Abstract
OBJECTIVE
To assess the effect of hormone therapy (HT) on coronary heart disease (CHD) events in younger and older postmenopausal women.
DESIGN
A comprehensive database search identified randomized-controlled trials of HT of at least 6 months' duration that reported CHD events, defined as myocardial infarction or cardiac death.
MEASUREMENTS
The pooled odds ratios (ORs) for CHD events were reported separately for younger and older women, defined as participants with mean time from menopause of less than or greater than 10 years, or mean age less than or greater than 60 years.
MAIN RESULTS
Pooled data from 23 trials, with 39,049 participants followed for 191,340 patient-years, showed that HT significantly reduced CHD events in younger women (OR 0.68 [confidence interval (C I), 0.48 to 0.96]), but not in older women (OR 1.03 [CI, 0.91 to 1.16]). Hormone therapy reduced events in younger women compared with older women (OR 0.66 [CI, 0.46 to 0.95]). In older women, HT increased events in the first year (OR 1.47 [CI, 1.12 to 1.92]), then reduced events after 2 years (OR 0.79 [CI, 0.67 to 0.93]).
CONCLUSIONS
Hormone therapy reduces the risk of CHD events in younger postmenopausal women. In older women, HT increases, then decreases risk over time.
Keywords: meta-analysis, coronary artery disease, hormone replacement, women, aging
There is continuing debate about the effect of hormone therapy (HT) on coronary heart disease (CHD) outcomes. Observational studies show that women who initiate HT shortly after menopause have 40% lower risk for CHD events than nonusers.1 In contrast, the Women's Health Initiative (WHI) found that older women who started treatment many years after menopause had an increased risk of CHD events when treated with estrogen–progestin, but not with estrogen alone.2,3 It is possible that HT has different effects in younger and older postmenopausal women. A meta-analysis of randomized trials showed that HT reduced total mortality by 40% in younger women, with a nonsignificant trend toward reduced cardiovascular deaths.4 The WHI trials have now provided data for CHD events separately for younger and older women. With the release of the WHI data, we are now able to evaluate the effect of HT on CHD outcomes in younger and older postmenopausal women.
METHODS
The MEDLINE, EMBASE, CINAHL and Cochrane databases were searched to identify relevant trials published between 1966 and November 2004. The search was augmented by scanning selected journals and references. We included randomized-controlled trials of at least 6 months duration that compared HT with placebo or no hormone therapy in postmenopausal women, and reported at least 1 CHD event. Attempts were made to obtain more information from investigators about cardiac events in the trials. Evaluation of those trials excluded for short duration revealed no CHD events.
The outcome measured was CHD events, defined as myocardial infarction or death thought to be due to cardiac causes. The methodological quality of each trial was assessed and used in a sensitivity analysis.4 The results for each trial were pooled to obtain a summary odds ratio (OR).4,5 The analysis was performed using Meta View 4.2 (Cochrane Library Software, Oxford). Only trials that reported at least 1 event could be used in the estimate of ORs.6
Trials were divided into those with younger or older women according to the mean time from menopause at baseline, with a cutoff set at 10 years. If that information was not available, trials were divided into those with mean age of participants less than or greater than 60 years. The results were reported separately for all ages and for the younger and older age groups, and compared with each other using the test of interaction.7 A separate analysis evaluated data that included only younger women2,3,8–13 or only older women,2,3,14–16 to eliminate overlap between groups. Another analysis evaluated events that occurred within the first year of treatment2,3,12,15,17–21 or after 2 years,2,3,18 to assess the effect of treatment initiation.
RESULTS
The literature search identified 23 trials that met inclusion criteria (Appendices 1 and 2).2,3,8–29 Additional information on the WHI trial was published separately.30 The analysis included 39,049 participants, with a mean trial duration of 4.9±1.7 years (range 0.5 to 10 years). The dropout rate was approximately 12.0% in the treatment group and 10.8% in the control group. Studies were excluded for the following reasons: 27 were not randomized, 115 provided data on patients already in the analysis, 89 were of less than 6 months' duration, 36 did not have a control group, and 128 did not report CHD events.
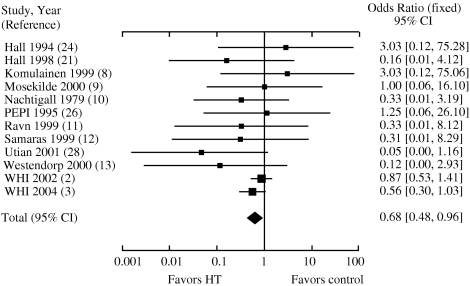
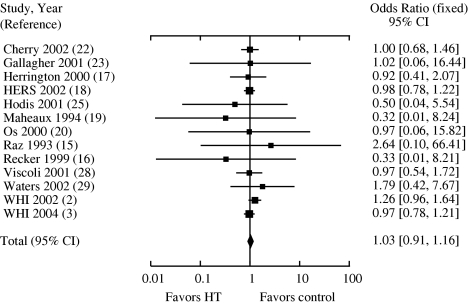
For all ages, there was no effect of HT on CHD events (OR 0.99 [confidence interval (CI), 0.88 to 1.11]). In the younger group, HT reduced CHD events by 32% (OR 0.68 [CI, 0.48 to 0.96; Fig. 1]). When trials with only younger women were evaluated, a similar reduction in events was found (OR 0.7 [CI, 0.49 to 1.0]). The OR for CHD events in the older group was 1.03 (CI, 0.91 to 1.16; Fig. 2). Trials with only older women showed similar results (OR 1.08 [CI, 0.91 to 1.27]). When the results of the 2 age groups were compared with each other, HT was associated with significantly lower CHD events in younger women compared with older women (OR 0.66 [CI, 0.46 to 0.95]).
FIGURE 1.
Odds ratios for coronary heart disease events associated with hormone replacement: trials with younger women.
FIGURE 2.
Odds ratios for coronary heart disease events associated with hormone replacement: trials with older women.
In the first year of treatment, HT had no effect on events in the younger group (OR 0.22 [CI, 0.02 to 2.26]), but significantly increased events in the older group (OR 1.47 [CI, 1.12 to 1.92]). After 2 years, HT significantly reduced CHD events in older women (OR 0.79 [CI, 0.67 to 0.93]), resulting in a neutral effect over time.2,3,18 A significant time trend was seen of increased events progressing to decreased events, P =.002.
In a sensitivity analysis, 5 trials with the lowest score on at least 1 quality domain were excluded and the OR changed by less than 0.01 points (P>0.9) for each of the analyses. There was no evidence of heterogeneity in any of the analyses, P>0.7.
DISCUSSION
In this meta-analysis, HT reduced CHD events in younger postmenopausal women by 32% compared with placebo or no treatment, and by 34% compared with treatment in older women. It should be noted that the risk for CHD events in younger postmenopausal women is low. This meta-analysis pooled over 70,000 patient-years of treatment in younger women, thus providing the power needed to observe significant results in this age group. In order to estimate the absolute risk reduction attributed to HT, those trials without reported events were included in the analysis, thus contributing to the denominator. The event rate over 4.6 years was estimated to be 0.29% in the treatment group and 0.65% in the control group, with a number needed to treat of 256 over 5 years and an absolute risk reduction of approximately 1 event per 1,000 patient-years.
In younger women, the reduction in CHD events seen with HT is similar to the reduction in total mortality that has been seen in pooled trial data (OR 0.6 [CI, 0.4 to 0.95]).4 This reduction in cardiac morbidity and mortality is similar to that found in the observational Nurses' Health Study, which followed a cohort of 120,000 women below the age of 55 years.1 After adjusting for potential confounding variables, such as age, cardiovascular risk factors, and socioeconomic status, HT use was associated with a 40% reduction in CHD events and total mortality.1,31
Two different cutoff criteria were used to divide the groups into younger and older women. Years from menopause is thought to be a stronger risk factor for CHD events than age, and was used first as the cutoff.32 In the WHI estrogen–progestin trial, women less than 10 years from menopause had a trend toward reduced CHD events, while women below the age of 60 years had a trend toward increased risk.2 This discrepancy could be explained if some women in the younger age group had been postmenopausal for greater than 10 years. Similar results are seen when this trial is excluded from the analysis (OR 0.53 [CI, 0.33 to 0.88]). In the WHI estrogen-alone trial, women with hysterectomies under the age of 60 years had a trend toward reduced CHD events.33
It is biologically plausible that HT, if started early after the development of menopause, can slow the progression of coronary atherosclerosis.34 Hormone therapy has been shown to reduce cardiovascular risk factors such as lipids, blood pressure, cell adhesion molecules, procoagulant factors, abdominal obesity, and insulin resistance, as well as to decrease the incidence of new-onset diabetes and improve glycemic control in those with diabetes.35 In older women, HT was associated with an increase in events in the first year, followed by a reduction after 2 years. In contrast, no initial increase in cardiac events was seen in younger women. Hormone replacement has been shown to increase C-reactive protein levels, which is a significant risk factor for myocardial infarction and death, especially in those with underlying atherosclerosis.36 It is possible that HRT has an initial prothrombotic effect in older women with underlying atherosclerosis, but that this effect is not apparent in those without significant disease.
This meta-analysis has several limitations. There was marked variability in the trial characteristics, with a wide range in study size, medication used, and method of administration. The age groups used in this study were defined according to the trials as a whole and not based on individual patients. However, similar results were found when only younger women were evaluated. Many of the trials did not include CHD events as a primary outcome, but rather as an adverse event, so it is not clear how ascertainment of events was made in each trial or whether there were any unreported events. The analysis was based only on published literature and therefore is subject to publication bias. However, funnel plots of effect size versus standard error for the trials in this analysis showed no evidence of bias.
In summary, HT reduces CHD events in younger postmenopausal women, indicating that it may have a primary preventive effect if started before atherosclerosis develops. Large randomized trials of younger women are needed to evaluate clinical CHD endpoints, as well as other outcomes such as cerebrovascular events and cancer. In the interim, clinicians should individualize decisions about the use of HT, taking into account the woman's age, menopausal symptoms, and any underlying risk factors.
Acknowledgments
The funding for this analysis came from salary support for Drs. Salpeter, Greyber, and Walsh. The authors thank Christopher Stave for coordinating the trials search.
REFERENCES
- 1.Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453–61. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- 2.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 4.Salpeter SR, Walsh JM, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J Gen Intern Med. 2004;19:791–804. doi: 10.1111/j.1525-1497.2004.30281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 6.Cochrane Collaboration. [November 4, 2005]; Cochrane Reviewer's Handbook 4.2.2. Section 8. Analysing and presenting results. 2004. Accessed at on http://www.cochrane.org/resources/handbook/section8.pdf.
- 7.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komulainen M, Kroger H, Tuppurainen MT, et al. Prevention of femoral and lumbar bone loss with hormone replacement therapy and vitamin D3 in early postmenopausal women: a population-based 5-year randomized trial. J Clin Endocrinol Metab. 1999;84:546–52. doi: 10.1210/jcem.84.2.5496. [DOI] [PubMed] [Google Scholar]
- 9.Mosekilde L, Beck-Nielsen H, Sorensen OH, et al. Hormonal replacement therapy reduces forearm fracture incidence in recent postmenopausal women—results of the Danish Osteoporosis Prevention Study. Maturitas. 2000;36:181–93. doi: 10.1016/s0378-5122(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 10.Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM. Estrogen replacement therapy II: a prospective study in the relationship to carcinoma and cardiovascular and metabolic problems. Obstet Gynecol. 1979;54:74–9. doi: 10.1097/00006250-197907000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Ravn P, Bidstrup M, Wasnich RD, et al. Alendronate and estrogen-progestin in the long-term prevention of bone: loss four-year results from the early postmenopausal intervention cohort study. A randomized, controlled trial. Ann Intern Med. 1999;131:935–42. doi: 10.7326/0003-4819-131-12-199912210-00005. [DOI] [PubMed] [Google Scholar]
- 12.Samaras K, Hayward CS, Sullivan D, Kelly RP, Campbell LV. Effects of postmenopausal hormone replacement therapy on central abdominal fat, glycemic control, lipid metabolism, and vascular factors in type 2 diabetes: a prospective study. Diabetes Care. 1999;22:1401–7. doi: 10.2337/diacare.22.9.1401. [DOI] [PubMed] [Google Scholar]
- 13.Westendorp IC, de Kleijn MJ, Bots ML, et al. The effect of hormone replacement therapy on arterial distensibility and compliance in perimenopausal women: a 2-year randomised trial. Atherosclerosis. 2000;152:149–57. doi: 10.1016/s0021-9150(99)00438-4. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher JC, Kable WT, Goldgar D. Effect of progestin therapy on cortical and trabecular bone: comparison with estrogen. Am J Med. 1991;90:171–8. [PubMed] [Google Scholar]
- 15.Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329:753–6. doi: 10.1056/NEJM199309093291102. [DOI] [PubMed] [Google Scholar]
- 16.Recker RR, Davies KM, Dowd RM, Heaney RP. The effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women. A randomized, controlled trial. Ann Intern Med. 1999;130:897–904. doi: 10.7326/0003-4819-130-11-199906010-00005. [DOI] [PubMed] [Google Scholar]
- 17.Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522–9. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- 18.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 19.Maheux R, Naud F, Rioux M, et al. A randomized, double-blind, placebo-controlled study on the effect of conjugated estrogens on skin thickness. Am J Obstet Gynecol. 1994;170:642–9. doi: 10.1016/s0002-9378(94)70242-x. [DOI] [PubMed] [Google Scholar]
- 20.Os I, Hofstad AE, Brekke M, et al. The EWA (estrogen in women with atherosclerosis) study: a randomized study of the use of hormone replacement therapy in women with angiographically verified coronary artery disease. Characteristics of the study population. Effects on lipids and lipoproteins. J Intern Med. 2000;247:433–41. doi: 10.1046/j.1365-2796.2000.00675.x. [DOI] [PubMed] [Google Scholar]
- 21.Hall G, Pripp U, Schenck-Gustafsson K, Landgren BM. Long-term effects of hormone replacement therapy on symptoms of angina pectoris, quality of life and compliance in women with coronary artery disease. Maturitas. 1998;28:235–42. doi: 10.1016/s0378-5122(97)00080-7. [DOI] [PubMed] [Google Scholar]
- 22.Cherry N, Gilmour K, Hannaford P, et al. Oestrogen therapy for prevention of reinfarction in postmenopausal women: a randomised placebo controlled trial. Lancet. 2002;360:2001–8. doi: 10.1016/s0140-6736(02)12001-0. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab. 2001;86:3618–28. doi: 10.1210/jcem.86.8.7703. [DOI] [PubMed] [Google Scholar]
- 24.Hall GM, Daniels M, Doyle DV, Spector TD. Effect of hormone replacement therapy on bone mass in rheumatoid arthritis patients treated with and without steroids. Arthritis Rheum. 1994;37:1499–505. doi: 10.1002/art.1780371014. [DOI] [PubMed] [Google Scholar]
- 25.Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–53. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 26.Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial PEPI Trial Writing Group. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 27.Utian WH, Shoupe D, Bachmann G, Pinkerton JV, Pickar JH. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril. 2001;75:1065–79. doi: 10.1016/s0015-0282(01)01791-5. [DOI] [PubMed] [Google Scholar]
- 28.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–9. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 29.Waters D, Alderman EL, Hsia J, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women. JAMA. 2002;288:2432–40. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 30.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 31.Grodstein F, Stampfer MJ, Colditz GA, et al. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;336:1769–75. doi: 10.1056/NEJM199706193362501. [DOI] [PubMed] [Google Scholar]
- 32.Kannel WB, Wilson PW. Risk factors that attenuate the female coronary disease advantage. Arch Intern Med. 1995;155:57–61. [PubMed] [Google Scholar]
- 33.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease The Women's Health Initiative. Arch Intern Med. 2006;166:357–65. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 34.Mikkola TS, Clarkson TB, Notelovitz M. Postmenopausal hormone therapy before and after the women's health initiative study: what consequences? Ann Med. 2004;36:402–13. doi: 10.1080/07853890410035430. [DOI] [PubMed] [Google Scholar]
- 35.Salpeter SR, Walsh JME, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2005 doi: 10.1111/j.1463-1326.2005.00545.x. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]