Abstract
Null mutations in the Drosophila Kinesin heavy chain gene (Khc), which are lethal during the second larval instar, have shown that conventional kinesin is critical for fast axonal transport in neurons, but its functions elsewhere are uncertain. To test other tissues, single imaginal cells in young larvae were rendered null for Khc by mitotic recombination. Surprisingly, the null cells produced large clones of adult tissue. The rates of cell proliferation were not reduced, indicating that conventional kinesin is not essential for cell growth or division. This suggests that in undifferentiated cells vesicle transport from the Golgi to either the endoplasmic reticulum or the plasma membrane can proceed at normal rates without conventional kinesin. In adult eye clones produced by null founder cells, there were some defects in differentiation that caused mild ultrastructural changes, but they were not consistent with serious problems in the positioning or transport of endoplasmic reticulum, mitochondria, or vesicles. In contrast, defective cuticle deposition by highly elongated Khc null bristle shafts suggests that conventional kinesin is critical for proper secretory vesicle transport in some cell types, particularly ones that must build and maintain long cytoplasmic extensions. The ubiquity and evolutionary conservation of kinesin heavy chain argue for functions in all cells. We suggest interphase organelle movements away from the cell center are driven by multilayered transport mechanisms; that is, individual organelles can use kinesin-related proteins and myosins, as well as conventional kinesin, to move toward the cell periphery. In this case, other motors can compensate for the loss of conventional kinesin except in cells that have extremely long transport tracks.
INTRODUCTION
Vesicle transport is important in eukaryotic cells for the addition of material to the plasma membrane, for secretion, and for cell polarity. Active vesicle transport is thought to be driven by mechanochemical enzymes (motor proteins). Motors attach to vesicle membranes and then use ATP hydrolysis to drive unidirectional movement along polar cytoskeletal filaments. Characterized members of the myosin family of motors move toward the barbed or fast-growing ends of actin filaments with the exception of myosin VI, which moves toward the pointed or slow-growing ends (Wells et al., 1999) (reviewed by Sellers and Goodson, 1995). Actin filaments in undifferentiated and in some differentiated cell types are highly concentrated in the cortex (Waterman-Storer et al., 1998; reviewed by Cramer, 1999). Cytoplasmic myosins may therefore be important for anchoring or moving vesicles when they are near the plasma membrane (Fath et al., 1994). Motors in the kinesin and dynein families move along microtubules. Characterized dyneins and members of the C-terminal kinesin subfamily move toward microtubule minus ends, whereas other kinesins that act as motors move toward microtubule plus ends (reviewed by Vale and Fletterick, 1997; Hirokawa, 1998; Goldstein and Yang, 2000).
Microtubules are relatively long, straight polymers that course throughout the cytoplasm. In undifferentiated and in fibroblastoid cell types, the minus ends of microtubules are usually near the cell center, whereas the plus ends are usually near the periphery (reviewed by Lippincott-Schwartz, 1998). This is consistent with the idea that most microtubule-based, outward vesicle movements (Golgi-to-endoplasmic reticulum [ER] or Golgi-to-plasma membrane) are driven by plus end–directed kinesins, and most inward movements are driven by cytoplasmic dyneins (reviewed by Lippincott-Schwartz, 1998). In differentiated cells, a variety of microtubule orientations are seen. For instance, in the axons of neurons, almost all plus ends are away from the cell center and toward the terminal (Heidemann et al., 1981). In some but not all polarized epithelial cells, microtubules are oriented with their plus ends toward the basal pole and their minus ends near the apical pole (reviewed by Mays et al., 1994; McNiven and Marlowe, 1999). In such situations, in which the polarity is relatively uniform, models have been developed that invoke appropriate microtubule motors for various steps in vesicle or other organelle transport.
Conventional kinesin (often referred to below simply as kinesin) is a ubiquitous and abundant plus end–directed microtubule motor protein. It is expressed in virtually all cells of both vertebrates and invertebrates (Saxton et al., 1988; Hollenbeck, 1989). The majority of kinesin appears to be free in cytosol, but various studies have shown that it can associate with ER, vesicles, mitochondria, and other organelles (Hollenbeck, 1989; Pfister et al., 1989; Brady et al., 1990; Hirokawa et al., 1991; Houliston and Elinson, 1991; Wright et al., 1991; Leopold et al., 1992; Yu et al., 1992; Marks et al., 1994; Elluru et al., 1995; Okada et al., 1995; Sturmer and Baumann, 1996). Function disruption tests indicate that it is critical for fast organelle transport in axons, although the set of cargoes it carries is not clearly defined (reviewed by Hirokawa, 1996; Martin et al., 1999; Goldstein and Yang, 2000). Other studies of non-neuronal cells or cell-free systems suggest that kinesin is important for the positioning of lysosomes, mitochondria, and ER. It is also thought to be important for vesicle transport from the Golgi to the plasma membrane, one of the late steps in the secretion pathway, and in Golgi-to-ER membrane recycling, which is an indirect but essential part of the early secretion pathway (reviewed by Goodson et al., 1997; Lane and Allan, 1998; Goldstein and Philp, 1999). For example, based on the effects of anti-kinesin heavy chain (KHC) antibodies or a KHC tail fragment microinjected into sea urchin embryos, kinesin has been proposed to be important for outward transport, to the cortex, of a class of vesicles used for rapid membrane repair (Bi et al., 1997). Furthermore, based on immunolocalization and on the effects of anti-KHC antibodies on brefeldin A-induced Golgi-to-ER membrane transport in vertebrate cultured cells, kinesin has been proposed to be the motor for Golgi-to-ER membrane recycling (Lippincott-Schwartz et al., 1995).
To address questions about kinesin function in an intact organism in motility processes other than axonal transport, we have examined the effects of recessive, Khc null mutations on various cell types in Drosophila. When the entire organism is homozygous for a null mutation, it becomes paralyzed and dies during the midlarval stage, preventing studies of KHC function during the remainder of development. To circumvent this lethality, we used a mitotic recombination strategy to generate chimeras with a few homozygous null cells in otherwise healthy heterozygous organisms. The fates of the descendants of such Khc null cells were then studied by light and electron microscopy. We focused on two predictions of the hypothesis that kinesin is an important motor for vesicle transport in the late secretory and recycling pathways. First, because disruption of vesicle traffic should block membrane growth and thus cell proliferation (Novick et al., 1980; Harrison et al., 1994; Lewis and Pelham, 1996), the proliferation of Khc null imaginal cells was assessed. Surprisingly, the null cells proliferated normally to produce large clones of adult cells. Second, because disruption of vesicle traffic can cause striking changes in the organization of ER and Golgi as well as defective secretion (Novick et al., 1980; Lewis and Pelham, 1996; Satoh et al., 1997), postmitotic cells that rely heavily on the secretory pathway were studied in detail. Defects consistent with a role for kinesin in the Golgi-to-ER recycling pathway were not seen. However, defects consistent with a role for kinesin in long-distance late secretory vesicle transport (Golgi-to-plasma membrane) were seen.
MATERIALS AND METHODS
Drosophila Culture and Mutant Alleles
Flies were cultured at 25°C with a 12-h light/12-h dark cycle on soft medium (0.5% agar, 7% molasses, 6% cornmeal, and 0.8% killed yeast) seeded with live yeast. Descriptions of some of the mutations used in this study can be found in an article by Lindsley and Zimm (1992). Descriptions of other mutations and strains used can be found in articles by Brendza et al. (1999), Xu and Rubin (1993), and Saxton et al. (1991), except for Khc20, which was isolated in a screen for new recessive lethal alleles of Khc (D.J. Rose and W.M. Saxton, unpublished data). The P{mini-w+, Khc+} rescue construct contains a genomic fragment that includes the Khc gene (Saxton et al., 1991). Although expression levels from this construct have not been rigorously quantified, it is controlled by the endogenous Khc promoter and rescues all defects tested to date. The P{ry+, hs-neo, FRT}42D c Khc20 and the P{ry+, hs-neo, FRT}42D c Khc27 chromosomes were constructed by meiotic recombination in females as described by Xu and Rubin (1993). Chromosomes carrying P{hs-FLP} and those carrying FRT sites linked to mutant Khc alleles were rendered isogenic by descent before use in the tests described here.
Electrophoresis and Immunoblotting
Cytosol was prepared as described previously from the following adult male flies: 1) w; Khc20/Khc20; P{w+, c-myc-Khc+}/+; 2) w; Khc27/Khc27; P{w+, c-myc-Khc+}/+; 3) w; Df(2R)JP6/+; P{w+, c-myc-Khc+}/+; or 4) w; Khc+/Khc+; +/+ (Saxton et al., 1991). P{w+, c-myc-Khc+} is a stable P-element insert that contains a wild-type Khc cDNA fused to a c-myc epitope tag (generously provided by L.S.B. Goldstein and M. deCuevas; deCuevas, 1993) with expression driven by a ubiquitin promoter. The expressed Myc-KHC fusion protein completely rescues the lethality caused by Khcnull mutations.
Total protein concentration was determined for each adult Drosophila extract using the Bio-Rad (Hercules, CA) protein assay. Equal loadings (170 μg of total protein) from each of the four extracts were run on an SDS-polyacrylamide gradient gel (5–10% acrylamide) as described previously (Saxton et al., 1988). The Myc-KHC fusion protein migrated more slowly than native KHC, allowing comparison of their relative levels by Western blotting. The Myc-KHC whose expression was driven by the ubiquitin promoter served as an internal loading control. Detection of both proteins was done with a mouse monoclonal anti-Drosophila KHC antibody, diluted 1∶50 (Flyk-2; Saxton et al., 1991) followed by incubation in an alkaline phosphatase–conjugated goat anti-mouse serum at 1∶1000 (Zymed, San Francisco, CA).
Mitotic Recombination
Recombination was induced in somatic cells according to the method of Golic and Lindquist (1989). Expression of yeast flip recombinase (FLP) from P{hs-FLP}, driven by heat shock, can generate crossover events between flip recombinase target (FRT) sites that have been inserted in Drosophila chromosomes (Golic and Lindquist, 1989; Golic, 1991). FRT sites at identical positions in homologous chromosomes yield reciprocal exchanges that link identical sister chromatid arms to the kinetochores of separate homologues. A mitotic division following such an exchange has a 50% chance of producing daughter cells that carry identical chromatid arms. Thus, in a dividing cell that is heterozygous for a Khc mutation, two mutant alleles can segregate to one daughter cell, and two wild-type alleles can segregate to the other (see Figure 2A).
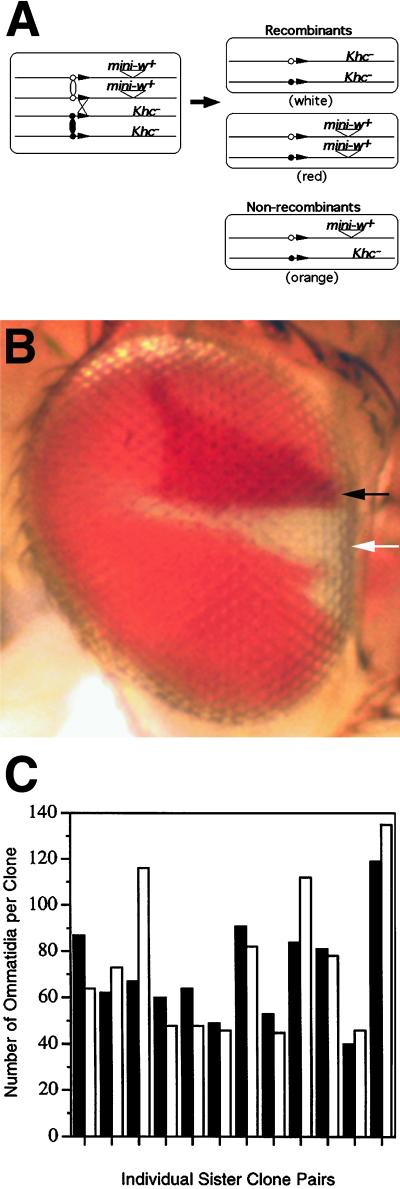
Figure 2.
Proliferative potential of Khcnull eye imaginal cells. (A) Diagram of the recombination strategy used to produce Khcnull and wild-type sister clones. Mitotic recombination was induced at FRT sites (black arrowheads) in first instar larvae that were homozygous for w− on the X chromosome and heterozygous for a Khcnull allele on chromosome 2. A single copy of mini-w+ was linked to the wild-type Khc allele (see MATERIALS AND METHODS for full genotypes). Thus, a recombinant Khc+/Khc+ cell gave rise to red eye tissue and its sister Khcnull/Khcnull cell gave rise to white eye tissue. Nonrecombinant Khcnull/Khc+ cells produced orange eye tissue. (B) Adult Drosophila eye containing a white Khc27/Khc27 eye clone (white arrow) and its red Khc+/Khc+ sister clone (black arrow). Based on their sizes, each clone contained ∼1500 cells derived from a single recombinant cell. (C) Comparison of the sizes of Khcnull/Khcnull clones (open bars) to their Khc+/Khc+ sister clones (filled bars) from 12 different sister clone pairs.
Recombination at FRT sites was induced in larvae of the appropriate age by immersing vial cultures in a 38°C water bath for 1 h. For twin spot analysis of adult eye development, first instar larvae of the following genotype were used: y w P{hs-FLP}; P{ry+, hs-neo, FRT}42D P{mini-w+, hs-NM}46F/P{ry+, hs-neo, FRT}42D c Khcnull (either Khc20 or Khc27) (Xu and Harrison, 1994). The mini-w+ transgene produced lower levels of pigment than the endogenous w gene. Consequently, homozygous Khcnull clones were white, homozygous wild-type sister clones (mini-w+/mini-w+) were deep red, and nonrecombinant heterozygous tissue (mini-w+/+) was orange. For all other analyses of Khcnull eye tissue, clones were induced in larvae that were either 1) y w P{hs-FLP}; P{ry+, hs-neo, FRT}42D P{ry+, w+}47A/P{ry+, hs-neo, FRT}42D c Khcnull or 2) the same genotype plus P{mini-w+, Khc+}/+ on chromosome 3. In flies that lacked P{mini-w+, Khc+}, Khcnull clones were white. In flies that carried P{mini-w+, Khc+}, Khcnull clones were orange. Because P{ry+; w+}47A produced high levels of pigment, the colors of +/+ clones and nonrecombinant heterozygous tissue were both red.
To generate Khcnull cuticular clones, y w; P{ry+, hs-neo, FRT}42D c Khcnull/P{ry+, hs-neo, FRT}42D c Khcnull; P{mini-w+, Khc+}/+ males were mated to y w P{hs-FLP}/y w P{hs-FLP}; P{ry+, hs-neo, FRT}42D P{y+}44B/P{ry+, hs-neo, FRT}42D P{y+}44B females. Recombination was induced in first instar larvae. Because y+ was linked to the wild-type Khc allele, Khcnull cuticular clones could be recognized by their pale yellow pigmentation. The presence or absence of P{mini-w+, Khc+} on the third chromosome was determined by eye color. The size distributions of test and control class wing clones were compared by log linear analysis using Systat 5.0 (Systat, Evanston, IL).
Electron Microscopy
For scanning electron microscopy (SEM), adult flies were stored in 70% (vol/vol) ethanol and then serially dehydrated to 100% ethanol. Specimens were desiccated in a Pelco (Redding, CA) model H critical point drier then mounted on stubs and sputter coated with gold for 4 min in a Polaron SEM coating unit. Specimens were imaged with a Cambridge (Cambridge, United Kingdom) Stereoscan 250 MK2 scanning electron microscope.
Tissue analyzed by transmission electron microscopy (TEM) was prepared according to the method of Baumann and Walz (1989) with minor modifications. Newly eclosed flies were dissected and fixed for 2 h at room temperature in 0.1 M Na-cacodylate buffer, pH 7.4, containing 2% glutaraldehyde, 2% paraformaldehyde, and 0.07% sucrose. Samples were then transferred into fresh fixative and incubated at 4°C overnight. They were washed twice for 30 min in 0.1 M Na-cacodylate buffer, pH 7.4, and postfixed in 2% OsO4 and 0.8% K3Fe(CN)6 in the same buffer. Specimens were then washed with distilled water, stained en bloc in 2% aqueous uranyl acetate, dehydrated in a graded acetone series, and embedded in Epon 812/Araldite 502 resin (McDonald, 1994).
Sections (90–100 nm) were cut using a Reichert UCT ultramicrotome, mounted on Formvar-coated slot grids, stained with uranyl acetate and Reynold's lead citrate, and examined with a JEOL (Tokyo, Japan) JEM 1010 transmission electron microscope. Accelerating voltages of 60 and 80 kV were used for eye and bristle sections, respectively. For eye analysis, 25 test class clones and 5 control class clones were sectioned. For SEM, bristles were analyzed in pairs. Bristles from test and control clones were compared with analogous bristle types from nonrecombinant heterozygous tissue located on the opposite site of the same fly. For TEM analysis, three test class, two control class, and three nonrecombinant bristles of the same type were sectioned and examined. Two test class bristles and one nonrecombinant bristle were serial sectioned completely to allow direct comparison of sections from the same positions along the lengths of the bristles.
Light Microscopy
Color micrographs of whole adult eyes were generated with a Nikon (Melville, NY) UFX-IIA stereomicroscope using Fujichrome 64T tungsten slide film (Fuji, Tokyo, Japan). Adult eye sections (500 nm) were prepared for light microscopy as described for transmission electron microscopy but were not stained with uranyl acetate and lead citrate. Eye sections were mounted on microscope slides with Permount and were examined using phase-contrast optics. Wings were dissected from adult flies and dehydrated in a graded ethanol series. Specimens were transferred into methyl salicylate and then mounted on slides in Gary's Magic Mountant (Lawrence et al., 1986). Wings were examined with bright-field and Nomarski optics. Wing bristle images were recorded on 35-mm film. They were later digitized and manipulated to generate figures using Adobe (Mountain View, CA) Photoshop 3.0.5.
RESULTS
Characterization of Khcnull Alleles
When studying the effects of disrupting the function of a protein, it is important to consider how specific the effects are and how thorough the disruption is. To control for the influence that genetic background might have on our analysis, all assays were performed using two independently isolated null alleles of the Kinesin heavy chain gene (Khc). Both produced the same results, and all defects could be rescued by a transgenic copy of wild-type Khc (P{mini-w+, Khc+}). Drosophila KHC consists of 975 amino acids (Yang et al., 1989). The Khc27 allele, which is recessive lethal, has a nonsense mutation at codon 65 that presumably halts translation and prevents KHC synthesis (Brendza et al., 1999). The molecular lesion in Khc20, another recessive lethal allele, has not been identified, but it causes a complete loss of function by genetic criteria and produces phenotypes identical to those of Khc27 in all assays to date. Null alleles of Khc should exert the same phenotypic effects as a deletion of the Khc locus (Saxton et al., 1991). Using the time course of lethality to assess levels of KHC function in homozygous or hemizygous animals, Khc20 and Khc27 are equivalent to a deletion (Df(2R)Jp6). A more sensitive method to determine whether Khc20 and Khc27 behave like a Khc deletion is to compare the effects of heteroallelic combinations with a hypomorphic Khc allele (e.g., Khc6). The lethal phase profiles for populations of larvae that were Khc6/Khc20, Khc6/Khc27 or Khc6/Df(2R)Jp6 were indistinguishable (Figure 1A). This confirms that the Khc20 and Khc27 alleles are functionally null, equivalent to a deletion of the Khc gene.
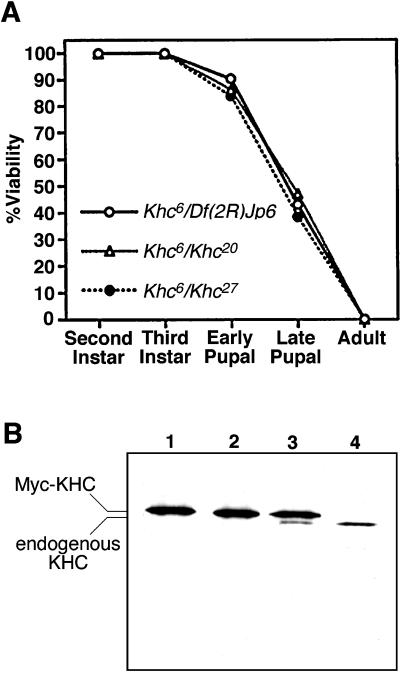
Figure 1.
Characterization of the Khc20 and Khc27 alleles. (A) An allele that causes a partial loss of function, Khc6, was used to compare the severities of Khc20, Khc27, and a deletion that removes the Khc locus (Df(2R)Jp6). Each point represents the percentage of animals that survived to the beginning of the indicated stage of development. The numbers of larvae tested for each combination were Khc6/Khc20, n = 138; Khc6/Khc27, n = 179; and Khc6/Df(2R)Jp6, n = 114. The lethal effects of Khc20, Khc27, and the deletion were indistinguishable. (B) Western blot of cytosol from male flies with the following genotypes: (lane 1) Khc20/Khc20; P{w+, c-myc-Khc+}/+; (lane 2) Khc27/Khc27; P{w+, c-myc-Khc+}/+; (lane 3) Df(2R)JP6/Khc+; P{w+, c-myc-Khc+}/+; (lane 4) Khc+/Khc+. Equal amounts of protein were loaded in each lane. The blot was probed with an antibody that binds to the amino-terminal motor domain of Drosophila KHC (Brendza et al., 1999). The P{w+, c-myc-Khc+} transgene produced a Myc-KHC fusion protein that rescued the Khc mutants. Its slower electrophoretic mobility allowed detection of KHC produced by endogenous genes (lane 3). No endogenous KHC was detected in flies that were homozygous for Khc20 or Khc27 (lanes 1 and 2).
To determine whether the Khc20 and Khc27 alleles are “protein nulls,” they were rendered homozygous in the presence of a P-element transposon that contains a wild-type Khc cDNA fused to a portion of c-myc (generously provided by M. deCuevas and L.S.B. Goldstein; deCuevas, 1993). The Myc-KHC fusion rescued the lethal effects of the Khc mutations, allowing the development of normal adult flies. In Western blots of SDS gels of adult fly cytosol, Myc-KHC could be distinguished from native KHC because of its slower electrophoretic mobility (Figure 1B, lane 3). Using Myc-KHC as a loading control, the relative amounts of native KHC present in flies bearing various Khc alleles could be judged. A heterozygous deletion appeared to reduce the amount of native KHC by ∼50% (Figure 1B, compare lanes 3 and 4), suggesting that protein dosage is directly proportional to gene dosage. In flies homozygous for either Khc20 or Khc27, no native KHC was detectable. This suggests that both are protein null alleles.
Kinesin Is Dispensable for the Growth and Division of Undifferentiated Cells
All Drosophila cells tested to date express KHC (Saxton et al., 1988). Tests of eye and wing discs by immunofluorescence indicate that KHC is distributed throughout the cytoplasm and is excluded from the nucleus (our unpublished results) as was previously demonstrated for embryonic cells (Saxton et al., 1991). Immunoelectron microscopy suggests an even distribution throughout the cytoplasm of adult photoreceptor cells and exclusion from rhabdomeres (S. Benzer, personal communication). To study the effects of a loss of KHC on the proliferation of various cell types, we used an FLP recombinase, site-specific mitotic recombination system with Khc20 and Khc27 (referred to interchangeably below as Khcnull) to generate single Khcnull/Khcnull cells in Khcnull/Khc+ larvae (Golic and Lindquist, 1989; Golic, 1991; Xu and Rubin, 1993). The proliferative capacities of single Khcnull/Khcnull cells were assessed by comparing the amount of adult tissue each could generate to the amount generated by equivalent control cells. The cells studied in detail included those in the eye imaginal disc, the wing imaginal disc, and the abdominal histoblast nests that form abdominal bristles.
To determine whether Khcnull cells in the developing eye could proliferate normally, mitotic recombination was used to generate pairs of sister cells, one homozygous null and the other homozygous wild type for Khc (Figure 2A). The induction of mitotic recombination (1–2 d after egg laying) led to adult progeny (10 d after egg laying) with Khcnull eye clones of substantial size (Figure 2B). Comparison of 40 individual sister clone pairs demonstrated that the sizes of Khcnull/Khcnull eye clones and their Khc+/Khc+ sister clones were often different. Differences in size are not surprising, because there is an element of randomness in the extent of proliferation of individual eye imaginal cells (Becker, 1957). Size comparisons for 12 of the largest sister clone pairs are shown in Figure 2C. There was no correlation between the relative sizes of sister clones and their genotypes. The maximum size observed for a Khcnull clone was ∼2800 cells or ∼20% of the eye. Thus, after the loss of Khc gene function, at least 11 rounds of cell growth and division could be completed at normal rates. This suggests that kinesin is not important for the growth or division of eye imaginal cells.
To determine whether the apparent dispensability of KHC applied to cells in a different developmental pathway and to conduct a more quantitative test of proliferation rates with transgenic controls, we studied Khcnull clones in the portion of the wing imaginal disc that gives rise to the anterior wing margin. To mark wing clones, a wild-type yellow transgene was linked to the Khc+ allele in a mutant yellow background. Consequently, bristles in the null clones were yellow, whereas bristles in sister clones and in nonrecombinant tissue were black. To generate control clones in parallel with test clones, the crosses were arranged to produce siblings with or without a wild-type Khc+ trans-gene in the genetic background. Homozygous Khcnull clones were found in the wings of both test and control classes (Figure 3). In test wings, 285 of 1650 wings (17%) contained null clones. In control wings, 274 of 1706 (16%) contained clones. The similarity in the frequencies of both classes of wing clones suggests that Khcnull cells in the wing imaginal disc can proliferate normally.
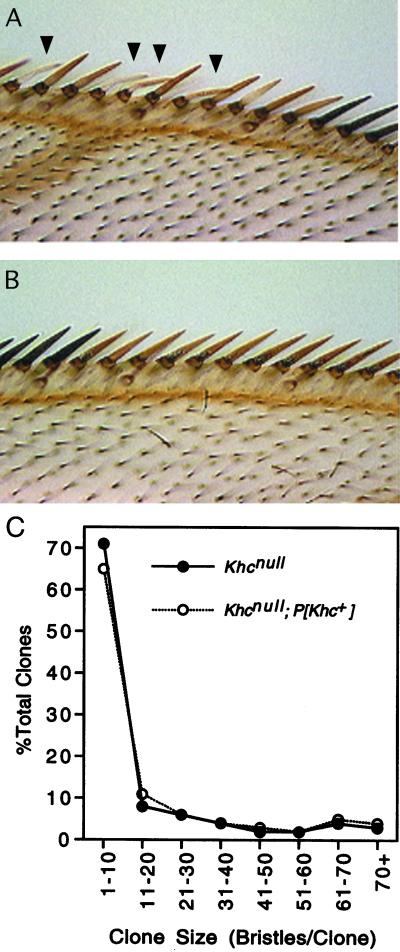
Figure 3.
Khcnull wing clones. Mitotic recombination was used to generate Khc27/Khc27 null imaginal cells in first instar larvae that were Khc27/Khc+ (test class) or Khc27/Khc+; P{mini-w+, Khc+}/+ (control class). (A) Anterior margin of a test class wing showing part of a clone derived from a null imaginal cell (yellow bristles). Some bristles were bent or kinked (arrowheads). (B) Part of a clone in a control class wing (yellow bristles). (C) Comparison of the size distributions of test class (Khcnull) and control class (Khcnull; P{mini-w+, Khc+}) wing clones. Sample sizes were 285 test clones (filled circles) and 274 control clones (open circles). Log linear analysis indicated that the two size distributions were indistinguishable. (See MATERIALS AND METHODS for full genotypes.)
A loss of KHC might slow rather than prevent cell proliferation. To explore this possibility, the size of each wing clone was determined by counting the number of yellow mechanosensory bristles on the anterior wing margin. The maximum sizes and size distributions of clones in the test and control classes were indistinguishable (Figure 3C and Table 1). The largest clone found in a test class wing contained 75 mechanosensory bristles. Based on previous studies, a wing clone that includes this number of mechanosensory bristles would encompass ∼600 total wing cells (Garcia-Bellido and Merriam, 1971). Therefore, at least nine rounds of division could be completed at normal rates after a wing imaginal cell became null. These results are consistent with those from the eye twin spot analysis; a severe depletion of KHC does not have any detectable effect on either the extent or the rate of imaginal cell proliferation.
Table 1.
Size distribution of Khcnull/Khcnull and Khcnull/Khcnull; P[mini-w+, Khc+] wing clones
| No. of mechanosensory bristles in clone | No. of cells in clone | Cell cycles required to produce clone | No. of Khcnull clones (%) | No. of Khcnull; P[Khc+] clones (%) |
|---|---|---|---|---|
| 1 | 5–8 | 3 | 61 (21) | 49 (18) |
| 2 | 9–16 | 4 | 60 (21) | 53 (19) |
| 3–4 | 17–32 | 5 | 32 (11) | 34 (12) |
| 5–8 | 33–64 | 6 | 35 (12) | 35 (13) |
| 9–16 | 65–128 | 7 | 33 (12) | 30 (11) |
| 17–32 | 129–256 | 8 | 24 (8) | 25 (9) |
| 33–64 | 257–512 | 9 | 24 (8) | 30 (11) |
| 65–79 | 513–632 | 10 | 16 (6) | 18 (7) |
The validity of the interpretation above depends on how completely KHC was eliminated in the mutant clones. The alleles we used were null; however, the founder of a Khcnull clone retained gene products inherited from its heterozygous precursor cell. A previous study of cytoplasmic dynein function by mitotic recombination in imaginal discs showed that this sort of perdurance allowed only one to three rounds of division in the wing disc, and that adult eye clones were not visible (Gepner et al., 1996). A similar study in eye imaginal cells of ROP, a Sec1p homologue important in the secretion pathway, suggested that perdurance might support one to two divisions but would not allow differentiation of adult cells (Harrison et al., 1994). In light of these and other clonal analysis studies, the 9–11 rounds of cell division and the differentiation that occur in Khcnull imaginal clones are striking results.
To estimate the degree to which KHC was eliminated in our experiments, we considered the rates of dilution of wild-type Khc gene products. A cell in which mitotic recombination occurred had one wild-type copy of the Khc gene and one null copy. Because KHC dosage in flies appears to depend on gene dosage (Figure 1B), we assume that the cell contained 50% of the normal concentration of KHC mRNA and protein. After the recombination event and cytokinesis, growth of the Khcnull daughter cell would dilute the inherited Khc products to 25% of the normal wild-type concentration. Further dilution (D) in this lineage attributable to successive rounds of cell growth and division can be estimated as D = 4n, where n is the number of progeny cells in the mutant clone. By this estimate, in the largest null eye clones studied (∼2800 cells) the dilution of the inherited KHC mRNA and protein was >10,000-fold. In clones as small as 128 cells (16 wing bristles or 6 eye facets) the dilution factor would be >500-fold. Beyond this simple calculation of dilution attributable to cell division and growth, one must consider the relationship between the synthesis of new KHC from the residual mRNA and the loss of KHC protein and mRNA caused by normal turnover. We suggest that these antagonistic processes, which should balance in normal cells, will remain balanced in the Khc mutant cells and thus are not serious concerns. If one accepts this and our premise that a 500- to 10,000-fold reduction in KHC by simple dilution would effectively eliminate its functions, our data argue that kinesin is not required for essential processes in proliferating imaginal cells.
Effects of Kinesin Loss on Photoreceptor Cells
To address the possibility that dividing imaginal cells might not challenge the secretion pathway sufficiently to reveal kinesin's functions, we analyzed post-mitotic differentiating cells that depend heavily on membrane growth and secretion. The Drosophila compound eye consists of approximately 750 ommatidia, each composed of a columnar cluster of eight elongated photoreceptor cells surrounded by a thin layer of pigment cells (see Figure 5A and review by Wolff and Ready, 1993). Each photoreceptor cell has a light-sensing rhabdomere, which is a tightly packed array of 60,000 microvilli that extends along the length of the ommatidial column (∼50–100 μm). The specification of the various cells of an ommatidium and their differentiation require precise cell-cell signaling and a massive expansion of plasma membrane; from the ∼150 μm2 of an imaginal cell to ∼10,000 μm2 for a mature photoreceptor (reviewed by Wolff and Ready, 1993). Based on these considerations, even moderate defects in the secretory pathway should have dramatic consequences during eye differentiation.
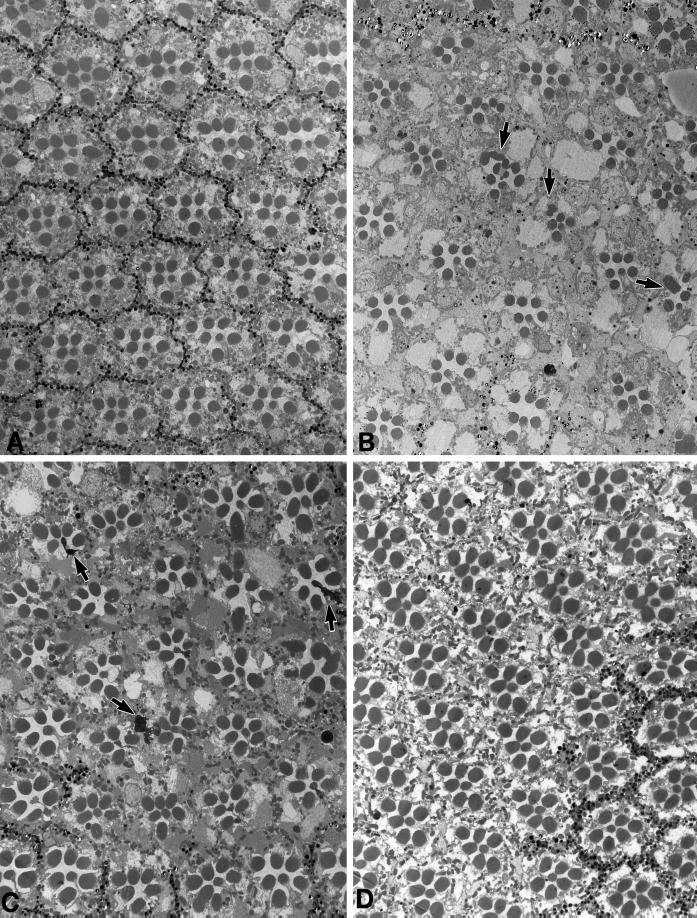
Figure 5.
Effects of a loss of KHC on photoreceptor cells. (A) Transmission electron micrograph of a cross section from a wild-type retina. Ommatidia normally have eight photoreceptor cells, each containing a photosensitive rhabdomere. The rhabdomeres of R1–6 are located around the outside of the ommatidium and extend along its entire length (∼100 μm). The rhabdomeres of R7 and R8 are located in the center with R7 stacked above R8. Thus, seven rhabdomere profiles are visible in most cross sections. (B) Portion of Khcnull eye clone from a newly eclosed fly. Khcnull ommatidia can be distinguished by the absence of the pigment granules that normally surround them. Note that a few null ommaditia have abnormal or missing photoreceptor cells (arrows). (C) Portion of a Khcnull eye clone from a fly aged 3 wk after eclosion. In addition to the structural defects seen in the photoreceptors of null clones in newly eclosed flies, some photoreceptors have degenerated (arrows). (D) Portion of a control Khcnull eye clone from a fly aged 3 wk after eclosion. The transgenic copy of wild-type Khc rescued the developmental defects as well as the age-dependent photoreceptor degeneration.
To look for signs of defects in the adult eye caused by a loss of kinesin function, we examined mitotic clones by electron microscopy. Matings were arranged to produce two types of sibling progeny that carried Khcnull clones: a control class with a rescuing Khc+ transgene and a test class without the transgene. Test and control clones were mapped and photographed with a light microscope and then examined with a scanning electron microscope (Figure 4). Slightly roughened eye surfaces were seen within test clones, suggesting defects in the underlying cells. To characterize those defects, 25 test and 5 control clones from newly eclosed flies were sectioned and examined by TEM (Figure 5). No defects were detected in control clones (Figure 5D). In test clones (Figure 5, B and C), ∼20% of the ommatidia (n = 1036 ommatidia) were missing one or two photoreceptors. This loss, which is characteristic of mild defects in postmitotic differentiation (Renfranz and Benzer, 1989), reduced the total number of photoreceptor cells in the clones by 5% (n = 8288 possible photoreceptors). In flies aged for >2 wk after eclosion, degenerating photoreceptors were seen at a low frequency (Figure 5C). This age-dependent degeneration may be a result of defective fast transport in photoreceptor axons (R.P. Brendza, unpublished observations work in progress). In addition, some photoreceptors in null clones showed structural defects, including disordered packing of microvilli and split or buckled rhabdomeres (Figure 5, B and C). The number of photoreceptors with such abnormal rhabdomeres varied from clone to clone but never exceeded 5–10%. The missing and malformed photoreceptors in newly eclosed flies altered the shapes of their ommatidia and hence caused disorder in the ommatidial array, which accounted for some of the surface roughness seen by SEM. These defects appeared equally severe in small and large clones, confirming that the decline of KHC function in a null clone was fairly complete after only a few cell cycles.
Figure 4.
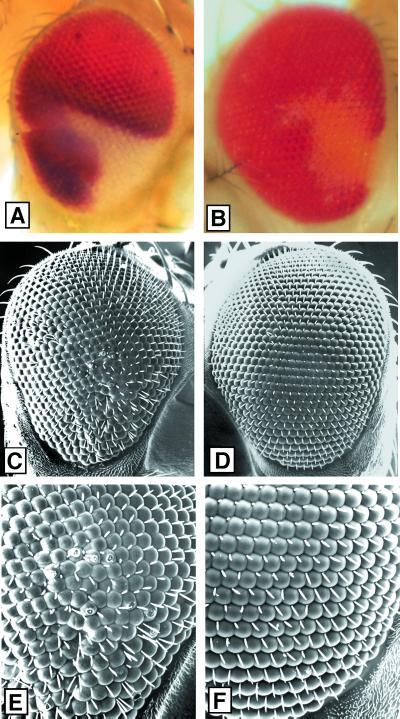
Effects of a loss of KHC on the eye surface. Mitotic recombination was used to generate Khc20/Khc20 null imaginal cells in first instar larvae that were Khc20/Khc+ (test class) or Khc20/Khc+; P{mini-w+, Khc+}/+ (control class). (A, C, and E) Clone in a test class eye (white facets). (B, D, and F) Clone in a control class eye (pale orange facets). (A and B) Light micrographs that show the positions of the clones. No roughening of the eye surface was detected in any clones at this level of resolution. (C–F) Scanning electron micrographs of the same eyes in the same orientations at low (C and D) and at high (E and F) magnification. Note that the test clone shows slight roughness attributable to misalignment of facets and bristles, whereas the control clone does not.
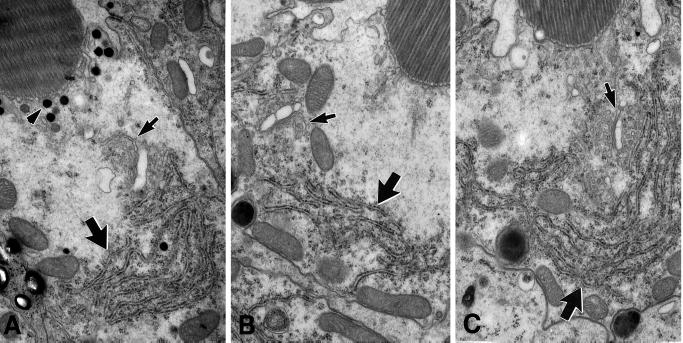
Based on studies in both Saccharomyces cereviseae and Drosophila, specific defects in ER and Golgi organization are expected when vesicle traffic in either the recycling pathway or the late secretory pathway is inhibited. Those defects include swelling or abnormal morphology of the ER, fragmentation or absence of the Golgi, increased accumulation of transport vesicles, and, in photoreceptor cells, a dramatic reduction of rhabdomere size (Novick et al., 1980; Colley et al., 1991; Lewis and Pelham, 1996; Mulholland et al., 1997; Satoh et al., 1997). To determine whether such abnormalities were present in Khcnull clones, we studied serial thin sections of photoreceptor cells from the level of the nucleus, where both Golgi and ER can be seen. No dramatic differences in the organization of rough ER or Golgi membranes were seen in null and neighboring wild-type cells (Figure 6). This was true for null photoreceptors with or without contorted rhabdomeres. Occasionally, Khcnull photoreceptors showed a slight increase in the abundance of ER near the Golgi (Figure 6C, large arrow) and slightly increased levels of vesicles and multivesicular bodies. However, dramatic defects in cytoplasmic structures and reduction of rhabdomere size, characteristic of mutations known to affect the secretory pathway (Colley et al., 1991; Satoh et al., 1997), did not occur in the null photoreceptors. Furthermore, no defects in the positioning of mitochondria or nuclei, were observed. Overall, although the organization of photoreceptor cell cytoplasm was mildly affected by a loss of KHC, ultrastructural changes known to correlate with serious disruptions of the recycling and secretory pathways were not seen.
Figure 6.
ER and Golgi organization in Khcnull photoreceptor cells. The three photoreceptor cells pictured here were from adjacent ommatidia located on the border of an eye clone that contained >1400 Khcnull cells. (A) Cross section of a wild-type photoreceptor cell. (B and C) Equivalent cross sections of null photoreceptor cells. Arrows indicate rough ER (large arrows) and Golgi bodies (small arrows). The genotypes of all cells examined were assessed by the presence (wild-type; Khc27/+ or +/+) or absence (null: Khc27/Khc27) of ommachrome pigment granules (A, arrowhead) adjacent to the base of the rhabdomere. Golgi morphology and ER organization in null and wild-type photoreceptors showed no consistent difference.
During eye development, cell specification is controlled through cell–cell signaling, which requires membrane proteins and secreted morphogens (Heberlein et al., 1993, 1995; Ma et al., 1995) (reviewed by Dickson and Hafen, 1993; Wolff and Ready, 1993). If photoreceptors in Khcnull test clones were aberrant or missing as a consequence of poor signaling, the effect should have been nonautonomous: mutant cells could cause defects in neighboring wild-type cells, or wild-type cells could rescue the defects in neighboring mutant cells. The margins of Khcnull eye clones contained mosaic ommatidia with mixtures of Khcnull and wild-type photoreceptor cells. Wild-type photoreceptors were distinguished by the presence of small ommachrome pigment granules at the bases of their rhabdomeres (Figure 6A, arrowhead). Pigment granules are absent in homozygous Khcnull photoreceptors because they lack a functional copy of the w gene, which is required for ommochrome pigment granule production. To assess autonomy, 246 mosaic ommatidia at clone borders (∼1700 photoreceptor cells) were examined. Defective rhabdomeres were seen only in Khcnull photoreceptors and never in adjacent wild-type cells. Also, there were no indications that wild-type cells could prevent the defects seen in neighboring Khcnull cells. In agreement with this, ommatidia with defective or missing photoreceptors were distributed randomly throughout Khcnull test clones; i.e., the frequency of defects was no greater at the centers of large clones than at their margins. These observations suggest that the defects seen were cell autonomous and thus were not due to defective delivery of membrane associated or secreted signaling molecules.
Effects of Kinesin Loss on Mechanosensory Bristles
A mechanosensory bristle shaft forms as a fluted cylinder of cuticle around a long cytoplasmic extension that projects outward from a trichogen cell body. During bristle shaft differentiation, which occurs in pupae, the core of the extension contains many parallel microtubules running from the base toward the tip (Tilney et al., 1995) (reviewed by Fristrom and Fristrom, 1993). Because the Golgi and nucleus of the trichogen are located in the cell body beneath the pupal epidermis, it is likely that secretion of the shaft cuticle components requires a great deal of vesicular traffic from the Golgi into and along the shaft-forming extension. The mechanosensory bristles in Khcnull wing clones were sometimes kinked (see Figure 3A, arrowheads). To examine possible cuticle secretion defects in more detail, we examined Khcnull clones throughout the adult epidermis. The longest null bristles had defects that were obvious even with a low-power light microscope. Some lay flat or twisted along the epidermal sheet rather than projecting outward from its surface. A number of those that did project outward were tested by direct mechanical manipulation. Beheaded flies will remain viable for several days in a moist chamber. They stand motionless but can respond to bristle deflections with reflex grooming behaviors (Vandervorst and Ghysen, 1980; Burg et al., 1993). Outside of test clones, the deflection of individual bristles with a tungsten needle caused normal pivoting at the base and elicited grooming reflexes. Inside test clones, bristles were so flaccid that attempted deflections usually caused a bend or kink rather than the pivoting needed to elicit a grooming reflex.
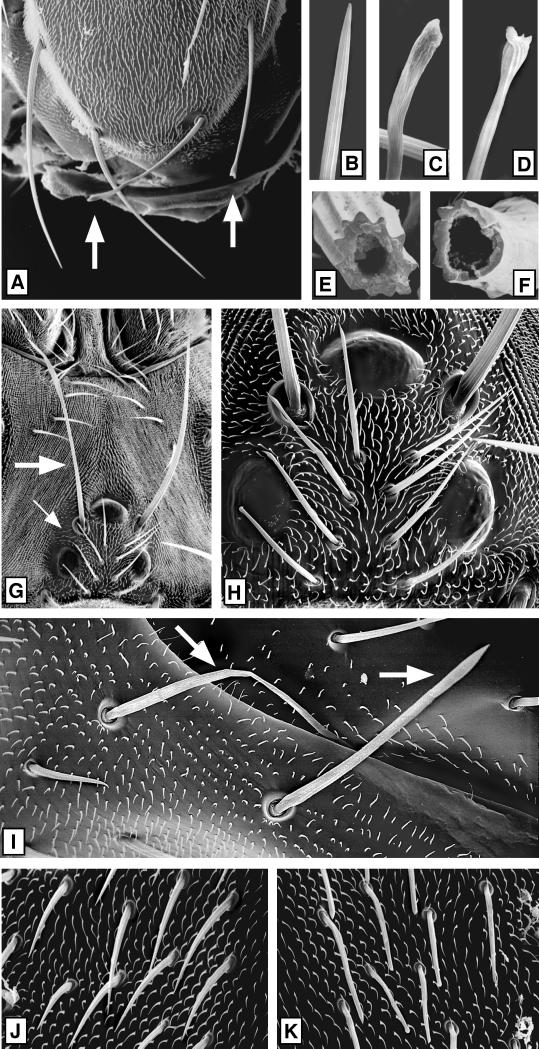
Bristles from null and control clones were studied in detail by SEM (Figure 7). Khcnull bristles had a variety of structural defects (e.g., Figure 7, C, D, and F), whereas wild-type or control bristles were normal (e.g., Figure 7, B and E). The longest test class bristles, the scutellar macrochaetae (300–400 μm), were usually ∼20% shorter than the analogous wild-type bristles (Figure 7A). This length defect was less evident in shorter macrochaetae and was not seen in microchaetae (Figure 7, H, J, and K). The tips of test bristles were often contorted, and the contortions were most severe at the tips of long machrochaetae, which always exhibited flattened, flared, or twisted tips (e.g., Figure 7, A, C, and D). Mutant microchaetae (65–70 μm) showed less dramatic defects, such as bluntness or slight tip swelling (Figure 7, H and K). No defects were observed in the remainder of the integument, including the bristle sockets, the tiny (10–15 μm) nonsensory hairs of the epidermal cells, or the epidermal cuticle sheet (Figures 3, A and B, and 7). As with the eye and wing clones, the severities of head, thoracic, and abdominal bristle defects were not detectably affected by clone size.
Figure 7.
Effects of a loss of KHC on secreted cuticle structures. Mitotic recombination was used to generate Khc20/Khc20 null clones marked with yellow bristles as for Figure 3. Clones were mapped by light microscopy and then examined in detail by SEM. (A) Scutellum with a large null clone covering the right half. Two null scutellar bristles (∼400 μm long) are marked by arrows. (B–D) High-magnification views of the tips of three of the scutellar bristles shown in A. (B) Wild type, (C and D) null. (E and F) Bases of wild-type (E) and Khcnull (F) scutellar bristle shafts that were broken off near their sockets. (G) Dorsal side of a head containing a Khcnull clone. The single macrochaete (∼280 μm long; large arrow) and the four microchaetae (small arrow) on the left side were null, whereas the analogous bristles on the right side were wild type. (H) A higher-magnification view shows that the null microchaetae (∼80 μm long) have slight tip abnormalities. (I) Mutant abdominal macrochaetae (∼170 μm long; white arrows). Note that the null sockets and surrounding sheet of cuticle appear normal here and in the other micrographs. (J) High-magnification view of wild-type microchaetae (∼70 μm long) and epidermal hairs (∼10 μm long) from the left side of a thorax. (K) Analogous area on the right side of the same thorax in the center of a large null clone. Note that mutant microchaetae in the clone have aberrant tips, whereas there are no apparent defects in the epidermal hairs.
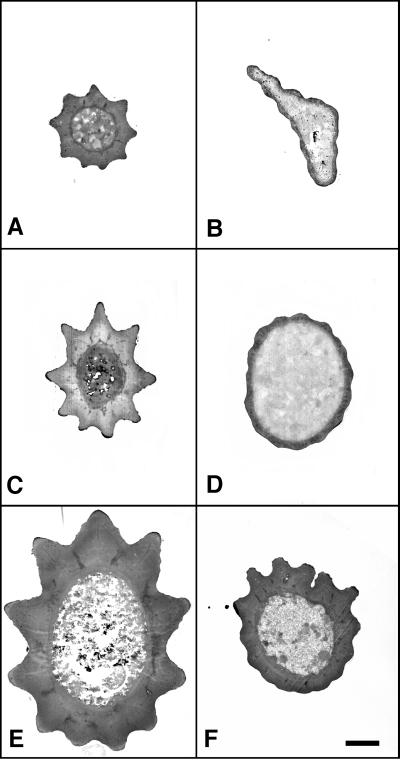
The evident weakness of Khcnull bristle shafts suggested that cuticle secretion from the shaft-forming extensions of trichogen cells was defective during differentiation. Consistent with this, SEM images showed defects in cuticle fluting (Figure 7, E and F). To study bristle cuticle pattern and thickness in more detail, serial cross-sections of wild-type and Khcnull scutellar bristles were compared by TEM (Figure 8). Overall, the cuticle layers of null bristles were quite thin. This effect was more pronounced at the tips of bristles than at their bases. Sections from bristles in null control clones carrying a wild-type Khc trans-gene were indistinguishable at all levels from wild type. These results suggest that KHC is critical for long-distance transport of secretory vesicles that bear cuticle precursors from the Golgi into and along the bristle shaft-forming extension. However, the fact that some cuticle secretion occurred even at the tips of the longest null bristles suggests that vesicle transport can continue at a low level despite the absence of KHC.
Figure 8.
Defects in the cuticle thickness of Khcnull bristles. Wild-type (A, C, and E; Khc20/Khc+ or Khc+/Khc+) and null (B, D, and F; Khc20/Khc20) anterior scutellar macrochaetae (∼340 μm long) were serial sectioned completely. Pairs of sections from equivalent positions along the lengths of the bristles are shown. (A and B) Tip; (C and D) middle; (E and F) base. In E and F, the images are oriented with the dorsal sides of the bristles at the top. Note that the cuticle of the Khcnull bristle is thin at the middle and tip. Bar, 500 nm.
DISCUSSION
Conventional kinesin has long been suspected of being a vesicle motor. Initially this stemmed from its discovery in axoplasm (Brady, 1985; Vale et al., 1985), which is rich in Golgi-derived transport vesicles, and its co-localization with vesicles in cultured cells (Pfister et al., 1989). Since then, a number of studies have focused on the identification of specific types of vesicles that kinesin might carry, but the results have not provided a consistent answer (reviewed by Lane and Allan, 1998). For example, in a study of vesicle/tubule transport in the recycling pathway, antibody inhibition of KHC blocked brefeldin A-induced movement of Golgi membranes into the ER in cultured NRK cells (Lippincott-Schwartz et al., 1995). Conversely, antisense oligonucleotide inhibition of KHC in cultured rat astrocytes (Feiguin et al., 1994) and gene disruption in cultured mouse extraembryonic cells (Tanaka et al., 1998) did not prevent brefeldin A-induced Golgi-to-ER membrane transfer. With regard to Golgi-to-plasma membrane vesicle transport, antisense oligonucleotide inhibition of KHC in cultured vertebrate neurons impaired delivery of vesicles containing certain synaptic proteins to axon terminals (Ferreira et al., 1992). In contrast, Khc mutations in Drosophila and Caenorhabditis elegans did not prevent the accumulation of normal levels of synaptic vesicles at axon terminals (Hall et al., 1991; Gho et al., 1992).
It has also been proposed that conventional kinesin is a motor for other elements of the cytoplasm, including mitochondria, lysosomes, ER, and intermediate filaments (Yabe et al., 1999) (reviewed by Goodson et al., 1997; Lane and Allan, 1998; Goldstein and Philp, 1999). However, function disruption tests have again yielded conflicting data. Antisense oligonucleotide inhibition of KHC in cultured rat astrocytes caused a retraction of the ER network (Feiguin et al., 1994). In contrast, antibody inhibition of KHC in sea urchin embryonic cells (Wright et al., 1993) or gene disruption in mouse extraembryonic cells (Tanaka et al., 1998) had no dramatic effects on ER organization. Antibody inhibition of KHC in human fibroblasts (Rodionov et al., 1993) and gene disruption in mouse extraembryonic cells (Tanaka et al., 1998) caused mitochondria, which are normally dispersed throughout the cytoplasm, to cluster near the cell center. However, no effect on mitochondrial distribution was seen in rat astrocytes injected with Khc antisense oligonucleotides (Feiguin et al., 1994) or in mutant strains of C. elegans (Hall et al., 1991) and fungi (Lehmler et al., 1997; Seiler et al., 1997).
In the studies mentioned above, some of the disagreements might be due to a lack of specificity in KHC function disruption, incomplete KHC inhibition, variability in the way brefeldin A perturbs different cell types, and variations in physiology caused by cell culture conditions. Attempting to minimize these potential caveats, we have studied the fates of clones of Khc null cells carried in otherwise healthy, intact Drosophila. The clonal mutant phenotypes we saw were rescued by introduction of a wild-type Khc trans-gene, indicating that the defects were indeed due to specific elimination of KHC. In addition, the loss of KHC function over the course of a given test appeared complete. The dilution of Khc gene products in the largest null clones was >10,000-fold, and the severity of differentiation defects seen in small clones was equal to that seen in the largest clones. Thus it appears that the loss of kinesin function was effectively complete after only a few cell division cycles. Overall, in the context of the cells and processes we have examined in Drosophila, our results suggest 1) that conventional kinesin is not essential for vesicle movement from the Golgi to either the ER or the plasma membrane in most cells, 2) that it is important for proper long-range vesicle transport in some elongated cells, and 3) that it is not required for the proper distribution of ER or mitochondria, at least in photoreceptor cells. Our data do not address the possibility that intermediate filaments and lysosomes are transported by conventional kinesin because Drosophila lack intermediate filament proteins and because lysosomes in the Drosophila tissues that we analyzed have not been characterized.
Our comparisons of control and Khcnull clones indicate that in the undifferentiated cells of imaginal discs, neither the rates nor the extents of cell proliferation are affected by a depletion of KHC. This should put to rest any lingering suspicion that conventional kinesin might have an essential role in mitosis. Furthermore, it shows that conventional kinesin is not essential for any of the interphase motility processes required for imaginal cell growth. Whether the positioning of ER, lysosomes, or mitochondria is critical for cell growth is not clear. However, it is clear that vesicle transport in the secretory pathway is essential. For instance, in S. cereviseae, mutations that inhibit the Golgi-to-ER recycling pathway (Novick et al., 1980; Lewis and Pelham, 1996) or the Golgi-to-plasma membrane “late” secretory pathway (Novick and Schekman, 1979; Novick et al., 1980; Ramirez et al., 1983) cause a rapid halt to cell growth and division. More directly relevant to our results, when mitotic recombination was used in Drosophila to create cells null for Rop, a homologue of the late secretory gene SEC1 of yeast (Novick et al., 1980), imaginal cells could not proliferate sufficiently to produce clones of adult cells (Harrison et al., 1994). Mutations in syntaxin, which encodes a t-SNARE thought to interact with ROP protein, also block imaginal cell proliferation (Schulze and Bellen, 1996). Thus, the proliferation and development of Drosophila imaginal cells is indeed sensitive to disruption of known elements of the secretion pathway (reviewed by Halachmi and Lev, 1996). The fact that in our tests imaginal cell proliferation was not affected by a loss of KHC suggests that both the recycling pathway and the late secretion pathway can operate at normal rates with little or no conventional kinesin in small cells (∼10 × 15 μm).
The defects in eye differentiation caused by a loss of KHC were mild and again are not consistent with major secretory pathway defects. Previous work has shown that disruption of the secretory pathway in photoreceptors should cause easily recognized changes in ultrastructure. For example, expression in the adult eye of a dominant negative form of a protein thought to function early in the secretory pathway, Rab1, causes hypertrophy and swelling of the ER, vesiculation or absence of the Golgi, and dramatic shrinkage of rhabdomeres (Satoh et al., 1997). Shrunken rhabdomeres are also caused by mutations that block the transport of vesicles bearing rhodopsin from the Golgi to the plasma membrane (Colley et al., 1991). Our data show that although a loss of KHC did cause some mild structural defects in a few photoreceptor cell bodies (∼10 × 100 μm), it did not cause shrunken rhabdomeres or any major changes in the organization of cytoplasmic organelles, including mitochondria, the nucleus, ER, and Golgi.
If conventional kinesin is dispensable for the secretion pathway in imaginal and retinal cells, as suggested by our results, how is vesicle movement away from the Golgi accomplished? Four alternative and nonexclusive force-generating systems come to mind: diffusion, minus end–directed microtubule motors, cytoplasmic myosins, and plus end–directed kinesin-related proteins.
Diffusion.
Imaginal cells are small and epithelial-like, with a microvillar tuft at the apical pole. Because of their small size, it is possible that simple diffusion of vesicles could compensate for a loss of plus end–directed, microtubule-based transport (reviewed by Bloom and Goldstein, 1998). However, studies of vesicles in live cells using quasielastic light scattering to view submicrometer motion or studies of green fluorescent protein–tagged vesicles to follow large-scale motion do not support the idea that simple vesicle diffusion is a major contributor to multimicrometer transport (Z. Kam, personal communication; Felder and Kam, 1994; Wacker et al., 1997).
Minus End Motors.
In some differentiated vertebrate epithelial cells, microtubule arrays have mixed polarity with some minus ends near the apical cortex and some near the cell center. Therefore, minus end–directed motors such as cytoplasmic dynein might be sufficient for vesicle transport in both directions (Fath et al., 1994). The polarity of interphase microtubules in rapidly dividing imaginal cells is not known, so we cannot formally eliminate this possibility. However, recent results suggest that in postmitotic eye imaginal cells, microtubules are oriented with their minus ends near the nucleus and their plus ends near the apical pole (Mosley-Bishop et al., 1999).
Myosins.
Many of the actin filaments present in proliferating cells are concentrated in the cortex (reviewed by Hartwig et al., 1985; Cramer, 1999). However, it is reasonable to suspect that enough actin filaments traverse the internal cytoplasm to provide tracks for long-distance myosin-driven vesicle transport. Also, it is clear that some myosins are active organelle motors (Fath et al., 1994; Tabb et al., 1998; Wu et al., 1998; reviewed by Mermall et al., 1998).
Plus End–directed Kinesin-related Motors.
This is an attractive possibility. There are several different types of motors in the kinesin superfamily that might function in the recycling and late secretion pathways, including kinesin-II (KRP85/95, KIF3A/B), UNC-104 (KIF1A), rab-kinesin 6, and others (reviewed by Goldstein and Philp, 1999). Whether these motors are expressed in imaginal cells is uncertain. However, it is reasonable to think that one or more of them is present and active in vesicle transport.
Our analysis shows that the elongated trichogen cells that create mechanosensory bristles (∼1–2 × 100–400 μm) clearly do require conventional kinesin for normal secretion of cuticle precursors. Previous studies of another elongated Drosophila cell type, the larval motor neuron (∼0.3 × 300–2000 μm), have shown that conventional kinesin is important in axons for membrane excitability, terminal growth, neurotransmitter secretion, and fast organelle transport (Gho et al., 1992; Hurd and Saxton, 1996; Hurd et al., 1996; Gindhart et al., 1998). In the shaft-forming extensions of trichogen cells, as in axons, parallel arrays of microtubules are prominent (Tilney et al., 1995; Hurd and Saxton, 1996; reviewed by Fristrom and Fristrom, 1993). In both motor axons and bristles, the loss of KHC has a graded effect; distal regions are most strongly affected, and the defects become more severe as cell length increases (Gho et al., 1992; Hurd and Saxton, 1996, this study). These observations suggest that conventional kinesin function is especially important for long-range vesicle movements, processes that are likely to demand efficient, highly processive transport machinery.
It is possible that conventional kinesin is a “specialty motor,” a major contributor only to long-distance transport in specialized cells, whereas more ordinary motility is accomplished by other motors, such as cytoplasmic myosins and kinesin-related proteins. The evolutionary conservation of KHC in metazoans and its ubiquitous expression in both undifferentiated and differentiated cell types argue against this hypothesis, but it remains possible. Alternatively, conventional kinesin could be a more general motor, combining with kinesin-related proteins and myosins as a contributor to both short- and long-range movement of a variety of organelles. In this case, the other organelle/vesicle motors could compensate for the absence of kinesin sufficiently to prevent dramatic defects in cells with transport tracks of normal length. However, as track length and the requirement for transport efficiency increases, the lack of kinesin would cause progressively more severe defects. That is consistent with what we have seen in this study. Some of the conflicting results seen in KHC function disruption tests (reviewed by Goodson et al., 1997; Lane and Allan, 1998) could then be due to differences in cell geometry and to variations in the sets of myosins and kinesin-related motors that are expressed in the different systems studied; that is, kinesin gets more or less support from other motors depending on cell size, cell type, and perhaps culture conditions. In either case, as has been found for mitotic chromosome movements, it is probable that interphase organelle/vesicle movements are driven by the cooperative activities of multiple types of motors and that monogamous motor–cargo relationships in common transport processes are rare. For both chromosomes and interphase organelles, a clear view of such cooperative or multilayered transport systems will require rigorous definitions of specific motor-cargo relationships, including linkage mechanisms and regulatory controls.
ACKNOWLEDGMENTS
We thank the members of the Saxton lab, Annette Parks, and Susan Strome for their insights and editing. This work was supported by a grant from the National Institutes of Health (R01 GM-46295). R.P.B. was supported by National Institutes of Health National Institute of General Medical Sciences predoctoral training grant T32 GM-07757-21. W.M.S. was supported by an Established Investigatorship from the American Heart Association with funds contributed in part by the American Heart Association, Indiana Affiliate.
REFERENCES
- Baumann O, Walz B. Topography of Ca2+-sequestering endoplasmic reticulum in photoreceptors and pigmented glial cells in the compound eye of the honeybee drone. Cell Tissue Res. 1989;255:511–522. [Google Scholar]
- Becker HJ. Uber rontgenmossaikflecken und defektmutationen am auge von Drosophila und die entwicklungsphysiologie des auges. Z Indukt Abstammungs Vererbungsl. 1957;88:333–373. [PubMed] [Google Scholar]
- Bi GQ, Morris RL, Liao G, Alderton JM, Scholey JM, Steinhardt RA. Kinesin- and myosin-driven steps of vesicle recruitment for Ca2+-regulated exocytosis. J Cell Biol. 1997;138:999–1008. doi: 10.1083/jcb.138.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom GS, Goldstein LS. Cruising along microtubule highways: how membranes move through the secretory pathway. J Cell Biol. 1998;140:1277–1280. doi: 10.1083/jcb.140.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ST. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985;317:73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Brady ST, Pfister KK, Bloom GS. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc Natl Acad Sci USA. 1990;87:1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendza KM, Rose DJ, Gilbert SP, Saxton WM. Lethal kinesin mutations reveal amino acids important for ATPase activation and structural coupling. J Biol Chem. 1999;274:31506–31514. doi: 10.1074/jbc.274.44.31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MG, Hanna L, Kim Y-T, Wu C-F. Development and maintenance of a simple reflex circuit in small-patch mosaics of Drosophila: effects of altered neuronal function and developmental arrest. J Neurobiol. 1993;24:803–823. doi: 10.1002/neu.480240608. [DOI] [PubMed] [Google Scholar]
- Colley NJ, Baker EK, Stamnes MA, Zuker CS. The cyclophilin homolog ninaA is required in the secretory pathway. Cell. 1991;67:255–263. doi: 10.1016/0092-8674(91)90177-z. [DOI] [PubMed] [Google Scholar]
- Cramer LP. Organization and polarity of actin filament networks in cells: implications for the mechanism of myosin-based cell motility. Biochem Soc Symp. 1999;65:173–205. [PubMed] [Google Scholar]
- deCuevas M. An Analysis of the Stalk of Drosophila Kinesin Heavy Chain. Ph.D. Thesis. Boston: Harvard University; 1993. [Google Scholar]
- Dickson B, Hafen E. In: Genetic dissection of eye development in Drosophila. In: The Development of Drosophila melanogaster. Bates M, Arias AM, editors. Plainview, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1327–1362. [Google Scholar]
- Elluru RG, Bloom GS, Brady ST. Fast axonal transport of kinesin in the rat visual system: functionality of kinesin heavy chain isoforms. Mol Biol Cell. 1995;6:21–40. doi: 10.1091/mbc.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath KR, Trimbur GM, Burgess DR. Molecular motors are differentially distributed on Golgi membranes from polarized epithelial cells. J Cell Biol. 1994;126:661–675. doi: 10.1083/jcb.126.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiguin F, Ferreira A, Kosik KS, Caceres A. Kinesin-mediated organelle translocation revealed by specific cellular manipulations. J Cell Biol. 1994;127:1021–1039. doi: 10.1083/jcb.127.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S, Kam Z. Human neutrophil motility: time-dependent three-dimensional shape and granule diffusion. Cell Motil Cytoskeleton. 1994;28:285–302. doi: 10.1002/cm.970280403. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Niclas J, Vale RD, Banker G, Kosik KS. Suppression of kinesin expression in cultured hippocampal neurons using antisense oligonucleotides. J Cell Biol. 1992;117:595–606. doi: 10.1083/jcb.117.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristrom D, Fristrom J. The metamorphic development of the adult epidermis. In: Bate M, Arias AM, editors. The Development of Drosophila melanogaster. Plainview, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 843–897. [Google Scholar]
- Garcia-Bellido A, Merriam JR. Parameters of the wing imaginal disc development of Drosophila melanogaster. Dev Biol. 1971;24:61–87. doi: 10.1016/0012-1606(71)90047-9. [DOI] [PubMed] [Google Scholar]
- Gepner J, Li M, Ludmann S, Kortas C, Boylan K, Iyadurai SJP, McGrail M, Hays TS. Cytoplasmic dynein function is essential in Drosophila melanogaster. Genetics. 1996;142:865–878. doi: 10.1093/genetics/142.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gho M, McDonald K, Ganetzky B, Saxton WM. Effects of kinesin mutations on neuronal functions. Science. 1992;258:313–316. doi: 10.1126/science.1384131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindhart JG, Desai CJ, Beushausen S, Zinn K, Goldstein LSB. Kinesin light chains are essential for axonal transport in Drosophila. J Cell Biol. 1998;141:443–454. doi: 10.1083/jcb.141.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LSB, Philp AV. The road less traveled: emerging principles of kinesin motor utilization. Annu Rev Cell Dev Biol. 1999;15:141–183. doi: 10.1146/annurev.cellbio.15.1.141. [DOI] [PubMed] [Google Scholar]
- Goldstein LSB, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci. 2000;23:39–72. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- Golic K. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- Golic KG, Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Goodson HV, Valetti C, Kreis TE. Motors and membrane traffic. Curr Opin Cell Biol. 1997;9:18–28. doi: 10.1016/s0955-0674(97)80147-0. [DOI] [PubMed] [Google Scholar]
- Halachmi N, Lev Z. The Sec1 family: a novel family of proteins involved in synaptic transmission and general secretion. J Neurochem. 1996;66:889–897. doi: 10.1046/j.1471-4159.1996.66030889.x. [DOI] [PubMed] [Google Scholar]
- Hall DH, Plenefisch J, Hedgecock EM. Ultrastructural abnormalities of kinesin mutant unc-116. J Cell Biol. 1991;115:389a. [Google Scholar]
- Harrison SD, Broadie K, van de Goor J, Rubin GM. Mutations in the Drosophila Rop gene suggest a function in general secretion and synaptic transmission. Neuron. 1994;13:555–566. doi: 10.1016/0896-6273(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Hartwig JH, Niederman R, Lind SE. Cortical actin structures and their relationship to mammalian cell movements. Subcell Biochem. 1985;11:1–49. doi: 10.1007/978-1-4899-1698-3_1. [DOI] [PubMed] [Google Scholar]
- Heberlein U, Singh CM, Luk AY, Donohoe TJ. Growth and differentiation in the Drosophila eye coordinated by hedgehog. Nature. 1995;373:709–711. doi: 10.1038/373709a0. [DOI] [PubMed] [Google Scholar]
- Heberlein U, Wolff T, Rubin G. The TGF-β homolog dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell. 1993;75:913–926. doi: 10.1016/0092-8674(93)90535-x. [DOI] [PubMed] [Google Scholar]
- Heidemann S, Lamders J, Hamborg M. Polarity orientation of axonal microtubules. J Cell Biol. 1981;91:661–665. doi: 10.1083/jcb.91.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Organelle transport along microtubules—the role of KIFs. Trends Cell Biol. 1996;6:135–140. doi: 10.1016/0962-8924(96)10003-9. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Kobayashi N, Pfister KK, Bloom GS, Brady ST. Kinesin associates with anterogradely transported membranous organelles in vivo. J Cell Biol. 1991;114:295–302. doi: 10.1083/jcb.114.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ. The distribution, abundance and subcellular localization of kinesin. J Cell Biol. 1989;108:2335–2342. doi: 10.1083/jcb.108.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houliston E, Elinson RP. Evidence for the involvement of microtubules, ER, and kinesin in the cortical rotation of fertilized frog eggs. J Cell Biol. 1991;114:1017–1028. doi: 10.1083/jcb.114.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd DD, Saxton WS. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics. 1996;144:1075–1085. doi: 10.1093/genetics/144.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd DD, Stern M, Saxton WM. Mutation of the axonal transport motor kinesin enhances paralytic and suppresses Shaker in Drosophila. Genetics. 1996;142:195–204. doi: 10.1093/genetics/142.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J, Allan V. Microtubule-based membrane movement. Biochim Biophys Acta. 1998;1376:27–55. doi: 10.1016/s0304-4157(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Johnston P, Morata G. Methods of marking cells. In: Roberts DB, editor. Drosophila: A Practical Approach. Oxford: IRL Press; 1986. pp. 229–242. [Google Scholar]
- Lehmler C, Steinberg G, Snetselaar KM, Schliwa M, Kahmann R, Bolker M. Identification of a motor protein required for filamentous growth in Ustilago maydis. EMBO J. 1997;16:3464–3473. doi: 10.1093/emboj/16.12.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold PL, McDowall AW, Pfister KK, Bloom GS, Brady ST. Association of kinesin with characterized membrane-bounded organelles. Cell Motil Cytoskeleton. 1992;23:19–33. doi: 10.1002/cm.970230104. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HR. SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell. 1996;85:205–215. doi: 10.1016/s0092-8674(00)81097-1. [DOI] [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG. The Genome of Drosophila melanogaster. San Diego: Academic Press; 1992. [Google Scholar]
- Lippincott-Schwartz J. Cytoskeletal proteins and Golgi dynamics. Curr Opin Cell Biol. 1998;10:52–59. doi: 10.1016/s0955-0674(98)80086-0. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Cole NB, Marotta A, Conrad PA, Bloom GS. Kinesin is the motor for microtubule-mediated Golgi-to-ER membrane traffic. J Cell Biol. 1995;128:293–306. doi: 10.1083/jcb.128.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Zhou Y, Beachy PA, Moses K. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell. 1995;75:927–938. doi: 10.1016/0092-8674(93)90536-y. [DOI] [PubMed] [Google Scholar]
- Marks DL, Larkin JM, McNiven MA. Association of kinesin with the Golgi apparatus in rat hepatocytes. J Cell Sci. 1994;107:2417–2426. doi: 10.1242/jcs.107.9.2417. [DOI] [PubMed] [Google Scholar]
- Martin ME, Hurd DD, Saxton WM. Kinesins in the nervous system. Cell Mol Life Sci. 1999;56:200–216. doi: 10.1007/s000180050422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays RW, Beck KA, Nelson WJ. Organization and function of the cytoskeleton in polarized epithelial cells: a component of the protein sorting machinery. Curr Opin Cell Biol. 1994;6:16–24. doi: 10.1016/0955-0674(94)90111-2. [DOI] [PubMed] [Google Scholar]
- McDonald KL. Electron microscopy and EM immunocytochemistry. Methods Cell Biol. 1994;44:411–444. doi: 10.1016/s0091-679x(08)60926-7. [DOI] [PubMed] [Google Scholar]
- McNiven MA, Marlowe KJ. Contributions of molecular motor enzymes to vesicle-based protein transport in gastrointestinal epithelial cells. Gastroenterology. 1999;116:438–451. doi: 10.1016/s0016-5085(99)70142-3. [DOI] [PubMed] [Google Scholar]
- Mermall V, Post PL, Mooseker MS. Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science. 1998;279:527–533. doi: 10.1126/science.279.5350.527. [DOI] [PubMed] [Google Scholar]
- Mosley-Bishop KL, Li Q, Patterson K, Fischer JA. Molecular analysis of the klarsicht gene and its role in nuclear migration within differentiating cells of the Drosophila eye. Curr Biol. 1999;9:1211–1220. doi: 10.1016/s0960-9822(99)80501-6. [DOI] [PubMed] [Google Scholar]
- Mulholland J, Wesp A, Riezman H, Botstein D. Yeast actin cytoskeleton mutants accumulate a new class of Golgi-derived secretory vesicle. Mol Biol Cell. 1997;8:1481–1499. doi: 10.1091/mbc.8.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P, Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1979;76:1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell. 1995;81:769–780. doi: 10.1016/0092-8674(95)90538-3. [DOI] [PubMed] [Google Scholar]
- Pfister KK, Wagner MC, Stenoien DL, Brady ST, Bloom GS. Monoclonal antibodies to kinesin heavy and light chains stain vesicle-like structures, but not microtubules, in cultured cells. J Cell Biol. 1989;108:1453–1463. doi: 10.1083/jcb.108.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez RM, Ishida-Schick T, Krilowicz BL, Leish BA, Atkinson KD. Plasma membrane expansion terminates in Saccharomyces cerevisiae secretion-defective mutants while phospholipid synthesis continues. J Bacteriol. 1983;154:1276–1283. doi: 10.1128/jb.154.3.1276-1283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfranz PJ, Benzer S. Monoclonal antibody probes discriminate early and late mutant defects in development of the Drosophila retina. Dev Biol. 1989;136:411–429. doi: 10.1016/0012-1606(89)90267-4. [DOI] [PubMed] [Google Scholar]
- Rodionov VI, Gyoeva FK, Tanaka E, Bershadsky AD, Vasiliev JM, Gelfand VI. Microtubule-dependent control of cell shape and pseudopodial activity is inhibited by the antibody to kinesin motor domain. J Cell Biol. 1993;123:1811–1120. doi: 10.1083/jcb.123.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh AK, Tokunaga F, Kawamura S, Ozaki K. In situ inhibition of vesicle transport and protein processing in the dominant negative Rab1 mutant of Drosophila. J Cell Sci. 1997;110:2943–2953. doi: 10.1242/jcs.110.23.2943. [DOI] [PubMed] [Google Scholar]
- Saxton WM, Hicks J, Goldstein LSB, Raff EC. Kinesin heavy chain is essential for viability and neuromuscular functions in Drosophila, but mutants show no defects in mitosis. Cell. 1991;64:1093–1102. doi: 10.1016/0092-8674(91)90264-y. [DOI] [PubMed] [Google Scholar]
- Saxton WM, Porter ME, Cohn SA, Scholey JM, Raff EC, McIntosh JR. Drosophila kinesin: characterization of microtubule motility and ATPase. Proc Natl Acad Sci USA. 1988;85:1109–1113. doi: 10.1073/pnas.85.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze KL, Bellen HJ. Drosophila syntaxin is required for cell viability and may function in membrane formation and stabilization. Genetics. 1996;144:1713–1724. doi: 10.1093/genetics/144.4.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler S, Nargang FE, Steinberg G, Schliwa M. Kinesin is essential for cell morphogenesis and polarized secretion in Neurospora crassa. EMBO J. 1997;16:3025–3034. doi: 10.1093/emboj/16.11.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers JR, Goodson HV. Motor proteins 2: myosin. Protein Profile. 1995;2:1323–1423. [PubMed] [Google Scholar]
- Sturmer K, Baumann O. Immunolocalization of kinesin and cytoplasmic dynein in the retina of the locust Schistocerca gregaria. Cell Tissue Res. 1996;286:547–549. doi: 10.1007/s004410050725. [DOI] [PubMed] [Google Scholar]
- Tabb JS, Molyneaux BJ, Cohen DL, Kuznetsov SA, Langford GM. Transport of ER vesicles on actin filaments in neurons by myosin. V J Cell Sci. 1998;111:3221–3234. doi: 10.1242/jcs.111.21.3221. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, Guild GM. F actin bundles in Drosophila bristles I. Two filament cross-links are involved in bundling. J Cell Biol. 1995;130:629–638. doi: 10.1083/jcb.130.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD, Fletterick RJ. The design plan of kinesin motors. Annu Rev Cell Dev Biol. 1997;13:745–777. doi: 10.1146/annurev.cellbio.13.1.745. [DOI] [PubMed] [Google Scholar]
- Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervorst P, Ghysen A. Genetic control of sensory connections in Drosophila. Nature. 1980;286:65–67. doi: 10.1038/286065a0. [DOI] [PubMed] [Google Scholar]
- Wacker I, Kaether C, Kromer A, Migala A, Almers W, Gerdes HH. Microtubule-dependent transport of secretory vesicles visualized in real time with a GFP-tagged secretory protein. J Cell Sci. 1997;110:1453–1463. doi: 10.1242/jcs.110.13.1453. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Desai A, Bulinski JC, Salmon ED. Fluorescent speckle microscopy, a method to visualize the dynamics of protein assemblies in living cells. Curr Biol. 1998;8:1227–1230. doi: 10.1016/s0960-9822(07)00515-5. [DOI] [PubMed] [Google Scholar]
- Wells AL, Lin AW, Chen LQ, Safer D, Cain SM, Hasson T, Carragher BO, Milligan RA, Sweeney HL. Myosin VI is an actin-based motor that moves backwards. Nature. 1999;401:505–508. doi: 10.1038/46835. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready D. Pattern formation in the Drosophila retina. In: Bates M, Arias AM, editors. The Development of Drosophila melanogaster. Plainview, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1277–1325. [Google Scholar]
- Wright BD, Henson JH, Wedaman KP, Willy PJ, Morand JN, Scholey JM. Subcellular localization and sequence of sea urchin kinesin heavy chain: evidence for its association with membranes in the mitotic apparatus and interphase cytoplasm. J Cell Biol. 1991;113:817–833. doi: 10.1083/jcb.113.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BD, Terasaki M, Scholey JM. Roles of kinesin and kinesin-like proteins in sea urchin embryonic cell division: evaluation using antibody microinjection. J Cell Biol. 1993;123:681–689. doi: 10.1083/jcb.123.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Bowers B, Rao K, Wei Q, Hammer JA. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. J Cell Biol. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Harrison SD. Mosaic analysis using FLP recombinase. Methods Cell Biol. 1994;44:655–681. doi: 10.1016/s0091-679x(08)60937-1. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin G. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Yabe JT, Pimenta A, Shea TB. Kinesin-mediated transport of neurofilament protein oligomers in growing axons. J Cell Sci. 1999;112:3799–3814. doi: 10.1242/jcs.112.21.3799. [DOI] [PubMed] [Google Scholar]
- Yang JT, Laymon RA, Goldstein LSB. A three-domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses. Cell. 1989;56:879–889. doi: 10.1016/0092-8674(89)90692-2. [DOI] [PubMed] [Google Scholar]
- Yu H, Toyoshima I, Steuer ER, Sheetz MP. Kinesin and cytoplasmic dynein binding to brain microsomes. J Biol Chem. 1992;267:20457–20464. [PubMed] [Google Scholar]