Abstract
BACKGROUND
Although the use of mammography on at regular intervals can save lives, not all women obtain the repeat mammography recommended in guidelines.
OBJECTIVE
To assess the associations between routine mammography use, perceived cancer risk, and actual projected cancer risk.
METHODS
We include women who were 45 to 75 years of age and who had responded to the 2000 National Health Interview Survey. Women who reported that they believed their risk of getting cancer in the future was “medium” or “high” were considered jointly as “medium/high-risk perception.”“Routine mammography use” was defined as having ≥3 mammograms in the previous 6 years. We used logistic regression to determine the independent relation between cancer risk perception, projected breast cancer risk, and routine mammography use.
RESULTS
Of the 6,002 women who met our inclusion criteria, 63.1% reported routine mammography use. About 76% of women in the highest quartile of projected breast cancer risk reported routine mammography use, compared with only 68%, 64%, and 51% in the third, second, and first quartiles, respectively (P<.001 chi-square test for trend). After adjusting for indicators of access to care, sociodemographic and behavioral factors, and perceived cancer risk, women in the highest quartiles of projected cancer risk were significantly more likely to report routine mammogram use than women in the lowest quartile (odds ratio [OR] of women in third and fourth quartiles were 1.57 [1.24 to 1.99], and 2.23 [1.73 to 2.87] vs the lowest quartile, respectively). Women with a higher perceived cancer risk were significantly more likely to undergo routine mammography (adjusted OR: 1.29 [1.12 to 1.48] P=.001). Cancer risk perceptions tended to be higher among women who were younger age, obese, smokers, depressed, or reported one of the following breast cancer risk factors: family breast cancer history, prior abnormal mammogram, and early age at menarche.
CONCLUSION
Actual and perceived risk were independent predictors of routine mammography use, suggesting that efforts to incorporate risk profiles into clinical decision making may need to involve more than just relaying information about projected risks to patients, but also to explore how risk perceptions can be affected by this information.
Keywords: breast cancer, screening, mammography, risk assessment
Breast cancer is a significant cause of morbidity and mortality in the United States, with approximately 217,000 women expected to be diagnosed in 2004.1 Despite recent controversy about the effectiveness of mammography, most organizations endorse regular mammography screening as a means of decreasing breast cancer mortality.2–6 Unfortunately, some studies suggest that only about two thirds of women who get a mammogram return for regular testing.7, 8 As repeat mammography at regular intervals is required for optimal mortality reduction,9 many of these women may not be receiving the benefit of screening.
Some have suggested that mammography utilization could be increased by tailoring screening recommendations to each woman's individualized risk of breast cancer.10 In women with a prior history of breast cancer, mammograms have a higher case finding rate and mammography-detected tumors are more likely to be earlier stage.11–14 As a result, current guidelines state that women with increased breast-cancer risk should begin consider initiating screening at an earlier age and return at regular intervals.2, 15–18
What type of risk information should be integrated into clinical decision making? Until recently, most guidelines and studies have relied exclusively on family history as the marker of breast cancer risk.19, 20 Indeed, women with a family history are more likely to receive mammograms than women without a family history, but as many as a third of women ≥50 years of age with a family history have been found to have not had a mammogram in the past year.19, 20
A broader concept of breast cancer risk can help to tailor risk stratification to individual patients far more accurately. Besides family history, other important risk factors for breast cancer include a history of prior breast abnormalities or hormone replacement therapy use, obesity, physical inactivity, age, ethnicity, and age at primary menarche, first live birth, and menopause.10, 21, 22 Comprehensive breast cancer risk assessment tools, such as the widely used model developed by Gail et al.21 incorporate multiple patient characteristics into a validated algorithm for informing the woman of her projected breast cancer risk.
Women's perceptions of breast cancer risk are not always consistent with their “objective” breast cancer risk estimates.23–25 These discrepancies are important because some data have suggested that perceived risk may be a stronger predictor of mammography use than quantitative estimates of projected risk.23, 26 Conversely, it is important not to overemphasize risk; some work has suggested that too much trepidation over one's risk may hinder screening.23, 27–29 Because women who misinterpret their risk may be less likely to make informed decisions about mammography use, it is important to understand factors that influence cancer risk perceptions and whether these perceptions impact mammography use at the population level.
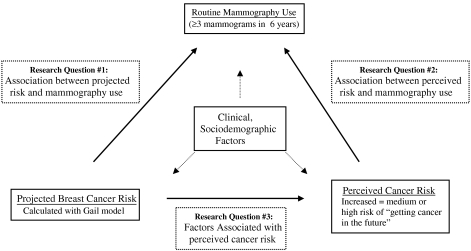
The objective of this study was to examine the interconnecting relationships between projected breast cancer risk, perceived cancer risk, and routine mammography use.30 We assessed predictors of routine mammography use using data from the 2000 National Health Interview Survey (NHIS) to address the following research questions (Fig. 1): First, what is the association between projected breast cancer risk and routine mammography use? Second, is perceived cancer risk independently associated with routine mammography use? Third, what factors are associated with perceived cancer risk in the general population, as well as among a subgroup of women with increased projected cancer risk?
FIGURE 1.
Study schema and research questions.
METHODS
Study Overview and Data Source
We performed a cross-sectional study using the 2000 NHIS data and its accompanying Cancer Control Module (CCM). The NHIS is a continuous survey of households in the United States that is conducted by the National Center for Health Statistics (Hyattsville, MD) and provides health information on the noninstitutionalized civilian population as a publicly available resource.31 The CCM is an additional set of questions incorporated into the NHIS periodically and includes a variety of questions about cancer risk factors and screening practices. The response rates for the eligible respondents to the 2000 NHIS core questionnaire and the CCM were 87.3% and 82.6%, respectively.31 Our study sample was restricted to female respondents who were 45 to 75 years of age, had no history of breast cancer, and had responded to the items about mammography use and perceived cancer risk.
Definition of Variables
Projected Breast Cancer Risk
The NHIS CCM included items necessary to estimate projected breast cancer risk using the breast cancer predictive model developed by Gail et al.32 This model estimates an individual woman's risk of developing breast cancer using several established breast cancer risk factors including age, age at first live birth, age at menarche, number of first-degree relatives with breast cancer, and number of breast biopsies.21, 33 We used this model (as modified by Anderson and Redmond) to calculate the 5-year projected breast cancer risk for each respondent.33, 34
Perceived Cancer Risk
The CCM included a single item which was phrased: “Would you say your risk of getting cancer in the future is low, medium, or high?” In our preliminary bivariate analysis, we found that there was no difference in reported mammography use between women with medium and high perceived cancer risk (P=.79), although reported mammography use in each of these groups was significantly greater than in women with low perceived cancer risk (68.5% [P=.0002] and 67.9% [P=.017] vs 62.6%, respectively). We therefore collapsed the medium-and high-risk responses into a single “medium/high risk” category.
Routine Mammography Use
The CCM included items asking whether women had ever received a mammogram, and those indicating “yes” were also asked how many they had received in the previous 6 years. For this analysis, we were most interested in whether women were undergoing regular mammography, as the benefits of mammography are generally achieved via routine testing.2, 35 Accordingly, we developed a “routine user” variable, defined as having ≥3 mammograms in the prior 6 years.
Other Characteristics
We categorized the women's smoking status into 4 groups based on their reported frequency of use: ≥1 pack per day (ppd),<1 ppd, former, or never. We also included other self-reported characteristics that have been found to be related to mammography use in prior studies, such as age, morbid obesity (body mass index >35), income (reported family income as a proportion of 1999 poverty thresholds), race, education, marital status, routine source of care for acute health problems, insurance status, health status (excellent/good/fair/poor), depressive symptoms family history of breast cancer, age at the first menstrual period, and history of abnormal mammogram or breast biopsy).36–40
Statistical Analysis
The Survey Data Analysis (SUDAAN) program was used for all analyses to account for the complex sampling design of the NHIS.41 Separate bivariate analyses were performed, comparing the predictor variables mentioned above for routine mammography use and perceived cancer risk using chi-square (χ2) and t-tests when appropriate. Candidate variables were selected based on clinical relevance as well as a review of the salient literature.36, 37, 38, 39, 40
For the main multivariable analysis, routine mammography use was the dependent variable and projected breast cancer risk (in quartiles) was the independent variable, with the covariates outlined above added into the model in a stepwise manner and retained if P<.05. As a sensitivity analysis, we repeated the bivariate and multivariate analysis of predictors of routine mammography use on the subgroup of women ages 50 to 75 years because of the controversy surrounding mammography use among women in their 40s. After confirming that perceived cancer risk was an independent predictor of routine mammography use, we then performed a secondary analysis to identify factors independently associated with medium/high perceived cancer risk. In this logistic regression, perceived cancer risk was the dependent variable (medium/high=1). We repeated this multivariable model in the subgroup of women who had a projected breast cancer risk >1.67%, as this was the cut point required for eligibility in the breast cancer prevention trial.42
RESULTS
Participant Demographics
The steps used to construct the study sample are outlined in Table 1. In the final study sample of 6,002 women, 4,237 (70.6%) were white, 849 (14.1%) black, and 766 (12.8%) Hispanic (Table 2). About 11.2% (671) of the respondents reported a family history of breast cancer. Overall, 63.1% of respondents reported routine mammography use.
Table 1.
Study Population
| Study Inclusion Criteria | Study Numbers |
|---|---|
| Initial cohort | 32,374 |
| Females | 18,388 (56.8%) |
| Age 45 to 75 | 7,354 (22.7%) |
| NO BRCA history | 7,107 (21.2%) |
| Total eligible subjects | 7,107 (21.2%) |
| Mammography information reported | 6,551 (92.2% of eligible) |
| Perceived Risk information reported | 6,161 (86.7% of eligible) |
| Total study sample | 6,002 (84.4% of eligible) |
Table 2.
Respondent Characteristics, Mammography Use, and Perceived Cancer Risk
| Demographic Characteristic | Total Respondents | Participants with … | |||
|---|---|---|---|---|---|
| Routine Mammography | Medium/High Perceived Cancer Risk | ||||
| % Total | P Value | % Total | P Value | ||
| Age | |||||
| 45 to 49 | 1,432 | 56.8 | <.0001 | 45.8 | <.0001 |
| 50 to 55 | 1,411 | 69.4 | 44.1 | ||
| 56 to 65 | 1,731 | 68.3 | 37.5 | ||
| 65+ | 1,428 | 64.6 | 36.5 | ||
| Ethnicity | |||||
| White | 4,237 | 67.7 | <.0001 | 43.6 | <.0001 |
| Hispanic | 766 | 50.5 | 26.6 | ||
| Black | 849 | 59.1 | 34.9 | ||
| All others | 150 | 48.3 | 28.1 | ||
| Ratio of family income to poverty (%) | |||||
| <125 | 870 | 47.8 | <.0001 | 42.4 | NS |
| 125 to 299 | 1,343 | 54.3 | 40.4 | ||
| ≥300 | 2,342 | 75.0 | 42.4 | ||
| NA | 1,447 | 62.7 | 39.4 | ||
| Marital status | |||||
| Married | 2,963 | 68.9 | <.0001 | 40.8 | NS |
| No married | 3,025 | 57.9 | 41.4 | ||
| Highest level of education | |||||
| <9th grade | 537 | 43.9 | <.0001 | 31.9 | .0008 |
| Between 9th and 12th grades | 711 | 50.5 | 41.0 | ||
| High school graduate | 3,520 | 66.1 | 42.5 | ||
| College or above | 1,198 | 76.1 | 39.6 | ||
| Routine source of care | |||||
| Yes | 5,584 | 67.3 | <.0001 | 41.0 | NS |
| No | 417 | 31.8 | 37.4 | ||
| Feeling sad (what percent of time): | |||||
| All to most of the time | 299 | 48.5 | 49.3 | ||
| Some to a little of the time | 1,448 | 61.3 | <.0001 | 49.0 | <.0001 |
| None of the time | 4,226 | 66.7 | 38.0 | ||
| Perceived cancer risk | |||||
| Medium/high | 2,415 | 68.3 | .0001 | ||
| Low | 3,587 | 62.6 | |||
| Five-year projected risk for breast cancer (Gail model) | |||||
| First quartile (<0.086%) | 1,494 | 50.7 | <.0001 | 39.4 | <.0001 |
| Second quartile (0.086% to 1.17%) | 1,476 | 63.6 | 38.7 | ||
| Third quartile (1.17% to 1.57%) | 1,506 | 67.7 | 35.0 | ||
| Fourth quartile (>1.57%) | 1,495 | 75.8 | 50.4 | ||
| Family history breast cancer | |||||
| Yes | 671 | 75.6 | <.0001 | 69.1 | <.0001 |
| No | 5,331 | 63.5 | 37.2 | ||
| Age at first menstrual period | |||||
| ≤10 | 435 | 63.1 | NS | 47.6 | .0416 |
| 11 to 12 | 2,085 | 67.1 | 41.8 | ||
| 13 to 18 | 3,158 | 65.1 | 40.3 | ||
| Age at first live birth | |||||
| ≤20 | 2,131 | 57.2 | 41.8 | NS | |
| 21 to 25 | 1,854 | 69.1 | <.0001 | 40.1 | |
| 26 to 45 | 1,052 | 71.3 | 39.9 | ||
| No children | 902 | 66.7 | 43.0 | ||
| Ever had abnormal mammogram | |||||
| No | 4,212 | 68.1 | <.0001 | 38.9 | <.0001 |
| Yes | 1,081 | 89.9 | 50.5 | ||
| Ever had biopsy | |||||
| No | 4,557 | 71.0 | <.0001 | 40.8 | <.0001 |
| Yes | 736 | 83.5 | 50.4 | ||
| Smoking | <.0001 | <.0001 | |||
| Never | 3,294 | 65.8 | 36.3 | ||
| Former | 1,532 | 72.6 | 41.8 | ||
| <1 ppd | 639 | 54.0 | 46.2 | ||
| ≥1 ppd | 521 | 49.2 | 62.0 | ||
| Obesity | |||||
| BMI≤35 | 5,107 | 65.7 | .0171 | 40.0 | .0029 |
| BMI>35 | 621 | 59.2 | 47.8 | ||
| NA | 274 | 61.6 | 45.6 | ||
| Health status | |||||
| “Good” to “excellent” | 4,873 | 66.4 | <.0001 | 39.8 | .0001 |
| “Fair” to “poor” | 1,126 | 57.4 | 46.8 | ||
Routine mammography use” is defined as ≥3 mammograms in prior 6 y.
NS, nonsignificant; NA, not available; ppd, pack per day; BMI, body mass index.
Predictors of Mammography Screening
Bivariate analysis indicated that increasing 5-year projected breast cancer risk was associated with a statistically significant increase in routine mammography use (Table 2). Approximately 50.7% of women in the lowest quartile of Gail risk reported routine mammography screening, with a corresponding increase in each subsequent quartile: 63.6%, 67.7%, and 75.8% in the second, third, and fourth quartiles, respectively (χ2 test for trend: P<.0001).
There was a significant relation between routine mammography use and race, with white women reporting a higher rate (67.7%) than Hispanic (50.5%) or Black (59.1%) women (P<.001). As expected, mammography use was significantly related to income, obesity, smoking status, education, and other factors pertaining to the individuals' health status and access to health care (Table 2). Women who had a history of abnormal mammograms, or who had ever had a breast biopsy, were significantly more likely to report routine mammogram use than women without these characteristics (P<.0001 for both comparisons).
In the multivariable model (Table 3), women 50 to 65 years of age were more likely to report mammogram use than women 45 to 49 years of age. There was no significant difference in reported mammogram use between women over 65 years of age and those 45 to 49 years of age, after accounting for breast cancer risk (odds ratio [OR]: 1.12; 0.89 to 1.42) Projected breast cancer risk was strongly correlated with routine mammography use (Table 3). With women in first (lowest) quartile of Gail risk held as the reference category, the odds of receiving regular mammograms for women in the second, third, and fourth quartiles were 1.38 (95% confidence interval [CI]: 1.12 to 1.69), 1.57 (1.24 to 1.99), and 2.23 (1.73 to 2.87), respectively. Women with greater education (OR: 2.06; 95% CI: 1.51 to 2.82 for women with at least a bachelors degree vs women with less than ninth grade education) and higher family incomes were also more likely to undergo regular mammograms. Having a regular source of care (OR: 2.92 vs those without; 95% CI: 2.21 to 3.86) and health insurance (OR: 2.21 vs those without; 95% CI: 1.73 to 2.84) were positive predictors of routine mammography use. Undergoing a previous breast biopsy also remained a positive predictor of mammography use in the multivariate. Although black women were less likely to report routine mammography (59.1%) than white women (67.7%) in bivariate analysis, after adjusting for access to care, socioeconomic status (SES), and other factors in the multivariable model, black women were slightly more likely to report routine mammography use (OR vs whites: 1.32; 95% CI: 1.06 to 1.65). Addressing research question 2 (the relation between perceived risk and mammography use), the multivariate model demonstrated that increased perceived cancer risk was significantly related to regular mammography use (OR: 1.30; 95% CI 1.13 to 1.49). Repeating the analysis using subgroup of women 50 to 75 years of age (excluding women in their forties for whom screening was controversial at the time) did not substantively change our findings.
Table 3.
Logistic Regression Model for Routine Mammography Use (N=5,921)
| Variables and Effects | OR | Lower 95% Limit OR | Upper 95% Limit OR |
|---|---|---|---|
| Age | |||
| 45 to 49 | 1.00 | ||
| 50 to 55 | 1.62 | 1.33 | 1.97 |
| 56 to 65 | 1.48 | 1.21 | 1.82 |
| 65+ | 1.12 | 0.89 | 1.42 |
| Projected breast cancer risk | |||
| First quartile (<0.086%) | 1.00 | ||
| Second quartile (0.086% to 1.17%) | 1.38 | 1.12 | 1.69 |
| Third quartile (1.17% to 1.57%) | 1.57 | 1.24 | 1.99 |
| Fourth quartile (>1.57%) | 2.23 | 1.73 | 2.87 |
| Smoking | |||
| Never | 1.00 | ||
| Former | 1.20 | ||
| <1 ppd | 0.70 | 0.56 | 0.87 |
| ≥1 ppd | 0.60 | 0.47 | 0.76 |
| Ratio of family income to poverty (%) | |||
| <125 | 1.00 | ||
| 125 to 299 | 0.99 | 0.78 | 1.25 |
| ≥300 | 1.74 | 1.36 | 2.22 |
| NA | 1.19 | 0.93 | 1.51 |
| Race | |||
| White | 1.00 | ||
| Hispanic | 1.16 | 0.91 | 1.48 |
| Black | 1.32 | 1.06 | 1.65 |
| Other | 0.61 | 0.39 | 0.94 |
| Education | |||
| <9 | 1.00 | ||
| 9 to 12 | 1.04 | 0.77 | 1.40 |
| Higher-secondary grade | 1.61 | 1.23 | 2.11 |
| Bachelors | 2.06 | 1.51 | 2.82 |
| Marital status | |||
| No married | 1.00 | ||
| Married | 1.34 | 1.17 | 1.55 |
| Routine source of care | |||
| No | 1.00 | ||
| Yes | 2.92 | 2.21 | 3.86 |
| Insurance status | |||
| No | 1.00 | ||
| Yes | 2.21 | 1.73 | 2.84 |
| Perceived cancer risk | |||
| Low risk | 1.00 | ||
| Med/high risk | 1.30 | 1.13 | 1.49 |
| Ever had biopsy | |||
| No | 1.00 | ||
| Yes | 5.69 | 3.19 | 10.15 |
OR, odds ratio; ppd, pack per day; NA, not available.
Given that perceived cancer risk was an independent predictor of routine mammography use, we explored factors associated with medium/high perceived overall cancer risk (research question 3; Table 2). In bivariate analysis, women in the highest quartile of projected breast cancer risk were significantly more likely to indicate that they perceived a medium/high risk of cancer during their lifetime (50.4%) than did women in the remaining 3 quartiles of projected risk (39.4%, 38.7%, and 35.0% for the first, second, and third quartiles, respectively; P<.0001). When we investigated individual breast cancer risk factors, family history of breast cancer (P<.0001), young age at first menstrual period (P<.042), history of an abnormal mammogram (P<.0001) past history of breast biopsy (P<.001), and morbid obesity (P=.0029) were each associated with increased perceived risk. Older age was associated with decreased perceived lifetime cancer risk (P<.001).
Perceived risk varied with race, with 43.6% of white women expressing increased perceived cancer risk, compared with 34.9% black women, 26.6% of Hispanic women, and 39.4% of other ethnicities (P<.0001). Approximately 62% of women who smoked >1 pack of cigarettes daily reported increased perceived risk (vs 36.3% of never-smokers reporting increased risk). Socioeconomic status, marital status, a routine source of care, age at first birth, and insurance status did not have a statistically significant effect on perceived cancer risk.
In the multivariable model, women with a family history of breast cancer were significantly more likely to have a medium/high perceived risk than those without a family history (OR: 3.64; 95%: 2.68 to 4.95; Table 4). Age was inversely related to perceived lifetime cancer risk. Women who had a history of an abnormal mammogram were significantly more likely to report medium/high perceived cancer risk than those without a prior abnormal mammogram (OR: 1.43 [1.03 to 1.98]).
Table 4.
Factors Associated with Medium/High Perceived Cancer Risk
| Variables | OR | Lower 95% Limit OR | Upper 95% Limit OR |
|---|---|---|---|
| Age | |||
| 45 to 49 | 1.00 | ||
| 50 to 55 | 0.83 | 0.40 | 1.72 |
| 56 to 65 | 0.65 | 0.36 | 1.17 |
| 65+ | 0.54 | 0.30 | 0.97 |
| Smoking | |||
| Never | 1.00 | ||
| Former | 1.12 | 0.83 | 1.51 |
| <1 ppd | 1.41 | 0.81 | 2.47 |
| ≥1 ppd | 1.76 | 0.97 | 3.19 |
| Obesity | |||
| BMI≤35 | 1.00 | ||
| BMI>35 | 1.09 | 0.66 | 1.78 |
| NA | 1.58 | 0.83 | 3.01 |
| Race | |||
| White | 1.00 | ||
| Hispanic | 0.49 | 0.23 | 1.07 |
| Black | 0.72 | 0.34 | 1.51 |
| Other | 0.44 | 0.16 | 1.26 |
| Health status (SF-1) | |||
| Good to excellent | 1.00 | ||
| Fair to poor | 1.30 | 0.88 | 1.91 |
| Feeling sad—what percent of time | |||
| None | 1.00 | ||
| Some/little | 1.46 | 1.02 | 2.08 |
| All/most | 1.55 | 0.62 | 3.91 |
| Family history breast cancer | |||
| No family history | 1.00 | ||
| Yes family history | 3.64 | 2.68 | 4.95 |
| Age at first menstrual period | |||
| ≤10 | 1.00 | ||
| 11 to 12 | 0.78 | 0.43 | 1.42 |
| 13 to 18 | 0.64 | 0.35 | 1.17 |
| Ever had abnormal mammogram | |||
| No | 1.00 | ||
| Yes | 1.43 | 1.03 | 1.98 |
| NA | 0.58 | 0.35 | 0.96 |
OR, odds ratio; ppd, pack per day; BMI, body mass index; NA, not available.
When we performed a subgroup analysis of women who were at increased breast cancer risk, defined by projected breast cancer risk >1.67%, we found that family breast cancer history was still the strongest predictor of perceived risk in this population (adjusted OR: 4.00; 95% CI: 3.22 to 4.96). Although race was unrelated to perceived cancer risk in the full study sample Table 4), in this high-risk subgroup black women (adjusted OR: 0.69; 95% CI: 0.57 to 0.85) and Hispanic women (adjusted OR: 0.57; 95% CI: 0.42 to 0.66) were less likely to report increased cancer risk perceptions than were white women.
DISCUSSION
In our analysis of a representative national sample, actual and perceived cancer risk were independent predictors of routine mammography use. Overall, the rate of routine mammography use (defined as ≥3 mammograms in the previous 6 years) reported by the study population was lower than expected—1 in 3 women did not have routine mammogram use. Even among the women with the highest projected breast cancer risk, approximately 25% did not report routine mammography use. The independent effects of projected and perceived risk on mammography use suggest that efforts to incorporate risk profiles into clinical decision making may need to involve more than just relaying information about projected risks to patients, but also to explore how risk perceptions can be affected by this information.
Our results are consistent with a recent analysis demonstrating insufficient use of routine mammography at the population level.43 We feel that our article adds a new level of insight. While we demonstrated a stepwise increase in routine mammography use with increasing quartile of projected cancer risk, we also found that even in the highest risk category, mammography use was suboptimal. Additionally, after demonstrating the perceived risk was an important predictor of mammography use, we also examined factors associated with increased perceived risk and found that family history was the only component of the Gail model that appeared to increase risk perceptions. This suggests that future campaigns to educate women about cancer risk should include information about other well-known cancer risks.
Unlike prior studies, we also explored risk perceptions among the subgroup of women with higher projected cancer risk. In this subgroup, we found that that white women were more likely than Black or Hispanic women to perceive themselves to be at increased cancer risk. This underscores the importance of examining the relation between race, risk communication, and mammography use, as these findings suggest that education about cancer risk could be a mechanism for reducing disparities in mammography use. Future studies should explore whether educating women about their own risk factors for breast cancer increases adherence to routine mammography.
Breast cancer risk assessment has evolved considerably in the past decade, and is no longer restricted to categorizing patients by family history. This is especially important because the majority of women who develop breast cancer do not have a family history of breast cancer.44 Using models such as those popularized by Gail et al. can help us to achieve a broader understanding of other risk factors in affected women, and potentially identify an even greater proportion of women likely to benefit from mammography. In our sample, family history was confirmed to be among the individual factors predicting routine mammography use, but several other factors did as well; women with superior health status, who did not smoke, and who were not morbidly obese were all more likely to report mammography use than were their counterparts.
There are several limitations to our study. The NHIS relies entirely upon participants' self-report, and the utilization or income data may not be accurate. Secondly, the NHIS CCM's item addressing perceived cancer risk inquired about the participant's perception of risk for “cancer,” not specifying breast cancer. Although this lack of specificity may have caused some variation, it seems unlikely that a systematic bias would result in either direction. We found that women with a family history of breast cancer were far more likely to report an increased perceived cancer risk (69%) than women without a family history (37%). This suggests that perceived breast cancer risk is strongly correlated with perceived overall cancer risk. Additionally, the number of biopsies (as a component of the Gail model), may effect not only individual projected risk estimates but also mammography utilization rates. That is, women who obtain routine mammograms are more likely to have abnormal findings and undergo biopsies than women who never have mammograms. This may lead to an overestimation of the impact of projected risk on mammography use. It is important to note that the mammograms received by women in our study included both diagnostic and screening mammography. Hence it is possible that routine mammography use for screening is overestimated in this sample. However, even despite this potential overestimate, overall mammography use was considerably lower than would be recommended.
It is unclear how much of the relationship found between projected breast cancer risk and routine mammography use is due to patient knowledge about risk factors for breast cancer or to other factors not measured such as receipt of a doctor's recommendation for mammography. Although perceived risk was independently associated with mammography use, physician counseling about cancer risk may have been accompanied by recommendations for mammography. In particular, the women age 45 to 49 years old in our sample may have been less likely to receive a physician's recommendation for mammography because of controversy about the role of mammography in this age group in the 1990s. Of note, when we repeated the analysis of factors associated with mammography use in a subgroup of women ages 50 to 75 years, there was no substantial change to the results.
In summary, we found that routine mammography is underused at the national level. Although it is reassuring that women with the highest breast cancer risk were more likely to report routine mammography use than low risk women, there is substantial room for improvement in this population as 1 in 4 women in the highest group did not report routine mammography use. We also found that perceived risk was independently associated with routine mammography use, and that among women with increased projected cancer risk, white women were more likely to report increased perceived risk than Black or Hispanic women. While family history of breast cancer was strongly associated with perceived cancer risk, other risk factors such as age at first menarche, obesity, or prior abnormal mammography were either weakly related or unrelated to perceived risk. Our results suggest that when clinicians approach patients to discuss the use of mammography, it is important not only to convey risk estimates to patients, but also to ascertain their understanding of their risk and how they incorporate their risk preceptions into their plan of care. Particularly as the concept of tailoring screening strategies based on individualized risk profiles gain momentum, our results underscore the importance of patient education and communication. Once women are motivated to return for regular mammography, informed of how mammography can help them, and are able to access the health care system, they will then be able to receive the benefit promised by evidence and guidelines.
Acknowledgments
Financial Support and Disclosure: Dr. Gross's efforts were supported by a Cancer Prevention, Control and Population Sciences Career Development Award (1K07CA-90402) and the Claude D. Pepper Older Americans Independence Center at Yale (P30AG21342). Dr. Farrell is supported by a Mentored Scientist Development Award in research ethics (7K01HL7253001).
REFERENCES
- 1.Ries L, Eisner M, Kosary C, National Cancer Institute et al. [December 1 2004]. SEER Cancer Statistics Review, 1975–2001.
- 2.U.S. Preventive Services Task Force. Screening for breast cancer: recommendations and rationale. Ann Intern Med. 2002;137:344–6. doi: 10.7326/0003-4819-137-5_part_1-200209030-00011. [DOI] [PubMed] [Google Scholar]
- 3.http://www.cancer.org/docroot/MED/content/MED_2_1x_American_Cancer_Society_Issues_Updated_Breast_Cancer_Screening_Guidelines.asp?sitearea=MED Vol. 2003; American Cancer Society
- 4.Olsen O, Gotzsche PC. Cochrane review on screening for breast cancer with mammography. Lancet. 2001;358:1340–2. doi: 10.1016/S0140-6736(01)06449-2. [DOI] [PubMed] [Google Scholar]
- 5.Gotzsche PC, Olsen O. Is screening for breast cancer with mammography justifiable? Lancet. 2000;355:129–34. doi: 10.1016/S0140-6736(99)06065-1. [DOI] [PubMed] [Google Scholar]
- 6.Ransohoff DF, Harris RP. Lessons from the mammography screening controversy: can we improve the debate? Ann Intern Med. 1997;127:1029–34. doi: 10.7326/0003-4819-127-11-199712010-00016. [DOI] [PubMed] [Google Scholar]
- 7.Yood M, McCarthy B, Lee N, Jacobsen G, Johnson C. Patterns and characteristics of repeat mammography among women 50 years and older. Cancer Epidemiol Biomarkers Prev. 1999;8:595–9. [PubMed] [Google Scholar]
- 8.Barr J, Franks A, Lee N, Herther P, Schachter M. Factors associated with continued participation in mammography screening. Prev Med. 2001;33:661–7. doi: 10.1006/pmed.2001.0942. [DOI] [PubMed] [Google Scholar]
- 9.Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom co-ordinating committee on cancer research. Eur J Cancer. 2002;38:1458–64. doi: 10.1016/s0959-8049(01)00397-5. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong K, Eisen A, Weber B. Assessing the risk of breast cancer. N Engl J Med. 2000;342:564–71. doi: 10.1056/NEJM200002243420807. [DOI] [PubMed] [Google Scholar]
- 11.Macmillan RD. Screening women with a family history of breast cancer—results from the British familial breast cancer group. Eur J Surg Oncol. 2000;26:149–52. doi: 10.1053/ejso.1999.0759. [DOI] [PubMed] [Google Scholar]
- 12.Moller P, Reis MM, Evans G, et al. Efficacy of early diagnosis and treatment in women with a family history of breast cancer. European familial breast cancer collaborative group. Dis Markers. 1999;15:179–86. doi: 10.1155/1999/805420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilanus-Linthorst MM, Bartels CC, Obdeijn AI, Oudkerk M. Earlier detection of breast cancer by surveillance of women at familial risk. Eur J Cancer. 2000;36:514–9. doi: 10.1016/s0959-8049(99)00337-8. [DOI] [PubMed] [Google Scholar]
- 14.Brekelmans CT, Seynaeve C, Bartels CC, et al. Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J Clin Oncol. 2001;19:924–30. doi: 10.1200/JCO.2001.19.4.924. [DOI] [PubMed] [Google Scholar]
- 15.Smith R, Saslow D, Sawyer K, Burke W, Costanza M, Evans W. American cancer society guidelines for breast cancer screening: update 2003. CA Cancer J Clin. 2003;54:141–69. doi: 10.3322/canjclin.53.3.141. [DOI] [PubMed] [Google Scholar]
- 16.Gail M, Rimer B. Risk-based recommendations for mammographic screening for women in their forties. J Clin Oncol. 1998;16:3105–14. doi: 10.1200/JCO.1998.16.9.3105. [DOI] [PubMed] [Google Scholar]
- 17.Gottsche P, Olsen O. Is screening for breast cancer with mammography justifiable? Lancet. 2000;355:129–34. doi: 10.1016/S0140-6736(99)06065-1. [DOI] [PubMed] [Google Scholar]
- 18.Humphrey L, Helfand H, Chan B, Woolf S. Breast cancer screening: a summary of the evidence for the U.S. preventive services task force. Ann Intern Med. 2002;137:347–60. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 19.Murabito J, Evans J, Larson M, Kreger B, Splansky G, Freund K. Family breast cancer risk and mammography. Am J Epidemiol. 2001;154:916–23. doi: 10.1093/aje/154.10.916. [DOI] [PubMed] [Google Scholar]
- 20.Lerman C, Kash K, Stefanek M. Younger women at increased risk for breast cancer: perceived risk, psychological well-being, and surveillance behavior. J Natl Cancer Inst Monogr. 1994;16:171–6. [PubMed] [Google Scholar]
- 21.Gail M, Brinton L, Byar D, Corle D, Green S, Schairer C. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 22.Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93:358–66. doi: 10.1093/jnci/93.5.358. [DOI] [PubMed] [Google Scholar]
- 23.Lerman C, Kash K, Stefanke M. Younger women at increased risk for breast cancer: perceived risk, psychological well-being, and surveillance behavior. J Natl Cancer Inst Monogr. 1994;16:171–6. [PubMed] [Google Scholar]
- 24.Evans DG, Blair V, Greenhalgh R, Hopwood P, Howell A. The impact of genetic counselling on risk perception in women with a family history of breast cancer. Br J Cancer. 1994;70:934–8. doi: 10.1038/bjc.1994.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans DG, Lalloo F. Risk assessment and management of high risk familial breast cancer. J Med Genet. 2002;39:865–71. doi: 10.1136/jmg.39.12.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diefenbach M, Miller S, Daly M. Specific worry about breast cancer predicts mammography use in women at risk for breast and ovarian cancer. Health Psychol. 1999;18:532–6. doi: 10.1037//0278-6133.18.5.532. [DOI] [PubMed] [Google Scholar]
- 27.Calvocoressi L, Kasl SV, Lee CH, Stolar M, Claus EB, Jones BA. A prospective study of perceived susceptibility to breast cancer and nonadherence to mammography screening guidelines in African American and White women ages 40 to 79 years. Cancer Epidemiol Biomarkers Prev. 2004;13:2096–105. [PubMed] [Google Scholar]
- 28.Vernon S. Risk perception and risk communication for cancer screening behaviors: a review. J Natl Cancer Inst. 1999;25:101–19. doi: 10.1093/oxfordjournals.jncimonographs.a024184. [DOI] [PubMed] [Google Scholar]
- 29.McCaul K, Branstetter A, Schroeder D, Glasgow R. What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health Psychol. 1996;15:423–9. doi: 10.1037//0278-6133.15.6.423. [DOI] [PubMed] [Google Scholar]
- 30.Sabatino SA, Burns RB, Davis RB, Phillips RS, Chen YH, McCarthy EP. Breast carcinoma screening and risk perception among women at increased risk for breast carcinoma: results from a national survey. Cancer. 2004;100:2338–46. doi: 10.1002/cncr.20274. [DOI] [PubMed] [Google Scholar]
- 31.Statistics NCfH (National Center for Health Statistics). National Health Interview Survey, 2000; 2001. Data file documentation. [Google Scholar]
- 32.Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526–32. doi: 10.1093/jnci/95.7.526. [DOI] [PubMed] [Google Scholar]
- 33.Constantino J, Gail M, Pee D, Anderson S, Redmond C, Benichou J. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91:1541–8. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 34.Anderson S, Ahnn S, Duff K. [August 14, 1992]. (NCI). NSABP Breast Cancer Prevention Trial risk assessment program, version 2. NSABP Biostatistical Center Technical Report.
- 35.Randolph W, Goodwin J, Mahnken J, Freeman J. Regular mammography use is associated with elimination of age-related disparities in size and stage of breast cancer at diagnosis. Ann Intern Med. 2002;137:783–90. doi: 10.7326/0003-4819-137-10-200211190-00006. [DOI] [PubMed] [Google Scholar]
- 36.Rakowski W, Clark MA, Ehrich B. Smoking and cancer screening for women ages 42–75: associations in the 1990–1994 national health interview surveys (6 Pt 1) Prev Med. 1999;29:487–95. doi: 10.1006/pmed.1999.0578. [DOI] [PubMed] [Google Scholar]
- 37.Burack RC, George J, Gurney JG. Mammography use among women as a function of age and patient involvement in decision-making. J Am Geriatr Soc. 2000;48:817–21. doi: 10.1111/j.1532-5415.2000.tb04759.x. [DOI] [PubMed] [Google Scholar]
- 38.Burack RC, Gurney JG, McDaniel AM. Health status and mammography use among older women. J Gen Intern Med. 1998;13:366–72. doi: 10.1046/j.1525-1497.1998.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernstein AB, Thompson GB, Harlan LC. Differences in rates of cancer screening by usual source of medical care. Data from the 1987 national health interview survey. Med Care. 1991;29:196–209. [PubMed] [Google Scholar]
- 40.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 national health interview survey. Cancer. 2003;97:1528–40. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 41.Shah B, Barnwell G, Bieler G. SUDAAN User's Manual, Release 8.0. Research Triangle Park: Research Triangle Park Institute; 2002. [Google Scholar]
- 42.Fisher B, Costantino J, Wickerham D, Redmond C, Kabannah M, Cronin W. Tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 43.Rakowski W, Breen N, Meissner H, et al. Prevalence and correlates of repeat mammography among women aged 55–79 in the year 2000 national health interview survey. Prev Med. 2004;39:1–10. doi: 10.1016/j.ypmed.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 44.Rockhill B, Weinberg CR, Newman B. Population attributable fraction estimation for established breast cancer risk factors: considering the issues of high prevalence and unmodifiability. Am J Epidemiol. 1998;147:826–33. doi: 10.1093/oxfordjournals.aje.a009535. [DOI] [PubMed] [Google Scholar]