results are calculated from the average tumor size 21 d after challenge and based on the average size of tumors in non-depleted mice. Significant increases in tumor size after depletion with monoclonal antibody are indicated by arrows (Wilcoxon rank sum test). The reduction in the number of tumor-free mice after antibody depletion is shown as percentage of tumor-free mice based on the number of tumor-free mice in the non-depleted
Replicase-based DNA induces qualitatively different effector mechanisms
Immunization with vaccinia-mTRP-1 or conventional DNA plasmids encoding human TRP-1 is accompanied by autoimmune depigmentation (vitiligo) of mice13,20. In our study, only pCMV-hTRP-1 immunized mice developed vitiligo (Fig. 3b). In contrast, mice immunized with either pSin-mTRP-1 or pSin-hTRP-1 showed no signs of vitiligo, even several months after immunization and successful rejection of B16-melanoma.
To further evaluate whether the two types of DNA vaccines trigger different effector mechanisms, CD4+ or CD8+ cells were depleted before challenge. CD4-cell depletion caused no significant increase in tumor growth of pCMV-hTRP-1 immunized mice. After immunization with replicase-based plasmid, CD4-cell depletion significantly reduced the efficacy of pSin-hTRP-1, but not pSin-mTRP-1 (Fig. 3c). CD8-cell depletion had no significant impact on tumor growth in mice immunized with the conventional plasmid (pCMV-hTRP-1), but significantly increased the tumor size in pSin-mTRP-1 immunized mice and abrogated tumor protection induced by pSin-hTRP-1 (Fig. 3c). Increased average tumor size after depletion was not simply the result of larger tumors, but was accompanied by a reduction in tumor-free mice (Fig. 3c). Our results point to a strong CD8+ T-cell component in the effector phase of the activity of the replicase-based pSin mouse or human TRP-1. This prompted us to examine the molecular background of the mechanisms triggered by replicase-based nucleic acid vaccines.
Replicase-based DNA triggers production of biologically active dsRNA species
In an alphavirus-infected cell, the activity of the viral replicase creates dsRNA species. We hypothesized that dsRNA molecules are also produced as a consequence of transfection with replicase-based DNA vaccines. To detect biologically active dsRNA, we used an assay that measures autophosphorylation of the protein-kinase-dsRNA-dependent (PKR). Cells transfected in vitro with pSin-EGFP (ref. 12) were sorted for enhanced green fluorescence protein (EGFP) expression and, lysed, and recombinant, unphosphorylated protein kinase R (PKR) was added. PKR autophosphorylation was measured by incorporation of radioactively labeled ATP ([γ-32P]ATP). Without PKR (purified glutathione S-transferase (GST) alone) no phosphorylation was detected indicating low levels of endogenous kinases. Autophosphorylation of wild-type PKR (PKR-WT) was strongly and specifically activated only by cell lysates prepared from cells that expressed EGFP from the replicase-based vector. The K296R mutation destroys PKR-kinase activity and this PKR-mutant was included as an additional control. The assay was performed with two cell lines (human SW480 (Fig. 4) and hamster BHK-21 cells (not shown)) and two transfection reagents (LipofectAMINE or Superfect). The finding that alphaviral replicase-based vectors mediated the autophosphorylation of dsRNA-dependent PKR raised the question of whether dsRNA produced by replicase-based vaccines also had a role in vivo in vaccine efficacy.
Fig. 4.
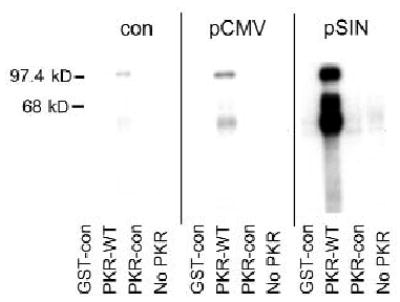
Production of biologically active dsRNA in cells transfected with replicase-based plasmids. PKR is activated by dsRNA in the lysate of transfected cells and autophosphorylates. pSin-EGFP or pCMV-EGFP transfected, sorted SW480 cells were lysed and a kinase assay was performed with purified GST, GST fused to wild-type PKR (GST–PKR), or GST fused to the dominant-negative K296 mutant of PKR (PKR-con) in the presence of [γ-32P]ATP. The ‘No PKR’ lane contains no added proteins and is a measure of endogenous kinase levels. Proteins were separated by SDS–PAGE and activated; autophosphorylated PKR was detected by autoradiography. Non-transfected cells (con) were obtained from transfection cultures to account for any transfection-induced PKR activation. The results obtained with SW480 cells were confirmed in BHK-21 cells using a different preparation of recombinant PKR protein (non-GST, data not shown).
Enhanced efficacy of replicase-based DNA vaccines is mediated by antiviral pathways
dsRNA molecules trigger antiviral pathways, which may provide an adjuvant effect for the vaccine and help initiate the release of ‘stress factors’ (such as type I interferons and heat shock proteins). Key molecules for two major dsRNA-triggered pathways are ribonuclease L (RNase L; refs. 14,21) and PKR (ref. 22). We previously demonstrated that RNase L-mutant mice have increased susceptibility to viral infections and are partially deficient in induced and spontaneous apoptosis23.
The in vivo impact of dsRNA pathways on the efficacy of replicase-based DNA vaccines was tested in RNase L-mutant (Rnasel−/−) mice24. The antibody response in pSin-mTRP-1 immunized Rnasel−/− mice was much lower than that in heterozygous litter-mates (Rnasel+/−; Fig. 5a). This was not due to the inability of the mutant mice to raise an immune response, as the antibody titer is comparable in Rnasel−/− and Rnasel+/− mice immunized with recombinant vaccinia encoding mouse TRP-1 (Fig. 5b; ref. 20). Next, we measured the efficacy of the TRP-1 vaccines in the Rnasel−/− mice using the B16 melanoma model. pSin-mTRP-1 only protected Rnasel+/− mice (compared to naive Rnasel+/− mice, P = 0.025 by ANOVA), whereas vaccine efficacy was impaired in the mutant mice, resulting in non-significant treatment (Figs. 6a and c). The endpoint difference in tumor size between Rnasel−/−and Rnasel+/− mice immunized with pSin-mTRP-1 was significant. In contrast to the DNA vaccine, immunization with vaccinia-mTRP-1 resulted in a high degree of tumor rejection in both Rnasel−/− and Rnasel+/− mice (Fig. 6b) compared with naive mice (P = 0.0001 and 0.018 for Rnasel−/− and Rnasel+/−, respectively, by ANOVA). Endpoint tumor size in the two immunized groups was not different (P = 0.544 by Wilcoxon rank sum test). pSin-β-gal did not induce protection (Fig. 6c).
Fig. 5.
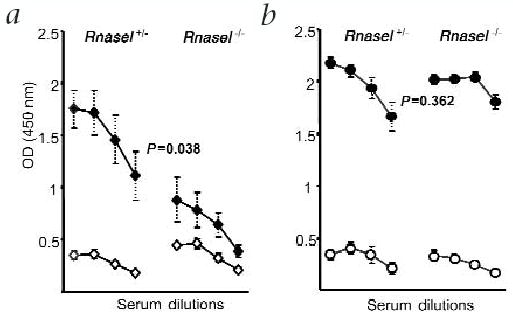
The involvement of dsRNA and dsRNA-dependent pathways in the immune response to replicase-based plasmids. a, Rnasel−/− mice and Rnasel+/− littermates were immunized weekly for 5 weeks (♦) and were bled before challenge together with non-immunized controls (⋄). Sera were diluted 1:5, 1:25, 1:125 and 1:625, and measured individually. Shown is the average antibody titer (n = 6) with standard errors. P value was calculated by ANOVA. The titer of antibody against TRP-1 was determined by ELISA as in Fig 2. b, To confirm the validity of the animal model, Rnasel+/− and Rnasel−/− mice were immunized twice with vaccinia-mTRP-1 (•). Two weeks after the second immunization, mice were bled together with non-immunized controls (○). The experiment was repeated with identical results.
Fig. 6.
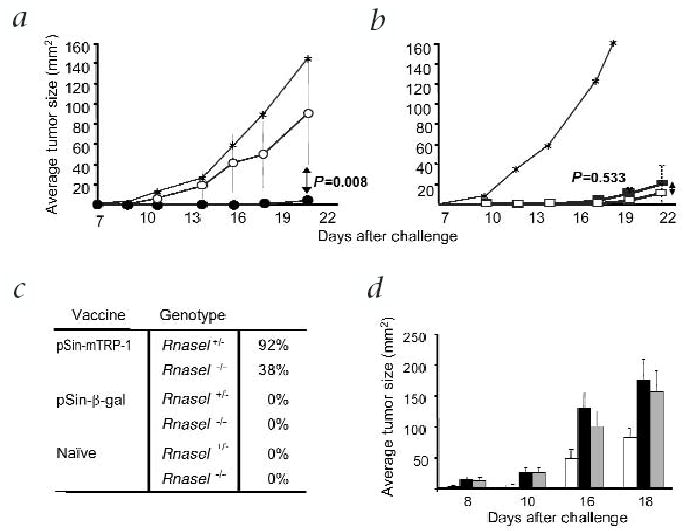
The involvement of dsRNA and dsRNA-dependent pathways in the immune response after immunization with replicase-based plasmids. a, Tumors are effectively prevented by pSin-mTRP-1 in Rnasel+/− (•), but not in Rnasel−/− mice (○). Shown are the average tumor sizes of mice (n = 6) immunized weekly with pSin-mTRP-1 5 times and challenged with B16.F10 7–10 days after the last immunization. No difference was observed in the tumor growth between Rnasel−/− and Rnasel+/− control mice immunized with pSin-β-gal (data not shown). The experiment was repeated with the same result. b, Average tumor sizes of Rnasel−/− (□) and Rnasel+/− (▪) mice immunized twice with recombinant vaccinia-mTRP-1 and challenged with B16 melanoma subcutaneously 2 weeks after the second immunization. Shown is the average tumor size of at least 6 mice. *Naive control mice. c, Compiled percentage of tumor-free mice (n = 12) immunized with replicase-based plasmid 21 d after subcutaneous challenge with B16.F10 melanoma. Mice were challenged 7–10 days after the last immunization. Shown are pooled data from two independent experiments. d, Tumor protection of Rnasel−/−mice receiving adoptively transferred CD8+ T cells from donors immunized with pSin-mTRP-1 (□). Challenge-control Rnasel−/− mice were only lymphocyte-depleted, but received no treatment (▪). Treatment-control mice were lymphocyte-depleted and treated in the same way as the experimental group with IL-2 and pSin-mTRP-1, but received purified CD8+ T cells from naive donors (▪). The difference between the experimental group and both control groups (n = 10 for all experiments) was statistically significant (P = 0.012 for challenge control, P = 0.027 for treatment control, by ANOVA). There was no difference between the two control groups (P = 0.526). In addition, there was a statistically significant difference (P < 0.05) between the group receiving immune CD8+ T cells and the challenge control on every single day of measurement (as determined by the F test). The experiment was repeated in wild-type mice with comparable results (data not shown) establishing the functionality of immune CD8+ T cells in Rnasel−/− mice.
To further explore the role of CD8+ T cells in the rejection of B16 melanoma after immunization with the replicase-based vectors, we carried out adoptive T-cell experiments. CD8+ cell enriched leukocytes from pSin-mTRP-1 immunized wild-type mice were transferred into naive, lymphocyte-depleted recipient Rnasel−/− mice. After the transfer, the growth of B16 melanoma was significantly decreased in mice receiving immune CD8+ T cells, but not in mice receiving control CD8+ T cells (Fig. 6d). The same result was obtained using C57BL/6 wild-type recipients (data not shown). Note that adoptively transferred T cells did not eliminate the tumor burden as efficiently as active immunization. This is most likely due to inherent inefficiencies with the adoptive transfer technique. Nevertheless, these findings show that antigen-specific CD8+ T cells were functional in Rnasel−/− hosts once they had been generated and activated in a normal host. Our data demonstrate for the first time that the increased immunogenicity of replicase-based DNA vaccines is mediated in part by the induction of the RNase L antiviral pathway.
Discussion
DNA vaccines have several theoretical and practical advantages over other types of experimental cancer vaccines, such as whole-cell vaccines, which deliver an array of known and uncharacterized tumor antigens25. Molecular vaccines encode defined antigens and provide off-the-shelf reagents that can easily be modified as needed, and they can be delivered by a variety of methods and routes3,4. However, DNA vaccines are insufficiently immunogenic for several antigens3,26. One possible reason for this shortcoming has been hypothesized to be low antigen production from the plasmid. Replicase-based DNA vaccines were developed in an effort to increase antigen production from nucleic acid vaccines6,7. In this new generation of DNA vaccines, antigen expression is under the control of an alphaviral replicase-enzyme, a molecule used by the alphavirus to produce a very large number of viral copies9. However, we did not find that alphaviral replicase-based vectors produced more antigen in transfected cells than conventional DNA vaccines in vitro or in vivo. Our findings are consistent with another report showing no over-production of antigen from a Sindbis replicase-based vector10. These results are at odds with several reports citing increased antigen production from replicase-based constructs7,27,28. However, the reported increase was much less than expected considering that intact alphaviruses monopolize the protein machinery of infected cells and produce up to 200,000 copies of positive-strand RNA from a single virus9. Nevertheless, several reports have demonstrated that replicase-based nucleic acid vaccines are significantly more immunogenic than conventional DNA vaccines requiring doses 100 to 1,000-times lower than conventional DNA plasmids to induce a comparable immune response10,12.
In this study, we sought to identify an alternative explanation for the superior efficacy of replicase-based DNA vaccines. Although the immunogenicity of a DNA vaccine can be improved by increasing the amount of antigen, antigen production is certainly not the only factor that determines immunogenicity. The immunological context in which the antigen is presented may be a more important aspect. For example, delivering a DNA vaccine together with a conventional adjuvant such as monophosphoryl lipid A (MPL) significantly decreases antigen production in transfected cells while the resulting immune response is strongly enhanced29. Similarly, causing apoptosis in DNA-vaccine–transfected cells reduces antigen production while significantly enhancing the immune response5. Finally, the immunogenicity of a plasmid could be increased 100-fold by co-injection of a GM-CSF-encoding “adjuvant-plasmid” (Z. Yu, pers. comm.). Thus, co-delivering a potent stimulator of the innate immune system can overcompensate for the reduced amount of antigen available to the adaptive immune system.
We thus investigated whether activation of innate, antiviral host mechanisms could account for the strong immunogenicity of replicase-based DNA vaccines. Such host pathways would be triggered by the production of dsRNA in transfected cells due to the activity of the alphaviral replicase. Here, we demonstrate for the first time the presence of such RNA species after transfection with an alphavirus replicase-based DNA plasmid (Fig. 4). The subsequent activation of dsRNA-dependent pathways (PKR and 2′-5′-A synthetase/RNase L pathways14,21,22) provides a mechanistic explanation for the observation that every cell transfected in vitro with replicase-based DNA or RNA undergoes apoptosis12,17,30. To demonstrate the role in the immunogenicity of replicase-based vaccines, we used Rnasel−/− mice24. The immunogenicity and efficacy of the replicase-based vaccine were markedly reduced. However, the activity of the DNA vaccine was not completely abrogated because only one of several dsRNA pathways was knocked out. To address the possibility that the immune response to TRP-1 or to tumor was compromised in Rnasel−/− mice, we used recombinant vaccinia that also encoded TRP-1 (Figs. 5b and 6b). This vaccine worked equally well in Rnasel−/− and Rnasel+/− mice. To further validate vaccine activity in Rnasel−/−mice, we adoptively transferred CD8+ T cells from wild-type mice immunized with pSin-mTRP-1. The recipients had significantly reduced B16 tumor burdens (Fig. 6d), similar to those observed in wild-type recipients (data not shown).
How does the activation of dsRNA-dependent, antiviral defense mechanisms increase immunogenicity? dsRNA molecules induce type I interferons and heat shock proteins, thus helping to initiate an immune response to a (perceived) viral infection. Furthermore, replicase-based DNA and RNA constructs cause apoptosis of transfected cells, most likely a consequence of the activation of the RNase L and the PKR pathways. Apoptosis has been shown by others to stimulate the immune system31–33, while apoptosis is reduced in mice lacking Rnasel after treatment with apoptosis-inducing drugs24. We argue that the context and the stimulus for the induction of apoptosis determine its impact on an immune response, distinguishing between bland, inflammatory and immune apoptosis34. Using pro-apoptotic genes co-delivered with a DNA vector, vaccine efficacy was significantly improved5. Dendritic cells can recognize and engulf (transfected) apoptotic cells and subsequently become activated35. We previously demonstrated that apoptosis triggered by replicase-based RNA stimulates dendritic cells17. Thus, the induction of apoptosis by replicase-based nucleic acid vaccines not only represents a welcome safety feature, but also seems to be critical for their function. Interfering with apoptosis by knocking out a pathway involved in inducing apoptosis after viral infection, such as the RNase L system (Figs. 5 and 6), or reducing apoptosis by co-immunization with an anti-apoptotic gene (manuscript in preparation) significantly reduces vaccine efficacy.
Why do replicase-based nucleic acid vaccines not necessarily over-produce antigen? Consequences of the transfection of cells with replicase-based DNA may account for this observation. The 2′-5′A synthetase/RNase L and the PKR pathways are activated by dsRNA and result in degradation of mRNA and blockade of protein synthesis, respectively, not only in virus-infected, but also in DNA-transfected, cells. Using dominant-negative mutants we have shown that at least one pathway involved in dsRNA recognition is responsible for limiting antigen production in cells transfected with replicase-based vaccines23. Furthermore, replicase-based DNA or RNA can trigger apoptosis36, thus limiting antigen production from the replicase-based vaccine. Analogues of mammalian anti-apoptotic molecules have been identified in several viruses37,38 and may be used by alphaviruses (but not replicase-based vaccines) to enhance viral replication.
Do conventional and replicase-based DNA vaccines use different mechanisms? The T-cell depletion and adoptive T-cell transfer experiments indicate a much stronger role of effector CD8+ cells for tumor protection induced by the replicase-based plasmids compared with the conventional DNA vaccine. Only the conventional plasmid (as well as vaccinia-mTRP-1) induced autoimmune depigmentation (vitiligo). Although the finding that that the vaccines that provide superior tumor protection induce no apparent auto-immunity may seem paradoxical, others have demonstrated that these two responses are mediated by different effector mechanisms13,39.
We have demonstrated that the immunogenicity and efficacy of DNA vaccines is improved significantly, not by providing more antigen for the adaptive immune response, but by delivering stronger ‘adjuvant-type’ signals to the innate immune system through activation of antiviral pathways. Together with immunostimulatory CpG motifs on bacterium-derived plasmids (recognized by Toll-like receptor 9 (TLR9); ref. 40), the production of dsRNA by replicase-based nucleic acid vaccines (recognized by TLR3; ref. 41) may represent a potent stimulation of the innate immune system. The RNase L pathway is potentially the first of several innate (antiviral) pathways shown to be involved in the high immunogenicity of replicase-based DNA vaccines. Tapping into such innate pathways may enable us to develop powerful vaccines while avoiding the side effects of highly immunogenic viruses or strong adjuvants.
Methods
Plasmids
Conventional CMV-promoter–based plasmids were pcDNA3-mTRP-1 (pCMV-mTRP-1; ref. 42) and pSport-hTRP-1 (pCMV-hTRP-1). pSport-hTRP-1 is based on pSport-β-gal12 (Invitrogen, Carlsbad, California). The lacZ gene was replaced with TYRP1 by blunt-end ligation. The replicase-based plasmids (pSin-mTRP-1, pSin-hTRP-1) are derived from pSin-β-gal (ref. 12). Plasmids were purified using EndoFree purification columns (Qiagen, Hilden, Germany). TRP-1 expression was confirmed for all plasmids by transfection of BHK-21 cells using LipofectAMINE PLUS (Invitrogen). After 24 h, the lysates of cells containing 2 × 104 transfected cells were loaded and TRP-1 was detected by immunoblot analysis (NuPage system, Novex, San Diego, California) using serum (diluted 1:250) from mice immunized with recombinant vaccinia-TRP-1 (ref. 20). pC1-EGFP (pCMV-EGFP, Clontech Laboratories, Palo Alto, California) was used as a negative control. In vivo expression of β-gal was determined in mechanically processed skin sections as described for transfected cells12.
Mice and immunization
C57BL/6 mice (National Cancer Institute (NCI), Frederick, Maryland) and Rnasel−/− mice24 (back-crossed on a C57BL/6 background) were used at 6–10 weeks of age. C57 wild-type and Rnasel+/− mice had comparable immune responses after immunization with β-gal or TRP-1 DNA plasmids. B16.F10-melanoma (National Cancer Institute-FCRDC) grew similarily in both (data not shown). Tyrp1−/−mice will be described (W.W.L. and N.P.R., manuscript in preparation) and can be distinguished from heterozygous (Tyrp1+/−) littermates by coat color (‘cappuccino’ versus black). Intramuscular injection yielded poor immunogenicity and efficacy (data not shown). Thus plasmid-coated gold particle43 were delivered using the Helios gene gun (BioRad, Hercules, California). Mice were challenged subcutaneously with 1 × 105 B16.F10 7 to 10 d after the last of 5 immunizations (approximately 3 μg of DNA/immunization) at weekly intervals. Tumor growth was monitored for at least 3 weeks after challenge in a blinded fashion. We immunized and challenged 5 to 8 mice per group in each experiment. Animal experiments were conducted according to approved animal protocols of the NCI (NIH, Bethesda, Maryland).
T-cell depletion and adoptive T-cell transfer
Four days after the last immunization, mice were injected intraperitoneally twice on consecutive days with 500 μg of GK1.4 (anti-CD4) or 2.43 (anti-CD8) antibody (ammonium sulfate precipitated and dialyzed; NCI-FCRDC, Frederick, Maryland). Efficacy of depletion was greater than 99% as determined by flow cytometry. For the adoptive T-cell transfer, C57BL/6 mice were immunized as described above. One week after the last immunization, spleens and lymph nodes from 10 mice were harvested. Adherent cells were removed by panning (1 h at 37 °C). B cells and CD4+ T cells were removed with antibodies against B220 and CD4 conjugated to magnetic particles in LD MACS columns (Miltenyi Biotec, Bergisch Gladbach, Germany). Recipient C57BL/6 or Rnasel−/− mice were depleted of endogenous T cells (500 rad, 1 day before cell transfer or 1 mg cyclophosphamide, intraperitoneally, 5 days before cell transfer). CD8+ T cells from 10 donors were injected intravenously into 10 recipient mice challenged as above on the day of transfer. Recipient mice were then immunized with pSin-mTRP-1 by gene gun on 3 consecutive days following the transfer and received 3 i.p. injections of 100,000 CU IL-2 (Chiron, San Diego, California).
Serology
Pre-challenge serum was diluted in PBS/1% BSA and tested by ELISA. Plates (Nunc, Roskilde, Denmark) were coated with 50 ng recombinant mouse TRP-1 per well44. Goat antibody against mouse/HRP (Amersham, Piscataway, New Jersey) was used at a dilution of 1:3,000. After addition of TMB substrate solution (Endogen, Woburn, Massachusetts) the reaction was measured at 450 nm.
PKR activation assay
SW480 or BHK-21 cells (ATCC, Manassas, Virginia) were transfected with pSin-EGFP or pCMV-EGFP using Superfect (Qiagen, Hilden, Germany) or LipofectAMINE PLUS, respectively. After 12 h, cells were double-sorted (Clinical Services Program, SAIC-Frederick, Maryland) using a FACStar Plus (Becton Dickinson, San Jose, California). The lysate of 1 × 105 cells was resuspended in 100 μl of PKR lysis buffer and incubated with recombinant PKR as described45. The sample was loaded onto a 10% SDS–PAGE gel and the dried gel was exposed to autoradiography. Purified GST–PKR-WT or GST–K296RPKR (dominant-negative form; 100 ng) were added to 5 μg of cell lysate for the kinase assay46.
Statistical analysis
Data were analyzed with ANOVA or Wilcoxon rank sum test using StatView (Abacus Concepts, Berkeley, California).
Acknowledgments
We thank L. Finch for FACS sorting, and E.S. Bergmann-Leitner and S. Frank for critical review of the manuscript. Supported in part by grant R01-AI34039 (B.R.G.W.) and CA44059 (R.H.S.) from the National Institutes of Health.
Footnotes
Competing interests statement ?
References
- 1.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Blum JS, Ma C, Kovats S. Antigen-presenting cells and the selection of immunodominant epitopes. Crit Rev Immunol. 1997;17:411–417. [PubMed] [Google Scholar]
- 3.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 4.Leitner WW, Hammerl P, Thalhamer J. Nucleic acid for the treatment of cancer: Genetic vaccines and DNA adjuvants. Curr Pharm Res. 2001;7:1641–1667. doi: 10.2174/1381612013397249. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki S, Amara RR, Oran AE, Smith JM, Robinson HL. Apoptosis-mediated enhancement of DNA-raised immune responses by mutant caspases. Nature Biotechnol. 2001;19:543–547. doi: 10.1038/89289. [DOI] [PubMed] [Google Scholar]
- 6.Zhou X, et al. Self-replicating Semliki Forest virus RNA as recombinant vaccine. Vaccine. 1994;12:1510–1514. doi: 10.1016/0264-410x(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 7.Herweijer H, et al. A plasmid-based self-amplifying Sindbis virus vector. Hum Gene Ther. 1995;6:1161–1167. doi: 10.1089/hum.1995.6.9-1161. [DOI] [PubMed] [Google Scholar]
- 8.Schlesinger S. Alphavirus vectors: Development and potential therapeutic applications. Exp Opin Biol Ther. 2001;1:177–191. doi: 10.1517/14712598.1.2.177. [DOI] [PubMed] [Google Scholar]
- 9.Schlesinger, R.W. in The Togaviruses (ed. Wengler, G.) 459 (Academic Press, New York, 1980).
- 10.Hariharan MJ, et al. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J Virol. 1998;72:950–958. doi: 10.1128/jvi.72.2.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berglund P, Smerdou C, Fleeton MN, Tubulekas I, Liljestrom P. Enhancing immune responses using suicidal DNA vaccines. Nature Biotechnol. 1998;16:562–565. doi: 10.1038/nbt0698-562. [DOI] [PubMed] [Google Scholar]
- 12.Leitner WW, Ying H, Driver DA, Dubensky TW, Restifo NP. Enhancement of tumor-specific immune response with plasmid DNA replicon vectors. Cancer Res. 2000;60:51–55. [PMC free article] [PubMed] [Google Scholar]
- 13.Weber LW, et al. Tumor immunity and autoimmunity induced by immunization with homologous DNA. J Clin Invest. 1998;102:1258–1264. doi: 10.1172/JCI4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou A, Hassel BA, Silverman RH. Expression cloning of 2-5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell. 1993;72:753–765. doi: 10.1016/0092-8674(93)90403-d. [DOI] [PubMed] [Google Scholar]
- 15.Overwijk WW, et al. gp100/pmel 17 is a murine tumor rejection antigen: Induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand . J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowne WB, et al. Coupling and uncoupling of tumor immunity and autoimmunity. J Exp Med. 1999;190:1717–1722. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying H, et al. Cancer therapy using a self-replicating RNA vaccine. Nature Med. 1999;5:823–827. doi: 10.1038/10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vijayasaradhi S, Bouchard B, Houghton AN. The melanoma antigen gp75 is the human homologue of the mouse b (brown) locus gene product. J Exp Med. 1990;171:1375–1380. doi: 10.1084/jem.171.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito S, Jimbow K. Quantitative analysis of eumelanin and pheomelanin in hair and melanomas. J Invest Dermatol. 1983;80:268–272. doi: 10.1111/1523-1747.ep12534616. [DOI] [PubMed] [Google Scholar]
- 20.Overwijk WW, et al. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci USA. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Player MR, Torrence PF. The 2-5A system: modulation of viral and cellular processes through acceleration of RNA degradation. Pharmacol Ther. 1998;78:55–113. doi: 10.1016/S0163-7258(97)00167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams BR. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 23.Terenzi F, et al. The antiviral enzymes PKR and RNase L suppress gene expression from viral and non-viral based vectors. Nucleic Acids Res. 1999;27:4369–4375. doi: 10.1093/nar/27.22.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou A, et al. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 1997;16:6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Elsas A, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: Comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leitner WW, Ying H, Restifo NP. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine. 1999;18:765–777. doi: 10.1016/s0264-410x(99)00271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubensky TWJ, et al. Sindbis virus DNA-based expression vectors: utility for in vitro and in vivo gene transfer. J Virol. 1996;70:508–519. doi: 10.1128/jvi.70.1.508-519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johanning FW, et al. A Sindbis virus mRNA polynucleotide vector achieves prolonged and high level heterologous gene expression in vivo. Nucleic Acids Res. 1995;23:1495–1501. doi: 10.1093/nar/23.9.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki S, et al. Monophosphoryl lipid A enhances both humoral and cell-mediated immune responses to DNA vaccination against human immunodeficiency virus type 1. Infect Immun. 1997;65:3520–3528. doi: 10.1128/iai.65.9.3520-3528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perri S, et al. Replicon vectors derived from Sindbis virus and Semliki forest virus that establish persistent replication in host cells. J Virol. 2000;74:9802–9807. doi: 10.1128/jvi.74.20.9802-9807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, et al. Efficient antitumor immunity derived from maturation of dendritic cells that had phagocytosed apoptotic/necrotic tumor cells. Int J Cancer. 2001;93:539–548. doi: 10.1002/ijc.1365. [DOI] [PubMed] [Google Scholar]
- 33.Shaif-Muthana M, McIntyre C, Sisley K, Rennie I, Murray A. Dead or alive: immunogenicity of human melanoma cells when presented by dendritic cells. Cancer Res. 2000;60:6441–6447. [PubMed] [Google Scholar]
- 34.Restifo NP. Vaccines to die for. Nature Biotechnol. 2001;19:527–528. doi: 10.1038/89255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chattergoon MA, et al. Targeted antigen delivery to antigen-presenting cells including dendritic cells by engineered Fas-mediated apoptosis. Nature Biotechnol. 2000;18:974–979. doi: 10.1038/79470. [DOI] [PubMed] [Google Scholar]
- 36.Der SD, Yang YL, Weissmann C, Williams BR. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarid R, Sato T, Bohenzky RA, Russo JJ, Chang Y. Kaposi’s sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nature Med. 1997;3:293–298. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- 38.Revilla Y, et al. Inhibition of apoptosis by the African swine fever virus Bcl-2 homologue: Role of the BH1 domain. Virology. 1997;228:400–404. doi: 10.1006/viro.1996.8395. [DOI] [PubMed] [Google Scholar]
- 39.Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch JV. Fc receptors are required in passive and active immunity to melanoma. Proc Natl Acad Sci USA. 1998;95:652–656. doi: 10.1073/pnas.95.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner H. Interactions between bacterial CpG-DNA and TLR9 bridge innate and adaptive immunity. Curr Opin Microbiol. 2002;5:62–69. doi: 10.1016/s1369-5274(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 41.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 42.Surman DR, et al. Generation of polyclonal rabbit antisera to mouse melanoma associated antigens using gene gun immunization. J Immunol Methods. 1998;214:51–62. doi: 10.1016/s0022-1759(98)00036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leitner WW, et al. Immune responses induced by intramuscular or gene gun injection of protective DNA vaccines that express circumsporozoite protein from Plasmodium berghei malaria parasites. J Immunol. 1997;157:6119. [PubMed] [Google Scholar]
- 44.Touloukian CE, et al. Expression of a “self-”antigen by human tumor cells enhances tumor antigen-specific CD4(+) T-cell function. Cancer Res. 2002;62:5144–5147. [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Z, Williams BR. The B56α regulatory subunit of protein phosphatase 2A is a target for regulation by double-stranded RNA-dependent protein kinase PKR. Mol Cell Biol. 2000;20:5285–5299. doi: 10.1128/mcb.20.14.5285-5299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar A, Haque J, Lacoste J, Hiscott J, Williams BR. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κ B by phosphorylating I κ B. Proc Natl Acad Sci USA. 1994;91:6288–6292. doi: 10.1073/pnas.91.14.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]


