Abstract
XLαs is a splice variant of the heterotrimeric G protein, Gαs, found on Golgi membranes in cells with regulated and constitutive secretion. We examined the role of the alternatively spliced amino terminus of XLαs for Golgi targeting with the use of subcellular fractionation and fluorescence microscopy. XLαs incorporated [3H]palmitate, and mutation of cysteines in a cysteine-rich region inhibited this incorporation and lessened membrane attachment. Deletion of a proline-rich region abolished Golgi localization of XLαs without changing its membrane attachment. The proline-rich and cysteine-rich regions together were sufficient to target the green fluorescent protein, a cytosolic protein, to Golgi membranes. The membrane attachment and Golgi targeting of the fusion protein required the putative palmitoylation sites, the cysteine residues in the cysteine-rich region. Several peripheral membrane proteins found at the Golgi have proline-rich regions, including a Gαi2 splice variant, dynamin II, βIII spectrin, comitin, and a Golgi SNARE, GS32. Our results suggest that proline-rich regions can be a Golgi-targeting signal for G protein α subunits and possibly for other peripheral membrane proteins as well.
INTRODUCTION
Heterotrimeric G proteins are classically known to couple cell surface receptors to various membrane-bound effectors (Gilman, 1987; Hamm and Gilchrist, 1996). Each heterotrimer consists of an α subunit, which exchanges GDP for GTP upon activation by a receptor, and βγ subunits, which are tightly bound together. The α subunits attach tightly to membranes through posttranslational lipid modifications and binding to the relatively hydrophobic βγ complex. Different α subunits segregate into specific subdomains of the plasma membrane (Stow et al., 1991) and are also associated with the membranes of different intracellular organelles (Jones, 1994; Denker et al., 1996; Stow and Heimann, 1998). Within the cell, G proteins are involved in vesicular transport between the endoplasmic reticulum and the Golgi (Beckers and Balch, 1989; Hidalgo et al., 1995), through the Golgi stack (Melançon et al., 1987; Leyte et al., 1992; Stow and Heimann, 1998), and other membrane-trafficking steps (Bomsel and Mostov, 1992). The functional consequences of receptor–G protein–effector systems at selective membranes presumably require the proper targeting of G proteins. The strong homology between α subunits makes their geographic diversity a puzzling trafficking problem.
Mutagenesis studies on targeting signals for Gαi2 and Gαi3 (de Almeida et al., 1994) and the discovery of splice variations of α subunits that change their intracellular localization provide clues to this problem. XLαs (extra-large αs), a splice variant of Gαs, is particularly interesting because it is found on Golgi membranes in cells with both regulated and constitutive pathways of protein secretion (Kehlenbach et al., 1994a,b). This protein, identified as a cholera toxin substrate, is identical to Gαs except for exon 1, at which a 347-amino acid amino terminus replaces 47 amino acids in Gαs. The XL portion has proline-rich and cysteine-rich regions and areas of EPAA and AARA repeats. Another α subunit splice variant, sGαi2, also resides on Golgi membranes and is identical to Gαi2 except for the last exon, which encodes a different 35-amino acid, proline-rich carboxy terminus (Montmayeur and Borrelli, 1994).
The signals involved in the Golgi targeting of peripheral membrane proteins, including G proteins, are poorly understood (reviewed by Stanley, 1996; Gleeson, 1998; Munro, 1998). Sorting signals on intrinsic membrane proteins act to retain these proteins in the Golgi apparatus or to retrieve them from the cell surface or other organelles. The transmembrane domain is critical for retention in the Golgi by proposed mechanisms that include oligomerization and sorting based on the length of the transmembrane domain. Retrieval signals such as the di-leucine motif are found on the cytoplasmic domains of integral membrane proteins. For peripheral membrane proteins at the Golgi, some transiently associate with ADP-ribosylation factor proteins leading to translocation to Golgi membranes. For several coiled-coil proteins, a Golgi-targeting domain, the GRIP domain, has been identified in their carboxy termini (Barr, 1999; Kjer-Nielsen et al. 1999; Munro and Nichols, 1999). For others, regions have been identified that are crucial for localization. Endothelial nitric oxide synthase (eNOS), SCG10, and glutamate decarboxylase have Golgi-localizing regions at their amino termini that also contain sites for two or more lipid modifications (Solimena et al., 1994; Di Paolo et al., 1997; Liu et al., 1997).
Palmitoylation, the reversible addition of palmitate to cysteine residues by a thioester bond, occurs on the amino terminus of G protein α subunits (Linder et al., 1993) and has several functions. Palmitoylation increases receptor–G protein coupling and the affinity of the α subunit for the βγ complex (Iiri et al., 1996; Ponimaskin et al., 1998). For Gαs, receptor activation causes a rapid turnover of palmitate, suggesting a role in signaling (Degtyarev et al., 1993b; Mumby et al., 1994; Wedegaertner and Bourne, 1994). The importance of palmitoylation in gross membrane attachment is controversial (Wedegaertner et al., 1993; Huang et al., 1999), but it may play a more subtle role in targeting G proteins to membrane microdomains (Arni et al., 1998; Melkonian et al., 1999). XLαs does not have the acylation site found on Gαs but does have a cysteine-rich domain containing six cysteines that are potential sites for palmitoylation.
We investigated the Golgi targeting of XLαs by studying two regions in its XL amino terminus: the cysteine-rich region (CRR) and the proline-rich region (PRR), which bears similarity to the proline-rich carboxy terminus of sGαi2. We wished both to better understand the mechanisms involved in G protein targeting and to test the generality of the Golgi-localization signals suggested for eNOS and sGαi2. We found that the PRR and the cysteines in the CRR were critical for the Golgi targeting of XLαs.
MATERIALS AND METHODS
Cell Culture
COS-7 and HEK-293 cells were grown in DMEM supplemented with penicillin (100 IU/ml), streptomycin (100 μg/ml), and 10% (vol/vol) FBS at 37°C in a humidified atmosphere of 5% CO2. PC12 cells were maintained in identical conditions except that the serum supplementation was 5% (vol/vol) FBS and 10% (vol/vol) heat-inactivated horse serum.
Plasmid Constructs and Mutagenesis
The cDNA coding the 715-amino acid rat XLαs (Kehlenbach et al., 1994a,b) in a pCDNA3.1 (+) plasmid (Invitrogen, Carlsbad, CA) underwent site-directed mutagenesis by a PCR-based method, QuikChange (Stratagene, La Jolla, CA), except that Pwo Polymerase (Boehringer Mannheim, Indianapolis, IN) was used. To generate green fluorescent protein (GFP) fusion proteins, cDNAs encoding the following regions of XLαs were amplified from wild-type XLαs-pcDNA3.1 or the 2C and 0C cysteine-to-serine mutants with the use of PCR: 1) PRR (amino acids 201–235); 2) PRR plus CRR (amino acids 201–312); and 3) CRR (amino acids 225–312). cDNAs were inserted into the pEGFP N1 vector (Clontech, Palo Alto, CA) at a BamHI site upstream and in frame with the cDNA coding EGFP. Mutations were confirmed with the use of ABI PRISM dye terminator cycle sequencing (Perkin Elmer-Cetus, Norwalk, CT).
Transient Transfection of the Cells
COS-7 cells, grown to subconfluence in 75-cm2 tissue culture flasks, were transfected with the use of 2 μg of plasmid DNA per flask and the DEAE-dextran method, as described (Butrynski et al., 1992). PC12 cells, suspended in 0.8 ml of RPMI-1640 at a density of 4 × 106 cells/ml, were transfected by electroporation at 960 μF and 360 V with the use of a Bio-Rad (Hercules, CA) Gene Pulser and 40–50 μg of plasmid DNA. Transfected PC12 cells were seeded into two-well chamber slides in RPMI-1640 supplemented with 10% FBS. The medium was changed to DMEM with 5% FBS and 10% heat-inactivated horse serum after 24 h. Metabolic labeling and immunodetection were performed 2 d after transfection. HEK-293 cells were transfected with the use of the LipofectAMINE PLUS reagent (GIBCO-BRL/Life Technologies, Grand Island, NY).
[3H]Palmitate Labeling and Cell Fractionation
For metabolic labeling, cells were first incubated for 2 h in serum-free DMEM and then in 5 ml of serum-free DMEM containing 500 μCi/ml [3H]palmitic acid (specific activity, 60 Ci/mmol; American Radiochemical, St. Louis, MO) for 50 min. Cells were harvested by scraping in 45 ml of ice-cold PBS and centrifuged at 2000 × g for 10 min, and cell pellets were stored at −70°C. The pellets were lysed, homogenized, and fractionated into particulate and soluble fractions by centrifugation at 125,000 × g for 1 h as described (Degtyarev et al., 1993a). Protein concentrations were determined with the use of the Bio-Rad assay (Bradford, 1976) with immunoglobulin G as the standard.
Immunoprecipitation and Immunoblotting
The polyclonal, affinity-purified antibody for Gαs (RM) and antibody for Gαi (AS), which recognize the carboxy-terminal decapeptide of Gαs and XLαs and the carboxy-terminal decapeptide of Gαi, respectively, were used (Jones et al., 1990). Equal amounts of protein (300–400 μg) from the particulate fractions were incubated with the antibody in 500 μl of solubilization buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% [vol/vol] Triton X-100, 0.2% [wt/vol] SDS, 1 mM EDTA) overnight at 4°C. The immunoprecipitates were recovered by a 2-h incubation with protein A–Sepharose CL-4B (Pharmacia LKB Biotechnology, Piscataway, NJ), washed, separated by SDS-PAGE, and prepared for fluorography as described previously (Jones et al., 1990). Densitometry of the fluorographs was performed with an AGFA Arcus II scanner (Pharmacia LKB Biotechnology) and NIH Image software (http://rsb.info.nih.gov/nih-image/). For immunoblots, 5 μg of protein from each particulate and soluble fraction was separated by SDS-PAGE and transferred to nitrocellulose paper. The proteins were detected with the RM antibody (1 μg/ml) or a polyclonal antibody raised against the GFP (1:3000) (Molecular Probes, Eugene, OR) and ECL (Amersham, Arlington Heights, IL) as described by the manufacturer.
Cholera Toxin Labeling and Detergent Solubilization
Particulate fractions (75 μg of protein) were incubated with 300 mM potassium phosphate, pH 7.0, 10 mM thymidine, 1 mM ATP, 0.1 mM GTP, 10 mM MgCl2, 1 mM EDTA, protease inhibitors, 6 μg of activated cholera toxin (Ribeiro-Neto et al., 1985), 1 mM DTT, 10 μg of purified brain βγ subunits (Sternweis and Robishaw, 1984), 5 μM NAD, and 10 μCi of [32P]NAD (specific activity, 1000 Ci/mmol; Amersham) in a volume of 60 μl for 45 min at 37°C. The reaction was stopped, and the samples were prepared for SDS-PAGE as described (Ribeiro-Neto et al., 1985). The gels were analyzed by autoradiography and a Phosphorimager (Molecular Dynamics, Sunnyvale, CA). Solubilization of proteins in the particulate fractions was performed with 1% (vol/vol) Triton X-100 as described previously (Jones and Gutkind, 1998).
Immunocytochemistry and Fluorescence Microscopy
Cells, grown and transfected in two-well chamber slides, were washed four times with PBS at room temperature, fixed and permeabilized by incubation in methanol at −20°C for 3 min, and washed four times with PBS. Cells expressing the GFP fusion proteins were fixed with 2% (wt/vol) paraformaldehyde in PBS for 20 min and permeabilized with 0.1% (wt/vol) Triton X-100 in PBS for 15 min. After incubation for 15 min in the blocking buffer (1% normal goat serum, 0.2% [vol/vol] Triton X-100 in PBS), cells were incubated for 1 h at room temperature in 0.1% (wt/vol) BSA in PBS with the RM antibody at 0.5 ng/ml and with the anti-58-kDa protein monoclonal mouse antibody (Sigma Chemical, St. Louis, MO) at a 1:100 dilution. After washing four times with 0.1% BSA in PBS, cells were incubated for 1 h at room temperature with one of the following secondary antibodies (Jackson ImmunoResearch, West Grove, PA): Cy3-labeled anti-rabbit (1:4000), FITC-labeled anti-mouse (1:100), or Cy3-labeled anti-mouse (1:100) antibody. Cells, washed four times with 0.1% BSA in PBS and once with distilled water, were mounted in Prolong antifade reagent (Molecular Probes) and visualized with the use of a Zeiss (Thornwood, NY) Axioskop microscope equipped for fluorescence microscopy with a 63×, 1.4 numerical aperture Plan-Apochromat oil immersion objective or a Leica (Wetzlar, Germany) LSM confocal microscope. For Golgi labeling of live PC12 cells with BODIPY FL C5-ceramide (Molecular Probes), the method described by Ktistakis et al. (1995) was used. For treatment with brefeldin A, transfected COS cells were incubated with BODIPY TR ceramide (1 μM, mixed with equimolar defatted BSA in serum-free DMEM; Molecular Probes) for 30 min at 4°C, washed three times with DMEM with 10% FBS, and incubated at 37°C for 30 min. Brefeldin A (Epicentre Technologies, Madison, WI) was added at a final concentration of 5 μM, and cells were incubated for another 15 min, washed three times with PBS, and mounted and visualized as described above.
Quantitative Analysis of the Immunofluorescence
Cells were counted if they were brightly stained with RM antibody or GFP fluorescence, indicating that they had undergone transfection. The cells showing staining that colocalized with the anti-58-kDa antibody staining of a dense, perinuclear area were counted as positive with respect to the Golgi localization of XLαs proteins. Transfections were done in parallel, and coded slides were used for this quantitation.
RESULTS
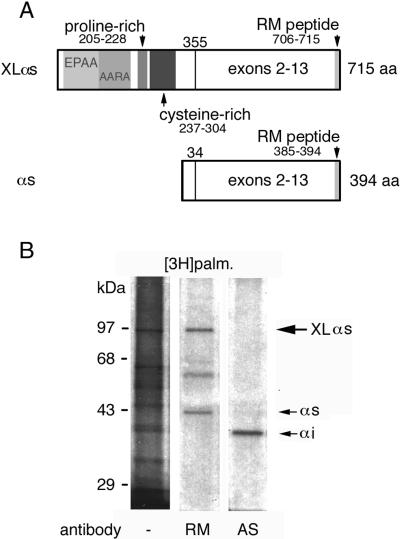
Endogenous XLαs Undergoes Palmitoylation in PC12 Cells
Gαs undergoes palmitoylation at its amino terminus (Linder et al., 1993). XLαs lacks the cysteine residue at the third position of Gαs that is critical for the modification (Degtyarev et al., 1993a) but has six cysteines in a cysteine-rich domain of its so-called XL portion (Figure 1A; Table 1). To determine if endogenous XLαs is palmitoylated, PC12 cells were metabolically labeled with [3H]palmitic acid and proteins were immunoprecipitated with the RM antibody, an antibody specific for the carboxy terminus of Gαs and XLαs (Figure 1A). Tritium incorporation was seen in 94- and 42-kDa protein bands, corresponding to the expected sizes of XLαs and Gαs, respectively (Figure 1B). Immunoprecipitation with the AS antibody, a Gαi-specific antibody prepared in the same way as the RM antibody, did not detect the 94-kDa band that is prominent in the fluorograph of the labeled membranes. Under these conditions, the tritium label incorporated into Gαs is [3H]palmitate (Degtyarev et al.,1993a), suggesting that XLαs is palmitoylated.
Figure 1.
(A) Domain organization of XLαs. XLαs is identical to Gαs with respect to exons 2–13. The alternatively spliced amino terminus contains areas with EPAA repeats (residues 37–104), ARAA repeats (residues 103–187), and the proline-rich (residues 205–228) and cysteine-rich (residues 237–304) domains. The RM antibody, which recognizes both XLαs and Gαs, was generated from the decapeptide sequence at the carboxy termini of these proteins. (B) Incorporation of [3H]palmitate into endogenous XLαs. PC-12 cells were incubated with [3H]palmitate, harvested, and separated into particulate and soluble fractions. One milligram of protein from the particulate fractions underwent immunoprecipitation with either the RM antibody or the AS antibody specific for Gαi. The immunoprecipitates and 20 μg of particulate fraction protein (lane 1) were analyzed by SDS-PAGE and indirect fluorography. Fluorographs were exposed for 1 mo at −70°C.
Table 1.
XLαs mutations
| XLαs | Sequence
|
Mutations | ||||
|---|---|---|---|---|---|---|
| 237 247 | 263 | 298 | 304 | |||
| WT | CCRYEAASGICEIE… . . ATGCFQW …FGSCFGLSEC | |||||
| 4C | SS | C237, 238S | ||||
| 3C | SS | S | C237, 238, 298S | |||
| 2C | SS | S | S | C237, 238, 298, 304S | ||
| 1C | SS | S | S | S | C237, 238, 263, 298, 304S | |
| 0C | SS S | S | S | S | C237, 238, 247, 263, 298, 304S | |
| XLαs | Sequence
|
Mutations | |
|---|---|---|---|
| 199 236 | |||
| WT | SRAHLRPPSPEIQVADPPTPRPAPRPSAWPDKYERGRS | ||
| ΔPRR | SRAHLRDKYERGRS | PRR deletion | |
| 2CΔPRR | SRAHLRDKYERGRS | 2C cysteine mutant and PRR deletion | |
(Top) Cysteines in the cysteine-rich domain of XLαs were replaced with serines by oligonucleotide-directed mutagenesis, and the mutants were named according to the number of cysteines remaining in this region. The underlined cysteine residues are conserved between the rat and human sequences (Hayward et al., 1998). (Bottom) The PRR (underlined) was deleted in both the wild-type XLαs and the 2C mutant by means of oligonucleotide-directed mutagenesis.
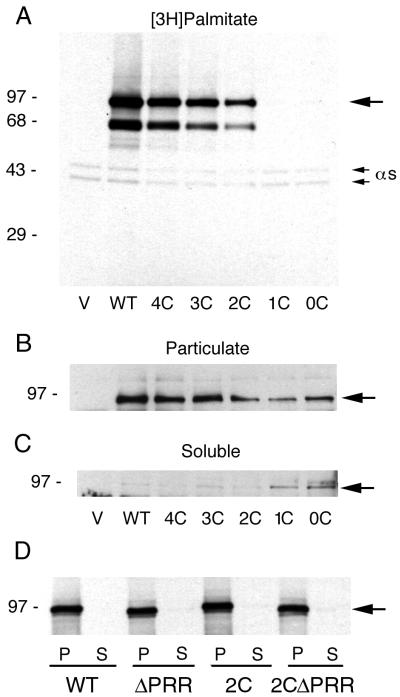
[3H]Palmitate Incorporation and Membrane Attachment of XLαs Mutants in COS-7 Cells
We investigated whether palmitoylation occurred on the cysteines in the CRR by creating a series of cysteine-to-serine mutants by site-directed mutagenesis (Table 1). COS-7 cells were transfected with vectors containing the wild-type and mutant XLαs cDNAs and incubated with [3H]palmitate. The Gαs and XLαs proteins in the particulate fraction were immunoprecipitated with the RM antibody. COS-7 cells express the long and short forms of Gαs seen as 45- and 42-kDa bands, respectively, in all the lanes but do not express XLαs endogenously (Figure 2A). Cells transfected with wild-type XLαs showed a band at 94 kDa strongly labeled with tritium. Mutation of the six cysteine residues in the wild type decreased the tritium incorporation into XLαs in a stepwise manner for mutants 4C, 3C, and 2C (Figure 2A). Densitometry readings of the 94-kDa bands for the 4C, 3C, and 2C mutants were 94, 85, and 60%, respectively, that of the wild-type XLαs. The 1C and 0C mutants did not show any tritium incorporation. A 64-kDa band seen in the transfected cells was probably the product of a late initiation site at residue 136. This protein band also showed a decrease in tritium incorporation because it contains the CRR.
Figure 2.
(A) Incorporation of [3H]palmitate into wild-type and cysteine mutants of XLαs. COS-7 cells were transiently transfected with the pCDNA3.1 (+) vector alone (V) or with the cDNA inserts of either the wild-type XLαs (WT) or its cysteine mutants (Table 1), incubated with [3H]palmitate, and separated into particulate and soluble fractions. A total of 500 μg of protein from the particulate fractions was immunoprecipitated with the RM antibody, followed by SDS-PAGE and fluorography. The fluorographs were exposed for 1 wk at −70°C. (B and C) Subcellular distribution of the cysteine mutants of XLαs. Five micrograms of protein from the particulate and soluble fractions of the transfected cells was separated by SDS-PAGE and underwent immunoblotting with the RM antibody and detection by ECL. (D) Expression of XLαs mutants with deletion of the PRR. COS-7 cells were transiently transfected with the pCDNA3.1 (+) vector alone or with the cDNA inserts of either the wild-type XLαs or mutants with a deletion of the PRR (ΔPRR) (Table 1). Cells were harvested and separated into particulate (P) and soluble (S) fractions, and proteins were analyzed by immunoblotting as described above. The large arrow points to XLαs.
The transfected cells were separated into particulate and soluble fractions followed by immunoblotting to test the membrane attachment of the reduced-palmitoylation mutants (Figure 2, B and C). The wild-type XLαs and 4C, 3C, and 2C mutants were primarily in the particulate fraction and had minimal amounts of protein localized to the soluble fraction. However, the 1C and 0C mutants, which did not incorporate [3H]palmitate, were found in both the soluble and particulate fractions. These results indicate that these cysteine residues in the CRR were critical for palmitoylation and facilitate membrane attachment, although palmitoylation must not be the sole factor in membrane attachment.
Deletion of the PRR
XLαs is localized primarily to the Golgi (Kehlenbach et al., 1994a). We deleted the PRR in the wild-type XLαs and the reduced-palmitoylation 2C mutant to create mutants that would test the role of this region in intracellular localization (Figure 1; Table 1). We chose the 2C mutant because its incorporation of [3H]palmitate was impaired compared with that of XLαs (Figure 2A), but it was still membrane attached (Figure 2, B and C). The mutants with a deletion of the PRR (ΔPRR) were slightly smaller than the wild-type XLαs but were expressed at levels equivalent to those of the wild-type XLαs in COS-7 cells (Figure 2D). The ΔPRR mutants were confined to the particulate fraction (Figure 2D) and showed no defects in incorporation of [3H]palmitate. The 2C and ΔPRR mutants underwent ADP-ribosylation catalyzed by cholera toxin and detergent solubilization with Triton X-100 to the same degree as the wild-type XLαs.
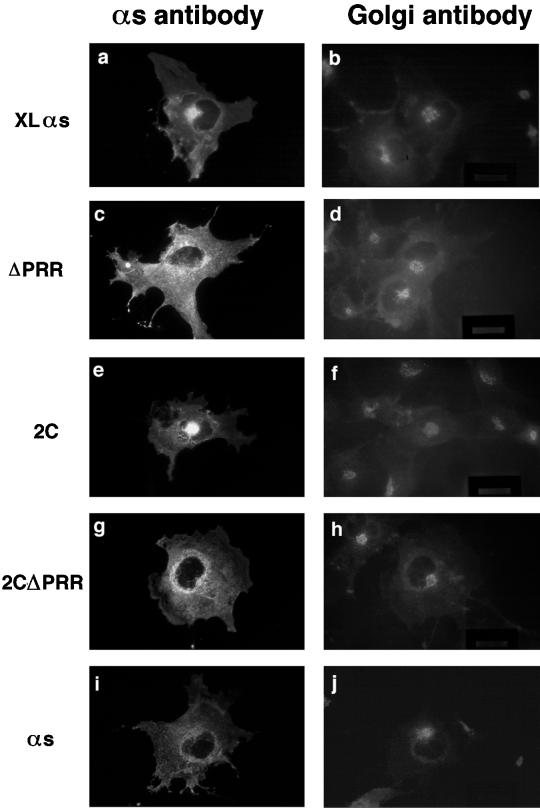
Intracellular Localization of XLαs Mutants
Localization of the reduced-palmitoylation and PRR-deletion mutants of XLαs was determined with the use of indirect immunofluorescence microscopy. We used an antibody specific to a protein associated with the cytoplasmic surface of the Golgi, the 58-kDa protein, to identify the Golgi structure (Bloom and Brashear, 1989). The RM antibody was used for the XLαs and Gαs proteins. In transfected COS cells, the wild-type XLαs was localized to a compact perinuclear area that can be identified as Golgi with the anti-58-kDa protein antibody (Figure 3, a and b). We also found colocalization of endogenous and overexpressed XLαs with another Golgi marker, BODIPY FL C5-ceramide (Ktistakis et al., 1995), in PC12 cells.
Figure 3.
Intracellular localization of the wild-type XLαs, the reduced-palmitoylation (2C) and PRR-deletion mutants of XLαs, and the wild-type Gαs. Two days after transfection with the indicated cDNAs, COS-7 cells were fixed and permeabilized with methanol at −20°C. Cells were double labeled with the rabbit polyclonal RM antibody to XLαs and Gαs and the monoclonal mouse anti-58-kDa antibody as a Golgi marker and subsequently with Cy3-labeled anti-rabbit and FITC-labeled anti-mouse antibodies. Each pair of panels shows an image made with a rhodamine filter and the corresponding image made with a FITC filter.
The reduced-palmitoylation mutants 4C, 3C, and 2C also showed bright, compact perinuclear staining that colocalized with the Golgi antibody staining (data shown for the 2C mutant in Figure 3, e and f). The 1C mutant, which did not undergo palmitoylation, exhibited a diffuse, cytosolic pattern consistent with its increased solubility but that obscured its membrane localization.
Deletion of the PRR in the XL portion of XLαs led to a significant decrease in Golgi localization (Figure 3, c, d, g, and h). After transfection with the ΔPRR and 2CΔPRR mutants, only 19% of 120 cells examined and 14% of 122 cells examined, respectively, showed a bright spot in the perinuclear area that colocalized with the Golgi antibody. In comparison, 50% of 136 cells transfected with the wild-type XLαs colocalized with the Golgi antibody staining in the perinuclear region. The distribution pattern of the ΔPRR mutants was comparable to the pattern of overexpressed Gαs, with staining predominantly on intracellular membranes and some plasma membrane staining (Figure 3, i and j). The ΔPRR mutants expressed in PC12 cells were primarily found on the plasma membrane, with some staining on other intracellular membranes but without prominent perinuclear staining.
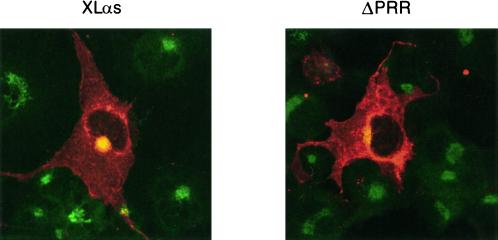
Images were also obtained by confocal microscopy that showed the colocalization of XLαs with anti-58-kDa Golgi staining (Figure 4A). The confocal images show more clearly that the ΔPRR mutant was found on various intracellular membranes, including a small amount on the Golgi that is seen in the limited colocalization with the Golgi antibody (Figure 4B).
Figure 4.
Confocal microscopic localization of the wild-type XLαs and PRR-deletion mutant (ΔPRR). COS-7 cells were transfected and stained for immunofluorescence as described in Figure 3 and examined with a confocal microscope. The anti-58-kDa antibody (Golgi) staining is green, and the RM antibody (XLαs and Gαs) staining is red. The extent of colocalization, assessed by superimposing red and green signals, is yellow.
Membrane Attachment and Intracellular Localization of GFP Fusion Proteins
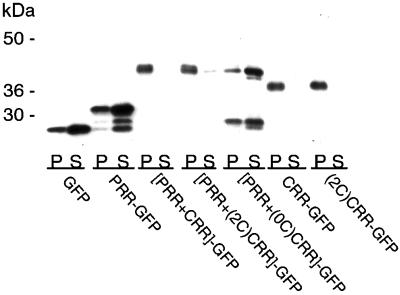
To further evaluate the roles of the PRR and the CRR as Golgi-targeting signals for XLαs, we constructed fusion proteins with these regions and the GFP (Table 2) and examined their membrane attachment and intracellular localization. Immunoblotting of the particulate and soluble fractions of transfected COS cell lysates with an antibody to GFP showed that GFP alone and PRR and PRR+(0C)CRR fused to GFP were primarily soluble proteins (Figure 5). In contrast, PRR+CRR, PRR+(2C)CRR, CRR, and (2C)CRR fused to GFP were primarily found in the particulate fraction, indicating that cysteines in the CRR were needed to direct a soluble protein to the membrane fraction.
Table 2.
GFP fusion proteins
| Name | Fused sequence | Residues |
|---|---|---|
| PRR-GFP | PRR | 201–235 |
| [PRR+CRR]-GFP | PRR and wild-type CRR | 201–312 |
| [PRR+(2C)CRR]-GFP | PRR and 2C mutant of CRR | 201–312 |
| [PRR+(0C)CRR]-GFP | PRR and 0C mutant of CRR | 201–312 |
| CRR-GFP | Wild-type CRR | 225–312 |
| (2C)CRR-GFP | 2C mutant of CRR | 225–312 |
To create the fusion proteins, the indicated sequences were amplified from XLαs or appropriate cysteine mutants of XLαs by PCR, purified, digested with BamHI, and inserted into the pEGFP-N1 vector at a BamHI site in frame and upstream of the sequence encoding the EGFP.
Figure 5.
Subcellular distribution of GFP fusion proteins. COS cells were transfected with the pEGFP N1 vector alone or with vectors containing the constructs of the PRR and the CRR fused to the amino terminus of the GFP (Table 2). Five micrograms of protein from the particulate (P) and soluble (S) fractions of the transfected cells was separated SDS-PAGE and underwent immunoblotting with a polyclonal antibody to GFP and detection by ECL.
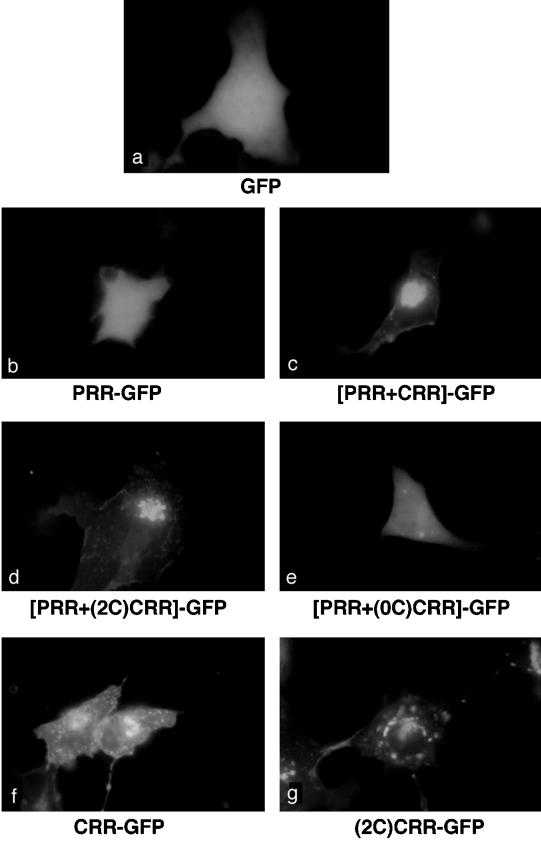
We then examined the intracellular distribution of fusion proteins by direct fluorescence microscopy in live COS and HEK-293 cells (data shown for COS cells in Figure 6). Cells expressing the GFP protein displayed a diffuse fluorescence throughout the cells (Figure 6a), consistent with previous reports demonstrating that GFP is expressed as a cytosolic protein. Diffuse cytosolic staining was also found for the PRR and PRR+(0C)CRR fusion proteins (Figure 6, b and e), consistent with the results from cell fractionation (Figure 5). In contrast, fusion proteins containing PRR together with CRR or (2C)CRR displayed a compact perinuclear localization (Figure 6, c and d) that was consistent with Golgi localization. For [PRR+CRR]-GFP, 87% of 101 cells examined showed bright, perinuclear staining, and for [PRR+(2C)CRR]-GFP, 81% of 110 cells examined showed bright, perinuclear staining. The CRR and (2C)CRR fusion proteins exhibited some perinuclear localization but also a scattered intracellular fluorescence, presumably caused by nonspecific localization of these fusion proteins to intracellular membranes (Figure 6, f and g).
Figure 6.
Intracellular localization of GFP fusion proteins. COS cells were transfected with the pEGFP N1 vector alone or with vectors containing the constructs of the PRR and the CRR fused to the amino terminus of the GFP (Table 2). GFP fluorescence was recorded on living cells 2 d after transfection.
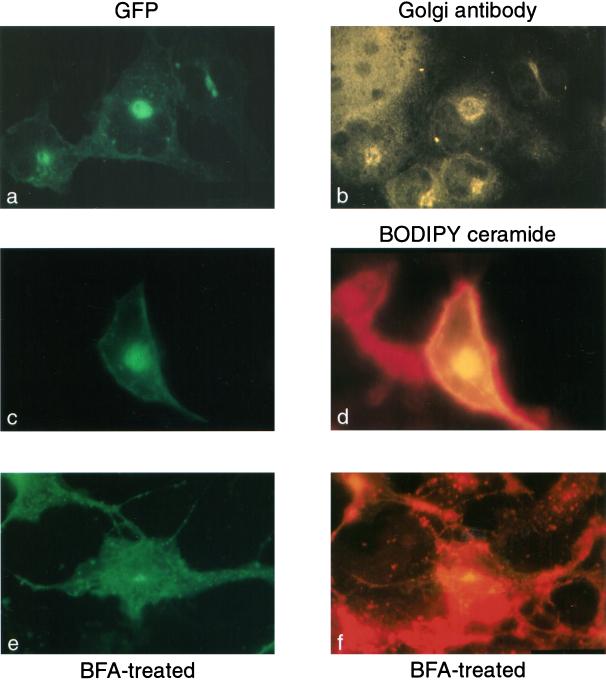
The Golgi localization of the PRR+CRR fusion protein was confirmed by observing that GFP fluorescence colocalized with staining for the 58-kDa Golgi protein antibody in fixed COS cells (Figure 7, a and b). In addition, the GFP fluorescence colocalized with BODIPY TR ceramide, a Golgi marker, in live cells (Figure 7, c and d). Treatment of cells with brefeldin A, which disintegrates the Golgi apparatus, abolished the perinuclear staining of the PRR+CRR fusion protein (Figure 7, e and f).
Figure 7.
Colocalization of the [PRR+CRR]-GFP with Golgi markers and treatment with brefeldin A. COS cells were transfected with a plasmid containing the cDNA for the [PRR+CRR]-GFP construct (Table 2). Two days after transfection, the cells were either fixed and prepared for immunohistochemistry with the anti-58-kDa antibody (a and b) or incubated with BODIPY TR ceramide (c–f) and brefeldin A (e and f) to label and disrupt, respectively, Golgi membranes. Cells were observed with fluorescence microscopy with the use of a FITC filter for detection of GFP (a, c, and e), a rhodamine filter for detection of the Cy3-labeled secondary antibody (b), or a Texas Red filter for detection of BODIPY TR ceramide (d and f).
Together, these data suggest that PRR could be a Golgi-targeting signal but that cysteines in the CRR were crucial for the attachment of otherwise soluble PRR to the Golgi membranes
DISCUSSION
Heterotrimeric G proteins work not only at the plasma membrane to couple cell surface receptors to intracellular effectors but also at the Golgi membrane to regulate vesicular transport (Gilman, 1987; Melançon et al., 1987; Leyte et al., 1992). Consequently, these proteins must be sorted to their appropriate intracellular sites. We found that the Golgi localization of XLαs, a Gαs splice variant, was determined by a PRR and a CRR in its amino terminus.
The PRR of XLαs
Deletion of the PRR in XLαs led to a loss of its Golgi localization after expression in PC12 cells with endogenous XLαs and in COS cells without the endogenously expressed protein. This protein was still attached to membranes and folded properly, as measured by its ability to undergo ADP-ribosylation catalyzed by cholera toxin. Fusion of the PRR and CRR to the GFP, a cytosolic protein, was sufficient for targeting to the Golgi membranes.
We initially chose to study this region because an alternatively spliced form of Gαi2 has a PRR in the carboxy terminus and is found at the Golgi rather than at the plasma membrane, as is Gαi2 (Montmayeur and Borrelli, 1994). The sequence of the human form of XLαs was identified recently, and the PRR was highly conserved between species (Table 3), although the intracellular distribution of the human XLαs is not known.
Table 3.
PRRs in peripheral membrane proteins at the Golgi
| Protein | Sequence | Percent prolinea |
|---|---|---|
| GTPases | 199 230 | |
| Rat XLαsb | SRAHLRPPSPEIQVADPPTPRPAPRPSAWPDK | 41.7 |
| 217 253 | ||
| Human XLαsc | PASGARRKI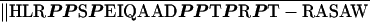 ‖RGK ‖RGK |
28.6 |
| 326 366 | ||
| sGαi2d | CATDTKSRKLFRETYLKLSGPDQHPHPSPAPAPPLSSDSVP | 38.1 |
| 743 | ||
| Dynamin IIe | PVPPPVDDTWLQSASSHSPTPQRRPVSSIHPPGRPPAVRGPTPGPPLIPVPV | 31.7 |
| GAAASFSAPPIPSRPGPQSVFANSDLFPAPPQIPSRPVRIPPGIPPGVPSRR | ||
| PPAAPSRPTIIRPAEPSLLD 866 | ||
| Autoantigensf | 20 37 | |
| GCP170g | PSSLPEAPLKPPGPLVPP | 44.4 |
| 58 94 | ||
| PDGPGQGGLCGNGPTPPFPDPPSSLDPTTSPVGPDASP | 31.6 | |
| GM130h | 356 378 | |
| LRSQMEEPPPPEPPTGPSEAEER | 70 | |
| 60 82 | ||
| Golgin-95h | LRNQMAEPPPPEPPAGPSEVEQQ | 70 |
| Cytoskeletal proteins | 2098 | |
| PPAPEPTASVPPGDLVGGQTASDTTWDGTQPRPPPSTQAPSVNGVCTDGEP | 20.3 | |
| βIII spectrini | SQPLLGQQRLEHSSFPEGPGPGSGDEANGPRGERQTRTRGPAPSAMPQSRS | |
| TESAHAATLPPRGPEP 2215 | ||
| 123 | ||
| Comitinj | PCTRDHLLSLPCAKPSGHPQSAYPPQQPGYGYPAQPGYPPQPGYPPQHGYP | 30 |
| PQHGYPQQP 182 | ||
| SNARE | 1 38 | |
| GS32k | MSGYPKSYNPFDDDVEDEDTRPAPWKDARDLPDGPDPP | 23.5 |
| 127 169 | ||
| PVEPPPEQNGSIVPQPSSRLKEAINQASHP | 23.3 |
The percentage of proline residues is calculated from the number of residues from the first to the last proline residues in these sequences.
The numbering of the residues is based on a cDNA with the start site at nucleotide 439–441, based on the original published sequence (Kehlenbach et al., 1994a), which was used in this study because it encodes a protein with an electrophoretic mobility identical to the endogenous protein (Kehlenbach et al., 1994b). The deleted residues in the PRR mutant are underlined.
The residue numbering is based on the work of Hayward et al. (1998). The boxed area is the area of strong homology with the rat sequence. The intracellular localization of the protein has not been determined.
The underlined residues differ from the Gαi2 sequence (Montmayeur and Borrelli, 1994).
The consensus sequences for SH3 domain binding are underlined with thick underlining for conserved residues found in dynamin I that bind SH3 domains (Okamoto et al., 1997).
A group of large proteins that were discovered using antibodies from people with autoimmune diseases. Their sequences predict areas of coiled coils, with the PRR either flanking or between the coiled coils. Functional information on these proteins is limited.
From Misumi et al. (1997).
GM130 and golgin-95 share considerable homology, including the PRR (Fritzler et al., 1993; Nakamura et al., 1995).
This PRR in βIII spectrin, a Golgi cytoskeletal protein, is near the carboxy terminus in a membrane association domain, based on studies with other spectrins (Stankewich et al., 1998). Earlier studies found that the amino terminus of βI spectrin was sufficient for Golgi targeting (Devarajan et al., 1997). Subsequently, βIII spectrin was identified and found to have extensive homology with β1Σ2, especially at the amino terminus. However, the PRR appears to be unique to βIII; therefore, its role in Golgi targeting has never been tested.
The PRR domain of this small actin-binding protein includes five tandem repeats of GYPXQ(P or H) (Weiner et al., 1993).
A 32-kDa Golgi SNARE protein that is related to SNAP-25 and SNAP-23. The two PRRs are unique to GS32. An alignment of the sequences places the second PRR of GS32 in the same area as the acylation sites of SNAP-25 (Wong et al., 1999).
Proline-rich peptides have unique properties based on the unusual side chain of proline that circles back to the backbone amide position (Williamson, 1994). PRRs serve both structural and binding functions for the diverse group of proteins that contain them. For XLαs, the PRR may be a rigid spacer between the amino-terminal domain and the cysteine-rich and αs domains. Deletion of the PRR bridge may then change the global conformation to diminish the interaction with the Golgi membrane and/or a Golgi protein. Alternatively, the PRR may be a site of direct binding to the Golgi. PRRs are found on a number of proteins, and their restricted mobility forms a “sticky arm” to facilitate protein–protein interactions that are rapid and less specific than typical receptor–ligand interactions that use “lock-and-key” binding (Williamson, 1994). This type of interaction is consistent with the results of our findings with a GFP fusion protein, in which the PRR and CRR alone could target the GFP fusion protein to the Golgi membranes.
Could the PRR be a Golgi-targeting signal for other proteins? A review of the literature uncovered several peripheral Golgi proteins that contain PRRs (Table 3). However, the functions of the PRRs and the Golgi-targeting signals (except for dynamin and βIII spectrin; see below and Table 3) are not known for these proteins. The abundance of proline residues, rather than a consensus pattern, distinguishes these regions. These proteins (except for dynamin) do not have known proline-enriched consensus sequences such as XPPXY or PPLP for WW domain binding, E/DFPPPXD/E for Ena-VASP homology domain 1 domain binding, or RXqPXqP or qPXqPXR for Src homology 3 domain binding (X is any amino acid, and q is a hydrophobic residue) (Chen and Sudol, 1995; Mayer and Eck, 1995; Niebuhr et al., 1997).
The PRR of dynamin has been carefully studied. Members of the dynamin family are high-molecular-weight GTPases involved in the vesiculation of clathrin-coated pits and are found on Golgi membranes as well as the plasma membrane and other intracellular membranes (Jones and Gutkind, 1998). Deletion of the proline-rich carboxy terminus abolishes targeting to coated pits (Shpetner et al., 1996), and an isoform of dynamin lacking the proline-rich carboxy terminus does not colocalize to the Golgi (Kamimoto et al., 1998). SH3 domain–binding regions have been identified in this PRR, but the role of these regions and other areas of the carboxy terminus in the intracellular localization of the different isoforms of the protein has not been reported (Okamoto et al., 1997). Interestingly, alternative splicing of dynamin, like XLαs and Gαs, can markedly change its intracellular distribution (Cao et al., 1998).
PRRs are involved in systems that require rapid and reversible association of several proteins into functional complexes (Williamson, 1994). Two well-characterized examples are the RNA polymerase II preinitiation complex and proteins associated with synaptic vesicles such as synapsins, VAMP-1, and synaptophysins. The PRRs of XLαs and possibly other Golgi proteins may bind them into a Golgi-associated “complex.” Specificity for Golgi membranes may arise through binding to isoforms of cytoskeletal proteins found at the Golgi (De Matteis and Morrow, 1998), because the binding target of PRRs is often the cytoskeleton (Williamson, 1994). For XLαs, the PRR alone was insufficient to target the GFP fusion protein to the Golgi membranes, suggesting that it required another factor, such as acylation, to establish or maintain membrane attachment. Given the number of proteins with PRRs, an additional signal may also be present within the PRR and CRR to direct the protein to the Golgi membranes.
Palmitoylation of XLαs
Like most other α subunits, XLαs underwent palmitoylation, as demonstrated by its ability to incorporate [3H]palmitate. Unlike other α subunits, in which the putative acylation sites are within the first 18 residues, the likely sites of palmitoylation for XLαs are on 6 cysteine residues within a CRR distant from the amino terminus, because mutation of these cysteines blocked [3H]palmitate incorporation. The nonpalmitoylated mutants were partially found in the soluble fraction, indicating that palmitoylation facilitated membrane attachment but was not required. The finding that the GFP fusion protein containing the PRR and the CRR lacking the cysteine residues was soluble suggests an additional site for membrane attachment outside of the PRR and CRR. Whether membrane attachment occurs through βγ subunits, as it does for other α subunits, is uncertain because the regions of α subunits that bind βγ differ between XLαs and Gαs.
Palmitoylation occurs on many membrane-bound proteins, and for that reason it cannot be a targeting signal by itself. However, palmitoylation is frequently found within or adjacent to a protein sequence that is critical for directing intracellular localization. For XLαs, the CRR starts nine residues from the PRR. The importance of acylation in Golgi targeting differs among proteins. For eNOS, acylation was critical for its Golgi localization (Liu et al., 1997). Yet for GAD65, mutation of all six cysteines adjacent to the amino-terminal Golgi-targeting domain did not change its Golgi localization (Solimena et al., 1994). Mutations to prevent palmitoylation in another Golgi protein, SCG10, led to a small increase in solubility and the concomitant difficulties in visualizing the intracellular localization (Di Paolo et al., 1997), as was the case for the nonpalmitoylated mutant of XLαs. In this study, the PRR was insufficient for Golgi localization, which required the putative acylation sites in the CRR for targeting of the GFP fusion protein. The proximity of acylation sites to targeting sequences may aid in orienting the targeting signal toward the membrane and preserving the contact. Differences among proteins in the role of palmitoylation in Golgi targeting may be due to differences in the affinity of the targeting sequence for the Golgi membrane.
Intracellular Localization and Function of XLαs
The intracellular localization of XLαs to the Golgi is based on colocalization with two Golgi markers, an antibody to the 58-kDa protein (Bloom and Brashear, 1989) and BODIPY-TR ceramide (Ktistakis et al.1995), and the loss of perinuclear staining after treatment with brefeldin A, a fungal metabolite that disrupts Golgi membranes. The compact pattern of the staining may indicate that it is localized to a discrete area within the Golgi complex. Some XLαs is also found at the plasma membrane (Figure 4A). The presence of XLαs only in cells with both regulated and constitutive secretion suggests that it may be involved in membrane trafficking. XLαs could cycle between the plasma membrane and the Golgi and use its Golgi-localization signals for targeting to a functional domain in the Golgi involved in vesicle transport.
These results are the first to indicate that a PRR was critical for Golgi localization and suggest that, at least for G proteins, insertion of this region by alternative splicing may be a general mechanism for sorting and specific targeting. More studies are needed to determine if PRRs are a general localization signal for Golgi peripheral membrane proteins and the means and function of this targeting.
ACKNOWLEDGMENTS
We thank Dr. Wieland B. Huttner and Dr. Ralph H. Kelhenbach for the XLαs cDNA, Dr. Regina Collins for cell culture expertise, Dr. William F. Simonds for providing purified brain βγ subunits, Dr. April Robbins for advice, and Dr. Leonid Margolis and the National Aeronautics and Space Administration/National Institutes of Health Center for Three Dimensional Tissue Culture for assistance with the confocal microscopy. This work was supported in part by a grant from the Turkish Scientific and Technical Research Council (SBAG-2105).
Abbreviations used:
- AS
antibody for Gαi
- FGF
fibroblast growth factor
- GFP
green fluorescent protein
- h
human
- IL
interleukin
- iLIF
intracellular leukemia inhibitory factor
- IRES
internal ribosome entry site
- LIF
leukemia inhibitory factor
- m
mouse
- PBST
PBS containing 0.1% Tween 20
- RM
antibody for Gαs
REFERENCES
- Arni S, Keilbaugh SA, Ostermayer AG, Brown DA. Association of GAP-43 with detergent-resistant membranes requires two palmitoylated cysteine residues. J Biol Chem. 1998;273:28478–28485. doi: 10.1074/jbc.273.43.28478. [DOI] [PubMed] [Google Scholar]
- Barr FA. A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins. Curr Biol. 1999;9:381–384. doi: 10.1016/s0960-9822(99)80167-5. [DOI] [PubMed] [Google Scholar]
- Beckers CJM, Balch WE. Calcium and GTP: essential components in vesicular trafficking between endoplasmic reticulum and Golgi apparatus. J Cell Biol. 1989;108:1245–1256. doi: 10.1083/jcb.108.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom GS, Brashear TA. A novel 58-kDa protein associates with the Golgi apparatus and microtubules. J Biol Chem. 1989;264:16083–16092. [PubMed] [Google Scholar]
- Bomsel M, Mostov K. Role of heterotrimeric G proteins in membrane traffic. Mol Biol Cell. 1992;3:1317–1328. doi: 10.1091/mbc.3.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butrynski JE, Jones TLZ, Backlund PS, Jr, Spiegel AM. Differential isoprenylation of carboxy-terminal mutants of an inhibitory G-protein α-subunit: neither farnesylation nor geranylgeranylation is sufficient for membrane attachment. Biochemistry. 1992;31:8030–8035. doi: 10.1021/bi00149a037. [DOI] [PubMed] [Google Scholar]
- Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HI, Sudol M. The WW domain of Yes-associated protein binds a proline-rich ligand that differs from the consensus established for Src homology 3-binding modules. Proc Natl Acad Sci USA. 1995;92:7819–7823. doi: 10.1073/pnas.92.17.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida JB, Holtzman EJ, Peters P, Ercolani L, Ausiello DA, Stow JL. Targeting of chimeric Gαi proteins to specific membrane domains. J Cell Sci. 1994;107:507–515. doi: 10.1242/jcs.107.3.507. [DOI] [PubMed] [Google Scholar]
- Degtyarev MY, Spiegel AM, Jones TLZ. The G protein αs subunit incorporates 3H-palmitic acid and mutation of cysteine-3 prevents this modification. Biochemistry. 1993a;32:8057–8061. doi: 10.1021/bi00083a001. [DOI] [PubMed] [Google Scholar]
- Degtyarev MY, Spiegel AM, Jones TLZ. Increased palmitoylation of the Gs protein α subunit after activation by the β-adrenergic receptor or cholera toxin. J Biol Chem. 1993b;268:23769–23772. [PubMed] [Google Scholar]
- De Matteis MA, Morrow JS. The role of ankyrin and spectrin in membrane transport and domain formation. Curr Opin Cell Biol. 1998;10:542–549. doi: 10.1016/s0955-0674(98)80071-9. [DOI] [PubMed] [Google Scholar]
- Denker SP, McCaffery JM, Palade GE, Insel PA, Farquhar MG. Differential distribution of α subunits and βγ subunits of heterotrimeric G proteins on Golgi membranes of the exocrine pancreas. J Cell Biol. 1996;133:1027–1040. doi: 10.1083/jcb.133.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan P, Stabach PR, De Matteis MA, Morrow JS. Na,K-ATPase transport from endoplasmic reticulum to Golgi requires the Golgi spectrin-ankyrin G119 skeleton in Madin Darby canine kidney cells. Proc Natl Acad Sci USA. 1997;94:10711–10716. doi: 10.1073/pnas.94.20.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, Lutjens R, Pellier V, Stimpson SA, Beuchat M-H, Catsicas S, Grenningloh G. Targeting of SCG10 to the area of the Golgi complex is mediated by its NH2-terminal region. J Biol Chem. 1997;272:5175–5182. doi: 10.1074/jbc.272.8.5175. [DOI] [PubMed] [Google Scholar]
- Fritzler MJ, Hamel JC, Ochs RL, Chan EKL. Molecular characterization of two human autoantigens: unique cDNAs encoding 95- and 160-kD proteins of a putative family in the Golgi complex. J Exp Med. 1993;178:49–62. doi: 10.1084/jem.178.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gleeson PA. Targeting of proteins to the Golgi apparatus. Histochem Cell Biol. 1998;109:517–532. doi: 10.1007/s004180050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm HE, Gilchrist A. Heterotrimeric G proteins. Curr Opin Cell Biol. 1996;8:189–196. doi: 10.1016/s0955-0674(96)80065-2. [DOI] [PubMed] [Google Scholar]
- Hayward BE, Kamiya M, Strain L, Moran V, Campbell R, Hayashizaki Y, Bonthron DT. The human GNAS1 gene is imprinted and encodes distinct paternally and biallelically expressed G proteins. Proc Natl Acad Sci USA. 1998;95:10038–10043. doi: 10.1073/pnas.95.17.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo J, Muniz M, Velasco A. Trimeric G proteins regulate the cytosol-induced redistribution of Golgi enzymes into the endoplasmic reticulum. J Cell Sci. 1995;108:1805–1815. doi: 10.1242/jcs.108.4.1805. [DOI] [PubMed] [Google Scholar]
- Huang C, Duncan JA, Gilman AG, Mumby SM. Persistent membrane association of activated and depalmitoylated G protein α subunits. Proc Natl Acad Sci USA. 1999;96:412–417. doi: 10.1073/pnas.96.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iiri T, Backlund PS, Jr, Jones TLZ, Wedegaertner PB, Bourne HR. Reciprocal regulation of Gsα by palmitate and the βγ subunit. Proc Natl Acad Sci USA. 1996;93:14592–14597. doi: 10.1073/pnas.93.25.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Howell KE, Henley JR, Cao H, McNiven MA. Role of dynamin in the formation of transport vesicles from the trans-Golgi network. Science. 1998;279:573–577. doi: 10.1126/science.279.5350.573. [DOI] [PubMed] [Google Scholar]
- Jones TLZ. Post-translational modifications and intracellular localization of α subunits. In: Spiegel AM, Jones TLZ, Simonds WF, Weinstein LS, editors. G Proteins. Austin, TX: R.G. Landes; 1994. pp. 49–65. [Google Scholar]
- Jones TLZ, Gutkind JS. Gα12 requires acylation for its transforming activity. Biochemistry. 1998;37:3196–3202. doi: 10.1021/bi972253j. [DOI] [PubMed] [Google Scholar]
- Jones TLZ, Simonds WF, Merendino JJ, Jr, Brann MR, Spiegel AM. Myristoylation of an inhibitory GTP-binding protein α subunit is essential for its membrane attachment. Proc Natl Acad Sci USA. 1990;87:568–572. doi: 10.1073/pnas.87.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimoto T, Nagai Y, Onogi H, Muro Y, Wakabayashi T, Hagiwara M. Dymple, a novel dynamin-like high molecular weight GTPase lacking a proline-rich carboxyl-terminal domain in mammalian cells. J Biol Chem. 1998;273:1044–1051. doi: 10.1074/jbc.273.2.1044. [DOI] [PubMed] [Google Scholar]
- Kehlenbach RH, Matthey J, Huttner WB. XLαs is a new type of G protein. Nature. 1994a;372:804–809. doi: 10.1038/372804a0. [DOI] [PubMed] [Google Scholar]
- Kehlenbach RH, Matthey J, Huttner WB. XLαs is a new type of G protein. Nature. 1994b;375:253. doi: 10.1038/372804a0. (correction). [DOI] [PubMed] [Google Scholar]
- Kjer-Nielsen L, Teasdale RD, van Vliet C, Gleeson PA. A novel Golgi-localization domain shared by a class of coiled-coil peripheral membrane proteins. Curr Biol. 1999;9:385–388. doi: 10.1016/s0960-9822(99)80168-7. [DOI] [PubMed] [Google Scholar]
- Ktistakis NT, Kao CY, Wang RH, Roth MG. A fluorescent lipid analogue can be used to monitor secretory activity and for isolation of mammalian secretion mutants. Mol Biol Cell. 1995;6:135–150. doi: 10.1091/mbc.6.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyte A, Barr FA, Kehlenbach RH, Huttner WB. Multiple trimeric G proteins on the trans-Golgi network exert stimulatory and inhibitory effects on secretory vesicle formation. EMBO J. 1992;11:4795–4804. doi: 10.1002/j.1460-2075.1992.tb05585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder ME, Middleton P, Hepler JR, Taussig R, Gilman AG, Mumby SM. Lipid modifications of G proteins: α subunits are palmitoylated. Proc Natl Acad Sci USA. 1993;90:3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hughes TE, Sessa WC. The first 35 amino acids and fatty acylation sites determine the molecular targeting of endothelial nitric oxide synthase into the Golgi region of cells: a green fluorescent protein study. J Cell Biol. 1997;137:1525–1535. doi: 10.1083/jcb.137.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer BJ, Eck MJ. SH3 domains: minding your p's and q's. Curr Biol. 1995;5:364–367. doi: 10.1016/s0960-9822(95)00073-x. [DOI] [PubMed] [Google Scholar]
- Melançon P, Glick BS, Malhotra V, Weidman PJ, Serafini T, Gleason ML, Orci L, Rothman JE. Involvement of GTP-binding “G” proteins in transport through the Golgi stack. Cell. 1987;51:1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- Melkonian KA, Ostermeyer AG, Chen JZ, Roth MG, Brown DA. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts: many raft proteins are acylated, while few are prenylated. J Biol Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- Misumi Y, Sohda M, Yano A, Fujiwara T, Ikehara Y. Molecular characterization of GCP170, a 170-kDa protein associated with the cytoplasmic face of the Golgi membrane. J Biol Chem. 1997;272:23851–23858. doi: 10.1074/jbc.272.38.23851. [DOI] [PubMed] [Google Scholar]
- Montmayeur J-P, Borrelli E. Targeting of Gαi2 to the Golgi by alternative spliced carboxyl-terminal region. Science. 1994;263:95–98. doi: 10.1126/science.8272874. [DOI] [PubMed] [Google Scholar]
- Mumby SM, Kleuss C, Gilman AG. Receptor regulation of G-protein palmitoylation. Proc Natl Acad Sci USA. 1994;91:2800–2804. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Localization of proteins to the Golgi apparatus. Trends Cell Biol. 1998;8:11–15. doi: 10.1016/S0962-8924(97)01197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Nichols BJ. The GRIP domain: a novel Golgi-targeting domain found in several coiled-coil proteins. Curr Biol. 1999;9:377–380. doi: 10.1016/s0960-9822(99)80166-3. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebuhr K, Ebel F, Frank R, Reinhard M, Domann E, Carl UD, Walter U, Gertler FB, Wehland J, Chakraborty T. A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J. 1997;16:5433–5444. doi: 10.1093/emboj/16.17.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto PM, Herskovits JS, Vallee RB. Role of the basic, proline-rich region of dynamin in Src homology 3 domain binding and endocytosis. J Biol Chem. 1997;272:11629–11635. doi: 10.1074/jbc.272.17.11629. [DOI] [PubMed] [Google Scholar]
- Ponimaskin E, Harteneck C, Schultz G, Schmidt MFG. A cysteine-11 to serine mutant of Gα12 impairs activation through the thrombin receptor. FEBS Lett. 1998;429:370–374. doi: 10.1016/s0014-5793(98)00638-3. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Neto FAP, Mattera R, Hildebrandt JD, Codina J, Field JB, Birnbaumer L, Sekura RD. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol. 1985;109:566–572. doi: 10.1016/0076-6879(85)09115-7. [DOI] [PubMed] [Google Scholar]
- Shpetner HS, Herskovits JS, Vallee RB. A binding site for SH3 domains targets dynamin to coated pits. J Biol Chem. 1996;271:13–16. doi: 10.1074/jbc.271.1.13. [DOI] [PubMed] [Google Scholar]
- Solimena M, Dirkx R, Jr, Radzynski M, Mundigl O, De Camilli P. A signal located within amino acids 1–27 of GAD65 is required for its targeting to the Golgi complex region. J Cell Biol. 1994;126:331–341. doi: 10.1083/jcb.126.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankewich MC, Tse WT, Peters LL, Ch'ng Y, John KM, Stabach PR, Devarajan P, Morrow JS, Lux SE. A widely expressed betaIII spectrin associated with Golgi and cytoplasmic vesicles. Proc Natl Acad Sci USA. 1998;95:14158–14163. doi: 10.1073/pnas.95.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley KK. Regulation of targeting signals in membrane proteins. Mol Membr Biol. 1996;13:19–27. doi: 10.3109/09687689609160570. [DOI] [PubMed] [Google Scholar]
- Sternweis PC, Robishaw JD. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- Stow JL, Heimann K. Vesicle budding on Golgi membranes: regulation by G proteins and myosin motors. Biochim Biophys Acta. 1998;1404:161–171. doi: 10.1016/s0167-4889(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Stow JL, Sabolic I, Brown D. Heterogeneous localization of G protein α-subunits in rat kidney. Am J Physiol. 1991;261:F831–F840. doi: 10.1152/ajprenal.1991.261.5.F831. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Bourne HR. Activation and depalmitoylation of Gsα. Cell. 1994;77:1063–1070. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Chu DH, Wilson PT, Levis MJ, Bourne HR. Palmitoylation is required for signaling functions and membrane attachment of Gqα and Gsα. J Biol Chem. 1993;268:25001–25008. [PubMed] [Google Scholar]
- Weiner OH, Murphy J, Griffiths G, Schleicher M, Noegel AA. The actin-binding protein comitin (p24) is a component of the Golgi apparatus. J Cell Biol. 1993;123:23–34. doi: 10.1083/jcb.123.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson MP. The structure and function of proline-rich regions in proteins. Biochem J. 1994;297:249–260. doi: 10.1042/bj2970249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Xu Y, Zhang T, Griffiths G, Lowe SL, Subramaniam VN, Seow KT, Hong W. GS32, a novel Golgi SNARE of 32 kDa, interacts preferentially with syntaxin 6. Mol Biol Cell. 1999;10:119–134. doi: 10.1091/mbc.10.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]