Abstract
In vitro studies suggest that the Barren protein may function as an activator of DNA topoisomerase II and/or as a component of the Xenopus condensin complex. To better understand the role of Barren in vivo, we generated conditional alleles of the structural gene for Barren (BRN1) in Saccharomyces cerevisiae. We show that Barren is an essential protein required for chromosome condensation in vivo and that it is likely to function as an intrinsic component of the yeast condensation machinery. Consistent with this view, we show that Barren performs an essential function during a period of the cell cycle when chromosome condensation is established and maintained. In contrast, Barren does not serve as an essential activator of DNA topoisomerase II in vivo. Finally, brn1 mutants display additional phenotypes such as stretched chromosomes, aberrant anaphase spindles, and the accumulation of cells with >2C DNA content, suggesting that Barren function influences multiple aspects of chromosome transmission and dynamics.
INTRODUCTION
Chromosomes undergo dramatic programmed changes in higher-order structure as a prerequisite to their proper segregation in mitosis. Replicated sister chromatids must remain attached to one another until anaphase, undergo significant condensation, and become disentangled from one another before complete segregation to opposite poles. Although many activities are required to ensure that these processes occur correctly, the characterization of key players remains at an early stage. A number of specialized components have been identified, either biochemically or genetically, and subsequently shown to be conserved among the different experimental systems. One of the more interesting but perplexing is the Barren protein.
Barren was originally discovered as a Drosophila mutation that caused defects in the nuclear division of neuronal precursor cells (Bhat et al., 1996). Further examination of the cellular phenotype of barren mutants revealed a morphology similar to that exhibited by DNA topoisomerase II mutants of Saccharomyces cerevisiae (Holm et al., 1985). Dividing cells frequently contain chromosomes that appear unable to separate from one another during anaphase; instead, the anaphase chromosomes are frequently stretched across the two daughter cells, and cytokinesis initiates with the plasma membrane pinching in toward the chromatin bridge. Because this stretched DNA phenotype did not appear to result from global defects in chromosome condensation, a role for Barren in topoisomerase II function was inferred from the observation that Barren and topoisomerase II coimmunoprecipitate and interact in a two-hybrid assay. Indeed, purified Barren influences the activity of purified topoisomerase II in vitro, suggesting that Barren may regulate topoisomerase II activity in vivo (Bhat et al., 1996). Although this is an exciting possibility, the hypothesis has yet to be tested directly in vivo.
A different model for Barren function was proposed from work in the Xenopus system based on the copurification of Barren with 13S condensin. This five-component complex, in which all of the proteins are conserved from Xenopus to yeast, was initially isolated from mitotic extracts of Xenopus eggs and shown to promote the condensation of chromatin in vitro (Hirano and Mitchison, 1994; Hirano et al., 1997). Two of the condensin subunits, XCAP-C and XCAP-E, are members of the SMC (structural maintenance of chromosomes) family of proteins (for recent reviews, see Jessberger et al., 1998; Strunnikov, 1998; Yanagida, 1998; Hirano, 1999). In vivo, inactivation of the yeast SMC homologues of XCAP-C, Smc2p and cut3, leads to defects in mitotic chromosome condensation (Saka et al., 1994; Strunnikov et al., 1995a). Because Barren is a condensin subunit in the Xenopus system, it has been postulated to be required for chromosome condensation.
Assigning a function for Barren based on the Drosophila or Xenopus studies is complicated by several factors. Because it has not been possible in the Xenopus system to inactivate individual subunits, their specific contribution to this in vitro condensation activity has yet to be established. Although a role for the SMC subunits in this activity is inferred from the condensation defect of yeast smc mutants (Saka et al., 1994; Strunnikov et al., 1995a), the in vivo role of individual non-SMC subunits in chromosome condensation has not been addressed. Thus, it remains an open question whether the non-SMC subunits mediate condensin activity or are involved in auxiliary functions, such as linking condensation with DNA topoisomerase II activity. At first, this latter model might seem particularly appealing given both the in vitro interaction between Drosophila Barren and DNA topoisomerase II and the failure to observe a condensation defect in the Drosophila barren mutants. However, studies in other systems have not revealed interactions between the condensin components and DNA topoisomerase II (Hirano and Mitchison, 1994; Hirano et al., 1997; Strunnikov, personal communication). Given the apparent discrepancy between the Drosophila and Xenopus observations, it is critical to test the in vivo role of Barren in cell cycle–dependent chromosome metabolism, particularly in chromosome topology and condensation.
With the development of cytological tools in budding yeast and its extremely powerful genetics, Saccharomyces cerevisiae has become an excellent model system for studying the in vivo function of proteins, such as Barren, involved in cell cycle–dependent chromosome dynamics. The coupling of these methods with biochemical analysis of DNA metabolism and topology allows a uniquely broad spectrum of questions in mitosis to be addressed. Here we exploit these approaches to analyze the Barren homologue in budding yeast, addressing its in vivo function in eukaryotic chromosome metabolism.
MATERIALS AND METHODS
Strains and Media
All strains used in this study have an S288c genetic background and are listed in Table 1. Standard genetic techniques were used in the construction and growth of these strains (Sherman et al., 1986). Cells were grown in YEPD (rich medium) or SD (synthetic dextrose or selective) medium. YEPD is 1% yeast extract, 2% bactopeptone, 2% dextrose, with or without 2% bacto-agar; SD medium is 0.67% yeast nitrogen base, 2% bacto-agar, and 2% dextrose. For synthetic complete (SC) medium, 20 mg of uracil and tryptophan and 60 mg of leucine were added to 1 l of SD medium. 5-FOA plates were made as described previously (Ausubel et al., 1999).
Table 1.
S. cerevesiae strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| CH325 | MATa lys2-801 ura3-52 his4-539 top2-4 | Holm et al. (1985) |
| CH335 | MATa lys2-801 ura3-52 his4-539 TOP2 | Holm et al. (1985) |
| CH345 | MATα ade2 ura3-52 top2-4 | Holm et al. (1985) |
| CH1580 | MATa leu2 trp1 ura3-52 | Holm laboratory |
| CH1861 | MATa/α ura3-52/ura3-52 leu2/leu2 trp1-63/trp1-63 | Holm laboratory |
| CH2518 | MATa ura3-52 leu2 trp1-63 brn1∷TRP1 [pCH1719 (BRN1 URA3)]a | This study |
| CH2523 | MATa ura3-52 leu2 trp1-63 BRN1.TRP1b | This study |
| CH2528 | MATα ura3-52 leu2 trp1-63 BRN1.TRP1b | This study |
| CH2524 | MATa ura3-52 leu2 trp1-63 brn1-9.TRP1b | This study |
| CH2529 | MATα ura3-52 leu2 trp1-63 brn1-9.TRP1b | This study |
| CH2525 | MATa ura3-52 leu2 trp1-63 brn1-16.TRP1b | This study |
| CH2530 | MATα ura3-52 leu2 trp1-63 brn1-16.TRP1b | This study |
| CH2534 | MATa ura3-52 leu2 trp1-63 BRN1.TRP1 [rho0]b | This study |
| CH2535 | MATa ura3-52 leu2 trp1-63 brn1-9.TRP1 [rho0]b | This study |
| CH2536 | MATa ura3-52 leu2 trp1-63 brn1-16.TRP1 [rho0]b | This study |
| 4aAS247 | MATa trp1-63 ura3-52 leu2 smc1-2 | Strunnikov et al. (1993) |
| 2bAS283 | MATa ade2 his3 leu2 lys2 ura3-52 smc2-6 | Stunnikov et al. (1995a) |
| 1aAS330 | MATa smc2-8 ura3 leu2 lys2 his3 ade2 | Strunnikov et al. (1995a) |
| CH2540 | MATa trp1-63 ura3-52 leu2 smc1-2 [rho0] | This study |
| CH2539 | MATa ade2 his3 leu2 lys2 ura3-52 smc 2-6 [rho0] | This study |
| CH2587 | MATa lys2-801 ura3-52 his4-539 top2-4 [rho0] | This study |
This strain carries a disruption of the BRN1 gene with the TRP1 gene as a marker.
These strains have TRP1 adjacent to a described gene but do not disrupt the gene.
Strains lacking mitochondrial DNA ([rho0]) were used for flow cytometry and were created by growing [rho+] strains in 25 μg/ml ethidium bromide in YEPD to stationary phase. These cells were plated for single colonies on YEPD and tested for their ability to grow on YEP plates containing 2% glycerol (a nonfermentable carbon source). In addition, fluorescence microscopy of DAPI-stained cells confirmed the absence of mitochondrial DNA in the [rho0] strains.
Plasmids
To construct plasmids pCH11720 (LEU2 CEN/ARS BRN1) and pCH1719 (URA3 CEN/ARS BRN1), a 2.3-kilobase (kb) fragment (containing the entire BRN1 gene, 5′ and 3′ untranslated sequences, and flanking BamHI and XbaI sites) was amplified by PCR with the use of primers prCH1140 (TTATGGCAGCACTGCATAATTCTCTTACTGCGCGGATCCTATGTGATGTTGATCACG) and prCH1148 (TAGCTCTAGAGATTTCGTAAGCCCGAAGGC). This fragment was ligated into the BamHI–XbaI sites of both pCH1097 (LEU2 CEN/ARS) and pCH1099 (URA3 CEN/ARS), which are also known as pRS315 and pRS316 vectors, respectively (Sikorski and Hieter, 1989). Plasmids pCH1748 (brn1-9) and pCH1749 (brn1-16) are derived by random in vitro mutagenesis from plasmid pCH1720; they were isolated in the screen for heat-sensitive brn1 alleles. To make plasmid pCH1721 (BRN1.TRP1; TRP1 is inserted next to BRN1), PCR was used to fuse the TRP1 allele with a fragment from downstream of the BRN1 locus with flanking EagI and SacII sites with the use of primers prCH1195 (GAATCGGCCGAAAGCCTTCGGGCTTACGAA) and prCH1196 (TAGGCCGCGGTCTTGGTCGCACTAATCAAC). Next, this EagI–SacII fragment was subcloned into the same sites of plasmids pCH1720, pCH1748, and pCH1749 to create plasmids pCH1721, pCH1725, and pCH1726.
Creation of Heat-sensitive brn1 Mutants
We first showed that BRN1 is essential by disrupting one allele of BRN1 with TRP1 in diploid strain CH1861. Although the control cross exhibited excellent spore viability, 11 of 12 tetrads from the diploid with the BRN1 disruption exhibited 2:2 segregation for viability; all living spores were Trp−, and the inviable spores gave rise to two to eight cells. We next used the plasmid-shuffle technique (Boeke et al., 1984) to identify 16 heat-sensitive brn1 mutations. The BRN1 gene in plasmid pCH1720 was mutagenized by PCR amplification in 12 independent reactions; each of these reactions contained a 75% lower concentration of dATP than of the rest of the nucleotides. The entire coding region was mutagenized with the use of primers prCH1149 and prCH1150. The amplified DNA containing the mutagenized BRN1 gene and a 6.0-kb BamHI–XbaI fragment of plasmid pCH1720 lacking the BRN1 coding region were then cotransformed into strain CH2518 to allow recombination during transformation (Muhlrad et al., 1992). The transformants were replica plated onto 5-FOA–containing plates to select for the loss of plasmid pCH1719 (BRN1 URA3) and were simultaneously screened for phenotypes at 37 and 14°C. Among 10,000 transformants, ∼10% contained knockout mutations for brn1. Although we screened for cold sensitivity at 14°C, no cold-sensitive mutants were recovered. Sixteen heat-sensitive mutants were recovered from transformants derived from 10 of the mutagenized pools of DNA. We confirmed that the heat-sensitive mutations were brn1 mutations by isolating and purifying the mutant plasmids and then transforming them into a brn1::TRP1 strain carrying a BRN1 URA3 plasmid (strain CH2518) (Robzyk and Kassir, 1992). In each case, eviction of the BRN1 plasmid led to temperature sensitivity.
To replace the normal chromosomal BRN1 allele with mutant brn1 alleles, the TRP1 gene was inserted just downstream of each plasmid-borne brn1 mutation, the brn1 genes plus flanking DNA were cut out of each plasmid with the use of BamHI and SacII, and the linear DNA was used to transfer the BRN1 gene into strain CH1580 (trp1 BRN1). After selecting the transformants for TRP1, integration was confirmed by PCR. In each case, the strain carrying the transferred brn1 gene was heat sensitive, as expected. Strains bearing integrated brn1 alleles were used for all physiological experiments.
Drug Sensitivity and Synthetic Lethality
To assess the drug sensitivity of strains, serial dilutions were spotted onto YEPD plates containing 0.02 or 0.04 M hydroxyurea, 0.005 or 0.017% methyl methane sulfonate, and 7.5 or 15 μg/ml benomyl. Sensitivity to UV light was evaluated by placing spots of cells on YEPD plates and then exposing them to 254-nm UV radiation doses from 0 to 200 J/m2 with the use of a Stratalinker 1800 (Stratagene, La Jolla, CA). For each test, the plates were incubated at 25°C for 2 d. Strains CH2523 (BRN1), CH2524 (brn1-9), and CH2525 (brn1-16) showed no unusual sensitivity to the DNA-damaging agents hydroxyurea, methyl methane sulfonate, and UV light or to the microtubule-depolymerizing drug benomyl (our unpublished results). Because previous reports have suggested that Brn1p may interact functionally with DNA topoisomerase II or SMC proteins, we performed crosses to determine whether brn1 mutations would exhibit synthetic lethality with mutations affecting these other proteins. Synthetic lethality was tested by crossing strain CH2529 (brn1-9) with strain CH325 (top2-4), 4aAS247 (smc1-2), or 2bAS283 (smc2-6). Brn1 mutations are not synthetic lethal with top2-4 (10 of 10 tetrads were 4:0 for viability), smc2-6 (7 of 7 tetrads were 4:0 for viability), or smc1-2 (5 of 7 tetrads were 4:0 for viability). These negative results are provided for completeness, but they provide little information about the function of Brn1p.
First-Cycle Arrest Experiments
Cell cycle progression was monitored at the restrictive temperature in plate assays as described (Weinert and Hartwell, 1993). Strains were grown to early log phase at 25°C. The cells were sonicated, diluted, and plated onto prewarmed YEPD plates and incubated at 25 or 37°C. The number of cell divisions was monitored over time by scoring the number of cell bodies (1, 2–4, 5–8, 9–16, and >17) in 100 microcolonies for up to 16 h. When incubated at 25°C, 88–100% of the microcolonies from each tested strain contained >16 cells after 16 h.
Viability Experiments
We examined viability in cultures that were growing exponentially at 25°C. To examine the effects of temperature on asynchronously dividing cells, each culture was divided, one aliquot was incubated at 25°C and one at 37°C, and the samples were checked for viability at 3 and 6 h. To check for viability, the samples were sonicated, diluted, and plated on YEPD plates that were incubated at 25°C for 3 d. To examine the effects of temperature on cells arrested in G1 or S phase, the exponentially growing cultures were initially incubated for 3 h at 25°C in 5 μg/ml α-factor or 0.1 M hydroxyurea in YEPD (pH 4.0). Each culture was then divided, an aliquot was incubated at 25 or 37°C for an additional 3 h, and the cultures were checked for viability.
Execution Point Experiments
To determine the time at which Barren function is required in the cell cycle, cultures growing exponentially at 25°C in YEPD (pH 4.0) were treated for 3 h with 5 μg/ml α-factor to arrest them in G1. After the α-factor was washed away, the cells were resuspended in YEPD and divided into three aliquots. Nothing was added to the first aliquot, 0.1 M hydroxyurea was added to the second aliquot, and 10 μg/ml nocodazole was added to the third aliquot. Each aliquot was divided into two portions, one of which was incubated at 25°C and the other at 37°C for 3 h. Samples were then taken and checked for viability as described above.
Cell Morphology and Indirect Immunofluorescence
Yeast nuclear DNA was visualized by DAPI staining of formaldehyde-fixed cells. Asynchronous cultures (2.5 × 106 cells/ml) were grown in YEPD at 23°C, shifted to 37°C for 3 h, and fixed in 3.6% formaldehyde (60 min, room temperature). Indirect immunofluorescence was done essentially as described (Kilmartin et al., 1982) with the use of the YOL1/34 mAb against α-tubulin (Serotec, Indianapolis, IN) and goat anti-rat FITC (Sigma, St. Louis, MO).
Catenation of Plasmids
Strains containing a 14-kb circular minichromosome (pDK243, ARS1 CEN3 LEU2 [Koshland et al., 1985]) were pregrown in selective medium, diluted into YEPD, and then grown to early log phase (3–4 × 106 cells/ml) at the permissive temperature (approximately five divisions). Cells were synchronized in G1 by adding 3 μM final α-factor, incubating at 23°C for 2.5 h, and preshifting to 37°C for 30 min (typically, >85% of cells showed a schmoo morphology). The cells were then released into prewarmed YEPD with 0.1 mg/ml pronase E in the presence of 20 μg/ml nocodazole. To keep the BRN1 cells arrested at early M for 3 h at the nonpermissive temperature, an additional 10 μg/ml nocodazole was added 1 h after release. Plasmid DNA was isolated from 10 ml of the α-factor– or nocodazole-arrested culture, and half was separated on a 0.6% agarose gel in 1× Tris-acetate-EDTA, transferred to GeneScreen (Du Pont, Boston, MA), and probed with random-primer-labeled pBR322 by standard techniques.
Chromosome Breakage Assay
Cultures growing exponentially in YEPD at 25°C were divided, and half was incubated at 25°C and half at 37°C for 5 h. Chromosomal DNA was isolated with the use of the method described (Spell and Holm, 1994). Chromosomes were separated on a 1% agarose gel in 0.5× Tris-borate-EDTA in a CHEF-DR pulsed-field gel apparatus (Bio-Rad, Richmond, CA). Electrophoresis was performed at 4°C with a 60-s switch time for 16.2 h and then a 90-s switch time for 9 h. Hybridization was performed on the dried, rehydrated gel, as described (Spell and Holm, 1994), with the use of a URA3 probe that was prepared by random primer labeling.
FACS Analysis
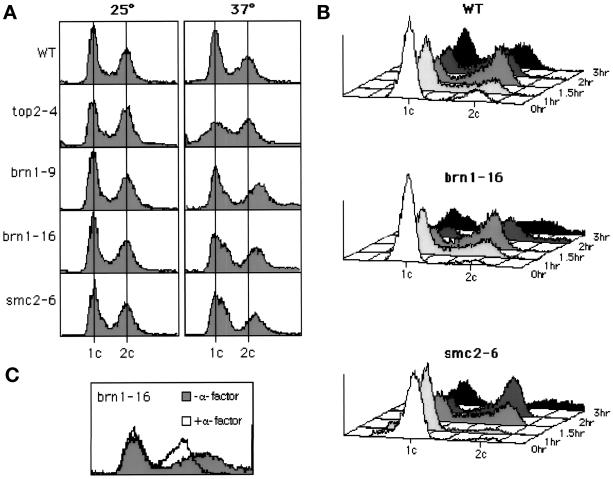
To prepare the cells for flow cytometry, samples were sonicated, diluted, and prepared for microfluorometric analysis by staining with propidium iodide, as described (Hutter and Eipel, 1979). A Becton-Dickinson (Franklin Lakes, NJ) FACS was used to determine the DNA content of the cells in each sample. A total of 10,000 cells of each type were analyzed for their DNA content. For cultures treated with drugs, the cells were grown in YEPD (pH 4.0) and treated with 5 μg/ml α-factor or 0.1 M hydroxyurea, as indicated in Figure 3.
Figure 3.
Mutant brn1 strains produce cells containing >2C DNA content after two generation times at the restrictive temperature. (A) Production of >2C cells in asynchronously dividing cultures. Strains CH2534 (BRN1), CH2587 (top2-4), CH2535 (brn1-9), CH2536 (brn1-16), and CH2539 (smc2-6) were grown exponentially at 25°C, the cultures were divided, and one aliquot was incubated at 25°C and one at 37°C for 3 h. At 37°C, strain CH2587 (top2-4) produces cells with both <1C and >2C DNA content. At 37°C, strains CH2535 (brn1-9), CH2536 (brn1-16), and CH2539 (smc2-6) exhibit a broadening of the 1C peak and a shift of the second peak to >2C DNA content. (B) Kinetics of production of >2C cells. Strains CH2534 (BRN1), CH2536 (brn1-16), and CH2539 (smc2-6) were grown exponentially at 25°C and then arrested in G1 with α-factor. The α-factor was removed and the synchronous cultures were allowed to divide at 37°C. Cells with >2C DNA content are produced in strains CH2536 (brn1-16) and CH2539 (smc2-6) in approximately the second generation time after initiation of the cell cycle. In a separate experiment, strain CH2535 (brn1-9) gave substantially similar results. (C) Preventing a second cell cycle prevents the production of cells with >2C DNA content. Strain CH2536 (brn1-16) was induced to traverse a cell cycle synchronously, as described for B. However, α-factor was added to one sample at 1.5 h after the initial release from α-factor, when most of the cells had completed DNA replication; a second sample was left untreated as a control. Samples were taken for FACS analysis 3 h after the cells were initially released from the synchronizing treatment with α-factor. Production of cells with >2C DNA content was prevented by preventing the cells from initiating a second division with the second α-factor treatment. Substantially similar results were obtained when treatment with hydroxyurea was substituted for the second treatment with α-factor.
Chromosome Condensation and Cohesion
BRN1, brn1, and smc2-8 cultures (2.5–3 × 106 cells/ml) were arrested in G1 (3, 3, and 1 μM final α-factor, respectively) for 2.5 h at 23°C, preshifted to 37°C, and released into nocodazole, as described above for the plasmid catenation assay. In all experiments, cells were fixed with 3.6% formaldehyde for 2 h and prepared for fluorescence in situ hybridization (FISH) analysis, as described (see Guacci et al. [1994] for methods and probe details). Digoxigenin (DIG)-labeled rDNA (chromosome XII) was used to visualize the condensation of repetitive DNA sequences. Condensation of unique sequences was monitored as described previously by measuring distances between DIG-CEN16 proximal and distal probes signals, which are separated by 255 kb (probes 1 and 3, respectively, from Guacci et al. [1994] and Strunnikov et al. [1995a]). Nuclei with a single signal (or more than two spots) were not included in our analysis because of the difficulty of assigning one spot to two overlapping signals or to hybridization of only one probe. It is noteworthy, however, that BRN1 cells contained roughly twice as many nuclei with a single spot than brn1-9 cells, suggesting that our calculated average distance for condensed DNA is somewhat overestimated, as is predicted from the distribution of the histogram (see Figure 4D). Sister chromatid cohesion was analyzed with the use of DIG-labeled CEN16 or CEN1 proximal probes, as described by Guacci et al. (1994). Mouse anti-DIG antibodies were from Sigma, and goat anti-mouse (FITC) and pig anti-goat (FITC) secondary antibodies were from Jackson Immunoresearch (West Grove, PA). Nuclei were counterstained with propidium iodide in antifade mounting medium (ONCOR; Intergen, New York, NY) and visualized with the use of a Zeiss (Thornwood, NY) epifluorescence microscope. Images were recorded digitally with the use of a Princeton (Trenton, NJ) charge-coupled device camera with Signal Analytics (Fairfax, VA) processing software, which allows image superimposition.
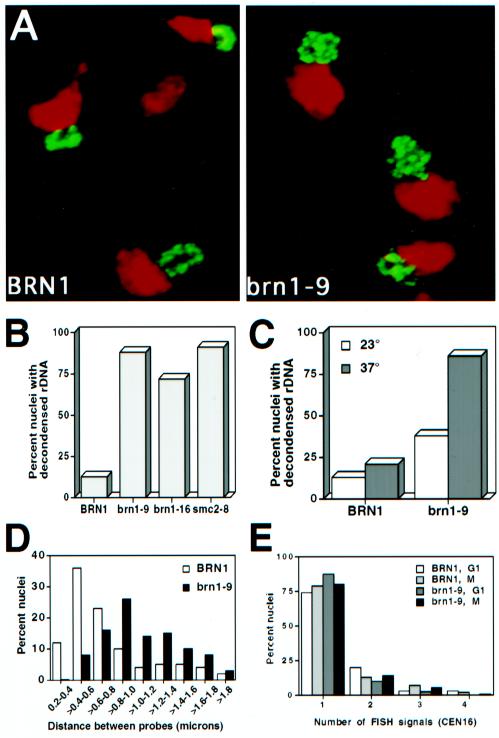
Figure 4.
Chromosome condensation and chromosome cohesion in brn1 mutants at the restrictive temperature. (A) FISH analysis of rDNA morphology in brn1 mutants. Exponentially growing strains CH2523 (BRN1) and CH2524 (brn1-9) were synchronized in G1, shifted to the nonpermissive temperature (37°C), and released into medium containing nocodazole (37°C) to rearrest the cells in mitosis. Cells were fixed and processed for FISH with the use of DIG-labeled rDNA as the probe with FITC-conjugated secondary antibodies (see MATERIALS AND METHODS). Micrographs show the rDNA signal in green and the bulk DNA (propidium iodide stain) in red (false colors). (B) Quantitation of rDNA morphology. Strains CH2523 (BRN1), CH2524 (brn1-9), CH2525 (brn1-16), and 1aAS330 (smc2-8) were treated as described for A. Amorphous rDNA signals were scored as decondensed, and clear loop or string-like structures were scored as condensed. In each sample, >100 nuclei were counted. (C) Maintenance of rDNA condensation. Strains CH2523 (BRN1) and CH2524 (brn1-9) were arrested in early mitosis in medium containing nocodazole for 2 h at the permissive temperature (23°C) to allow chromosome condensation to occur. The cultures were then shifted to the restrictive temperature (37°C) for 1 h. The state of rDNA condensation was monitored by FISH as described for A both before (23°C) and after the shift to restrictive temperature (37°C). Scoring of samples was as described for B. More than 100 nuclei were scored per sample. (D) Chromosome condensation of unique chromosome XVI sequences. Cells were prepared as described for A and probed by FISH with the use of a CEN16 proximal probe and a second chromosome XVI probe 250 kb away (see MATERIALS AND METHODS). Distances between FISH signals are shown as the percentage of total measurements in each category among nuclei exhibiting two signals. The average distance between signals is 0.75 ± 0.08 μm (n = 83) for BRN1 and 1.07 ± 0.11 μm (n = 101) for brn1-9. Nuclei with greater or fewer than two signals were not included in the analysis. (E) FISH analysis of chromosome cohesion. Nocodazole-arrested cells (37°C) prepared as described for A were analyzed with a CEN16 proximal probe, and the number of signals per nucleus was scored. At least 100 nuclei were counted per sample.
For the rDNA condensation maintenance experiment, cells were arrested in G1 with the use of α-factor (as described above) and then released into medium containing nocodazole for 2 h at the permissive temperature (23°C). An aliquot was taken to assess the initial state of rDNA condensation. The remainder of the culture was then shifted to 37°C for 1 h in the continued presence of nocodazole, after which the cells were harvested. Condensation was assessed with the use of DIG-labeled rDNA, as described above.
RESULTS
Heat-sensitive brn1 Mutations Were Produced in the Essential BRN1 Gene
To examine the in vivo role of Barren, we generated conditional alleles in the structural gene for the budding yeast homologue Brn1p (for details, see MATERIALS AND METHODS). We first showed that BRN1 is essential by disrupting one allele in a diploid cell; after sporulating the heterozygous diploid, all of the haploid progeny carrying the disrupted gene were inviable, giving rise to only two to eight progeny cells. We next used the plasmid-shuffle technique (Boeke et al., 1984) to isolate 16 heat-sensitive brn1 mutations. Stable heat-sensitive brn1 mutants were made by introducing 5 of the 16 brn1 mutations into chromosomes in place of the normal BRN1 gene. We chose two alleles (brn1-9 and brn1-16) for additional experimentation; these alleles caused no unusual drug sensitivity or synthetic lethality with top2, smc1, or smc2 mutations (see MATERIALS AND METHODS).
Brn1 Mutations Cause Arrest of Cell Division and Cell Cycle–dependent Loss of Viability
To examine the effects of the brn1 mutations on cell division, we investigated whether brn1 strains undergo an arrest of cell division in the first cycle after they are placed at the restrictive temperature. To allow comparison of this phenotype with other strains, we examined strains CH2523 (BRN1), CH2524 (brn1-9), CH2525 (brn1-16), CH325 (top2-4), 4aAS247 (smc1), and 2bAS283 (smc2) in the same experiments. Exponentially growing cultures at 25°C were sonicated and spread on prewarmed YEPD plates at 25 or 37°C, and the number of cells in each microcolony was counted over time. The top2 and smc1 strains exhibited a first-cycle arrest; none of the microcolonies contained more than 4 cell bodies even when incubated at 37°C for 9 h. The majority of brn1 and smc2 cells underwent arrest in the first division, but a small proportion of the cells exhibited a multicycle arrest; ∼25% of the microcolonies contained more than 4 cells after 7.5 h of incubation at 37°C. Even the cells in these microcolonies ultimately arrested, however, because none of the microcolonies from these strains contained more than 16 cells after an overnight incubation at 37°C. Thus, although a minority of brn1 cells continue to divide, the majority of the cells cease dividing in the first generation after they are shifted to the restrictive temperature.
brn1 mutants also exhibit a loss of viability when incubated at the restrictive temperature. When exponentially growing cultures are shifted to the restrictive temperature, ∼75% of the brn1 cells become inviable after 3 h (1.5 generation times) and ∼95% become inviable after 6 h (3 generation times) (Table 2). This result is quantitatively similar to results observed with smc1 and smc2 mutants, which maintain moderate viability after 1 generation time at the restrictive temperature (Strunnikov et al., 1993, 1995a). However, the result is noticeably different from what is observed with top2 mutants, which lose viability much more rapidly at the restrictive temperature (Holm et al., 1985).
Table 2.
Viability at the restrictive temperature of dividing cells and cells arrested in the cell cycle
| Strain | Asynchronousa
|
Drug treatedb
|
||
|---|---|---|---|---|
| 3 h | 6 h | +α-Factor | + Hydroxyurea | |
| CH2523 (BRN1+) | 140% | 220% | 94% | 114% |
| CH2524 (brn1-9) | 29% | 4% | 88% | 96% |
| CH2525 (brn1-16) | 27% | 8% | 92% | 97% |
| 4aAS247 (smc1-2) | 22% | 1% | 89% | 100% |
| 2bAS283 (smc2-6) | 23% | 10% | 95% | 94% |
| CH325 (top2-4) | 12% | 1% | 116% | 91% |
For each experiment, viability is expressed as a ratio of the number of colonies produced from the 37°C incubation to the number of colonies produced from the 25°C incubation. Values shown are averages of two experiments that produced similar results.
Cultures growing exponentially at 25°C were divided into two aliquots, which were incubated at 25 and 37°C for the indicated times and then plated for viability.
Cultures growing exponentially at 25°C were treated with α-factor or hydroxyurea for 3 h and then divided into two portions, which were incubated at 25 or 37°C for an additional 3 h and then plated for viability.
Loss of viability at the restrictive temperature could be caused by the requirement for Brn1p at a specific time during the cell cycle, or it could be caused by a more general requirement for Brn1p in cell metabolism. If the former is true, then brn1 mutants should remain viable at the restrictive temperature when they are prevented from traversing the cell cycle; both smc2 and top2 mutants exhibit this phenotype. Exponentially growing brn1, smc1, smc2, and top2 cultures were arrested in G1 phase of the cell cycle with α-factor or in S phase with hydroxyurea. The cultures were divided into two aliquots, and the cells were incubated at either the restrictive or the permissive temperature for an additional 3 h. All cultures maintained good viability (at least 84%) when cell cycle arrest was maintained (Table 2). This result demonstrates that brn1 mutants must pass through the cell cycle to incur irreversible damage. It also demonstrates that arrested G1- and S-phase cells do not require ongoing Brn1p activity.
Brn1p Is Required between Early S Phase and Early M Phase
To determine the time in the cell cycle during which Brn1p is required, we examined the viability of synchronously dividing populations of cells. Because brn1 mutants show only a moderate loss of viability in the first generation time at the restrictive temperature, we examined cell viability in two “windows” of the cycle. In each case, exponentially growing cells were treated with α-factor to arrest the cells in G1; the α-factor was then removed, and the cells were shifted to the restrictive temperature and allowed to begin cycling. In one sample, the cultures were treated with hydroxyurea to stop the cell cycle at early S phase; in a second sample, the cells were instead treated with nocodazole to allow them to pass through S phase and arrest at G2/M; a third sample was not treated with drugs and served as a control. After 3 h at the restrictive temperature, an aliquot of each sample was spread on rich medium at the permissive temperature to evaluate the viability of the cells. Because viability was assessed at the permissive temperature, this regimen reveals when the irreversible lethal event occurs during the cell cycle, regardless of when the actual death of the cell occurs.
Our results suggest that Barren is required between the beginning of S phase and the beginning of mitosis. When a synchronously dividing brn1 strain is prevented from entering S phase with hydroxyurea, the viability of the culture remains high, even though it is incubated at the restrictive temperature for 3 h (Table 3). If the strain is instead allowed to pass though S and G2 phases, the viability of the culture decreases to the same extent whether it is treated with nocodazole or not. (The efficacy of the nocodazole treatment was confirmed by observations of cell morphology [our unpublished results].) This result is in sharp contrast with the result observed with a top2 strain, which maintains good viability as long as it is prevented from passing through mitosis by treatment with either hydroxyurea or nocodazole. These results suggest that the activity or assembly of Brn1p is required between early S phase and early M phase.
Table 3.
Viability at the restrictive temperature of cells traversing specific landmarks in the cell cycle
| Strain | Drug added at initiation of synchronous cell cycle
|
||
|---|---|---|---|
| None | Hydroxyurea | Nocodazole | |
| CH2523 (BRN1+) | 169% | 95% | 92% |
| CH2524 (brn1-9) | 34% | 98% | 37% |
| CH2525 (brn1-16) | 48% | 98% | 72% |
| CH325 (top2-4) | 8% | 96% | 93% |
Cultures synchronized in G1 with α-factor were divided into aliquots, half of which were incubated at 25°C and half at 37°C. At each temperature, one aliquot was immediately treated with hydroxyurea, one was immediately treated with nocodazole, and one was left untreated. After 3 h, the cultures were plated at 25°C for viability, which is expressed as a ratio of the number of colonies produced from the 37°C culture to the number produced by the 25°C culture of each type.
Brn1 Mutants Arrest Heterogeneously and Exhibit Aberrant Mitotic Spindles
Although it appears that Brn1p is required to allow cells to traverse the cell cycle successfully, brn1 mutants, like top2 and smc2 mutants, do not show a classic cell-division-cycle phenotype at the restrictive temperature. BRN1 and brn1 cultures were grown exponentially at 25°C and then shifted to 37°C for 3 h. As expected, the BRN1 strain showed a distribution of cell morphologies, representing cells at all normal stages of the cell cycle (Figure 1A). This distribution was also observed with control BRN1 and brn1 cultures that were retained at 25°C (our unpublished results). In comparison, the brn1 strain at 37°C was somewhat depleted of cells with small buds. In their place was an increased number of large-budded cells, almost half of which appeared to be in early anaphase.
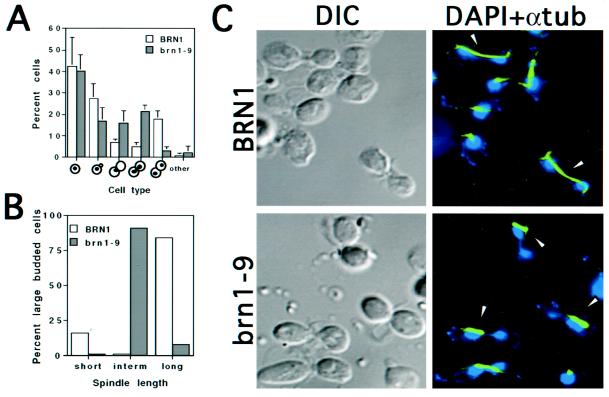
Figure 1.
Phenotypic characteristics of brn1 mutants at the restrictive temperature. (A) Distribution of cell types. Asynchronous cultures of strains CH2523 (BRN1) and CH2524 (brn1-9) were shifted to 37°C for 3 h, fixed, and stained with DAPI to visualize nuclei. Cells were scored according to bud morphology and position/distribution of nuclear DNA, as indicated. (B) Spindle length in large-budded BRN1 and brn1-9 cells. Large-budded cells from strains grown as described for A were examined for spindle length. Short spindles refers to the 1- to 2-μm length typical of a metaphase cell, intermediate spindles are 4–5 μm in length, and long spindles range from 7 to 15 μm, typical of anaphase/telophase cells. At least 100 large-budded cells were scored per sample. (C) Nuclear and spindle morphology. Cells were grown as described for A, fixed, prepared for indirect immunofluorescence of tubulin (see MATERIALS AND METHODS), and stained with DAPI to visualize DNA. Micrographs were false colored in blue (DNA) and green (anti-tubulin). Arrowheads denote extended spindles in dividing BRN1 (wild-type) cells in contrast to the thick, intermediate spindles found in the large-budded brn1-9 cells. Note that in the mutant, the spindle is often associated with only one side of the bilobed DNA mass and/or grossly mispositioned (see DISCUSSION). In addition, the bilobed nuclei present in BRN1 cells are morphologically distinct from those in the mutant; in general, the BRN1 cells show very little chromosomal material joining two equal DNA masses, suggesting the rapid progression of anaphase to telophase in BRN1 cells. In the brn1 mutant, however, anaphase appears to initiate normally but proceeds with difficulty, yielding cells with two connected chromatin masses. In these early to mid anaphase cells, >40% of bilobed nuclear masses showed uneven DAPI staining. DIC, differential interference contrast microscopy.
To characterize the morphology of the large-budded cells more thoroughly, cells from similarly grown cultures were fixed, prepared for indirect immunofluorescence of tubulin, and treated with DAPI to stain DNA. More than half of the large-budded BRN1 cells contained two well-separated nuclei (anaphase and telophase cells) with nuclear microtubules extending along the entire DNA mass (Figure 1, B and C). In contrast, roughly half of the large-budded brn1 cells possessed a dumbbell-shaped DNA mass that extended through the bud neck and was frequently distributed asymmetrically. The nuclear spindles in these cells were intensely stained, only partially extended (4–5 μm), and predominantly localized at or near the bud neck, although typically failing to traverse it. That the cells with stretched nuclei initiate a true anaphase was confirmed by a separate experiment in which hemagglutinin-tagged Pds1p, an inhibitor of the metaphase-to-anaphase transition, was observed to be depleted (our unpublished results). Surprisingly, 62% of brn1 cells with bilobed nuclei displayed aberrant spindle localization, with tubulin staining localized to only one of the two DNA masses. Together, these data suggest that brn1 cells undergo an aberrant anaphase.
Brn1 Mutants Do Not Accumulate Catenated Plasmids or Broken Chromosomes
Although brn1 mutants appear phenotypically distinct from top2 mutants both in the efficiency and morphology of their arrest and in the loss of viability at the restrictive temperature, the apparent interaction of Barren and DNA topoisomerase II in Drosophila prompted us to test brn1 mutants for two phenotypes that are defining characteristics of top2 mutants, catenation of circular plasmids and the production of broken chromosomes.
Because DNA topoisomerase II is the only cellular enzyme capable of disentangling catenated circles of double-stranded DNA, circular plasmids remain catenated after replication when top2 strains are incubated at the restrictive temperature (Koshland et al., 1985). If Barren is required for DNA topoisomerase II activity, as has been suggested in Drosophila, then brn1 mutants might exhibit a similar accumulation of catenated plasmids when they are incubated at the restrictive temperature. Wild-type and mutant strains containing a 14-kb circular minichromosome were synchronized in G1 in medium containing α-factor, then released into nocodazole-containing medium at the nonpermissive temperature. The topological state of purified minichromosomes was then analyzed by agarose gel electrophoresis (Figure 2A). As expected, higher-molecular-weight catenated circles accumulate in nocodazole-treated top2 cells. In contrast, the brn1 mutants display no change in plasmid topology at the restrictive temperature, indicating that brn1 mutants effectively resolve the catenanes produced during replication, even at the restrictive temperature.
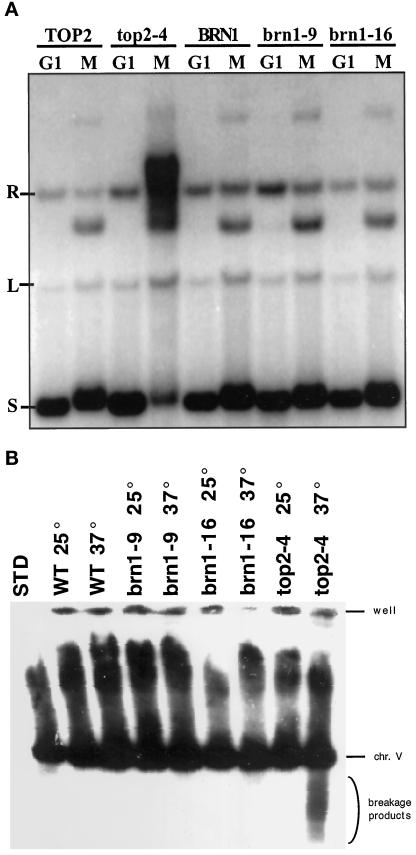
Figure 2.
Neither catenated plasmids nor broken chromosomes are apparent in brn1 strains at the restrictive temperature. (A) Plasmid catenation in top2 and brn1 mutants. Strains CH2523 (BRN1), CH2524 (brn1-9), CH2525 (brn1-16), CH335 (TOP2), and CH325 (top2-4), each containing a 14-kb CEN3 ARS1 plasmid (pDK243), were synchronized in G1 with α-factor, shifted to the restrictive temperature, and then released into prewarmed medium containing nocodazole (37°C) to rearrest cells in mitosis (M). Isolated plasmid DNA was separated by agarose gel electrophoresis and visualized by Southern blotting with the use of a pBR322-based probe. S, L, and R denote the supercoiled, linear, and relaxed forms, respectively. Catenated circular minichromosomes run as a high-molecular-weight smear of topoisomers (see top2-4, lane M). The unlabeled M-specific band between L and R has been ascribed to two closed circular intertwined monomers (C forms), as described by Koshland and Hartwell (1987). (B) Assay for broken chromosomes in brn1 and top2 mutants. Strains CH2523 (BRN1 TOP2), CH2524 (brn1-9),CH2525 (brn1-16), and CH325 (top2-4) were grown exponentially at 25°C, the cultures were divided, and half of each culture was incubated at 25°C and half at 37°C for 5 h. Chromosomes were separated on a CHEF gel, and chromosome V was visualized by hybridization with a URA3 probe. The smear of hybridization that lies between the well and the chromosome V band is not reproducible. Characteristic fragments of chromosome V appear only in strain CH325 (top2-4) at 37°C. WT, wild type. STD, chromosomal molecular weight standard.
When full-length chromosomes are segregated in the absence of DNA topoisomerase II activity, approximately one-third of chromosomes larger than 300 kb suffer a distinctive pattern of breakage (Spell and Holm, 1994). If Barren is required for DNA topoisomerase II activity, then brn1 mutants might be expected to exhibit the same kind of chromosome breakage. To test this hypothesis, BRN1, brn1-9, brn1-16, and top2-4 strains were grown exponentially at 25°C, and the cultures were divided and incubated for an additional 5 h at either 25 or 37°C. Although a clear smear of broken chromosomes was visible in the lane with DNA from the top2 culture grown at 37°C, no such smear was visible in the lanes containing DNA from brn1 strains (Figure 2B). Thus, it appears unlikely that Barren is required to activate DNA topoisomerase II in vivo.
Brn1 Mutants Accumulate an Unusual Population of Cells with an Apparent >2C DNA Content at the Restrictive Temperature
To examine the state of the DNA in brn1 mutants at the restrictive temperature, we used FACS analysis of cells lacking mitochondrial DNA. Exponentially growing cultures at 25°C were divided and placed at 25 or 37°C for various periods. Surprisingly, there is a broadening and flattening of the G2 peak in brn1 cells incubated at the restrictive temperature, and many cells appear to have a DNA content >2C (Figure 3A). This phenomenon is not observed in the BRN1 control strain, and it is not observed in a variety of temperature-sensitive yeast strains incubated at the restrictive temperature. For example, both late-nuclear-division mutants cdc5 and cdc15 and many DNA-replication mutants (e.g., cdc44/rfc1 and pol30) undergo cell cycle arrest with a uniform 2C DNA content (Howell et al., 1994; Amin and Holm, 1996; Oh and Holm, unpublished data). Notably, however, a suggestion of the same unusual phenomenon occurs when an smc2 strain is incubated at the restrictive temperature; this phenotype becomes more pronounced after prolonged incubation times (our unpublished results; see also Figure 3B). This FACS profile differs, however, from that produced by top2 mutant cells, which suffer chromosome breakage and nondisjunction, thereby producing cells with both <1C and >2C DNA contents (Figure 3A) (Holm et al., 1989). Thus, it appears that in both brn1 and smc2 mutants incubated at the restrictive temperature, a significant fraction of cells display an increase in DNA content.
To examine the timing of this phenomenon, we arrested exponentially growing cells in G1 with α-factor, released them to the restrictive temperature, and then examined them by FACS at various times. For the brn1-16 strain, DNA replication was apparent 1 h after release from α-factor, which is similar to what is observed with BRN1 cells (Figure 3B). The initial appearance of >2C cells occurred 2 h after release, and the population of >2C cells was much more apparent 3 h after release from α-factor (Figure 3B). This cell type appears to be produced during the second cell cycle after release from α-factor, because its production was entirely prevented by treating the culture with either α-factor or hydroxyurea 1.5 h after release from the initial α-factor treatment (Figure 3C; our unpublished results). A similar phenomenon was seen with smc2 mutants, except that progress through the cell cycle was somewhat slower with this strain. Thus, it appears that brn1 and smc2 cells begin to exhibit this unusual staining in the second cell cycle after a shift to the restrictive temperature. One possibility is that this staining pattern reflects aberrant excessive DNA replication (see DISCUSSION).
Brn1 Mutants Are Defective in Chromosome Condensation
The phenotypic similarities between brn1 and smc2 mutants suggest that, like smc2 mutants, brn1 mutants may have defects in chromosome condensation. This predicted phenotype of brn1 mutants is also suggested from the copurification of Xenopus Barren with the 13S condensin complex (Hirano et al., 1997). To test this hypothesis, exponentially growing brn1 and BRN1 cultures were arrested in G1 with α-factor, shifted to the restrictive temperature, and then released into nocodazole to allow the chromosomes to attain maximal condensation at the restrictive temperature. Under this regimen, Brn1p should be inactivated during both the establishment and the maintenance of condensation. Chromosome condensation was monitored with the use of FISH with an rDNA probe (Guacci et al., 1994), revealing dramatic differences among the strains. Chromosome XII in BRN1 strains condensed as expected to a discrete linear array, but brn1 mutants revealed a disorganized rDNA array (Figure 4, A and B). Similarly, an smc2 mutant showed a clear defect in rDNA condensation when subjected to this regimen (Figure 4B) (Strunnikov, personal communication). Thus, Barren, like Smc2p, is involved in mitotic chromosome condensation.
To determine whether Barren function is limited to an early stage in chromosome condensation (i.e., as for an assembly factor) or whether ongoing Barren activity is required to maintain mitotic chromosome structure, we inactivated Barren after chromosome condensation had occurred. BRN1 and brn1 cultures were arrested in early mitosis with the use of nocodazole at the permissive temperature to allow rDNA condensation. The cultures were then shifted to the restrictive temperature to inactivate Barren specifically in mitosis, and the state of the rDNA was monitored by FISH with the use of an rDNA probe. As expected, both BRN1 and brn1 chromosomes were condensed at the permissive temperature. In contrast, although the BRN1 chromosomes remained condensed at 37°C, rDNA condensation was disrupted in the brn1-9 strain (Figure 4C). These data indicate that ongoing Barren activity is required for the maintenance of chromosome condensation during mitosis.
To address whether the condensation defects in brn1 mutants are limited to repetitive DNA, we examined chromosome condensation in unique sequences on chromosome XVI. Exponentially growing brn1 and BRN1 cultures were arrested with α-factor, shifted to the restrictive temperature, and then released into nocodazole to block the cells in mitosis. Chromosome condensation was examined by using FISH and measuring the distance between two sequences that map 255 kb apart on chromosome XVI (Figure 4D). In BRN1 nuclei, the average distance between CEN16 proximal and distal probes was 0.75 ± 0.08 μm (experiment 1; Figure 4D) and 0.77 ± 0.09 μm (experiment 2; our unpublished results), which are similar to values reported previously for condensed DNA (Strunnikov et al., 1995a). In contrast, in brn1-9 nuclei, the average distance between probes was 1.07 ± 0.11 μm (experiment 1) and 0.97 ± 0.11 μm (experiment 2); these values are very similar to what is seen in uncondensed chromosomes in G1 cells (1.0 ± 0.01 to 1.1 ± 0.1 μm; Strunnikov et al., 1995a). Thus, little or no chromosome condensation occurs on unique DNA sequences in the absence of functional Barren protein.
Because a number of mutations that affect chromosome cohesion also cause alterations in chromosome condensation (Guacci et al., 1997; Strunnikov, personal communication), we tested brn1 mutants arrested in mitosis for precocious separation of sister chromatids in the absence of microtubules. Although chromosome condensation was impaired under these conditions, sister chromatid cohesion appeared normal when analyzed with a CEN16 probe (Figure 4E) and a CEN1 probe (our unpublished results). Thus, an underlying defect in cohesion is unlikely to be the source of the observed condensation defects. Instead, brn1 cells are specifically impaired in their ability to establish and/or maintain chromosome condensation at the restrictive temperature. Together, these results strongly suggest that Barren plays an essential role in chromosome condensation in yeast.
DISCUSSION
To begin to unravel the biological function of Barren in eukaryotic cells, we used genetic, cytological, and molecular biological methods to initiate studies of the Barren homologue in budding yeast. The Barren product in budding yeast is essential for viability and cell division. The analysis of heat-sensitive brn1 mutants revealed that cells compromised in Barren function exhibit partially elongated chromosome masses similar to the anaphase defect observed for barren mutants in Drosophila, which suggests a conserved function for Barren in chromosome transmission between yeast and flies. This chromosome phenotype is also very similar to the phenotype of smc2 mutants of budding yeast, cut3 and cut14 mutants of Schizosaccharomyces pombe, and mix1 mutants of Caenorhabditis elegans (Saka et al., 1994; Strunnikov et al., 1995a; Lieb et al., 1998). All of these genes encode homologues of components of Xenopus condensin, a complex essential for chromosome condensation in vitro. Furthermore, we directly show that brn1 mutants are defective in chromosome condensation in vivo, as has been demonstrated for smc2, cut3, and cut14. Consistent with this observation, Barren performs an essential function between early S phase and early M phase, the period of the cell cycle during which condensation is established and maintained. Together, our results reveal that the Barren product of budding yeast is critical for mitotic chromosome segregation and condensation.
The demonstration that yeast Barren functions in vivo in chromosome condensation both corroborates and extends the previous analysis of the role of Barren. In Xenopus, Barren copurifies with the five-subunit condensin complex, and as such it was inferred to play an important role in the condensation process (Hirano et al., 1997). Because the contribution of individual condensin subunits to the in vitro condensation reaction was not addressed, however, the physiological significance of Barren's association with condensin was unclear. That yeast Barren is essential for viability and is required for chromosome condensation in vivo suggests that it is an integral component of condensin function. Although we do not know the molecular function of Barren in the condensin complex, our genetic results provide two important constraints. Because loss of Barren function yields uncondensed chromosomes, Barren is likely to be critical to the core function of condensin rather than to serve as a minor auxiliary factor. Second, the absence of chromosome condensation when Barren activity is compromised implies that the loss of Barren function inactivates the cell's condensation machinery, i.e., Barren is not a negative regulatory subunit of condensin. Recent work with the Xenopus condensin complex has shown that, in vitro, the condensin complex wraps DNA in a right-handed manner (Kimura and Hirano, 1997; Kimura et al., 1999). That this reaction requires phosphorylated Barren and/or XCAP-D2 and/or XCAP-G suggests that some or all of these proteins play a role in the activation of condensin (Kimura et al., 1998). Consistent with this suggestion, anti-Eg7 (XCAP-D2) antibodies were used to demonstrate a role for this subunit in the in vitro condensation assay (Cubizolles et al., 1998). Additional experiments will be needed to assess whether Barren functions as a regulatory switch or as a direct mediator of altering chromosome topology.
In vivo, ongoing Barren activity is required to maintain mitotic chromosome structure. The most simple interpretation of this result is that Barren plays a structural role in higher-order chromosome organization. Alternatively, the maintenance of chromosome condensation could be a highly dynamic process involving a constant reestablishment of the condensed state. Regardless of the mechanism, it is clear that inactivation of Brn1p in early M causes an untimely loss of chromosome condensation, probably through inactivation of the cellular condensation machinery. Because chromosome condensation is a reversible process that is initiated in early M and reversed in late M/early G1, the regulation of condensin activity through Brn1p could provide an effective mechanism for both condensing and decondensing DNA.
An intriguing question is whether both repetitive and unique DNA condense through similar mechanisms. Although previous work with smc2 mutant cells failed to show a defect in rDNA condensation (Strunnikov et al., 1995a), this was most likely due to a less stringent inactivation regimen (see Figure 4B). We now show that both Smc2p and Brn1p are required for the condensation of repetitive as well as unique DNA sequences. These data are consistent with a model whereby chromosome compaction of both repetitive and unique DNA sequences occurs through a common molecular machinery and thus through a conserved mechanism. Although defects in rDNA condensation are more dramatic by FISH than those seen with unique DNA probes, this difference may reflect the reiterative nature of the rDNA array and/or a change in the relative distribution of condensin proteins. Indeed, indirect immunofluorescence of Smc4p and Ycd2p, yeast homologues of two additional condensin subunits, suggests that they are enriched on the rDNA (Strunnikov, personal communication; Zheng and Koshland, unpublished results).
It is noteworthy that condensation of both unique and repetitive DNA is perturbed in the first mitosis after Brn1p inactivation. At this time, topological links between sisters are resolved, as judged by the absence of catenated circular minichromosomes and chromosome V breaks. Thus, condensation does not appear to be obligatory in driving out DNA tangles, as has been proposed previously (Wood and Earnshaw, 1990). Rather, anaphase itself may be sufficient to promote DNA untangling by topoisomerase II (Holm et al., 1985; Holm, 1994). However, a modest delay in anaphase is detectable in brn1 mutants at the restrictive temperature (our unpublished results), suggesting that the kinetics of chromosome untangling may be facilitated by condensation, and this effect may be particularly important for the larger chromosomes of higher eukaryotes.
Although a condensation defect is the earliest observable phenotype of brn1 mutants, there is a later appearance of additional phenotypes, including an anaphase failure that is cytologically similar to DNA topoisomerase II mutants. Although earlier in vitro analysis of Drosophila Barren suggested that it might serve as an activator of topoisomerase II function, our results suggest that yeast Barren is probably not an essential activator of topoisomerase II in vivo. Because topoisomerase II is the only enzyme known to disentangle catenated circular plasmids, a failure by the mutant Barren protein to activate topoisomerase II should have led to the accumulation of catenanes. In brn1 mutants at the restrictive temperature, however, catenanes are efficiently resolved and chromosomes are segregated without breakage. Nonetheless, it remains possible that Barren could serve to regulate topoisomerase II activity in a more subtle manner. Barren may serve as an inhibitor of topoisomerase II activity in vivo, as was observed in vitro when Barren was present at high concentrations. Cytological evidence suggests that Barren and topoisomerase II reside in close proximity in condensed and condensing chromosomes (Bhat et al., 1996). Perhaps when associated with chromosomes they are members of a large multiprotein complex and perform their functions simultaneously but independently.
An unexpected phenotype of cells defective for Barren function is an accumulation of cells containing >2C DNA content. This phenotype may be general for chromosome condensation mutants, because smc2 mutants also produce >2C cells. When Barren or Smc2p is inactivated at the beginning of the cell cycle, chromosome condensation is perturbed at the time of the first mitosis (Figure 4; our unpublished results). In contrast, the appearance of cells with >2C DNA content occurs much later (Figure 3). In fact, the appearance of >2C cells can be entirely abolished if the brn1 cells are prevented from proceeding through a second cell cycle with either α-factor or hydroxyurea (Figure 3C; our unpublished results). It is unlikely that this phenotype is caused by some aberrant binding of propidium iodide to the uncondensed DNA, because a sharp 2C peak is observable in both mutants in the first M phase. Instead, the >2C FACS profiles are reminiscent of FACS profiles produced by a cdc6 mutant that displays promiscuous initiation of DNA replication (Liang and Stillman, 1997). This observation brings up the interesting possibility that improper condensation of the chromosomes in the first cell cycle subsequently leads to inappropriate initiation of DNA replication. Because the prereplication complex is formed at the M-to-G1 transition (Diffley et al., 1994), one possibility is that its normal assembly is affected by the condensation state of the chromosomes.
A second unexpected phenotype of brn1 mutants is the absence of spindle and chromosome colocalization. One possible explanation is that decondensation results in intertwining that causes detachment of chromosomes from the spindle when the cell enters anaphase. This possibility seems unlikely, however, because topological links and chromosome breaks do not accumulate in brn1 mutants. Furthermore, top2 mutants complete anaphase by breaking chromosomes, suggesting that kinetochore-spindle attachments resist a challenge from topologically linked DNA (Holm et al., 1989). A more attractive model is that Barren (or the chromosome condensation it maintains) plays a novel role in centromere structure and/or function. In support of this idea, cytological observations of mitotic chromosomes suggest an unusual condensed state of centromeric regions (Rattner and Lin, 1985). In addition, mutations in Cep3p, an established kinetochore component, result in a similar displacement of the chromosomes from the spindle, presumably resulting from a failure of the chromosomes to maintain a stable attachment to the dynamic spindle (Strunnikov et al., 1995b). Furthermore, preliminary evidence suggests that Brn1p genetically interacts with a subset of kinetochore proteins (Lavoie and Koshland, unpublished observation). It will be interesting to determine whether this putative role for Barren at the centromere reflects a general role for condensin proteins. However, neither smc2 mutations nor mutations affecting the homologue of XCAP-G cause an accumulation of mid anaphase nuclei (Lavoie and Koshland, unpublished observation). Thus, it is tempting to speculate that specific cellular processes may be differentially sensitive to defects in the functions of condensin proteins. Additional study will be necessary to determine the precise functional roles of these proteins, which play a pivotal role in the condensation of chromosomes.
Note added in proof. In the accompanying paper by Ouspenski et al., brn1 mutants do not exhibit excess DNA replication during the time course of the FACS experiment. This disparity probably reflects the roughly twofold difference in doubling times of the strains used by the two laboratories.
ACKNOWLEDGMENTS
We are indebted to A. Strunnikov for providing the 1aAS330 (smc2-8) strain and for communication of unpublished results. We thank A. Philp and members of our laboratories for critical reading of the manuscript. B.D.L. and K.M.T. contributed equally to this work. This work was supported by grants GM36510 (to C.H.) and GM41718 (to D.K.) from the National Institutes of Health. B.D.L. is the recipient of a Centennial Fellowship from the Medical Research Council of Canada.
REFERENCES
- Amin NS, Holm C. In vivo analysis reveals that the interdomain region of the yeast proliferating cell nuclear antigen is important for DNA replication and DNA repair. Genetics. 1996;144:479–493. doi: 10.1093/genetics/144.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1999. [Google Scholar]
- Bhat MA, Philp AV, Glover DM, Bellen HJ. Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with topoisomerase II. Cell. 1996;87:1103–1114. doi: 10.1016/s0092-8674(00)81804-8. [DOI] [PubMed] [Google Scholar]
- Boeke JD, Lacroute F, Fink GR. A positive selection for mutants lacking orotidine 5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;181:288–291. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Cubizolles F, Legagneux V, Le Guellec R, Chartrain I, Uzbekov R, Ford C, Le Guellec K. pEg7, a new Xenopus protein required for mitotic chromosome condensation in egg extracts. J Cell Biol. 1998;143:1437–1446. doi: 10.1083/jcb.143.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley JF, Cocker JH, Dowell SJ, Rowley A. Two steps in the assembly of complexes at yeast replication origins in vivo. Cell. 1994;78:303–316. doi: 10.1016/0092-8674(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Guacci V, Hogan E, Koshland D. Chromosome condensation and sister chromatid pairing in budding yeast. J Cell Biol. 1994;125:517–530. doi: 10.1083/jcb.125.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guacci V, Koshland D, Strunnikov A. A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell. 1997;91:47–57. doi: 10.1016/s0092-8674(01)80008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. SMC-mediated chromosome mechanics: a conserved scheme from bacteria to vertebrates? Genes Dev. 1999;13:11–19. doi: 10.1101/gad.13.1.11. [DOI] [PubMed] [Google Scholar]
- Hirano T, Kobayashi R, Hirano M. Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell. 1997;89:511–521. doi: 10.1016/s0092-8674(00)80233-0. [DOI] [PubMed] [Google Scholar]
- Hirano T, Mitchison TJ. A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell. 1994;79:449–458. doi: 10.1016/0092-8674(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Holm C. Coming undone: how to untangle a chromosome. Cell. 1994;77:955–957. doi: 10.1016/0092-8674(94)90433-2. [DOI] [PubMed] [Google Scholar]
- Holm C, Goto T, Wang JC, Botstein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985;41:553–563. doi: 10.1016/s0092-8674(85)80028-3. [DOI] [PubMed] [Google Scholar]
- Holm C, Stearns T, Botstein D. DNA topoisomerase II must act at mitosis to prevent nondisjunction and chromosome breakage. Mol Cell Biol. 1989;9:159–168. doi: 10.1128/mcb.9.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell EA, McAlear MA, Rose D, Holm C. CDC44: a putative nucleotide-binding protein required for cell cycle progression that has homology to subunits of replication factor C. Mol Cell Biol. 1994;14:255–267. doi: 10.1128/mcb.14.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter KJ, Eipel HE. Microbial DNA determinations by flow cytometry. J Gen Microbiol. 1979;113:369–375. doi: 10.1099/00221287-113-2-369. [DOI] [PubMed] [Google Scholar]
- Jessberger R, Frei C, Gasser SM. Chromosome dynamics: the SMC protein family. Curr Opin Genet Dev. 1998;8:254–259. doi: 10.1016/s0959-437x(98)80149-4. [DOI] [PubMed] [Google Scholar]
- Kilmartin JV, Wright B, Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982;93:576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Hirano M, Kobayashi R, Hirano T. Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science. 1998;282:487–490. doi: 10.1126/science.282.5388.487. [DOI] [PubMed] [Google Scholar]
- Kimura K, Hirano T. ATP-dependent positive supercoiling of DNA by 13S condensin: a biochemical implication for chromosome condensation. Cell. 1997;90:625–634. doi: 10.1016/s0092-8674(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Kimura K, Rybenkov VV, Crisona NJ, Hirano T, Cozzarelli NR. 13S condensin actively reconfigures DNA by introducing global positive writhe: implications for chromosome condensation. Cell. 1999;98:239–248. doi: 10.1016/s0092-8674(00)81018-1. [DOI] [PubMed] [Google Scholar]
- Koshland D, Hartwell LH. The structure of sister minichromosome DNA before anaphase in Saccharomyces cerevisiae. Science. 1987;238:1713–1716. doi: 10.1126/science.3317838. [DOI] [PubMed] [Google Scholar]
- Koshland D, Kent JC, Hartwell LH. Genetic analysis of the mitotic transmission of minichromosomes. Cell. 1985;40:393–403. doi: 10.1016/0092-8674(85)90153-9. [DOI] [PubMed] [Google Scholar]
- Liang C, Stillman B. Persistent initiation of DNA replication and chromatin-bound MCM proteins during the cell cycle in cdc6 mutants. Genes Dev. 1997;11:3375–3386. doi: 10.1101/gad.11.24.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb JD, Albrecht MR, Chuang PT, Meyer BJ. MIX-1: an essential component of the C. elegans mitotic machinery executes X chromosome dosage compensation. Cell. 1998;92:265–277. doi: 10.1016/s0092-8674(00)80920-4. [DOI] [PubMed] [Google Scholar]
- Muhlrad D, Hunter R, Parker R. A rapid method for localized mutagenesis of yeast genes. Yeast. 1992;8:79–82. doi: 10.1002/yea.320080202. [DOI] [PubMed] [Google Scholar]
- Rattner JB, Lin CC. Radial loops and helical coils coexist in metaphase chromosomes. Cell. 1985;42:291–296. doi: 10.1016/s0092-8674(85)80124-0. [DOI] [PubMed] [Google Scholar]
- Robzyk K, Kassir Y. A simple and highly efficient procedure for rescuing autonomous plasmids from yeast. Nucleic Acids Res. 1992;20:3790. doi: 10.1093/nar/20.14.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka Y, Sutani T, Yamashita Y, Saitoh S, Takeuchi M, Nakaseko Y, Yanagida M. Fission yeast cut3 and cut14, members of the ubiquitous protein family, are required for chromosome condensation and segregation in mitosis. EMBO J. 1994;13:4938–4952. doi: 10.1002/j.1460-2075.1994.tb06821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F, Fink G, Hicks J. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spell RM, Holm C. Nature and distribution of chromosomal intertwinings in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:1465–1476. doi: 10.1128/mcb.14.2.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunnikov AV. SMC proteins and chromosome structure. Trends Cell Biol. 1998;8:454–459. doi: 10.1016/s0962-8924(98)01370-1. [DOI] [PubMed] [Google Scholar]
- Strunnikov AV, Hogan E, Koshland D. SMC2, a Saccharomyces cerevisiae gene essential for chromosome segregation and condensation, defines a subgroup within the SMC family. Genes Dev. 1995a;9:587–599. doi: 10.1101/gad.9.5.587. [DOI] [PubMed] [Google Scholar]
- Strunnikov AV, Kingsbury J, Koshland D. CEP3 encodes a centromere protein of Saccharomyces cerevisiae. J Cell Biol. 1995b;128:749–760. doi: 10.1083/jcb.128.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunnikov AV, Larionov VL, Koshland D. SMC1: an essential yeast gene encoding a putative head-rod-tail protein is required for nuclear division and defines a new ubiquitous protein family. J Cell Biol. 1993;123:1635–1648. doi: 10.1083/jcb.123.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert TA, Hartwell LH. Cell cycle arrest of cdc mutants and specificity of the RAD9 checkpoint. Genetics. 1993;134:63–80. doi: 10.1093/genetics/134.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ER, Earnshaw WC. Mitotic chromatin condensation in vitro using somatic cell extracts and nuclei with variable levels of endogenous topoisomerase II. J Cell Biol. 1990;111:2839–2850. doi: 10.1083/jcb.111.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida M. Fission yeast cut mutations revisited: control of anaphase. Trends Cell Biol. 1998;8:144–149. doi: 10.1016/s0962-8924(98)01236-7. [DOI] [PubMed] [Google Scholar]