Abstract
The heat shock chaperones mortalin/mitochondrial heat shock protein 70 (mtHsp70) and Hsp60 are found in multiple subcellular sites and function in the folding and intracellular trafficking of many proteins. The chaperoning activity of these 2 proteins involves different structural and functional mechanisms. In spite of providing an excellent model for an evolutionarily conserved molecular “brotherhood,” their individual functions, although overlapping, are nonredundant. As they travel to various locations, both chaperones acquire different binding partners and exert a more divergent involvement in tumorigenesis, cellular senescence, and immunology. An understanding of their functional biology may lead to novel designing and development of therapeutic strategies for cancer and aging.
INTRODUCTION
The mitochondrion generates copious amounts of free radicals. In addition to the mitochondrial antioxidant defense consortium that quenches and neutralizes free radicals, mitochondrial chaperones prevent the aggregation and promote refolding of redox-modified proteins (Parcellier et al 2003). Chaperones play crucial roles in mitochondrial biogenesis that involve the import, and partitioning of nuclear-encoded precursor proteins within the matrix and 2 mitochondrial membranes. We focus on the 2 major mitochondrial chaperones in this review: the first part presents a structural–mechanistic view of the 2 chaperones working together within the confines of their native cellular locale, the mitochondria. This life-essential “brotherhood” between mortalin and Hsp60 is best appreciated by their major complementing roles in maintaining mitochondrial biogenesis and energetics. The second part reviews aspects related to their extramitochondrial biology, putting to fore their acquisition of novel cellular functions. Surprisingly, these 2 chaperones have begun to display entirely unique behaviors. Their functional divergence as sentinels of longevity and as death effectors is discussed.
PART I. CELL STRESS, THE MITOCHONDRIA, AND ITS CHAPERONES
The heat shock proteins (Hsps) or molecular chaperones are present in all organisms and play an important role in cell survival. They are highly conserved proteins and constitute one of the most ancient cellular defense systems on the planet. The term “heat shock protein” was so coined due to their strong induction of gene expression in response to heat stress in Drosophila larvae (Ritossa 1962). The activities of chaperones in both housekeeping and stress response are based on their ability to interact with unfolded (nascent) and misfolded (denatured) proteins. They transiently associate with short peptide segments of the folding intermediates while preventing their aggregation by shielding-off exposed hydrophobic patches (Gosslau et al 2001).
The functionality of chaperones, such as the recognition of substrates and their refolding activity, interactions with cochaperones, clients, and other cofactors (adenosine triphosphate [ATP] and cations), depends on their own unique architectures. Two of the best-known mitochondrial chaperone machines, in terms of structure and dynamics, are mortalin/mitochondrial Hsp70 (mtHsp70) and Hsp60.
Architectural individuality of the mitochondrial chaperones
Mortalin/mtHsp70
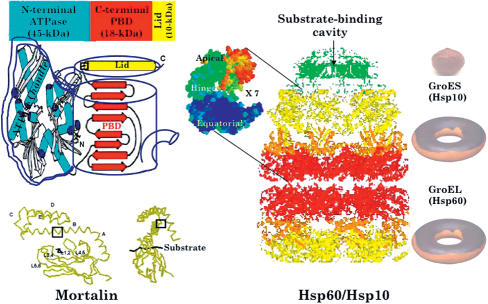
Mortalin is a heat-uninducible, novel member of Hsp70 family of proteins initially identified from the cytoplasmic fractions of normal mouse fibroblasts. It is 679 amino acids long with molecular weight 73 913 Da. It has a high degree of identity with other members of the Hsp70 family, including Escherichia coli DnaK (51%), Saccharomyces cerevisiae SSC1p (65%), the constitutive cytosolic Hsp70 from rat, Hsc70 (46%), and the rat endoplasmic reticulum (ER) isoform BiP (49%). The precursor protein has a 46-amino acid–long mitochondrial-targeting signal peptide. It undergoes Ca-dependent autophosphorylation, has multiple subcellular sites and binding partners, and has functions related to the control of cell proliferation and stress (Kaul et al 1993; Wadhwa et al 1993a; 2002). The crystal structure of mortalin has not been elucidated so far. Based on its evolutionary conservation within the Hsp70 family (Macario and de Macario 1999; Mayer and Bukau 1998), it is expected to have structural characteristics similar to that of other Hsp70s.
The Hsp70 contains 2 principal domains joined by a protease-sensitive site, ie, the N-terminal adenosine triphosphatase (ATPase) and C-terminal regions (Fig 1). Chaperonizing activities of the Hsp70 family proteins are intimately linked with ATP hydrolysis. The structure of 44-kDa ATPase domain, modeled after that of the bovine Hsc70, consists of 4 subdomains that fold into a pair of lobes forming a deep catalytic cleft (Sriram et al 1997). Studies on E coli Hsp70, DnaK, have demonstrated that its ATPase activity can be cyclically stimulated by cochaperones DnaJ and GrpE. DnaJ permits the hydrolysis of Hsp70-bound ATP allowing the adenosine 5′-diphosphate (ADP)-bound Hsp70 to interact more strongly with unfolded proteins. The nucleotide exchange factor GrpE enables the recycling of Hsp70 back into an ATP-bound state, permitting the efficient release of its substrate (Harrison et al 1997). Multiple GrpE-like proteins and a unique human GrpE homolog, HMGE, have been reported to be restricted to the mitochondria and to form chaperone pairing with mortalin/mtHsp70 (Naylor et al 1998; Choglay et al 2001). Besides the known mtDnaJ (Zhao et al 2002), dj2 and dj3 could be candidate cochaperones of mortalin, because these cytosolic DnaJs are also detected in the mitochondria (Terada and Mori 2000). As these cycles of ATP hydrolysis occur, allosteric changes in the N terminus are transmitted to the peptide-binding domain (PBD). The 18-kDa PBD is made up of 2 sets of 4-stranded antiparallel beta sheets that form a twisted sandwich. Whereas the ATPase domains are strictly conserved, the PBD domain shows a greater sequence variation resulting in the diversification of the client peptides and substrate specificity. This also reflects the adaptive specialization of each member of the Hsp70 family (Rudiger et al 2000). Flanking the PBD is a 10-kDa-long C-terminal helix “lid” of 5 distinct helical domains (A–E) that do not directly come to contact with the peptide substrate. The helix is kinked in the middle and is bent upward, resulting in the loss of interaction with the outer pair of loops that, in turn, enhances the lid's capacity to flip-flop. This structure has also been proposed to function as a molecular “latch” that closes the peptide-binding pocket and makes peptide binding less dynamic in the ADP-bound state (Zhu et al 1996). Whereas helix A has high sequence conservation between Hsp70 proteins, the second half of helix B is divergent. Although the function of this variable C-terminal domain remains unclear, it has been shown to bind to some cofactors in order to modulate its activity (Horton et al 2001) and possess immunomodulatory functions (Lehner et al 2004).
Fig 1.
(A) A “kettle pot” model for the structure of mortalin. Above, For simplicity, we can compare the structure of the mortalin with a kettle pot showing its handle (ATPase domain), which consists of 4 subdomains that fold into a pair of lobes to form a deep catalytic cleft and regulates the opening of the lid, and the pot (substrate-binding cleft), which contains the substrate. Upon binding to ATP, the Hsp70 ATPase confers the lid an altered conformation, opening it by bending the hinge (shown by an empty square), and allows the substrate to enter the cleft. Below, Ribbon diagrams of the peptide-binding domain (in standard stereo [left] and one rotated 90° counterclockwise [right]) (from the Protein Data Bank, 1dkx.pdb). Four loops emanate within this β-subdomain: an inner pair (L1,2 and L3,4) that establishes a hydrophobic substrate cleft, and a flanking outer pair (L4,5 and L5,6) that stabilizes the substrate-binding cleft. (B) A “donut and munchkin” model for Hsp60. Shown in side view is the structure of the GroEL–GroES–(ADP)7 complex with the 4-banded appearance of the “doubly stacked donut” GroEL and its apical spherical-shaped GroES (munchkin) with a central substrate-binding cavity. Each “donut” is composed of a ring of 7 subunits. Each subunit (right) is composed of 3 domains: apical, intermediate, and equatorial. Upon binding of 7 molecules of ATP to the equatorial domains, sequential conformational changes occur in both the intermediate and apical domains, and allosteric interactions are transmitted from one ring to the other causing the expansion of the cavity size that gives an illusion that the complex “breathes.” If substrate is not folded properly, it can rebind to the same or different GroEL molecule, and the cycle of regurgitation is repeated until the protein substrate attains its native state (from the Protein Data Bank, 1a6d.pdb).
There is a substantial understanding of what substrates are recognized by Hsp70 from the studies of 3 research teams that have used proteomic and combinatorial peptide technologies. First, with a 15-mer phage display library, Takenaka et al (1995) demonstrated that Hsc70 prefers 2 distinct sets of sequence motifs important for its dual chaperoning roles: one as an organelle-translocator motif (NIVRKKK-like) and the other as sequences recognized to enable protein refolding (FYQLALT-like). Second, a more comprehensive screen was conducted for DnaK substrates using a library of 4360 cellulose-bound 13-mer peptides. This screen deduced that the binding motif possesses a central hydrophobic core of 4 or 5 residues enriched particularly with Leu and to a lesser extent with Ile, Val, Phe, and Tyr. It has 2 flanking regions enriched with basic residues while disfavoring acidic residues (Rudiger et al 1997). And third, by liquid chromatography–ion trap mass spectrophotometry (LC-ITMS) of endogenously bound peptides to Hsp70, the database of this comprehensive proteomic screening reveals that Hsp70-binding motifs must contain both basic and acidic residues at critical positions (1, 3, 5, 7, and 9), disfavoring large aromatic residues (Grossmann et al 2004).
Two allelic forms of mortalin were discovered in mice. One, being an uncanny exception to the gero-protective paradigms of Hsp70 chaperones, is the pancytoplasmic mortalin (mot-1). Initially christened as a ‘mortality factor’ due to its marked presence in normal murine fibroblasts and mortal cybrids (Wadhwa et al 1993a), overexpression of the protein (mot-1) induced aging in immortalized fibroblasts (Wadhwa et al 1993b). In contrast, its other allelic form, the perinuclear mortalin (mot-2), supported malignant transformation of mouse fibroblasts (Kaul et al 1998) and extended life span of human fibroblasts (Kaul et al 2003) and worms (Yokoyama et al 2002). Human cells have only the mot-2 (hmot-2) that is distributed pancytoplasmically in normal cells. When human cells become immortal and/or tumorigenic, the subcellular distribution of hmot-2 shifts to a perinuclear form and gains the ability to inactivate p53 and Ras–Raf pathways (Wadhwa et al 1998, 2003). Although these events appear to be a universal feature of immortalization, the molecular factors that govern this switch remain to be defined.
Hsp60/Hsp10
Described as a “giant breathing machine” (Richardson et al 1998), the mammalian Hsp60/Hsp10 (chaperonin) complex engulfs misfolded proteins and regurgitates them into properly folded structures. Due to the stability of the bacterial chaperonin system and the relative ease with which GroEL can be purified and crystallized, most of our biophysical understanding of Hsp60 are based on the E coli GroEL chaperonin (Ranson et al 2001). This oligomer, weighing nearly a million daltons, has the cochaperonin GroES/Hsp10 resting on its apex (Hunt et al 1996). GroEL's body is a 14 subunit-toroidal assembly that creates a large central cavity to which the unfolded protein substrate binds via hydrophobic interactions. Each GroEL subunit can be divided into 3 domains, ie, (1) the apical domain that binds to both its substrate and GroES, (2) the equatorial domain that contains a binding site for ATP and the ring, and (3) the intermediate domain that functions as a mobile linker to these 2 domains (Fig 1). The central cavity is an assemblage of rings that processes misfolded proteins at 2 transitional states, ie, (1) peptide-accepting state: when it is open, exposing a flexible hydrophobic “lip” that captures non-native species and funnels them in the cavity (Weissman et al 1995;Sigler et al 1998; Fenton and Horwich 2003) and (2) peptide-folding state: once the protein is ingested, the lid-like chaperonin GroES caps the end and triggers unscrambling of the secondary structure of the substrate by multivalent mechanical vacillations of its hydrophobic/hydrophilic interior (Farr et al 2000).
Similar to Hsp70, ATP binding to 1 of the 2 rings of the GroEL complex drives its protein-folding cycle. Simultaneously, as GroES caps the complex, the interior of the cavity starts achieving a global physical change, in terms of shape and hydrophobicity. The cavity of the bound ring enlarges by more than 2-fold while its hydrophobic binding surface twists away from the polypeptide throwing off the engulfed protein into the cavity. As a result of the allosteric communication among its subunits, the interactive surface of the apical domains of GroEL switches from hydrophobic to hydrophilic, and vice versa. The different subunits with their coordinated movements engulf the protein with exposed hydrophobic surface and reorients until it attains a native state (Ranson et al 2001). The binding of nucleotide alone, in the absence of GroES, has not been observed to produce such an extent of apical movement (Bukau and Horwich 1998; Rye et al 1999). This captivating choreography of GroES– GroEL–ATP complex has been captured in a movie and can be viewed from the site courtesy of the University of London's Helen Saibil (http://people.cryst.bbk.ac.uk/~ubcg16z/cpn/elmovies.html).
Despite the high sequence homology of mammalian mitochondrial Hsp60 to bacterial GroEL, this chaperonin possesses unique features (Levy-Rimler et al 2002). The oligomeric state of mammalian Hsp60 is different from that of bacterial, yeast mitochondrial and chloroplast chaperonins. As the last 3 types exist as tetradecamers of 2 heptameric rings, the mammalian mitochondrial chaperonin, depending on its concentration, maintains a dynamic equilibrium between its monomeric, heptameric, and tetradecameric states (Levy-Rimler et al 2001). Furthermore, they differ in their requirement for cochaperones (Levy-Rimler et al 2002).
Roles in mitochondrial biogenesis
Mortalin in preprotein translocation
Because the mitochondrial genome encodes just a handful of proteins, it relies heavily on the import of proteins from the cytosol. It has, therefore, evolved an elaborate translocation system for efficient import of nuclear-coded proteins and also for export of proteins coded by its own genome (for recent reviews, see Koehler 2004; Rehling et al 2004; Wiedemann et al 2004). During the import, most proteins, eg, those targeting the matrix, would need to pass through the outer membrane (translocase of the outer membrane [TOM]) followed by the inner membrane (translocase of the inner membrane [TIM]) channels (Neupert 1997). Because bulky proteins need to unfold during shuttling and then refold back to their native conformations, mitochondrial molecular chaperones are assumed to play the central role in mitochondrial biogenesis. It is noteworthy that most of our understanding of mitochondrial transport mechanisms is from the study of the simple yeast (S cerevisiae) cell model due, in part, to the highly conserved nature of mitochondrial import and translocation processes (Voos and Rottgers 2002). A yeast cell can survive when starved of energy (the main function of the mitochondrion) but, ironically, perishes under all conditions when it harbors deletion in Ssc1, the yeast homolog of mortalin (Craig et al 1987). In the following discussion, mortalin has been used interchangeably with mtHsp70 and Ssc1 for consistency and ease.
Precursor proteins contain targeting and sorting information to reach the mitochondrion, whereas the translocons recognize the information and direct the precursor to the correct compartment. The outer membrane contains the TOM complex for translocation and the sorting and assembly machinery (SAM) complex for assembly. Translocation of positively charged N terminus of the preprotein through the TIM channel is driven by the energy from the membrane potential generated in the matrix (Geissler et al 2000, 2001). Albeit, membrane potential effects may not be sufficient to transport an entire protein, the import process is fueled by another energy source, ATP. Mortalin/mtHsp70 has been identified as the only ATPase component of this preprotein mitochondrial import complex and is required for the translocation of most mitochondrial inner membrane and matrix proteins (Schneider et al 1994; Brunner et al 1995). The protein transport machinery of the inner mitochondrial membrane contains 4 essential Tim proteins: Tim17, Tim23, Tim50, and Tim44. The TIM channel comprises 3 integral membrane proteins, Tim17, Tim50, and Tim23. While tethered onto the inner face of the inner mitochondrial membrane, Tim44 is transiently associated with mortalin/mtHsp70, the 2 cochaperone J-proteins, Pam18 and Pam16 (a degenerate J-protein), and the nucleotide exchange factor Mge1 (homolog of the bacterial GrpE), to form an ATP-driven import complex, called the presequence translocase-associated motor (PAM) (D'Silva et al 2004). The binding sites of Mge1 and Tim44 to mortalin have been assigned at the variable region along C-terminal α-helical lid (Strub et al 2003). During the translocation, Mge1 enhances the otherwise low intrinsic ATPase activity of mortalin via the release of ADP and Pi (Dekker and Pfanner 1997). Interestingly, whereas mortalin is an essential component for import of both tightly and loosely folded preproteins, Tim44 is believed to be a nonessential structural component. Instead, Tim44 may play a more specialized role in translocation of tightly folded domains (Bomer et al 1998). Recent data have provided evidence that Pam16, lacking the canonical tripeptide motif His-Pro-Asp (HPD), heterodimerizes with Pam18 and antagonizes its function modulating the interaction of mortalin with precursor proteins (Li et al 2004).
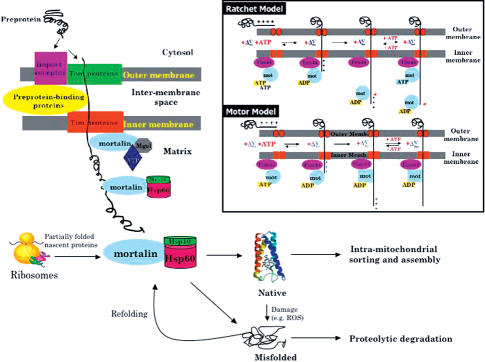
With the role of mortalin/mtHsp70 as the core of the mitochondrial transport machinery, its appreciation through the years has put forth 2 controversial hypotheses regarding its mechanism(s): mortalin/mtHsp70 as a molecular ratchet and as a dynamo in the “trapping” and “motor” models, respectively (Voos and Rottgers 2002). In the trapping model, brownian motion is the initial driving force that becomes converted into vectorial movement of the preprotein. Subsequently, as the N-terminal part of the preprotein is inserted into the inner membrane, pulled by the membrane potential, the exposed hydrophobic stretches of the protein within the matrix are then recognized as substrate by mortalin/mtHsp70. The binding with mortalin/mtHsp70 results in the holding of the protein and prevents its backflow. The fact that several Hsp70-binding sites are known to exist within a single protein, gradual trapping of the peptides completes the translocation process. The enhanced trapping by mortalin/mtHsp70 also reduces dependence on the import-driving activity of the membrane potential (Strub et al 2000; Geissler et al 2001). A more recent paper by Liu et al (2003) proposed that ATP hydrolysis may be unnecessary during the regulated interactions of mortalin/ mtHsp70 by Tim44. Furthermore, the release of Tim44 is also more rapid than the rate of ATP hydrolysis. As this be the case, the otherwise proposed existence of the translocation motor complex, as discussed later, may not run concurrent with the peptide translocation (Fig 2).
Fig 2.
Mortalin and Hsp60 cooperate during mitochondrial biogenesis and maintenance. After protein synthesis, preproteins enter the mitochondria through the outer membrane (TOM) followed by the inner membrane (TIM) translocation channels. Mortalin, bound to Tim44, brings the preprotein into the matrix by acting either as a molecular ratchet or motor (see box and see Schneider et al 1994; Neupert and Brunner 2002). During its transit, the preprotein is simultaneously refolded by mortalin, or is handed over to the Hsp60 complex for further cycles of protein refolding. Alternatively, some proteins are synthesized by resident mitochondrial ribosomes, and the nascent proteins are also assisted to fold by mortalin and Hsp60. Upon reaching the native state, mature mitochondrial proteins are further trafficked, assembled, and sorted to their proper places to start functioning in various mitochondrial processes. Because proteins may be damaged, either by wear-and-tear or by ROS attack during their lifetime, the repair of misfolded domains or degradation is achieved with the assistance of the 2 chaperones.
In the motor model, using Tim44 as a fulcrum on the membrane, with hydrolysis of ATP, mortalin/mtHsp70 generates a “power-pull” for the polypeptide (Voos et al 1996). Experimental data to support the existence of a “mortalin/mtHsp70 dynamo” demonstrate both the requirement for preprotein unfolding during import and the generation of an inward-directed translocation force. Due to the geometry of the import channel, precursor proteins are required to cross the mitochondrial membranes in a completely unfolded, or even stretched conformation. Components of the outer membrane, eg, surface receptors or the import pore have no unfoldase activity (Huang et al 2000). The mitochondrial import machinery, therefore, must be able to unfold a preprotein simultaneously as it transports (Voos and Rottgers 2002). Unfolding of a tightly folded preprotein, such as cytochrome b2, requires ATP hydrolysis in the matrix, indicating the direct involvement of mortalin/mtHsp70 (Glick et al 1993; Wachter et al 1994). Unfolding of a precursor at the mitochondrial surface is dramatically accelerated when its presequence is long enough to span both membranes and interact with mortalin/mtHsp70 in the mitochondrial matrix. Furthermore, the rates of unfolding are also orders of magnitude faster than for spontaneous unfolding, suggesting that mortalin/mtHsp70 could be the ATP-driven force-generating motor during protein import (Matouschek et al 1997). In the mitochondrial protein import motor, mortalin/mtHsp70 first hydrolyzes ATP, and then associates tightly with Tim44 and a precursor protein, and finally undergoes a conformational change to drive translocation (Horst et al 1996). The motor does not exert a constant pulling force but instead releases a translocating polypeptide chain such that the precursor can then slide back and refold on the surface of the mitochondria. Since refolding competes with translocation, the (polypeptide) cargo may undergo several rounds of unfolding and refolding prior to their import (Gaume et al 1998). In contrast to this model, a prerequisite for import protein via the passive trapping mode is that the preprotein must unfold spontaneously before entering the translocation channel so that it can slither into the matrix and get trapped by mortalin/mtHsp70. Current evidence, albeit, points out that both the mechanisms cooperate in order to obtain maximal import efficiency (Voos et al 1999; Voos and Rottgers 2002). Although the Hsp90–Hsp70 complex has been isolated and shown to stimulate mitochondrial import of precursor protein (Scherrer et al 1993), the involvement of Hsp90 as well as its mitochondrial counterpart, TRAP-1, on protein translocation remains obscure so far.
Folding and assembly by Hsp60
After entry into the mitochondrial matrix, the preproteins are initiated to refold and assemble. Hsp60 is one of the most important components of the protein-folding system within the mitochondrial matrix. It was demonstrated that yeast cells carrying a null mutation of Hsp60 are nonviable due to severe defects in folding of mitochondrial proteins, whereas those with conditional mutants tend to accumulate misfolded proteins that are unable to form active enzyme complexes (Cheng et al 1989). It was later shown that newly imported mitochondrial preproteins interact with Hsp60 upon its entry into the matrix compartment (Mahlke et al 1990; Langer and Neupert 1991; Hartl et al 1992). A rare genetic disorder, congenital lactic acidemia, is found associated with a general decline in Hsp60 activity, leading to multiple mitochondrial enzyme deficiencies (Briones et al 1997). Similarly, patients with hereditary spastic paraplegia SPG13 harbor a mutation in the gene encoding the mitochondrial Hsp60 (Hansen et al 2002) and in the short chain acyl-CoA dehydrogenase (SCAD) that both result in mitochondrial folding anomalies (Pedersen et al 2003). Hsp60 deficiency has also been reported in fibroblasts derived from a patient with a fatal, systemic mitochondrial disease leading to deficiency of multiple (10 out of 11) mitochondrial enzymes as well as abnormality of mitochondrial structure (Agsteribbe et al 1993).
Roles in the mitochondrial stress response
Exposure of cells to stress results in a global misfolding and impairment of cellular processes leading to a transactivation of genes with a heat shock element (HSE). However, cells can also respond to localized or organelle-specific stresses. The mitochondrial-specific stress response (MSR) in mammalian cells has recently been elucidated (Martinus et al 1996; Zhao et al 2002). Deletion of mtDNA from mammalian cells induced a stress response by stimulating the transcription of the nuclear genes encoding Hsp60 and Hsp10. Of note, both Hsp60 and Hsp10, but not mortalin, are also upregulated by heat stress (Naylor 1996). The possible explanations of the uninducible feature of mortalin may lie in its abundance and multiplicity of its roles.
PART II. BEYOND THE MITOCHONDRIAL BORDER
Despite the fact that they are popular mainstays of the mitochondria, the story of these 2 cooperating chaperones does not end within this organelle. In recent years, evidence has emerged indicating that they are (unexpectedly) found in other locations, evoking questions as to whether these wandering proteins fulfill other essential functions as well (for review, see Soltys and Gupta 1999, 2000).
The mitochondrial Hsp60 precursor protein contains an N-terminal mitochondrial targeting sequence (MTS) that gets cleaved during its import into the matrix compartment (Singh et al 1990; Venner and Gupta 1990; Venner et al 1990). Hsp60 was initially discovered in mammalian cells as a protein specifically altered in Chinese hamster ovary (CHO) cells resistant to the microtubule (MT) inhibitor podophyllotoxin. Hsp60 and tubulin have been independently detected on the plasma membrane (Soltys and Gupta 2000). The extramitochondrial localization of Hsp60 in a variety of mammalian cells and tissues was also confirmed by electron microscopy (EM) (Soltys and Gupta 1996, 1997) and its role as amino acid transporter has been suggested (Jones et al 1994). Hsp60 is also present in mature secretory granules of beta cells. Interestingly, in nonobese diabetic mice, accompanying the progression of insulitis is the loss of Hsp60 in the insulin-secretory granules. A role of Hsp60 in insulin biosynthesis/packaging and in pathogenesis of insulin-dependent diabetes has been proposed (Brudzynski 1992).
Membrane-associated Hsp60 forms a complex with histone 2B and is regulated via site-specific phosphorylation by type I protein kinase in a human leukemic T cell line (Khan et al 1998). Ikawa and Weinberg (1992) have identified an association of Hsp60 with plasma membrane– resident p21ras protein. More recently, a study on biotinylation of cell surface proteins in cancer vs normal cell lines and subsequent analysis with mass spectrometry reported that both Hsp60 as well as mortalin are highly abundant in the surface of cancer cells when matched against normal cells (Shin et al 2003).
Compelling evidence has emerged indicating that mortalin/mtHsp70, just like Hsp60, docks at extramitochondrial sites. Visual studies of the protein with specific antibodies in a variety of cell lines revealed its existence in multiple extramitochondrial sites that include the endoplasmic reticulum, cytoplasmic vesicles, and cytosol (Wadhwa et al 2002). How mortalin is being targeted to these other compartments is not clear at present, but one possibility is that binding of mortalin to residents of different organelles may assist in its relocation. Far Western screening had identified glucose-regulated ER chaperone (GRP94) as one of its binding partners. Mortalin–GRP94 interaction has been confirmed by mammalian 2-hybrid assays, in vitro and in vivo coimmunoprecipitations (Takano et al 2001). In addition, mortalin is also found to bind to various other proteins including p53, membrane-associated proteins such as fibroblast growth factor 1 (FGF-1), interleukin receptor 1α (for review, see Wadhwa et al 2002), cytoskeleton elements, mitochondrial protein p66shc, and peroxisomal protein mevalonate pyrophosphate decarboxylase (MPD) (Wadhwa et al 2003; Choi et al 2004; Orsini et al 2004). Some complement-activated cells have been shown to release mortalin by vesiculation that has been interpreted as a protective response against complement-mediated lysis (Pilzer and Fishelson 2005; Pilzer et al 2005). In a manner re-enacting its role in mitochondrial import, mortalin assumes a function in trafficking nonmitochondrial proteins too. Interestingly, its interaction with FGF-1 assists the growth factor's intracellular uptake and organellar routing. This process is coupled to the cell cycle–dependent tyrosine phosphorylation of mortalin (Mizukoshi et al 1999, 2001). A similar phenomenon is noted in the ATP-sensitive association of mortalin with the receptor for the proinflammatory cytokine interleukin 1 (IL-1) that leads to receptor internalization and downstream signaling cascades (Sacht et al 1999).
Gaining new roles in neoplasia
Apoptosis or programmed cell death is a central regulator of tissue homeostasis. Genetic disturbances of apoptotic signaling pathways are found in almost all cancers and are linked to tumor development and progression. Resistance to apoptosis is also one of the main contributors to bad prognosis and insensitivity to conventional cancer therapies. Elevated expression of Hsp90, Hsp70, and Hsp27 has been widely reported in various cancers. Their abundance measured from tumor biopsies is indicative of worse prognosis (Jolly and Morimoto 2000). Dundas et al (2005) demonstrated the prognostic value of mortalin/mtHsp70 overexpression in a large series of colorectal cancers by a comparative proteomic. Of note, elevated levels of mortalin have also been noted in many in vitro–immortalized and tumor-derived cells, and tumor tissues (Wadhwa et al 2006). Consistently, the reduction in mortalin level by antisense and ribozymes in immortal and cancer cells led to their growth arrest (Wadhwa et al 2004).
On the other hand, studies describing clinical associations with the up-regulation of Hsp60 in tumor biopsies appear to conflict. For patients with acute myeloid leukemia (AML), overall survival and complete remission rates correlate with lower expression of Hsp27 and Hsp60. When confounded with the amount of cytogenetic abnormalities accrued by the cancer cells, a more pejorative outcome begin to emerge (Thomas et al 2005), a finding that is consistent with the role of Hsps as survival/microevolutionary buffers for genomic instability (Soti and Csermely 2002). And like mortalin, cellular distribution of Hsp60 and Hsp10 are potentially informative in tumor diagnosis. For instance, both Hsp60 and Hsp10 proteins are localized only in cells of basal and parabasal layers of low-grade cervical tumor lesions, whereas in higher grades, a diffused pattern in all layers is seen (Cappello 2003). Hsp60 has also been found to be of some utility in predicting disease progression in patients with cancers of the bladder (Lebret et al 2003) and uterus– cervix (Cappello et al 2002), but not in tumors of the prostate (Cornford et al 2000), tongue (Ito et al 1998), and bone (Uozaki et al 2000). Contradicting results have been reported for the prognostic value of Hsp60 in ovarian (Schneider et al 1999) and esophageal squamous cell carcinomas (Faried et al 2004). As this is the case, Hsp60 may seem to act in a cell type–specific manner; its function in the induction of cell death is believed to be operative in cancer types that show good prognosis, contradicting the generally accepted prosurvival percept of Hsp60 as a “chaperoning angel” (from the vantage point of a tumor cell).
Mortalin as a prosurvival chaperone
In a proteome analysis of oubain-treated vascular smooth muscle cells (VSMCs), mortalin/mtHsp70 was identified as one of the important antiapoptotic genes (Taurin et al 2002). Its overexpression suppresses apoptosis from various stressors, eg, arsenite in rat lung epithelial cells (Lau et al 2004), differentiation agent 1,25-dihydroxyvitamin D3 in rat gliomas (Baudet et al 1998), and glucose starvation and ischemia reperfusion in Chinese hamster lung (CHL) cells (Gao et al 2003). It causes inactivation of p53 function by cytoplasmic sequestration (Wadhwa et al 1998) and protects cells from ATP depletion and energy deprivation associated with cell death by preventing the rapid rise in mitochondrial reactive oxygen species (ROS) (Liu et al 2005).
As a native of the mitochondrion, mortalin is envisioned to play an even more expansive role in its modulation of apoptosis pathways. Treatment of cells with ultraviolet radiation induces the release of monomeric p66Shc from its inhibitory complex with mortalin and triggers a collapse of the mitochondrial trans-membrane potential. After engagement of Fas receptor with its ligand, the N-terminal portion of cyclin dependent kinase 11 (CDK11 p60) translocates from the nucleus to the mitochondria and associates with mortalin/mtHsp70 (Feng et al 2005). Similarly, p53 has also been reported to translocate to the mitochondria and induce apoptosis independent of its well-known transcriptional functions (Mihara et al 2003). Furthermore, overexpression of antiapoptotic Bcl-2 or Bcl-xL abrogates stress signal-mediated mitochondrial p53 accumulation and apoptosis (Marchenko et al 2000). The overall picture of the role of the mitochondrial p53 in apoptosis relies on the direct interaction of Bcl2 and Bcl-xL with p53 as detected by coimmunoprecipitation experiments (Mihara et al 2003). In addition, p53 can “kidnap” Bcl-xL and Bcl2 from tBid, Bak, and Bax, creating apoptotic homodimers (Chipuk et al 2004). Whether mortalin manipulates p53 by stealing it from the p53–Bcl-xL/2 complex and finally dragging it for proteasomal degradation would be an interesting plot in the prosurvival story of this mitochondrial chaperone.
Proapoptotic and antiapoptotic Hsp60
Hsp60/Hsp10 complex bears an unusual role in apoptosis. Overexpression of Hsp60 prevents apoptosis by protecting mitochondrial functions after ischemic injury in both cardiac and muscle cells (Lau et al 1997; Lin et al 2001). Cytosolic Hsp60 forms a macromolecular complex with both Bax and Bak, blocking their ability to effect apoptosis. Reduction in Hsp60 levels in these cells either by antisense or hypoxic treatment precipitates to the translocation of Bax (Gupta and Knowlton 2002; Kirchhoff et al 2002). Adenoviral transduction of Hsp60/Hsp10 in cardiac myocytes attenuated doxorubicin-induced cardiac muscle death by inhibiting ubiquitination of Bcl-xL, increasing the abundance of the Bcl-xL and Bcl-2, without altering expression of Bad (Shan et al 2003). Others have proposed that Hsp60 also protects epithelial cells from stress-induced death via activation of extracellular signal-regulated kinase (ERK) and inhibition of caspase-3 (Zhang et al 2004). Hsp60 and mortalin/mtHsp70 are also 2 of the major cellular proteins that become covalently modified after treatment with certain nephrotoxic agents, such as tetrafluoroethyl cysteine analogs (Bruschi et al 1993; Bruschi and Lindsay 1994).
There have been accumulating reports on the proapoptotic role for Hsp60/Hsp10 complex during apoptosis. One can account for 3 different twists for Hsp60's proapoptotic personalities. First (in the cytoplasm), after release from the mitochondria, Hsp60 and Hsp10 activate caspase-3 in an ATP-dependent fashion in response to camptothecin treatment (Samali et al 1999; Xanthoudakis et al 1999). A similar phenomenon of caspase-3 activation has also been previously identified from ultrasound-induced cell death in Walker 256 carcinosarcoma cells (Tian et al 2005). Second (in the mitochondria), Hsp60 has been discovered, from proteomic studies, to be an important target by the hepatitis B virus X protein (HBx). Instead of preventing HBx-induced apoptosis, overexpression of Hsp60 further facilitates HBx-induced apoptosis in hepatic cells (Tanaka et al 2004). And third, (on the plasma membrane) of endothelial cells, Hsp60 displays cross-reactivity to antiendothelial cell antibodies (AECAs) from patients with systemic autoimmune diseases, ie, vasculitis and lupus erythematosus. By an ambiguous mechanism, apoptosis was triggered by anti-Hsp60–containing AECA-positive sera and was inhibited by neutralization with free recombinant Hsp60 (Jamin et al 2005).
Mortalin and Hsp60 in the senescence pathways
As with apoptosis, senescence (cellular aging) represents another major barrier to tumorigenesis. Most human normal somatic cells permanently stop dividing after a finite number of cell divisions in culture and enter a state of “permanent growth arrest,” termed as cellular or replicative senescence (Hayflick and Moorehead 1961). Tumor cells, on the other hand, acquire the means to bypass this limit to extend their life span and achieve immortality. Being immortal has its own drawbacks; a tumor cell suffers an accumulation of genetic mutations, partly, as a result of the absence of proliferation breaks that counter genomic instability. It is noteworthy that many of their key components of the senescence pathways are regulated by interactions with the molecular chaperones. Hsps serve as safeguards to maintain homeostasis and integrity of these critical protein interactions. The observation that tumor cells often have elevated levels of Hsps may be associated with a premalignant cell's response to the selection process occurring during tumorigenesis. By virtue of their activities as molecular chaperones, Hsps contribute to cellular immortalization and provide survival advantages for neoplasia.
The Hsp60 does not seem to show an impact on cellular life span. Whereas overexpression of mortalin extends the in vitro life span of normal human fibroblasts (Kaul et al 2003), population doublings (PDs) of human fibroblasts overexpressing Hsp60 remained unchanged (Wadhwa et al 2005). What could account for this discrepancy? Looking back at the molecular chaperone model of Hsp60, coimmunoprecipitation experiments with GroEL showed that fewer than 15% of proteins could bind to Hsp60 in vitro (Ewalt et al 1997) and no more than 2% misfolded proteins can be processed by this chaperonin in vivo (Todd et al 1996). Whereas Hsp60 possess a fastidious central cavity that discerns a smaller fraction of proteins, the structure of mortalin may accommodate exposed hydrophobic protein strands in its promiscuous peptide-binding domain. This might explain an apparent “dominance” of mortalin during mitochondrial biogenesis: preproteins are first processed (ie, unfolded, trapped, translocated, refolded) by mortalin/mtHsp70 prior to passing them on to Hsp60 for mitochondrial protein triage. The functional distinctions between the 2 chaperones are likely to account for the fact that overexpression of mortalin, but not of Hsp60, leads to life span extension. This observation also underscores the independence of senescence and apoptosis pathways. Some stress chaperones, Hsp90, Hsp27, and Hsp70 (Hsc70 and the heat-inducible Hsp70) have been assigned roles in resistance of many cancers to anticancer drugs (Rashmi et al 2004) estimated to cause treatment failure in over 90% of patients. With the exception of Hsp27, very little is known yet about the roles of the mitochondrial chaperones in chemoresistance.
The parable of the 2 brothers—a perspective
This review is akin to a parable that tells a story of 2 brothers (proteins) that have distinctive structural characteristics and behaviors, and have been entasked to fulfill evolutionarily conserved, life-essential roles in the mitochondria for the past 2 billion years, marking the beginning of eukaryotic transition to aerobic metabolism (Kurland and Andersson 2000; Gabaldon and Huynen 2004). As a general assumption in biology that proteins bound for specific organelles do not leave their destined compartments, the recent spate of reports refocus the spotlight to their extraordinary extramitochondrial biology. We have depicted the 2 prodigal chaperones as displaying remarkably divergent personalities that accompany the breakdown of their fraternity. It is easy to see such contrasts when presented in terms of their newly acquired functions in aging and carcinogenesis as well as their emergent binding partners. Their ectopic expressions permitted them to assume more expansive roles in signal transduction, cell communication, and neoplastic development. And partly, as a result of their neolocalizations, these chaperones possess the ability to modulate the immune system.
Mortalin/mtHsp70 and Hsp60 both have intrinsic antigenicities and are reported to be a potent activator of innate immunity. Aberrant expression of these chaperones in certain organs promotes immunopathology (Wick 2000). For example, binding of Hsp60 to high-density lipoproteins may explain the known association between immunity developed against Hsp60 and atherosclerosis (Bocharov et al 2000; De Bruyn et al 2000). Hsp60 also serves as a ligand to Toll-like receptors (TLRs), the molecular sensors of innate immune system (Vabulas et al 2002). Their deregulated expression may also result in autoimmune pathologies. Yokota et al (2000) showed the presence of autoantibodies against Hsp60 in patients with autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematodes, Sjögren syndrome, and mixed connective tissue disease. It is not remote for both mortalin and Hsp60 to show immunotherapeutic potential as was shown for Hsp70 (Todryk et al 2003).
In principle, we can exploit many of the new properties acquired by these chaperones in biotechnology and use them as therapeutic targets and agents. However, before that, we need to explore reasons for their uncanny diaspora and frame our acquired knowledge into coherently broad biological and even philosophical perspectives.
Acknowledgments
We thank Dr. Nicholas Hoogenraad (La Trobe University, Melbourne, Australia) and Dr. Radhey S. Gupta (McMaster University, Hamilton, Ontario, Canada) for their valuable comments.
REFERENCES
- Agsteribbe E, Huckriede A, and Veenhuis M. et al. 1993 A fatal, systemic mitochondrial disease with decreased mitochondrial enzyme activities, abnormal ultrastructure of the mitochondria and deficiency of heat shock protein 60. Biochem Biophys Res Commun. 193:146–154. [DOI] [PubMed] [Google Scholar]
- Baudet C, Perret E, Delpech B, Kaghad M, Brachet P, Wion D, Caput D. Differentially expressed genes in C6.9 glioma cells during vitamin D-induced cell death program. Cell Death Differ. 1998;5:116–125. doi: 10.1038/sj.cdd.4400327.1350-9047(1998)005[0116:DEGICG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bocharov AV, Vishnyakova TG, Baranova IN, Remaley AT, Patterson AP, Eggerman TL. Heat shock protein 60 is a high-affinity high-density lipoprotein binding protein. Biochem Biophys Res Commun. 2000;277:228–235. doi: 10.1006/bbrc.2000.3663.0006-291X(2000)277[0228:HSPIAH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bomer U, Maarse AC, and Martin F. et al. 1998 Separation of structural and dynamic functions of the mitochondrial translocase: Tim44 is crucial for the inner membrane import sites in translocation of tightly folded domains, but not of loosely folded preproteins. EMBO J. 17:4226–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones P, Vilaseca MA, Ribes A, Vernet A, Lluch M, Cusi V, Huckriede A, Agsteribbe E. A new case of multiple mitochondrial enzyme deficiencies with decreased amount of heat shock protein 60. J Inherit Metab Dis. 1997;20:569–577. doi: 10.1023/a:1005303008439.0141-8955(1997)020[0569:ANCOMM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brudzynski K, Martinez V, Gupta RS. Immunocytochemical localization of heat-shock protein 60-related protein in beta-cell secretory granules and its altered distribution in non-obese diabetic mice. Diabetologia. 1992;35:316–324. doi: 10.1007/BF00401198.0012-186X(1992)035[0316:ILOHPR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brunner M, Schneider HC, Lill R, Neupert W. Dissection of protein translocation across the mitochondrial outer and inner membranes. Cold Spring Harb Symp Quant Biol. 1995;60:619–627. doi: 10.1101/sqb.1995.060.01.066.0091-7451(1995)060[0619:DOPTAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bruschi SA, Lindsay JG. Mitochondrial stress protein actions during chemically induced renal proximal tubule cell death. Biochem Cell Biol. 1994;72:663–667. doi: 10.1139/o94-087.1208-6002(1994)072[0663:MSPADC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bruschi SA, West KA, Crabb JW, Gupta RS, Stevens JL. Mitochondrial HSP60 (P1 protein) and a HSP70-like protein (mortalin) are major targets for modification during S-(1,1,2,2-tetrafluoroethyl)-l-cysteine-induced nephrotoxicity. J Biol Chem. 1993;268:23157–23161.0021-9258(1993)268[23157:MHPPAA]2.0.CO;2 [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9.0092-8674(1998)092[0351:THAHCM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cappello F. HSP60 and HSP10 as diagnostic and prognostic tools in the management of exocervical carcinoma. Gynecol Oncol. 2003;91:661. doi: 10.1016/j.ygyno.2003.08.009.0090-8258(2003)091[0661:HAHADA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cappello F, Bellafiore M, and Palma A. et al. 2002 Expression of 60-kD heat shock protein increases during carcinogenesis in the uterine exocervix. Pathobiol. 70:83–88. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Hartl FU, and Martin J. et al. 1989 Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 337:620–625. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734.0193-4511(2004)303[1010:DAOBBP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Choglay AA, Chapple JP, Blatch GL, Cheetham ME. Identification and characterization of a human mitochondrial homologue of the bacterial co-chaperone GrpE. Gene. 2001;267:125–134. doi: 10.1016/s0378-1119(01)00396-1.0378-1119(2001)267[0125:IACOAH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Choi J, Forster MJ, McDonald SR, Weintraub ST, Carroll CA, Gracy RW. Proteomic identification of specific oxidized proteins in ApoE-knockout mice: relevance to Alzheimer's disease. Free Radic Biol Med. 2004;36:1155–1162. doi: 10.1016/j.freeradbiomed.2004.02.002.0891-5849(2004)036[1155:PIOSOP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cornford PA, Dodson AR, and Parsons KF. et al. 2000 Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 60:7099–7105. [PubMed] [Google Scholar]
- Craig EA, Kramer J, Kosic-Smithers J. SSC1, a member of the 70-kDa heat shock protein multigene family of Saccharomyces cerevisiae, is essential for growth. Proc Natl Acad Sci U S A. 1987;84:4156–4160. doi: 10.1073/pnas.84.12.4156.0027-8424(1987)084[4156:SAMOTK]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyn J, Soetaert K, and Buyssens P. et al. 2000 Evidence for specific and non-covalent binding of lipids to natural and recombinant Mycobacterium bovis BCG hsp60 proteins, and to the Escherichia coli homologue GroEL. Microbiology. 146:1513–1524. [DOI] [PubMed] [Google Scholar]
- Dekker PJ, Pfanner N. Role of mitochondrial GrpE and phosphate in the ATPase cycle of matrix Hsp70. J Mol Biol. 1997;270:321–327. doi: 10.1006/jmbi.1997.1131.0022-2836(1997)270[0321:ROMGAP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- D'Silva P, Liu Q, Walter W, Craig EA. Regulated interactions of mtHsp70 with Tim44 at the translocon in the mitochondrial inner membrane. Nat Struct Mol Biol. 2004;11:1084–1091. doi: 10.1038/nsmb846.1545-9985(2004)011[1084:RIOMWT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ewalt KL, Hendrick JP, Houry WA, Hartl FU. In vivo observation of polypeptide flux through the bacterial chaperonin system. Cell. 1997;90:491–500. doi: 10.1016/s0092-8674(00)80509-7.0092-8674(1997)090[0491:IVOOPF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Faried A, Sohda M, Nakajima M, Miyazaki T, Kato H, Kuwano H. Expression of heat-shock protein Hsp60 correlated with the apoptotic index and patient prognosis in human oesophageal squamous cell carcinoma. Eur J Cancer. 2004;40:2804–2811. doi: 10.1016/j.ejca.2004.08.013.0959-8049(2004)040[2804:EOHPHC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Farr GW, Furtak K, Rowland MB, Ranson NA, Saibil HR, Kirchhausen T, Horwich AL. Multivalent binding of nonnative substrate proteins by the chaperonin GroEL. Cell. 2000;100:561–573. doi: 10.1016/s0092-8674(00)80692-3.0092-8674(2000)100[0561:MBONSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Feng Y, Ariza ME, Goulet AC, Shi J, Nelson MA. Death signal induced relocalization of cyclin dependent kinase 11 to mitochondria. Biochem J. 2005;392:65–73. doi: 10.1042/BJ20050195.0264-6021(2005)392[0065:DSIROC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton WA, Horwich AL. Chaperonin-mediated protein folding: fate of substrate polypeptide. Q Rev Biophys. 2003;36:229–256. doi: 10.1017/s0033583503003883.0033-5835(2003)036[0229:CPFFOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gabaldon T, Huynen MA. Shaping the mitochondrial proteome. Biochim Biophys Acta. 2004;6:2–3. doi: 10.1016/j.bbabio.2004.07.011.0006-3002(2004)006[0002:STMP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gao CX, Zhang SQ, Yin Z, Liu W. Molecular chaperone GRP75 reprove cells from injury caused by glucose deprivation. Shi Yan Sheng Wu Xue Bao. 2003;36:381–387.0001-5334(2003)036[0381:MCGRCF]2.0.CO;2 [PubMed] [Google Scholar]
- Gaume B, Klaus C, Ungermann C, Guiard B, Neupert W, Brunner M. Unfolding of preproteins upon import into mitochondria. EMBO J. 1998;17:6497–6507. doi: 10.1093/emboj/17.22.6497.1460-2075(1998)017[6497:UOPUII]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler A, Krimmer T, Bomer U, Guiard B, Rassow J, Pfanner N. Membrane potential-driven protein import into mitochondria. The sorting sequence of cytochrome b(2) modulates the deltapsi-dependence of translocation of the matrix-targeting sequence. Mol Biol Cell. 2000;11:3977–3991. doi: 10.1091/mbc.11.11.3977.1059-1524(2000)011[3977:MPPIIM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler A, Rassow J, Pfanner N, Voos W. Mitochondrial import driving forces: enhanced trapping by matrix Hsp70 stimulates translocation and reduces the membrane potential dependence of loosely folded preproteins. Mol Cell Biol. 2001;21:7097–7104. doi: 10.1128/MCB.21.20.7097-7104.2001.0270-7306(2001)021[7097:MIDFET]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS, Wachter C, Reid GA, Schatz G. Import of cytochrome b2 to the mitochondrial intermembrane space: the tightly folded heme-binding domain makes import dependent upon matrix ATP. Protein Sci. 1993;2:1901–1917. doi: 10.1002/pro.5560021112.0961-8368(1993)002[1901:IOCBTT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosslau A, Ruoff P, Mohsenzadeh S, Hobohm U, Rensing L. Heat shock and oxidative stress-induced exposure of hydrophobic protein domains as common signal in the induction of hsp68. J Biol Chem. 2001;276:1814–1821. doi: 10.1074/jbc.M008280200.0021-9258(2001)276[1814:HSAOSE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Grossmann ME, Madden BJ, Gao F, Pang YP, Carpenter JE, McCormick D, Young CY. Proteomics shows Hsp70 does not bind peptide sequences indiscriminately in vivo. Exp Cell Res. 2004;297:108–117. doi: 10.1016/j.yexcr.2004.02.030.0014-4827(2004)297[0108:PSHDNB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gupta S, Knowlton AA. Cytosolic heat shock protein 60, hypoxia, and apoptosis. Circulation. 2002;106:2727–2733. doi: 10.1161/01.cir.0000038112.64503.6e.0009-7322(2002)106[2727:CHSPHA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hansen WJ, Ohh M, Moslehi J, Kondo K, Kaelin WG, Welch WJ. Diverse effects of mutations in exon II of the von Hippel-Lindau (VHL) tumor suppressor gene on the interaction of pVHL with the cytosolic chaperonin and pVHL-dependent ubiquitin ligase activity. Mol Cell Biol. 2002;22:1947–1960. doi: 10.1128/MCB.22.6.1947-1960.2002.0270-7306(2002)022[1947:DEOMIE]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CJ, Hayer-Hartl M, Di Liberto M, Hartl F, Kuriyan J. Crystal structure of the nucleotide exchange factor GrpE bound to the ATPase domain of the molecular chaperone DnaK. Science. 1997;276:431–435. doi: 10.1126/science.276.5311.431.0193-4511(1997)276[0431:CSOTNE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartl FU, Martin J, Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453.1056-8700(1992)021[0293:PFITCT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorehead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6.0014-4827(1961)025[0585:TSCOHD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Horst M, Oppliger W, Feifel B, Schatz G, Glick BS. The mitochondrial protein import motor: dissociation of mitochondrial hsp70 from its membrane anchor requires ATP binding rather than ATP hydrolysis. Protein Sci. 1996;5:759–767. doi: 10.1002/pro.5560050421.0961-8368(1996)005[0759:TMPIMD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton LE, James P, Craig EA, Hensold JO. The yeast hsp70 homologue Ssa is required for translation and interacts with Sis1 and Pab1 on translating ribosomes. J Biol Chem. 2001;276:14426–14433. doi: 10.1074/jbc.M100266200.0021-9258(2001)276[14426:TYHHSI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Huang S, Murphy S, Matouschek A. Effect of the protein import machinery at the mitochondrial surface on precursor stability. Proc Natl Acad Sci U S A. 2000;97:12991–12996. doi: 10.1073/pnas.230243097.0027-8424(2000)097[12991:EOTPIM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt JF, Weaver AJ, Landry SJ, Gierasch L, Deisenhofer J. The crystal structure of the GroES co-chaperonin at 2.8 A resolution. Nature. 1996;379:37–45. doi: 10.1038/379037a0.1476-4687(1996)379[0037:TCSOTG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ikawa S, Weinberg RA. An interaction between p21ras and heat shock protein hsp60, a chaperonin. Proc Natl Acad Sci U S A. 1992;89:2012–2016. doi: 10.1073/pnas.89.6.2012.0027-8424(1992)089[2012:AIBPAH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Kawabe R, Kurasono Y, Hara M, Kitamura H, Fujita K, Kanisawa M. Expression of heat shock proteins in squamous cell carcinoma of the tongue: an immunohistochemical study. J Oral Pathol Med. 1998;27:18–22. doi: 10.1111/j.1600-0714.1998.tb02085.x.0904-2512(1998)027[0018:EOHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jamin C, Dugue C, Alard JE, Jousse S, Saraux A, Guillevin L, Piette JC, Youinou P. Induction of endothelial cell apoptosis by the binding of anti-endothelial cell antibodies to Hsp60 in vasculitis-associated systemic autoimmune diseases. Arthritis Rheum. 2005;52:4028–4038. doi: 10.1002/art.21401.0004-3591(2005)052[4028:IOECAB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92:1564–1572. doi: 10.1093/jnci/92.19.1564.0027-8874(2000)092[1564:ROTHSR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jones M, Gupta RS, Englesberg E. Enhancement in amount of P1 (hsp60) in mutants of Chinese hamster ovary (CHO-K1) cells exhibiting increases in the A system of amino acid transport. Proc Natl Acad Sci U S A. 1994;91:858–862. doi: 10.1073/pnas.91.3.858.0027-8424(1994)091[0858:EIAOPH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul SC, Duncan EL, Englezou A, Takano S, Reddel RR, Mitsui Y, Wadhwa R. Malignant transformation of NIH3T3 cells by overexpression of mot-2 protein. Oncogene. 1998;17:907–911. doi: 10.1038/sj.onc.1202017.0950-9232(1998)017[0907:MTONCB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kaul SC, Wadhwa R, Komatsu Y, Sugimoto Y, Mitsui Y. On the cytosolic and perinuclear mortalin: an insight by heat shock. Biochem Biophys Res Commun. 1993;193:348–355. doi: 10.1006/bbrc.1993.1630.0006-291X(1993)193[0348:OTCAPM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kaul SC, Yaguchi T, Taira K, Reddel RR, Wadhwa R. Overexpressed mortalin (mot-2)/mthsp70/GRP75 and hTERT cooperate to extend the in vitro lifespan of human fibroblasts. Exp Cell Res. 2003;286:96–101. doi: 10.1016/s0014-4827(03)00101-0.0014-4827(2003)286[0096:OMGAHC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Khan IU, Wallin R, Gupta RS, Kammer GM. Protein kinase A-catalyzed phosphorylation of heat shock protein 60 chaperone regulates its attachment to histone 2B in the T lymphocyte plasma membrane. Proc Natl Acad Sci U S A. 1998;95:10425–10430. doi: 10.1073/pnas.95.18.10425.0027-8424(1998)095[10425:PKAPOH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation. 2002;105:2899–2904. doi: 10.1161/01.cir.0000019403.35847.23.0009-7322(2002)105[2899:CHSPAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Koehler CM. New developments in mitochondrial assembly. Annu Rev Cell Dev Biol. 2004;20:309–335. doi: 10.1146/annurev.cellbio.20.010403.105057.1081-0706(2004)020[0309:NDIMA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kurland CG, Andersson SG. Origin and evolution of the mitochondrial proteome. Microbiol Mol Biol Rev. 2000;64:786–820. doi: 10.1128/mmbr.64.4.786-820.2000.1092-2172(2000)064[0786:OAEOTM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer T, Neupert W. Heat shock proteins hsp60 and hsp70: their roles in folding, assembly and membrane translocation of proteins. Curr Top Microbiol Immunol. 1991;167:3–30. doi: 10.1007/978-3-642-75875-1_1.0070-217X(1991)167[0003:HSPHAH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lau AT, He QY, Chiu JF. A proteome analysis of the arsenite response in cultured lung cells: evidence for in vitro oxidative stress-induced apoptosis. Biochem J. 2004;382:641–650. doi: 10.1042/BJ20040224.0264-6021(2004)382[0641:APAOTA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S, Patnaik N, Sayen MR, Mestril R. Simultaneous overexpression of two stress proteins in rat cardiomyocytes and myogenic cells confers protection against ischemia-induced injury. Circulation. 1997;96:2287–2294. doi: 10.1161/01.cir.96.7.2287.0009-7322(1997)096[2287:SOOTSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lebret T, Watson RW, Molinie V, O'Neill A, Gabriel C, Fitzpatrick JM, Botto H. Heat shock proteins HSP27, HSP60, HSP70, and HSP90: expression in bladder carcinoma. Cancer. 2003;98:970–977. doi: 10.1002/cncr.11594.0765-7846(2003)098[0970:HSPHHH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lehner T, Wang Y, Whittall T, McGowan E, Kelly CG, Singh M. Functional domains of HSP70 stimulate generation of cytokines and chemokines, maturation of dendritic cells and adjuvanticity. Biochem Soc Trans. 2004;32:629–632. doi: 10.1042/BST0320629.0300-5127(2004)032[0629:FDOHSG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Levy-Rimler G, Bell RE, Ben-Tal N, Azem A. Type I chaperonins: not all are created equal. FEBS Lett. 2002;529:1–5. doi: 10.1016/s0014-5793(02)03178-2.0014-5793(2002)529[0001:TICNAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Levy-Rimler G, Viitanen P, and Weiss C. et al. 2001 The effect of nucleotides and mitochondrial chaperonin 10 on the structure and chaperone activity of mitochondrial chaperonin 60. Eur J Biochem. 268:3465–3472. [DOI] [PubMed] [Google Scholar]
- Li Y, Dudek J, Guiard B, Pfanner N, Rehling P, Voos W. The presequence translocase-associated protein import motor of mitochondria. Pam16 functions in an antagonistic manner to Pam18. J Biol Chem. 2004;279:38047–38054. doi: 10.1074/jbc.M404319200.0021-9258(2004)279[38047:TPTPIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lin KM, Lin B, Lian IY, Mestril R, Scheffler IE, Dillmann WH. Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation. 2001;103:1787–1792. doi: 10.1161/01.cir.103.13.1787.0009-7322(2001)103[1787:CAIMHA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liu Q, D'Silva P, Walter W, Marszalek J, Craig EA. Regulated cycling of mitochondrial Hsp70 at the protein import channel. Science. 2003;300:139–141. doi: 10.1126/science.1083379.0193-4511(2003)300[0139:RCOMHA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu W, Song XD, Zuo J. Effect of GRP75/mthsp70/ PBP74/mortalin overexpression on intracellular ATP level, mitochondrial membrane potential and ROS accumulation following glucose deprivation in PC12 cells. Mol Cell Biochem. 2005;268:45–51. doi: 10.1007/s11010-005-2996-1.0300-8177(2005)268[0045:EOMOOI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Macario AJ, de Macario EC. The archaeal molecular chaperone machine: peculiarities and paradoxes. Genetics. 1999;152:1277–1283. doi: 10.1093/genetics/152.4.1277.0016-6731(1999)152[1277:TAMCMP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlke K, Pfanner N, Martin J, Horwich AL, Hartl FU, Neupert W. Sorting pathways of mitochondrial inner membrane proteins. Eur J Biochem. 1990;192:551–555. doi: 10.1111/j.1432-1033.1990.tb19260.x.0014-2956(1990)192[0551:SPOMIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202.0021-9258(2000)275[16202:DSLOPP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Martinus RD, Garth GP, Webster TL, Cartwright P, Naylor DJ, Hoj PB, Hoogenraad NJ. Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur J Biochem. 1996;240:98–103. doi: 10.1111/j.1432-1033.1996.0098h.x.0014-2956(1996)240[0098:SIOMCI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Matouschek A, Azem A, Ratliff K, Glick BS, Schmid K, Schatz G. Active unfolding of precursor proteins during mitochondrial protein import. EMBO J. 1997;16:6727–6736. doi: 10.1093/emboj/16.22.6727.1460-2075(1997)016[6727:AUOPPD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperone systems: diversity of cellular functions and mechanism of action. Biol Chem. 1998;379:261–268.1431-6730(1998)379[0261:HCSDOC]2.0.CO;2 [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9.1097-2765(2003)011[0577:PHADAR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mizukoshi E, Suzuki M, and Loupatov A. et al. 1999 Fibroblast growth factor-1 interacts with the glucose-regulated protein GRP75/ mortalin. Biochem J. 2:461–466. [PMC free article] [PubMed] [Google Scholar]
- Mizukoshi E, Suzuki M, Misono T, Loupatov A, Munekata E, Kaul SC, Wadhwa R, Imamura T. Cell-cycle dependent tyrosine phosphorylation on mortalin regulates its interaction with fibroblast growth factor-1. Biochem Biophys Res Commun. 2001;280:1203–1209. doi: 10.1006/bbrc.2001.4225.0006-291X(2001)280[1203:CDTPOM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Naylor DJ, Hoogenraad NJ, Hoj PB. Isolation and characterisation of a cDNA encoding rat mitochondrial GrpE, a stress-inducible nucleotide-exchange factor of ubiquitous appearance in mammalian organs. FEBS Lett. 1996;396:181–188. doi: 10.1016/0014-5793(96)01100-3.0014-5793(1996)396[0181:IACOAC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Naylor DJ, Stines AP, Hoogenraad NJ, Hoj PB. Evidence for the existence of distinct mammalian cytosolic, microsomal, and two mitochondrial GrpE-like proteins, the co-chaperones of specific Hsp70 members. J Biol Chem. 1998;273:21169–21177. doi: 10.1074/jbc.273.33.21169.0021-9258(1998)273[21169:EFTEOD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863.0066-4154(1997)066[0863:PIIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Neupert W, Brunner M. The protein import motor of mitochondria. Nat Rev Mol Cell Biol. 2002;3:555–565. doi: 10.1038/nrm878.1471-0080(2002)003[0555:TPIMOM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Orsini F, Migliaccio E, and Moroni M. et al. 2004 The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem. 279:25689–25695. [DOI] [PubMed] [Google Scholar]
- Parcellier A, Gurbuxani S, Schmitt E, Solary E, Garrido C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun. 2003;304:505–512. doi: 10.1016/s0006-291x(03)00623-5.0006-291X(2003)304[0505:HSPCCT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Bross P, Winter VS, Corydon TJ, Bolund L, Bartlett K, Vockley J, Gregersen N. Misfolding, degradation, and aggregation of variant proteins. The molecular pathogenesis of short chain acyl-CoA dehydrogenase (SCAD) deficiency. J Biol Chem. 2003;278:47449–47458. doi: 10.1074/jbc.M309514200.0021-9258(2003)278[47449:MDAAOV]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pilzer D, Fishelson Z. Mortalin/GRP75 promotes release of membrane vesicles from immune attacked cells and protection from complement-mediated lysis. Int Immunol. 2005;17:1239–1248. doi: 10.1093/intimm/dxh300.0953-8178(2005)017[1239:GPROMV]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immunopathol. 2005;27:375–387. doi: 10.1007/s00281-005-0004-1.0344-4325(2005)027[0375:EOMVRI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ranson NA, Farr GW, Roseman AM, Gowen B, Fenton WA, Horwich AL, Saibil HR. ATP-bound states of GroEL captured by cryo-electron microscopy. Cell. 2001;107:869–879. doi: 10.1016/s0092-8674(01)00617-1.0092-8674(2001)107[0869:ASOGCB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rashmi R, Kumar S, Karunagaran D. Ectopic expression of Hsp70 confers resistance and silencing its expression sensitizes human colon cancer cells to curcumin-induced apoptosis. Carcinogenesis. 2004;25:179–187. doi: 10.1093/carcin/bgh001.1460-2180(2004)025[0179:EEOHCR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rehling P, Brandner K, Pfanner N. Mitochondrial import and the twin-pore translocase. Nat Rev Mol Cell Biol. 2004;5:519–530. doi: 10.1038/nrm1426.1471-0080(2004)005[0519:MIATTT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Richardson A, Landry SJ, Georgopoulos C. The ins and outs of a molecular chaperone machine. Trends Biochem Sci. 1998;23:138–143. doi: 10.1016/s0968-0004(98)01193-1.0376-5067(1998)023[0138:TIAOOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ritossa FA. A new puffing pattern induced by temperature shock and DNP in Drosophila. Exeperimentia. 1962;18:571–573.0014-4754(1962)018[0571:ANPPIB]2.0.CO;2 [Google Scholar]
- Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 1997;16:1501–1507. doi: 10.1093/emboj/16.7.1501.1460-2075(1997)016[1501:SSOTDC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudiger S, Mayer MP, Schneider-Mergener J, Bukau B. Modulation of substrate specificity of the DnaK chaperone by alteration of a hydrophobic arch. J Mol Biol. 2000;304:245–251. doi: 10.1006/jmbi.2000.4193.0022-2836(2000)304[0245:MOSSOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rye HS, Roseman AM, Chen S, Furtak K, Fenton WA, Saibil HR, Horwich AL. GroEL-GroES cycling: ATP and nonnative polypeptide direct alternation of folding-active rings. Cell. 1999;97:325–338. doi: 10.1016/s0092-8674(00)80742-4.0092-8674(1999)097[0325:GCAANP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sacht G, Brigelius-Flohe R, Kiess M, Sztajer H, Flohe L. ATP-sensitive association of mortalin with the IL-1 receptor type I. Biofactors. 1999;9:49–60. doi: 10.1002/biof.5520090107.0951-6433(1999)009[0049:AAOMWT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of Jurkat cells. EMBO J. 1999;18:2040–2048. doi: 10.1093/emboj/18.8.2040.1460-2075(1999)018[2040:POAPCO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer LC, Picard D, Massa E, Harmon JM, Simons SS, Yamamoto KR, Pratt WB. Evidence that the hormone binding domain of steroid receptors confers hormonal control on chimeric proteins by determining their hormone-regulated binding to heat-shock protein 90. Biochemistry. 1993;32:5381–5386. doi: 10.1021/bi00071a013.0006-2960(1993)032[5381:ETTHBD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schneider HC, Berthold J, Bauer MF, Dietmeier K, Guiard B, Brunner M, Neupert W. Mitochondrial Hsp70/MIM44 complex facilitates protein import. Nature. 1994;371:768–774. doi: 10.1038/371768a0.1476-4687(1994)371[0768:MMCFPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schneider J, Jimenez E, Marenbach K, Romero H, Marx D, Meden H. Immunohistochemical detection of HSP60-expression in human ovarian cancer. Correlation with survival in a series of 247 patients. Anticancer Res. 1999;19:2141–2146.0250-7005(1999)019[2141:IDOHIH]2.0.CO;2 [PubMed] [Google Scholar]
- Shan YX, Liu TJ, Su HF, Samsamshariat A, Mestril R, Wang PH. Hsp10 and Hsp60 modulate Bcl-2 family and mitochondria apoptosis signaling induced by doxorubicin in cardiac muscle cells. J Mol Cell Cardiol. 2003;35:1135–1143. doi: 10.1016/s0022-2828(03)00229-3.0022-2828(2003)035[1135:HAHMBF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shin BK, Wang H, and Yim AM. et al. 2003 Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem. 278:7607–7616. [DOI] [PubMed] [Google Scholar]
- Sigler PB, Xu Z, Rye HS, Burston SG, Fenton WA, Horwich AL. Structure and function in GroEL-mediated protein folding. Annu Rev Biochem. 1998;67:581–608. doi: 10.1146/annurev.biochem.67.1.581.0066-4154(1998)067[0581:SAFIGP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Singh B, Patel HV, Ridley RG, Freeman KB, Gupta RS. Mitochondrial import of the human chaperonin (HSP60) protein. Biochem Biophys Res Commun. 1990;169:391–396. doi: 10.1016/0006-291x(90)90344-m.0006-291X(1990)169[0391:MIOTHC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Soltys BJ, Gupta RS. Immunoelectron microscopic localization of the 60-kDa heat shock chaperonin protein (Hsp60) in mammalian cells. Exp Cell Res. 1996;222:16–27. doi: 10.1006/excr.1996.0003.0014-4827(1996)222[0016:IMLOTK]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Soltys BJ, Gupta RS. Cell surface localization of the 60 kDa heat shock chaperonin protein (hsp60) in mammalian cells. Cell Biol Int. 1997;21:315–320. doi: 10.1006/cbir.1997.0144.1065-6995(1997)021[0315:CSLOTK]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Soltys BJ, Gupta RS. Mitochondrial-matrix proteins at unexpected locations: are they exported? Trends Biochem Sci. 1999;24:174–177. doi: 10.1016/s0968-0004(99)01390-0.0376-5067(1999)024[0174:MPAULA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Soltys BJ, Gupta RS. Mitochondrial proteins at unexpected cellular locations: export of proteins from mitochondria from an evolutionary perspective. Int Rev Cytol. 2000;194:133–196. doi: 10.1016/s0074-7696(08)62396-7.0074-7696(2000)194[0133:MPAUCL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Soti C, Csermely P. Chaperones and aging: role in neurodegeneration and in other civilizational diseases. Neurochem Int. 2002;41:383–389. doi: 10.1016/s0197-0186(02)00043-8.0197-0186(2002)041[0383:CAARIN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sriram M, Osipiuk J, Freeman B, Morimoto R, Joachimiak A. Human Hsp70 molecular chaperone binds two calcium ions within the ATPase domain. Structure. 1997;5:403–414. doi: 10.1016/s0969-2126(97)00197-4.0969-2126(1997)005[0403:HHMCBT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Strub A, Lim JH, Pfanner N, Voos W. The mitochondrial protein import motor. Biol Chem. 2000;381:943–949. doi: 10.1515/BC.2000.115.1431-6730(2000)381[0943:TMPIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Strub A, Zufall N, Voos W. The putative helical lid of the Hsp70 peptide-binding domain is required for efficient preprotein translocation into mitochondria. J Mol Biol. 2003;334:1087–1099. doi: 10.1016/j.jmb.2003.10.023.0022-2836(2003)334[1087:TPHLOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Takano S, Wadhwa R, Mitsui Y, Kaul SC. Identification and characterization of molecular interactions between glucose-regulated proteins (GRPs) mortalin/GRP75/peptide-binding protein 74 (PBP74) and GRP94. Biochem J. 2001;357:393–398. doi: 10.1042/0264-6021:3570393.0264-6021(2001)357[0393:IACOMI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka IM, Leung SM, McAndrew SJ, Brown JP, Hightower LE. Hsc70-binding peptides selected from a phage display peptide library that resemble organellar targeting sequences. J Biol Chem. 1995;270:19839–19844. doi: 10.1074/jbc.270.34.19839.0021-9258(1995)270[19839:HPSFAP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kanai F, and Kawakami T. et al. 2004 Interaction of the hepatitis B virus X protein (HBx) with heat shock protein 60 enhances HBx-mediated apoptosis. Biochem Biophys Res Commun. 318:461–469. [DOI] [PubMed] [Google Scholar]
- Taurin S, Seyrantepe V, and Orlov SN. et al. 2002 Proteome analysis and functional expression identify mortalin as an antiapoptotic gene induced by elevation of [Na+]i/[K+]i ratio in cultured vascular smooth muscle cells. Circ Res. 91:915–922. [DOI] [PubMed] [Google Scholar]
- Terada K, Mori M. Human DnaJ homologs dj2 and dj3, and bag-1 are positive cochaperones of hsc70. J Biol Chem. 2000;275:24728–24734. doi: 10.1074/jbc.M002021200.0021-9258(2000)275[24728:HDHDAD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Thomas X, Campos L, Mounier C, Cornillon J, Flandrin P, Le QH, Piselli S, Guyotat D. Expression of heat-shock proteins is associated with major adverse prognostic factors in acute myeloid leukemia. Leuk Res. 2005;29:1049–1058. doi: 10.1016/j.leukres.2005.02.010.0145-2126(2005)029[1049:EOHPIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tian ZM, Wan MX, Lu MZ, Wang XD, Wang L. The alteration of protein profile of Walker 256 carinosarcoma cells during the apoptotic process induced by ultrasound. Ultrasound Med Biol. 2005;31:121–128. doi: 10.1016/j.ultrasmedbio.2004.09.008.0301-5629(2005)031[0121:TAOPPO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Todd MJ, Lorimer GH, Thirumalai D. Chaperonin-facilitated protein folding: optimization of rate and yield by an iterative annealing mechanism. Proc Natl Acad Sci U S A. 1996;93:4030–4035. doi: 10.1073/pnas.93.9.4030.0027-8424(1996)093[4030:CPFOOR]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todryk SM, Gough MJ, Pockley AG. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology. 2003;110:1–9. doi: 10.1046/j.1365-2567.2003.01725.x.0019-2805(2003)110[0001:FOHSPS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozaki H, Ishida T, Kakiuchi C, Horiuchi H, Gotoh T, Iijima T, Imamura T, Machinami R. Expression of heat shock proteins in osteosarcoma and its relationship to prognosis. Pathol Res Pract. 2000;196:665–673. doi: 10.1016/S0344-0338(00)80118-1.0344-0338(2000)196[0665:EOHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of Toll-like receptors. Curr Top Microbiol Immunol. 2002;270:169–184. doi: 10.1007/978-3-642-59430-4_11.0070-217X(2002)270[0169:HSPALO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Venne TJ, Gupta RS. Nucleotide sequence of rat hsp60 (chaperonin, GroEL homolog) cDNA. Nucleic Acids Res. 1990;18:5309. doi: 10.1093/nar/18.17.5309.0305-1048(1990)018[5309:NSORHC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venner TJ, Singh B, Gupta RS. Nucleotide sequences and novel structural features of human and Chinese hamster hsp60 (chaperonin) gene families. DNA Cell Biol. 1990;9:545–552. doi: 10.1089/dna.1990.9.545.1044-5498(1990)009[0545:NSANSF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Voos W, Martin H, Krimmer T, Pfanner N. Mechanisms of protein translocation into mitochondria. Biochim Biophys Acta. 1999;16:235–254. doi: 10.1016/s0304-4157(99)00007-6.0006-3002(1999)016[0235:MOPTIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Voos W, Rottgers K. Molecular chaperones as essential mediators of mitochondrial biogenesis. Biochim Biophys Acta. 2002;2:51–62. doi: 10.1016/s0167-4889(02)00264-1.0006-3002(2002)002[0051:MCAEMO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Voos W, von Ahsen O, Muller H, Guiard B, Rassow J, Pfanner N. Differential requirement for the mitochondrial Hsp70-Tim44 complex in unfolding and translocation of preproteins. EMBO J. 1996;15:2668–2677.1460-2075(1996)015[2668:DRFTMH]2.0.CO;2 [PMC free article] [PubMed] [Google Scholar]
- Wachter C, Schatz G, Glick BS. Protein import into mitochondria: the requirement for external ATP is precursor-specific whereas intramitochondrial ATP is universally needed for translocation into the matrix. Mol Biol Cell. 1994;5:465–474. doi: 10.1091/mbc.5.4.465.1059-1524(1994)005[0465:PIIMTR]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa R, Kaul SC, Ikawa Y, Sugimoto Y. Identification of a novel member of mouse hsp70 family. Its association with cellular mortal phenotype. J Biol Chem. 1993a;268:6615–6621.0021-9258(1993)268[6615:IOANMO]2.0.CO;2 [PubMed] [Google Scholar]
- Wadhwa R, Kaul SC, Sugimoto Y, Mitsui Y. Induction of cellular senescence by transfection of cytosolic mortalin cDNA in NIH 3T3 cells. J Biol Chem. 1993b;268:22239–22242.0021-9258(1993)268[22239:IOCSBT]2.0.CO;2 [PubMed] [Google Scholar]
- Wadhwa R, Taira K, Kaul SC. An Hsp70 family chaperone, mortalin/mtHsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaperones. 2002;7:309–316. doi: 10.1379/1466-1268(2002)007<0309:ahfcmm>2.0.co;2.1466-1268(2002)007[0309:AHFCGW]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa R, Takano S, and Kaur K. et al. 2005 Identification and characterization of molecular interactions between mortalin/mtHsp70 and HSP60. Biochem J. 391:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa R, Takano S, Kaur K, Deocaris CC, Pereira-Smith OM, Reddel RR, and Kaul SC 2006 Upregulation of mortalin/mtHsp70/ Grp75 contributes to human carcinogenesis. Int J Cancer, in press. [DOI] [PubMed] [Google Scholar]
- Wadhwa R, Takano S, Robert M, Yoshida A, Nomura H, Reddel RR, Mitsui Y, Kaul SC. Inactivation of tumor suppressor p53 by mot-2, a hsp70 family member. J Biol Chem. 1998;273:29586–29591. doi: 10.1074/jbc.273.45.29586.0021-9258(1998)273[29586:IOTSPB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wadhwa R, Takano S, Taira K, Kaul SC. Reduction in mortalin level by its antisense expression causes senescence-like growth arrest in human immortalized cells. J Gene Med. 2004;6:439–444. doi: 10.1002/jgm.530.1099-498X(2004)006[0439:RIMLBI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wadhwa R, Yaguchi T, Hasan MK, Taira K, Kaul SC. Mortalin-MPD (mevalonate pyrophosphate decarboxylase) interactions and their role in control of cellular proliferation. Biochem Biophys Res Commun. 2003;302:735–742. doi: 10.1016/s0006-291x(03)00226-2.0006-291X(2003)302[0735:MMPDIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Weissman JS, Hohl CM, and Kovalenko O. et al. 1995 Mechanism of GroEL action: productive release of polypeptide from a sequestered position under GroES. Cell. 83:577–587. [DOI] [PubMed] [Google Scholar]
- Wick G. Atherosclerosis—an autoimmune disease due to an immune reaction against heat-shock protein 60. Herz. 2000;25:87–90. doi: 10.1007/pl00001957.0340-9937(2000)025[0087:AADDTA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wiedemann N, Frazier AE, Pfanner N. The protein import machinery of mitochondria. J Biol Chem. 2004;279:14473–14476. doi: 10.1074/jbc.R400003200.0021-9258(2004)279[14473:TPIMOM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Xanthoudakis S, Roy S, and Rasper D. et al. 1999 Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 18:2049–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota SI, Hirata D, Minota S, Higashiyama T, Kurimoto M, Yanagi H, Yura T, Kubota H. Autoantibodies against chaperonin CCT in human sera with rheumatic autoimmune diseases: comparison with antibodies against other Hsp60 family proteins. Cell Stress Chaperones. 2000;5:337–346. doi: 10.1379/1466-1268(2000)005<0337:aaccih>2.0.co;2.1466-1268(2000)005[0337:AACCIH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K, Fukumoto K, Murakami T, Harada S, Hosono R, Wadhwa R, Mitsui Y, Ohkuma S. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett. 2002;516:53–57. doi: 10.1016/s0014-5793(02)02470-5.0014-5793(2002)516[0053:ELOCEB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhang L, Pelech S, Uitto VJ. Bacterial GroEL-like heat shock protein 60 protects epithelial cells from stress-induced death through activation of ERK and inhibition of caspase 3. Exp Cell Res. 2004;292:231–240. doi: 10.1016/j.yexcr.2003.08.012.0014-4827(2004)292[0231:BGHSPP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21:4411–4419. doi: 10.1093/emboj/cdf445.1460-2075(2002)021[4411:AMSSRI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606.0193-4511(1996)272[1606:SAOSBB]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]