Abstract
Objective
Suboptimal treatment of hyperlipidemia in patients with coronary artery disease (CAD) is well documented. We report the impact of a computer-assisted physician-directed intervention to improve secondary prevention of hyperlipidemia.
Design and Setting
Two hundred thirty-five patients under the care of 14 primary care physicians in an academically affiliated practice with an electronic health record were enrolled in this proof-of-concept physician-blinded randomized, controlled trial. Each patient with CAD or risk equivalent above National Cholesterol Education Program-recommended low-density lipoprotein (LDL) treatment goal for greater than 6 months was randomized, stratified by physician and baseline LDL. Physicians received a single e-mail per intervention patient. E-mails were visit independent, provided decision support, and facilitated “one-click” order writing.
Measurements
The primary outcomes were changes in hyperlipidemia prescriptions, time to prescription change, and changes in LDL levels. The time spent using the system was assessed among intervention patients.
Results
A greater proportion of intervention patients had prescription changes at 1 month (15.3% vs 2%, P=.001) and 1 year (24.6% vs 17.1%, P=.14). The median interval to first medication adjustment occurred earlier among intervention patients (0 vs 7.1 months, P=.005). Among patients with baseline LDLs >130 mg/dL, the first postintervention LDLs were substantially lower in the intervention group (119.0 vs 138.0 mg/dL, P=.04). Physician processing time was under 60 seconds per e-mail.
Conclusion
A visit-independent disease management tool resulted in significant improvement in secondary prevention of hyperlipidemia at 1-month postintervention and showed a trend toward improvement at 1 year.
Keywords: hyperlipidemia, electronic health records, reminder systems, randomized-controlled trial
The shortfall in the application of evidence-based clinical guidelines toward the prevention and management of cardiovascular disease, described as a “quality chasm” by the Institute of Medicine, is well reported.1–3 Despite the importance of hypercholesterolemia as a modifiable risk factor for coronary artery disease (CAD), fewer than 1 in 5 patients treated reach National Cholesterol Education Program (NCEP)-defined goals of therapy. Data from our own institution among diabetics confirm these trends of inadequate cholesterol control in high-risk patients.4,5
Why does such a disparity between guidelines and practice exist? Contributors to this “knowledge-performance gap” include time limitations during the clinical encounter,6 difficulty in managing an increasing burden of clinical data,7 and suboptimal medication adherence.8 Advances in clinical informatics provide opportunities to improve the management of problems such as hyperlipidemia. However, physician-directed interventions using computerized clinical decision support system (CDSS) have had limited impact on clinical outcomes.9–18 In a recent review of 68 trials published between 1974 and 1998 evaluating the effects of CDSS, only two-thirds of studies showed that CDSS actually improved physician performance.19
Two shortcomings shared by current electronic health record (EHR) applications include the following: (1) they often provide clinical information in the form of physician reminders without transforming information into action and (2) information is generally only accessed by the physician during a clinical encounter (e.g., when meeting with a patient and referring to the patient's chart), and thus cannot improve care for patients without current clinic visits.
“Cholesterol FastTrack,” specifically designed to address these 2 limitations, used automated population surveillance for high-risk patients with elevated low-density lipoprotein (LDL) cholesterol levels to trigger an e-mail dynamically linked to the EHR. This e-mail served as a stand-alone interactive document that provided clinical context, decision support, and “one-click” order writing—all independent of a face-to-face encounter.
Although computerized reminders (CRs) have been promoted as a strategy to improve clinical care,20–25 CRs are historically “real-time” clinical tools that encourage practitioners to consider guideline recommendations when a patient's chart, and usually the patient, is in front of the provider. While CRs are most valuable when augmenting a frequently interrupted clinician's saturated memory during a time-pressured patient visit,26 clinicians most often simply ignore embedded CRs.27
Rather than a simple reminder system, our intervention represents an outreach and intervention system where an ambulatory provider order entry (POE) is delivered to the physician's inbox outside of a constrained clinical encounter. We conducted a proof-of-concept randomized, controlled trial to determine whether this “asynchronous” disease management (DM) program would be used by physicians and would improve lipid management by significantly reducing delays in the process of medication adjustment.
METHODS
Eligibility and Setting
We identified primary care physicians (PCPs) who practiced within an academic adult practice and relied on an EHR28,29 for the majority of clinical care. From usage logs, we identified physicians as potential study participants if the provider used the EHR for the majority of clinic visits (>80%) and if the electronic charts had active medication and problem lists. We identified 14 physicians meeting these criteria. All eligible PCPs consented to enroll in the study. For each consenting physician, we identified within their primary care panels all high-risk patients with elevated and outdated LDL results. Specifically, eligible patients were adults over age 30 with CAD or CAD risk equivalent,30 seen by their PCP in the past 18 months, with the most recent LDL result both above the NCEP goal of 100 mg/dL30 and obtained between 6 and 24 months prior to study initiation. There were 235 patients who fulfilled all eligibility criteria.
The Intervention
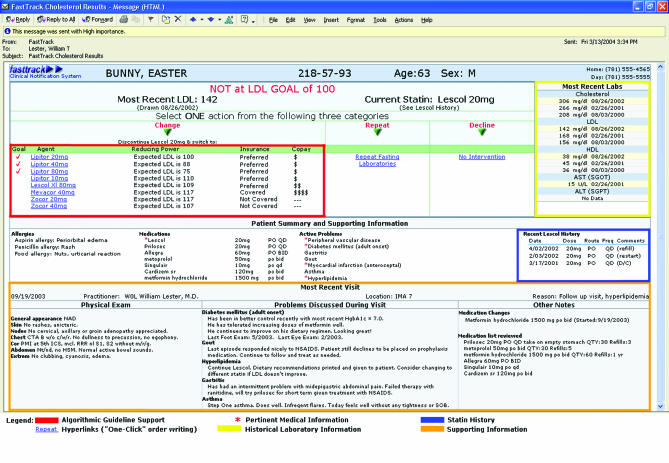
The intervention consisted of a single “FastTrack” e-mail (Fig. 1) sent to enrolled physicians on the first day of the study. Physicians received a customized e-mail for each patient randomized to intervention status. Each e-mail was HTML encoded and delivered securely via intranet by our hospital's Microsoft Exchange e-mail system. To protect patient confidentiality, no identifying information was included in the subject line and all POE hyperlinks were nonfunctional when accessed outside the hospital's firewall. The FastTrack system acted at several steps to improve cholesterol management:
FIGURE 1.
Sample FastTrack e-mail
Outreach and identification: E-mail receipt alerted physicians of high-risk, above-goal patients within their clinic panels. Our intent was to consolidate in 1 e-mail all the available and relevant information necessary for making a sound medical decision, although this effort was limited by any data (such as allergies, etc.) that may have been missing from the EHR.
-
Best-Practice decision support: Possible medication choices were algorithmically rank ordered from among all 3-hydroxy-methylglutaryl-coenzyme A-reductase inhibitors (statins) according to:
Anticipated postintervention NCEP-goal achievement,
Predicted postintervention LDL result,
Patient insurance formulary preference, and
Patient copay information.
Predicted postintervention LDL result was determined by first estimating the patient's LDL if his or her current statin was discontinued, and then accounting for the effect of adding the new statin prescription. Statin effects were calculated using published estimates of action for different agents and doses.
-
“One-click” POE: In response to the clinical information provided, study physicians selected via single-click 1 of 3 mutually exclusive actions, represented as 3 separate icons on the e-mail:
Change: This hyperlink generated a specific statin prescription and updated the patient's EHR. The prescription, an explanatory letter, a “Statins Frequently Asked Questions” sheet, and a tailored hyperlipidemia patient information page were automatically printed to be signed by the physician and mailed to the patient.
Repeat: This link automatically generated a patient letter requesting a repeat fasting lipid profile as well as a completed laboratory requisition and hyperlipidemia patient information page.
Decline: When providers declined to change medical management, a questionnaire captured the physician's clinical rationale for not changing care in that particular patient.
Integration into the existing EHR: Although clinical action occurred external to the EHR, complete documentation was automatically entered into the EHR.
Objectives and Hypothesis
We hypothesized that this facilitated approach to hyperlipidemia management utilizing asynchronous outreach and intervention would be accepted and used by physicians and result in improved clinical outcome measures. Our specific aims were to test this system's impact on: (1) changes in statin prescriptions and (2) LDL levels over time.
Outcomes
For each participating physician, the intervention occurred on Day 1 of the study when the physician received all corresponding intervention e-mails in a single batch. All e-mails were sent within an 8-week period beginning July 26, 2003. Clinical care was monitored for 12 months. The primary clinical outcome was the proportion of patients with changes in statin prescriptions 1 and 12 months after study Day 1; our secondary outcome was change in LDL levels. Baseline LDL values were defined as the most recent test prior to initiation of the study. Endpoint LDL values were defined as follows: (1) the first LDL result recorded greater than 6 weeks after the FastTrack e-mail and (2) the final LDL result during the 12-month follow-up period. A priori, we also analyzed prescription and LDL changes in the subset of high-risk patients with baseline LDL levels >130 mg/dL.
Among intervention patients, we identified provider explanations when no clinical action was taken and assessed 2 process measures of the intervention: (1) proportion of e-mails opened and acted upon and (2) time spent reading e-mail (measured as the time elapsed between successive e-mail actions).
Nine months after the intervention date, letters requesting a fasting lipid profile were generated for PCPs to electively sign and mail to all patients (intervention and control) without a repeat fasting cholesterol test as the study inception.
Randomization
Randomization occurred at the patient level using a computer-generated number list, stratified by physician and baseline LDL (dichotomized at 110 mg/dL). Thus, each participating physician received FastTrack e-mails for half of his or her eligible study panel (intervention patients), while the other half of the study panel received usual care (control patients). Physicians were aware that they had roughly an equal number of control patients receiving usual care but were blinded to the identity of these patients at study inception. Because our intervention tested a novel tool to translate established care guidelines into clinical action, and because physicians individually signed all materials prior to being sent to patients, informed consent was not obtained from individual patients. This study was approved by the Massachusetts General Hospital Human Research Committee.
Statistical Methods
All analyses were completed according to the preestablished analysis plan. The intervention was individualized for each patient, and outcomes were measured at the patient level. Among a panel of patients cared for by the same physician, correlation in outcomes would be expected to arise primarily because of individual physician practice patterns. The effect of this intraphysician correlation was minimized by our study design, which randomized patients within each physician cluster rather than randomizing physician clusters, thereby accounting for individual variations in the practice of hyperlipidemia management.
We used t-tests for normally distributed and Wilcoxon rank sum tests for nonnormal continuous data, and compared proportions using χ2tests with continuity correction or Fisher's exact test where appropriate. Multivariate analyses were conducted with logistic regression. Primary analysis was intention-to-treat and involved all randomly assigned patients. The study had an 80% power to detect a 20 mg/dL difference in cholesterol levels between-study groups with a 0.05 2-sided significance level. To address the potential clustering effect of patients grouped by physician (which would reduce our effective sample size proportional to the degree of correlation within physician clusters), we used the PROC GENMOD procedure (SAS, version 9.0, SAS Institute Inc.) with physician as the clustering variable in the analyses of our primary outcomes. This analysis did not substantially change our results, implying that there was no significant correlation in patent-related outcomes by provider. Two-sided significance tests were used throughout. P-values less than .05 were considered as statistically significant.
RESULTS
Study Subjects
Fourteen staff physicians consented to participate in our study. The physician cohort was 36% female, with a mean (±SD) of 15.1 (±8.7) years of experience as attending physicians. Resident physicians were excluded from our study. Physicians worked from 2 to 7 half-day clinic sessions per week. In the prior year, these physicians had 1,760 (±1,031) scheduled patient visits and cared for 686 (±371) unique patients.
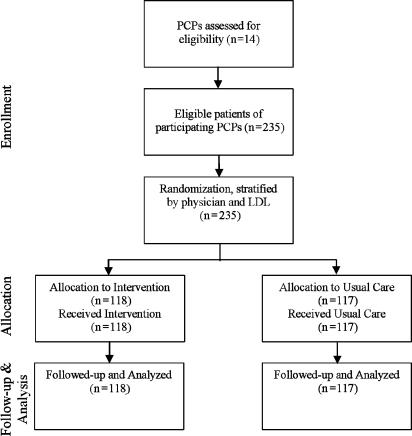
We identified 235 patients (21.4 [±13.8] patients per study physician) meeting the eligibility criteria. After randomization, there were 118 intervention and 117 control patients. Study participant flow is shown in Figure 2. Baseline patient characteristics were similar across all measures and are summarized in Table 1 The mean LDL for this high-risk group, last measured as a median of 9.7 months prior to the start of the study, was 126 mg/dL.
FIGURE 2.
Participant flow
Table 1.
Baseline Characteristics of Intervention and Control Patients
| Intervention (n=118) | Control (n=117) | P Value | |
|---|---|---|---|
| Age, years (SD) | 64.3 (14.5) | 62.4 (13.3) | .3 |
| Female, n(%) | 57 (48.3) | 60 (51.3) | .7 |
| Nonwhite, n(%) | 17 (14.4) | 22 (18.8) | .4 |
| Married, n(%) | 51 (43.2) | 49 (41.9) | .8 |
| High-risk diagnoses, n(%) | |||
| CAD | 49 (41.5) | 43 (36.7) | .5 |
| Diabetes | 48 (40.7) | 46 (39.3) | .9 |
| Other macrovascular disease | 60 (50.8) | 51 (43.6) | .3 |
| Multiple high-risk diagnoses | 24 (20.3) | 19 (16.2) | .5 |
| Baseline LDL, mg/dL (SD) | 125.2 (22.1) | 127.1 (24.6) | .5 |
| Months from baseline LDL result to study start, median (IQR) | 9.8 (7.0–17.1) | 9.6 (7.2–13.8) | .5 |
| On statins at baseline, n(%) | 52 (44.1) | 51 (43.6) | .9 |
| Months of statin treatment prior to study start, median (IQR) | 17.3 (5.7–43.6) | 25.5 (13.2–41.6) | .1 |
| Patients with any follow-up in 12 months after intervention, n(%) | 95 (80.5) | 98 (83.8) | .5 |
Data are numbers and proportions, means and standard deviations (SD), or medians and interquartile ranges (IQR). CorrespondingP-values are from χ2tests, Student'st-tests, or Wilcoxon Rank Sum. High-risk diagnosis, diagnosis at baseline qualifying patient for high-risk status; CAD, coronary artery disease; Other macrovascular disease, includes cerebral and peripheral vascular disease and aortic aneurysm; LDL, low-density lipoprotein cholesterol; statins, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors.
Actions in Response to FastTrack E-mails
Of 118 FastTrack e-mails sent, 117 (99%) were opened, read, and completed by the participating PCP. One e-mail was overlooked and deleted by 1 provider. The median time to complete a FastTrack e-mail (including the questionnaire generated when no management change was chosen) was 90 seconds (range: 15 seconds to 49 minutes). As physicians became accustomed to the e-mail's user interface, time-to-completion declined by an average of 34% between the first and last e-mails. Thus, after an initial learning curve, physicians spent less than 60 seconds per patient to complete both the FastTrack e-mail and, when necessary, the linked questionnaire.
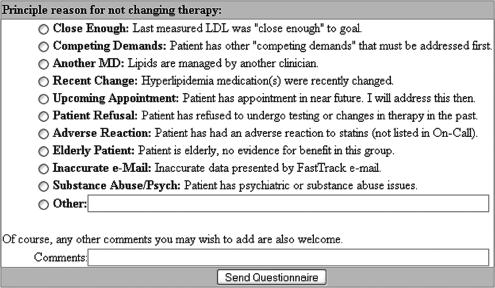
Thirty-four e-mails (29%) resulted in a clinical management change, defined as either medication adjustment (statin initiation, substitution, or dose change) or a repeat fasting lipid profile request being mailed to the patient (Table 2) Physicians completed a brief questionnaire (Fig. 3) for each of the remaining 84 e-mails (71%) where no clinical action was taken. The most common explanation given for not changing a patient's cholesterol management was that the measured LDL was “close enough” (n=33, 39% of deferred patients). The average LDL in this “close enough” group was 109 mg/dL with 50% of patients having LDL levels less than 105 mg/dL. Explanations for the remaining 51 patients included the following: lipids managed by another physician (14; 17%), inaccurate problem list (10; 12%), upcoming appointments (10; 12%), competing medical demands (9; 11%), prior patient refusal (4; 5%), and adverse response to statins (2; 4%).
Table 2.
Actions Taken in Response to FastTrack e-mails, Intervention Patients (n=118)
| PCP Action | N(% of total e-mails) | Baseline LDL |
|---|---|---|
| Statin change | 19 (16) | 137.3±18.9 |
| Repeat LDL | 15 (13) | 129.9±20.9 |
| Defer action | 84 (71) | 121.6±22.0 |
| Reasons for Deferred Action | N(% of deferred e-mails) | Baseline LDL |
|---|---|---|
| Close enough | 33 (39) | 108.5±7.4 |
| Lipids managed by another provider | 14 (17) | 133.4±37.0 |
| Inaccurate problem list | 10 (12) | 126.7±16.7 |
| Proximal visit | 10 (12) | 130.1±14.4 |
| Elderly or too sick | 9 (11) | 126.9±20.8 |
| Patient refusal | 4 (5) | 138.8±26.0 |
| Adverse statin effect | 2 (4) | 139.5±0.7 |
| Maximal statin dose | 1 (1) | 111.0 |
| E-mail not opened | 1 (1) | 116.0 |
LDL, low lipoprotein cholesterol, presented in mg/dL±SD.
FIGURE 3.
Questionnaire generated when no changes in management were made
Impact of the Intervention on Statin Prescriptions
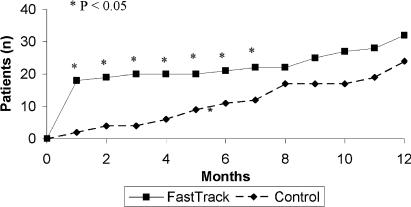
Compared with controls, more intervention patients had statin prescription changes at 1 month postrandomization (15.3% vs 2%, P=.001). After the full 1-year follow-up period, there remained a greater proportion of statin changes in intervention versus control patients, although the differences were no longer statistically significant (24.6% vs 17.1%, P=.14). The median interval to first medication adjustment for intervention patients occurred more than a half year earlier than for control patients (0 vs 7.1 months, P=.005) (Fig. 4).
FIGURE 4.
Time to statin initiation or dose change, FastTrack versus Controls
Impact of the Intervention on LDL Cholesterol
Low-density lipoprotein levels improved for all patients over the study's duration (Table 3)Although not statistically significant, time-to-first-measured-LDL after study initiation was 22 days shorter in the intervention group [median (interquartile ranges) 99 (48 to 171) days] compared with the control group [121 (45 to 208) days, P=.48]. Although follow-up LDL levels were consistently lower in the intervention group, these differences were not statistically significant for the overall study cohort. In the subgroup of patients with baseline LDLs greater than 130 mg/dL, however, first postintervention LDL levels were substantially lower in the intervention group compared with controls (119 vs 138 mg/dL, P=.04). This difference between groups persisted through the 1-year follow-up period (111 vs 129 mg/dL, P=.055).
Table 3.
Impact of Intervention on Statin Prescriptions and LDL Levels
| Intervention N=118 (% of total) | Control N=117 (% of total) | P Value | |
|---|---|---|---|
| Statin prescriptions | |||
| Statin change within 1 month, n(%) | 18 (15.3) | 2 (2) | .001 |
| Statin change within 1 year, n(%) | 29 (24.6) | 20 (17.1) | .14 |
| Months until statin change among patient with changes, median (IQR) | 0 (0–8.5) | 7.1 (3.9–10.4) | .005 |
| LDL changes | |||
| All Patients with LDL results, n(%) | 81 (68.6) | 82 (70.1) | .8 |
| First LDL after intervention | 111.7±30.2 | 118.1±32.1 | .2 |
| Final LDL | 106.8±26.8 | 111.5±30.0 | .3 |
| Patients with LDL>130 mg/dL at baseline, n(%) | 41 (34.7) | 39 (33.3) | .9 |
| Baseline LDL | 150.1±17.0 | 155.9±20.4 | .2 |
| First LDL after intervention | 119.0±32.1 | 138.0±35.6 | .04 |
| Final LDL | 111.4±29.3 | 128.3±35.7 | .055 |
Data are numbers and proportions, means and standard deviations (SD), or medians and interquartile ranges (IQR). LDL, low-density lipoprotein cholesterol, presented in mg/dL±SD; statins, 3-hydrox-3-methylglutaryl coenzyme A reductase inhibitors; for intervention patients ordered repeat testing, first LDL was defined as the next LDL after repeated level, and for all other patients, first LDL was defined as the next measured LDL after intervention date. P-value is for pairedt-tests for change in LDL from baseline.
DISCUSSION
While EHRs have become integral to discussions of health care policy and reform,31–35 they remain underused. Correspondingly, more effective applications of their clinical use are required.27 By challenging the notion that care delivered via an EHR occurs only during a clinical visit, our “FastTrack” intervention demonstrated in a proof-of-concept randomized-controlled trial an application of informatics technology within a health system that has already adopted an EHR.
We successfully demonstrated in this trial that PCPs are willing to use a visit-independent DM tool to intervene on guideline noncompliant patients. While LDL levels improved among intervention patients after a single FastTrack e-mail, differences between study arms were attenuated over the 12-month follow-up period, indicating that usual care converged toward care in the intervention arm. This relative improvement in the control arm contrasts with prior CR studies in which the reminder's effect typically attenuates over time.36 Our intervention's lack of iteration may be 1 explanation for the eventual convergence between usual care and intervention groups. Our intervention consisted of a single e-mail sent once per patient. Given our system's ability to report laboratory results in real time, repeated FastTrack e-mails may have led to more sustained improvements among intervention patients over time.
Another possible explanation for the convergence between study arms is contamination of the effect of the intervention in the control arm. For our study, however, information contamination was not likely to be a significant problem for the following reasons: (1) unlike traditional CRs, our intervention did not simply provide nonspecific information about LDL control (which could be transferred by the physician to control patients, leading to contamination) but rather served to facilitate rapid action change tailored specifically to an individual patient, (2) contamination would tend to bias results toward the null, and thus any significant findings would occur despite this tendency, (3) study physicians were very well aware of the LDL guidelines, and therefore our intervention provided little additional educational impact, and (4) physicians were unaware of the identities of their control patients. Therefore, it is more likely that over the 12-month study period, the slower pace of visit-based usual care in the control arm “caught up” with the care facilitated by our visit-independent intervention.
Our finding of decreased delay until medication adjustment in the intervention group supports this assertion and also suggests that reminder-like interventions, when delivered asynchronously from a clinical visit and effectively linked with POE, can shorten the delay from problem identification (i.e., elevated LDL) to corresponding clinical action (i.e., statin prescription).
In addition to recorded changes in statin prescriptions, the primary measured outcome in this study was LDL level, which was obtained during routine care rather than by study investigators. Because these results were not obtained uniformly for all patients, our results may be confounded by characteristics associated with obtaining timely LDL tests.
While some might argue that the shortened delay to hyperlipidemia medication adjustment is not of major clinical importance, identifying and overcoming delays to care represent an important advance in informatics-supported clinical management that, with wider application, may have a substantial clinical impact on populations of patients over time.
For our secondary prevention target, we selected a highly prevalent disease with unambiguous diagnostic criteria, well-established DM guidelines, and well-tolerated, efficacious treatment options. Although our patient population had an average LDL well above the NCEP-defined goal (126 mg/dL), and nearly a year elapsed (9.7 months) since hyperlipidemia had even been addressed, no statin prescription changes were made in the majority of intervention patients. The questionnaire linked to the moment of the clinical decision making yielded a powerful tool in identifying barriers to guideline compliance. While many patients were considered by physicians to be simply “close enough,” we often found that physicians had valid reasons for not changing care in patients who appeared not to be meeting evidence-based LDL goals. For example, 1 patient had a myocardial infarction secondary to traumatic coronary dissection, while another had limited life expectancy because of metastatic cancer. Given the increasing reliance of quality improvement efforts on physician report cards,37,38 this finding reemphasizes the fact that “one-size-fits-all” guidelines cannot be uniformly applied to real patient populations.
Our intervention represented a simple and efficient means by which physicians could make well-informed clinical management changes. In designing our intervention, we applied the following principles:
Preserve physician autonomy. By providing a range of medication choices algorithmically ranked by target LDL/NCEP-goal attainment, formulary/copay information, we preserve physician choice, thereby addressing physician attitudes that guidelines undermine physician authority and result in oversimplification or “cookbook” medicine.39,40
Respect the workflow. The primary explanation for CR underutilization is physician workload.41 Even an incremental addition of a single screening or diagnostic test adds over 5 minutes to a clinical visit's duration.42 Because of FastTrack's self-contained nature, physicians could confidently alter therapy without reference to the EHR. A single “click”—consuming less than 60 seconds of physician time—initiated the entire medication adjustment workflow sequence and accelerated care by over 7 months.
Involve the patient. Although our intervention occurred external to a constrained clinical encounter, we incorporated patient outreach and education through the use of individually tailored patient mailings.
One limitation of our study was the relatively small patient sample size, which left us underpowered to demonstrate statistically significant LDL differences in our patient population at 1 year. Further, our small physician sample size led to a dilemma in our randomization scheme. We chose to randomize patients within providers rather than randomize providers for this study because with only 14 study physicians, randomization at the physician level would not have adequately addressed the primary reason for randomization, which is to distribute both measured and unmeasured confounders evenly. In this case, as physician practice style (e.g., “aggressive” vs “less-aggressive” approaches to medication management) could present a critical unmeasured confounder that might have invalidated our results, we randomized patients stratified by physician. The advantage of this approach is that—with equal numbers of case and control patients—each physician serves as his or her own control, thereby controlling for “practice style” and other physician-level unmeasured confounders. In addition, our intervention served to provide physicians with more than generic information about LDL control: FastTrack also provided physicians with a means to transform that information into clinical action, which does not easily diffuse to control patients.
Another limitation of our study existed in our measurement of e-mail completion time. We estimated the time that physicians spent reading e-mails as the time elapsed between successive e-mail actions, which may skew our results toward longer times in cases where physicians were interrupted or otherwise distracted. Despite this limitation, we found that physicians generally required less than a minute to complete each e-mail.
This trial represents proof-of-concept of an extension to an EHR. As a result, the intervention depended on both the advanced EHR within our system, as well as the physicians who used the EHR for clinical care, both of which limit immediate generalizability of our application. In addition, implementation of advanced clinical support tools such as the prototype tested in our study will be limited by the quality of EHR data available. Care will be required to prevent potentially preventable adverse events because of incomplete information in the EHR.
For this clinical trial, physicians received all of their intervention FastTrack e-mails in a single batch. Future longer-term studies will need to address physician responsiveness to advanced care alerts that are received intermittently. One strategy might be to gather all such alerts on a single physician-specific web page that can be accessed by the provider on a regular basis.
Study limitations regarding sample size, randomization, and clustering will be addressed in future studies with larger physician populations, such that physician randomization can be afforded. As proof-of-concept, however, our system demonstrates the potential of informatics-based applications to change the current practice of medicine substantially, and therefore supports the national effort to increase EHR adoption.
In summary, we implemented and tested a novel extension to an EHR. In highest-risk patients with elevated and outdated LDL levels, our “asynchronous” intervention had a significant and clinically meaningful impact on pharmacotherapy and subsequent LDL results. Yet, based on our findings, clearly, there is still a need to better understand the process of clinical decision making and to account for individual patient uniqueness in the context of evidence-based guidelines. Once designed and implemented, informatics applications are relatively inexpensive to use for large patient populations on an iterative basis, and therefore have the potential for significantly improving the efficiency and effectiveness of care for large patient populations.
Acknowledgments
We thank Daniel E. Singer, MD for insightful comments on an earlier draft of this manuscript, E. John Orav PhD for biostatistical advice, and Nancy Wong for assistance with data management.
REFERENCES
- 1.Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century Health Care Services. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 2.Braunwald E, et al. Shattuck lecture—cardiovascular medicine at the turn of the millennium:triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–9. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 3.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 4.Grant RW, Cagliero E, Murphy-Sheehy P, Singer DE, Nathan DM, Meigs JB, et al. Comparison of hyperglycemia, hypertension, and hypercholesterolemia management in patients with type 2 diabetes. Am J Med. 2002;112:603–9. doi: 10.1016/s0002-9343(02)01103-8. [DOI] [PubMed] [Google Scholar]
- 5.Grant RW, Cagliero E, Dubey A, et al. Clinical inertia in the management of type 2 diabetes metabolic risk factors. Diabetic Med. 2004;21:150–5. doi: 10.1111/j.1464-5491.2004.01095.x. [DOI] [PubMed] [Google Scholar]
- 6.Kottke TE, Brekke ML, Solberg LI, et al. Making time for preventative services. Mayo Clin Proc. 1993;68:785–91. doi: 10.1016/s0025-6196(12)60638-7. [DOI] [PubMed] [Google Scholar]
- 7.Hibble A, Kanka D, Pencheon D, Pooles E, et al. Guidelines in general practice:the new tower of Babel? BMJ. 1998;317:862–3. doi: 10.1136/bmj.317.7162.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearson TA, McBride PE, Miller NH, Smith SC, Jr, et al. 27th Bethesda conference:matching the intensity of risk factor management with the hazard for coronary disease events. Task Force 8. Organization of preventive cardiology service. J Am Coll Cardiol. 1996;27:1039–47. doi: 10.1016/0735-1097(96)87736-9. [DOI] [PubMed] [Google Scholar]
- 9.Johnston ME, Langton KB, Haynes B, Mathieu A, et al. Effects of computer-based clinical decision support systems on clinician performance and patient outcome. Ann Intern Med. 1994;120:135–42. doi: 10.7326/0003-4819-120-2-199401150-00007. [DOI] [PubMed] [Google Scholar]
- 10.Classen D, et al. Clinical decision support systems to improve clinical practice and quality of care. JAMA. 1998;280:1360–1. doi: 10.1001/jama.280.15.1360. [DOI] [PubMed] [Google Scholar]
- 11.Smith S, Murphy M, Huschka T, et al. Impact of a diabetes electronic management system on the care of patients seen in a subspecialty diabetes clinic. Diabetes Care. 1998;21:972–6. doi: 10.2337/diacare.21.6.972. [DOI] [PubMed] [Google Scholar]
- 12.McDonald C, et al. Use of a computer to detect and respond to clinical events:its effect on clinician behavior. Ann Int Med. 1976;84:162–7. doi: 10.7326/0003-4819-84-2-162. [DOI] [PubMed] [Google Scholar]
- 13.McDonald CJ, Hui SL, Smith DM, et al. Reminders to physicians from an introspective computer medical record:a two-year randomized trial. Ann Intern Med. 1984;100:130–8. doi: 10.7326/0003-4819-100-1-130. [DOI] [PubMed] [Google Scholar]
- 14.Tierney WM, Overhage JM, Murray MD, et al. Effects of computerized guidelines for managing heart disease in primary care. J Gen Intern Med. 2003;18:967–76. doi: 10.1111/j.1525-1497.2003.30635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maviglia SM, Teich JM, Fiskio J, Bates DW, et al. Using an electronic medical record to identify opportunities to improve compliance with cholesterol guidelines. J Gen Intern Med. 2001;16:531–7. doi: 10.1046/j.1525-1497.2001.016008531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamos TD, Shaltoni H, Girard SA, Parrillo JE, Calvin JE, et al. Effectiveness of chart prompts to improve physician compliance with the National Cholesterol Education Program guidelines. Am J Cardiol. 2001;88:1420–3. doi: 10.1016/s0002-9149(01)02125-7. A8. [DOI] [PubMed] [Google Scholar]
- 17.Murray MD, Harris LE, Overhage JM, et al. Failure of computerized treatment suggestions to improve health outcomes of outpatients with uncomplicated hypertension:results of a randomized controlled trial. Pharmacotherapy. 2004;24:324–37. doi: 10.1592/phco.24.4.324.33173. [DOI] [PubMed] [Google Scholar]
- 18.Grimshaw JM, Russell IT, et al. Effect of clinical guidelines on medical practice:a systematic review of rigorous evaluations. Lancet. 1993;342:1317–22. doi: 10.1016/0140-6736(93)92244-n. [DOI] [PubMed] [Google Scholar]
- 19.Hunt D, Haynes R, Hanna S, Smith K, et al. Effects of computer-based clinical decision support systems on physician performance and patient outcomes:a systematic review. JAMA. 1998;280:1339–46. doi: 10.1001/jama.280.15.1339. [DOI] [PubMed] [Google Scholar]
- 20.McDonald CJ, Hui SL, Smith DM, et al. Reminders to physicians from an introspective computer medical record. A two-year randomized trial. Ann Intern Med. 1984;100:130–8. doi: 10.7326/0003-4819-100-1-130. [DOI] [PubMed] [Google Scholar]
- 21.Dexter PR, Perkins S, Overhage JM, Maharry K, Kohler RB, McDonald CJ, et al. A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med. 2001;345:965–70. doi: 10.1056/NEJMsa010181. [DOI] [PubMed] [Google Scholar]
- 22.Overhage JM, Tierney WM, Zhou XH, McDonald CJ, et al. A randomized trial of “corollary orders” to prevent errors of omission. J Am Med Inform Assoc. 1997;4:364–75. doi: 10.1136/jamia.1997.0040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald C, et al. Use of a computer to detect and respond to clinical events:its effect on clinician behavior. Ann Intern Med. 1976;84:162–7. doi: 10.7326/0003-4819-84-2-162. [DOI] [PubMed] [Google Scholar]
- 24.Litzelman DK, Dittus RS, Miller ME, Tierney WM, et al. Requiring physicians to respond to computerized reminders improves their compliance with preventive care protocols. J Gen Intern Med. 1993;8:311–7. doi: 10.1007/BF02600144. [DOI] [PubMed] [Google Scholar]
- 25.Tierney WM, Hui SL, McDonald CJ, et al. Delayed feedback of physician performance versus immediate reminders to perform preventive care. Effects on physician compliance. Med Care. 1986;24:659–66. doi: 10.1097/00005650-198608000-00001. [DOI] [PubMed] [Google Scholar]
- 26.McDonald CJ, et al. Computer reminders, the quality of care and the nonperfectability of man. N Engl J Med. 1976;295:1351–5. doi: 10.1056/NEJM197612092952405. [DOI] [PubMed] [Google Scholar]
- 27.Schellhase KG, Koepsell TD, Norris TE, et al. Providers' reactions to an automated health maintenance reminder system incorporated into the patient's electronic medical record. J Am Board Fam Pract. 2003;16:312–7. doi: 10.3122/jabfm.16.4.312. [DOI] [PubMed] [Google Scholar]
- 28.Barnett GO, et al. The application of computer based medical record systems in ambulatory practice. N Engl J Med. 1984;310:1643–50. doi: 10.1056/NEJM198406213102506. [DOI] [PubMed] [Google Scholar]
- 29.Rabbani U, Morgan M, Barnett O, et al. A COSTAR interface using WWW technology. Proc AMIA Symp. 1998:703–7. [PMC free article] [PubMed] [Google Scholar]
- 30.NCEP Expert Panel. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 31.Roper WL, Baker EL, Dyal WW, Nicola RM, et al. Strengthening the public health system. Public Health Rep. 1992;107:609–15. [PMC free article] [PubMed] [Google Scholar]
- 32.Bakken S, et al. An informatics infrastructure is essential for evidence-based practice. J Am Med Inform Assoc. 2001;8:199–201. doi: 10.1136/jamia.2001.0080199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Committee on Vital and Health Statistics. Information for health: a strategy for building the national health information infrastructure report and recommendations. [December 20, 2004]; Available at http://aspe.hhs.gov/sp/nhii/Documents/nhiilayo.pdf.
- 34.Transforming health care: the President's health information technology plan. [December 20, 2004]; Available at http://aspe.hhs.gov/sp/nhii/news/WhiteHouseITPlan.pdf.
- 35.Research in Action, Issue 6. Medical Informatics for Better and Safer Health Care Agency for Healthcare Research and Quality. [December 20, 2004]; Available at http://www.ahrq.gov/data/informatics/informatria.pdf.
- 36.Demakis JG, Beauchamp C, Cull WL, et al. Improving residents' compliance with standards of ambulatory care:results from the VA cooperative study on computerized reminders. JAMA. 2000;284:1411–6. doi: 10.1001/jama.284.11.1411. [DOI] [PubMed] [Google Scholar]
- 37.Hofer TP, Hayward RA, Greenfield S, Wagner EH, Kaplan SH, Manning WG, et al. The unreliability of individual physician “report cards” for assessing the costs and quality of care of a chronic disease. JAMA. 1999;281:2098–105. doi: 10.1001/jama.281.22.2098. [DOI] [PubMed] [Google Scholar]
- 38.Balas EA, Boren SA, Brown GD, Ewigman BG, Mitchell JA, Perkoff GT, et al. Effect of physician profiling on utilization. Meta-analysis of randomized clinical trials. J Gen Intern Med. 1996;11:584–90. doi: 10.1007/BF02599025. [DOI] [PubMed] [Google Scholar]
- 39.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines?. A framework for improvement. JAMA. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 40.Tunis SR, Hayward R, Wilson MC, et al. Internists' attitudes about clinical practice guidelines. Ann Intern Med. 1994;120:956–63. doi: 10.7326/0003-4819-120-11-199406010-00008. [DOI] [PubMed] [Google Scholar]
- 41.Patterson ES, Nguyen AD, Halloran JP, Asch SM, et al. Human factors barriers to the effective use of ten HIV clinical reminders. J Am Med Inform Assoc. 2004;11:50–9. doi: 10.1197/jamia.M1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blumenthal D, Causino N, Chang Y, et al. The duration of ambulatory visits to physicians. J Fam Pract. 1999;48:264–71. [PubMed] [Google Scholar]