Abstract
MAGP-1 and fibrillin-1, two protein components of extracellular microfibrils, were shown by immunoprecipitation studies to interact with the chondroitin sulfate proteoglycan decorin in the medium of cultured fetal bovine chondrocytes. Decorin interacted with each protein individually and with both proteins together to form a ternary complex. Expression of truncated fibrillin-1 proteins in Chinese hamster ovary cells localized proteoglycan binding to an amino-terminal region near the proline-rich domain. A spatially analogous fibrillin-2 truncated protein did not coprecipitate the same sulfated molecule, suggesting that chondroitin sulfate proteoglycan binding in this region is specific for fibrillin-1. An interaction between fibrillin and MAGP-1 was also observed under culture conditions that abrogated decorin secretion, suggesting that the two microfibrillar proteins can associate in the absence of the proteoglycan. Sulfation of matrix proteins is important for elastic fiber assembly because inhibition of sulfation was shown to prevent microfibrillar protein incorporation into the extracellular matrix of cultured cells.
INTRODUCTION
The elasticity of tissues that undergo repeated cycles of extension and recoil is dependent on the proper assembly of soluble tropoelastin monomers into an insoluble elastic fiber. Although there is some evidence that tropoelastin can self-assemble in vitro (Bressan et al., 1986; Vrhovski et al., 1997), it is generally accepted that elastin assembly requires the assistance of microfibrils (Ross and Bornstein, 1969; Brown-Augsburger et al., 1996). Microfibrils are 10- to 12-nm filaments that were first identified as components of elastic fibers, although they have subsequently been identified in nonelastic tissues, such as in the ciliary zonules of the eye.
When examined by rotary shadowing electron microscopy, microfibrils are seen as linear arrays of electron-dense “beads” separated by ∼50 nm with filamentous “strings” (Kielty et al., 1991). Fibrillin-1 and fibrillin-2 are the main structural proteins in microfibrillar architecture, contributing to both the beads and the filamentous arms that connect the beads (Sakai et al., 1986; Zhang et al., 1994). Another protein that has been localized to the bead structures of nearly all microfibrils is microfibril-associated glycoprotein-1 (MAGP-1) (Kumaratilake et al., 1989; Henderson et al., 1996). The function of this low-molecular-weight sulfated protein, however, is not known (Gibson et al., 1991). Several other macromolecules have been found to associate with microfibrils as nonintegral components (Mecham and Davis, 1994; Kielty and Shuttleworth, 1995), including several members of the proteoglycan family.
Proteoglycans constitute a number of genetically unrelated multidomain proteins that have covalently attached glycosaminoglycan (GAG) chains (Iozzo and Murdoch, 1996). The large modular proteoglycans, such as versican and aggrecan, are capable of multiple simultaneous interactions that enable indirect associations between many matrix molecules. The smaller proteoglycans, such as decorin and biglycan, perform a number of different functions, including regulation of matrix assembly (Scott, 1988; Danielson et al., 1997). Early evidence for an association between proteoglycans and elastic fiber components was based mainly on electron microscopic observations in which proteoglycans were visualized with the use of polycationic dies (Kadar et al., 1972; Pasquali-Ronchetti et al., 1984; Baccarani Contri et al., 1985). Subsequently, the presence of proteoglycans within elastic fibers has been confirmed with the use of specific antibodies. Baccarani Contri et al., (1990) used the antipeptide antibodies PG I and PG II (specific to biglycan and decorin, respectively) to demonstrate the presence of these two small, leucine-rich chondroitin sulfate proteoglycans (CSPGs) in the elastic fibers of normal human dermis. Biglycan was localized to the amorphous elastin component of mature elastic fibers and decorin to the elastin-associated microfibrils. Versican, a large CSPG, was also found to localize to microfibrils in skin (Zimmermann et al., 1994). Kielty et al. (1996) used a biochemical and ultrastructural approach to show that proteoglycans associated with microfibrils localized to the bead structures.
A direct association between proteoglycans and specific microfibrillar proteins was first observed at the molecular level in the medium of cultured smooth muscle cells. Kielty et al. (1996) demonstrated that immunoprecipitation of fibrillin from culture medium resulted in the coprecipitation of a small, ∼100-kDa chondroitin sulfate-rich proteoglycan. This interaction was suggested to be important for fibrillin function because treatment of purified microfibrils with chondroitinase ABC resulted in disruption of the beaded microfibrillar architecture (Kielty et al., 1996). The nature of the CSPG, however, was not determined.
In this study, we show that the microfibrillar protein MAGP-1 associates with a sulfated molecule that is identified as the CSPG decorin. The core protein of this proteoglycan rather than the GAG side chain is demonstrated to mediate the interaction. Fibrillin secreted by these cells also interacts with a CSPG of similar size as well as with MAGP-1. Immunoprecipitation of truncated fibrillin proteins expressed in Chinese hamster ovary (CHO) cells localized a decorin-binding site to an amino-terminal region of fibrillin-1 near the proline-rich domain. The interaction between fibrillin-1, MAGP-1, and a CSPG suggests the formation of a ternary complex that may be important in the assembly of elastic fibers.
MATERIALS AND METHODS
All reagents were obtained from Sigma Chemical (St. Louis, MO) unless specified otherwise. Restriction enzymes were purchased from Boehringer Mannheim (Indianapolis, IN), and cloning was performed according to protocols described by Sambrook et al. (1989). Tissue culture supplies were obtained from Fisher Scientific (Pittsburgh, PA). Mammalian cells were maintained at 37°C in a humidified incubator in an atmosphere of 5% CO2. Radioisotopes were purchased from ICN (Costa Mesa, CA). Fetal bovine chondrocytes (FBCs) were isolated and maintained as described previously (Mecham, 1987).
Mammalian Expression of Fibrillin-1 Amino-terminal Fibrillin Constructs
The truncated fibrillin constructs (PET/fibrillin-1 and Aik/fibrillin-2) have been described previously (Trask et al., 1999). PET spans base pairs 1168–2165 (Ser-390–Ser-722) of fibrillin-1 and includes the first residue of the proline-rich domain and ends with the last residue predicted for the second eight-cysteine domain. Aik begins five residues into the glycine-rich region of fibrillin-2 and extends into the second eight-cysteine domain, ending three residues short of the eighth cysteine. Both recombinant proteins contain the fibrillin-2 signal peptide. Fibrillin-1 cDNA sequences are numbered according to Pereira et al. (1993) (accession number L13923), and the fibrillin-2 sequences are numbered according to GenBank sequence number U03272. PET and Aik were cloned into the pEE14 mammalian expression vector as described (Trask et al., 1999). Expression of the insert in this vector is driven by the strong cytomegalovirus promoter/enhancer. A glutamine synthase mini-gene, whose expression is driven by the simian virus-40 promoter, is used in the selection of stable transfectants with methionine sulfoximine. With the use of the protocol described by Crouch et al. (1994), 27 μg of the truncated fibrillin-containing mammalian expression plasmid was used to transfect three 60-mm tissue culture dishes of CHO K1 cells with Lipofectin (Life Technologies/BRL, Grand Island, NY), according to the manufacturer's instructions. After 2 d, transfected cells were split into p100 tissue culture dishes in Glasgow's modified Eagle's medium without glutamine (Life Technologies/BRL) to which 10% dialyzed FCS and 25 μM methionine sulfoximine had been added. Medium was replaced every 3–5 d. After ∼15 d, colonies were selected and, after reaching confluence, were switched to serum-free Glasgow's modified Eagle's medium for screening of recombinant protein production by immunoblotting, as described below. Positive colonies were cloned by serial dilution, and subclones were again screened by immunoblotting.
Antibodies
Antibodies to the human fibrillin-1 carboxyl-terminal domain (amino acids 2686–2871) were described by Ritty et al. (1999) and those to the proline-rich domain were described by Trask et al. (1999). Antibody to the truncated fibrillin-2 construct has also been described by Trask et al. (1999). All antisera were enriched for immunoglobulin G by caprylic acid precipitation (Harlow and Lane, 1988). LF-94 and LF-96, antibodies raised to peptide sequences found in bovine decorin and bovine biglycan, respectively (Fisher et al., 1995), were the kind gift of Dr. Thomas Wight (University of Washington, Seattle). mAb to tropoelastin was from clone BA4 (Wrenn et al., 1986). Ascites was enriched for immunoglobulin G by caprylic acid precipitation. Polyclonal anti-bovine fibronectin antiserum was purchased from Chemicon (Temecula, CA).
Antibodies to MAGP-1 were made against recombinant protein. Full-length bovine MAGP-1 cDNA (Gibson et al., 1991) in pBS was digested with PstI and ApaLI, yielding a 270-base pair fragment. Recessed ends were filled in with the large fragment of DNA polymerase (Klenow, Life Technologies/BRL) and religated into PstI- and EcoRV-cut pBS. This construct was digested with BamHI and XhoI, and the insert was ligated into the pGEX vector (Pharmacia, Piscataway, NJ) cut with the same enzymes. This protocol resulted in a fusion protein with GST coupled to amino acids 14–103 of MAGP-1. Vector-derived sequences resulted in the addition of six amino acids, Gly-Ile-Pro-Arg-Ala-Ala, between the factor Xa cleavage site that follows the GST moiety and amino acid 14 of MAGP-1. XL1-Blue Escherichia coli (Pharmacia) were transformed with the vector, and colonies were screened for the presence of the appropriate plasmid by restriction digestion.
To make recombinant protein, an overnight culture of E. coli containing this plasmid was inoculated into 1 l of Luria-Bertani broth containing 50 μg/ml ampicillin in an environmental shaker at 37°C. When bacteria reached an OD600 of 0.4, fusion-protein synthesis was induced by the addition of 0.2 mM isopropylthio-β-galactoside (Life Technologies/BRL) for 4 h. Bacteria were pelleted and subsequently lysed by repeated freeze-thaw cycles followed by the addition of 0.1% Triton X-100 and sonication. Bacterial lysate was cleared of debris by centrifugation for 30 min at 14,000 rpm in a Sorvall (Newtown, CT) RC-5B centrifuge with the use of an SA-600 rotor. The supernatant was adsorbed onto a 2.5-ml glutathione–Sepharose 4B column (Pharmacia) that had been preequilibrated in PBS. The column was washed with 40 column volumes of PBS, and bound fusion protein was eluted with 6 column volumes of PBS containing 10 mM glutathione.
Polyclonal antibodies were produced by injection of these purified recombinant proteins diluted with adjuvant into rabbits (Harlow and Lane, 1988). Serum was collected, and antibodies were affinity purified over a Sepharose 4B (Pharmacia) column onto which elastic fiber proteins extracted from bovine nuchal ligament had been coupled (Gibson et al., 1989).
Immunoblotting
For screening of recombinant protein production, confluent stably transfected CHO cells in 24-well plates were washed three times with serum-free Glasgow's modified Eagle's medium before overnight incubation in 300 μl of the same medium. The conditioned medium was harvested and subjected to reducing SDS-PAGE. Proteins were then transferred to nitrocellulose (Schleicher & Schuell, Keene, NH) for 1 h at 70 V in a buffer containing 100 mM 3-[cyclohexylamino]-1-propanesulfonic acid, 10% methanol (vol/vol). The nitrocellulose with transferred protein was blocked in PBS:3% nonfat dry milk (wt/vol; PBS:milk) for 1 h at room temperature and incubated with primary antibody overnight at 4°C in PBS:milk. Recombinant fibrillin proteins were detected with the use of the antibodies described above, both at 1:1000 dilutions. After incubation with primary antibody, the blots were washed three times with PBS:milk and incubated with a HRP-conjugated secondary antibody (Amersham) at a 1:2000 dilution for 1 h at room temperature in PBS:milk. The blots were then washed three more times with PBS:milk, and immunoreactive proteins were visualized by ECL.
Immunoprecipitations
Confluent transfected CHO cells or FBCs in p100 tissue culture dishes were starved for 1 h in 5 ml of serum-free medium depleted of the component used for labeling. Cells were then incubated for 5–6 h in 3 ml of cysteine-free DMEM containing 5% dialyzed FBS and 50 μCi/ml [35S]cysteine (specific activity > 800 Ci/mmol) or sulfate-free Joklik-modified minimal essential medium (S-MEM; Life Technologies/BRL) containing 5% dialyzed FBS plus 150 μCi/ml Na[35S]O4 (specific activity ∼ 43 Ci/mg S). For all immunoprecipitations, radiolabeled conditioned medium was precleared to diminish nonspecific binding by incubating with 40 μl of protein A immobilized on Trysacryl (Pierce, Rockford, IL) rotating end-over-end for 1 h at room temperature. Beads were pelleted, and the supernatant was transferred to a new tube containing antibody (35 μl of the anti-MAGP-1 antibody and 20 μl of the anti-human fibrillin-1 carboxyl-terminal domain antibody). Protein A–Trysacryl (35 μl) was added, and the mixture was rotated end-over-end at 4°C overnight. Beads were then pelleted by centrifugation and washed four times with detergent buffer containing 0.1% SDS, 0.25% deoxycholate, 2 mM EDTA, 0.5% Triton X-100, 150 mM NaCl, and 50 mM Tris, pH 8. Cultured FBCs were labeled as described above, and immunoprecipitates were washed with the same buffer lacking SDS.
For treatment with chondroitinase, immunoprecipitated protein on Trysacryl beads was resuspended in 25 μl of 10 mM Tris, pH 8.3, after the final wash. Chondroitinase ABC (1 mU) was added, and samples were incubated at 37°C for 1 h. Reactions were stopped by the addition of reducing sample buffer. For deglycosylation of proteoglycans in chondrocyte medium, 20 mU/ml chondroitinase ABC was added to serum-free DMEM incubated overnight with FBC cells. Proteins were immunoprecipitated as described above. To reduce the level of proteoglycan synthesis by FBCs, cells were thawed and plated in DMEM containing 10% FBS. One day after plating, the medium was changed to serum-free DMEM supplemented with insulin, transferrin, and selenium (ITS) and cells were allowed to reach confluence before radiolabeling and immunoprecipitation as described above. Inhibition of sulfation was achieved by the addition of 10 mM KClO3 to the medium during both the starvation and radiolabeling periods.
Immunoprecipitated proteins were separated on 10% acrylamide gels by reducing SDS-PAGE. For radioactive proteins, gels were fixed, treated with En3Hance (Amersham), and dried before visualization of immunoprecipitated proteins by fluorography. Nonradioactive immunoprecipitated proteins were transferred to nitrocellulose and immunoblotted as described above with the use of either the LF-94 anti-decorin antibody or the anti-MAGP-1 antibody at a dilution of 1:1000.
Sulfation Inhibition and Indirect Immunofluorescence
FBCs (d 145–160 of gestation) were plated at a density of 104 cells/well on four-well Lab-Tek slides (Nalge Nunc International, Rochester, NY) in DMEM plus 10% dialyzed FCS. Beginning 2 d after plating, when cells were still subconfluent, the medium was replaced with sulfate-free S-MEM plus 10% dialyzed FCS. Experimental cells were treated twice each day with 10 mM KClO3 to inhibit sulfation of proteins and proteoglycans. Control cells were grown in S-MEM plus 10% dialyzed FCS to which MgSO4 had been added to a final concentration of 0.1 mg/ml. Medium was changed once each day. After 7 d, when cells were approximately 4 d postconfluent, the cultures were washed three times with PBS and fixed for 20 min with 2% paraformaldehyde (wt/vol) in PBS, pH 8. The fixed cells were then washed with PBS before a 15-min treatment with 6 M guanidine HCl, 20 mM Tris, 50 mM DTT, pH 8, for better visualization of matrix proteins. After this treatment, cells were washed three times with 20 mM Tris, pH 8, and were treated with 100 mM iodoacetamide, 20 mM Tris in the dark for 15 min to alkylate free sulfhydryls. Cells were then washed two times with 20 mM Tris, pH 8, followed by a short incubation with PBS containing 0.1% BSA and 1% normal goat serum (PBS/BSA/NGS) (Vector Laboratories, Burlingame, CA). Primary antibodies diluted 1:100 (1:400 for fibrillin) in PBS/BSA/NGS were allowed to incubate with the cell layers for at least 30 min at room temperature. The samples were then washed three times with PBS/BSA/NGS, and FITC-conjugated secondary antibody (Cappel, West Chester, PA), diluted 1:400, was added for 30 min. Slides were washed three times with PBS/BSA/NGS, mounted with a 50% glycerol solution containing two to three grains of p-phenylenediamine per milliliter, and examined with the use of a Zeiss (Thornwood, NY) Axioskop microscope.
RESULTS
Coprecipitation of Decorin with MAGP-1
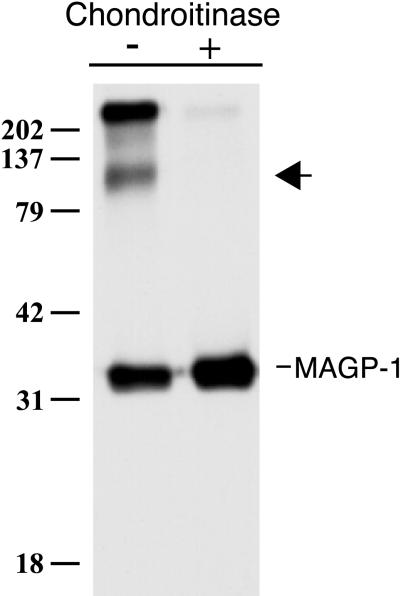
FBCs assemble elastic fibers in vitro, as shown by their deposition of both microfibrillar proteins and cross-linked elastin into the extracellular matrix. The interactions that occur between elastic matrix proteins in this cell culture model are thought to be representative of interactions that occur in vivo. Using coimmunoprecipitation to identify binding partners within the family of elastic fiber proteins, we noticed an ∼100-kDa protein that coprecipitated with MAGP-1 when [35S]sulfate was used to radiolabel secreted proteins (Figure 1). (MAGP-1 contains a tyrosine sulfation consensus sequence and has been shown to incorporate sulfate label [Brown-Augsburger et al., 1994].) Larger-molecular-mass sulfate-labeled bands were often in the immunoprecipitate, although in many cases they were found to associate nonspecifically with the protein A beads used to precipitate immune complexes. The identity of these bands was not investigated in this study.
Figure 1.
Immunoprecipitation of MAGP-1 from the medium of [35S]sulfate-labeled FBCs showing the coprecipitation of a sulfated molecule (arrow) that is sensitive to chondroitinase ABC digestion. Shown is an autoradiogram. Molecular mass markers are in kilodaltons.
The diffuse nature of the coprecipitating 100-kDa band, along with its intense labeling with radioactive sulfate, suggested that it might be a proteoglycan. The sensitivity of the immunoprecipitated material to digestion with chondroitinase ABC confirmed this suspicion and also demonstrated that the coprecipitating proteoglycan contained chondroitin and/or dermatan sulfate GAG(s). Because chondrocytes synthesize proteoglycans containing primarily chondroitin rather than dermatan sulfate carbohydrates, the coprecipitating proteoglycan was suspected to be a CSPG. The possibility that it contained dermatan sulfate GAG(s), however, was not formally excluded.
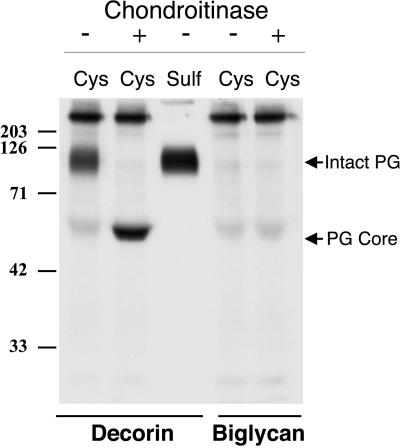
The molecular mass of the coprecipitating CSPG is typical of the small, leucine-rich family of proteoglycans. To determine whether FBCs secrete either of the two most common members of this family, decorin or biglycan, cells were labeled with sulfate and immunoprecipitations for each proteoglycan were performed. Figure 2 shows that decorin is synthesized in relatively high quantities, whereas little if any biglycan is secreted by these cells. It seemed likely, therefore, that the CSPG coprecipitating with MAGP-1 was bovine decorin. To determine if the interaction between MAGP-1 and the CSPG was occurring via the proteoglycan core protein or through its GAG component, serum-free conditioned medium collected from FBCs was treated with chondroitinase ABC to remove GAG side chains. Immunoprecipitation for MAGP-1 was then performed, and MAGP-1–precipitated material was subjected to immunoblotting for bovine decorin. The results shown in Figure 3 confirm that the proteoglycan coprecipitating with MAGP-1 is, indeed, decorin. Furthermore, coprecipitation of the proteoglycan from chondroitinase-treated medium indicates that its association with MAGP-1 occurs via the decorin core protein.
Figure 2.
Decorin and biglycan production by FBCs. Immunoprecipitation with the use of antibodies specific for decorin and biglycan from sulfate-labeled cells demonstrates that FBCs secrete decorin but not biglycan. Shown is an autoradiogram of immunoprecipitated products. Arrows indicate the gel positions of the intact proteoglycan and the core protein after chondroitinase ABC digestion. [35S]cysteine and [35S]sulfate were used to radiolabel the core protein and GAG side chains, respectively. Molecular mass markers are in kilodaltons.
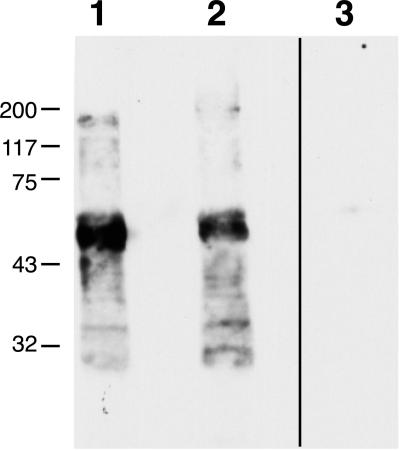
Figure 3.
Combined immunoprecipitation-immunoblotting shows that decorin coprecipitates with MAGP-1. Proteins immunoprecipitated from chondroitinase-treated chondrocyte medium with antibodies to either decorin (lane 1) or MAGP-1 (lanes 2 and 3) were separated by SDS-PAGE, transferred to nitrocellulose, and subjected to immunoblotting. Anti-decorin immunoblotting of the decorin-immunoprecipitated material serves as a positive control (lane 1). Immunoblotting of the MAGP-1–immunoprecipitated proteins with anti-decorin antibody (lane 2) confirms the coprecipitating proteoglycan's identity and demonstrates that its association with MAGP-1 is mediated by the core protein. An immunoblot of MAGP-1–immunoprecipitated material with secondary antibody alone serves as a negative control (lane 3). Molecular mass markers are in kilodaltons.
Fibrillin, MAGP, and Decorin Form a Ternary Complex in FBC Culture Medium
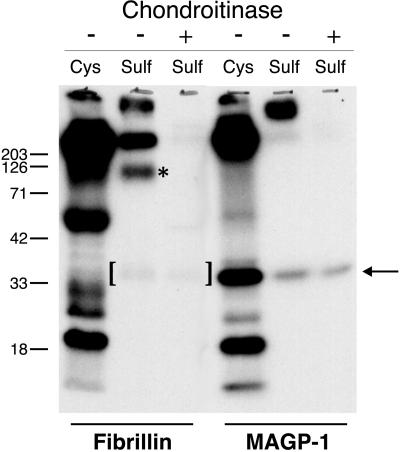
Kielty et al. (1996) observed that a CSPG the size of decorin coprecipitated with fibrillin from the medium of fetal bovine aortic smooth muscle cells. To assess whether a similar interaction could be identified in the FBC culture system, fibrillin was immunoprecipitated from the medium of sulfate-labeled cells. Figure 4 shows that a sulfate-labeled protein at ∼100 kDa was present with fibrillin in the immunoprecipitate. This band was lost upon treatment of the immunoprecipitated material with chondroitinase ABC, demonstrating that it is a CSPG. Interestingly, in addition to the CSPG, another sulfated protein coprecipitated with fibrillin. This protein was resistant to chondroitinase digestion, indicating that it was not a CSPG. Its molecular mass and its ability to incorporate sulfate label directly into the protein suggested that the protein's identity was MAGP-1. On SDS-PAGE, the protein migrated identically to authentic MAGP-1.
Figure 4.
MAGP-1 and decorin coimmunoprecipitate with fibrillin. Fibrillin was immunoprecipitated from the medium of [35S]sulfate-labeled or [35S]cysteine-labeled FBC cells, and the precipitated proteins were analyzed by SDS-PAGE before and after treatment with chondroitinase ABC. Treatment of the immunoprecipitated protein with chondroitinase ABC degrades the GAG associated with decorin (asterisk) but does not affect MAGP-1, whose migration position is indicated by the arrow. Brackets denote coprecipitated MAGP-1. Fibrillin is not able to be resolved on this 10% acrylamide gel. Shown are autoradiograms. Molecular mass markers are in kilodaltons.
An Amino-terminal Domain of Fibrillin-1 Expressed in CHO Cells Coprecipitates a CSPG
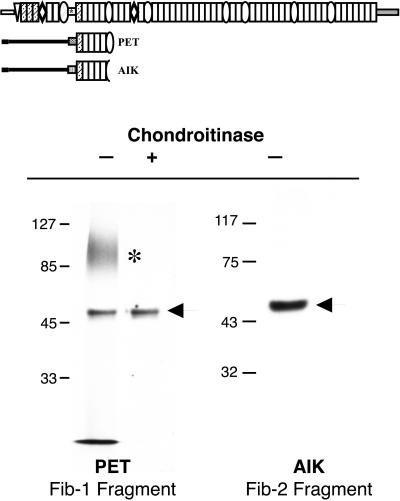
To identify which of the fibrillins associate with the CSPG, and to localize the site of its interaction on the fibrillin molecule, portions of fibrillin-1 and fibrillin-2 were expressed in CHO cells. Cells stably expressing amino-terminal regions of these proteins were radiolabeled with [35S]O4, and immunoprecipitations for these recombinant proteins were performed. Figure 5 shows that a sulfated band coprecipitates with a truncated fibrillin-1 protein (PET) that consists of the proline-rich and downstream EGF domains. The spatially analogous fibrillin-2 truncated protein does not coprecipitate the same sulfated molecule, suggesting that CSPG binding in this region is specific for fibrillin-1. As with FBCs, this sulfated band is susceptible to chondroitinase treatment, confirming that it is a CSPG.
Figure 5.
A proteoglycan-binding site is located near the proline-rich domain of fibrillin-1. (Top) Scheme of the fibrillin-1 and fibrillin-2 constructs used in this study and their relative positions within the full-length molecules. (Bottom) Immunoprecipitation of truncated fibrillin proteins from the medium of [35S]sulfate-labeled transfected CHO cells demonstrates that a sulfated proteoglycan (*) sensitive to chondroitinase ABC digestion coprecipitates with PET, the truncated fibrillin-1 protein (left), but not with Aik, an analogous region of fibrillin-2 (right). Arrows indicate gel positions of PET and Aik. Shown are autoradiograms. Molecular mass markers are in kilodaltons.
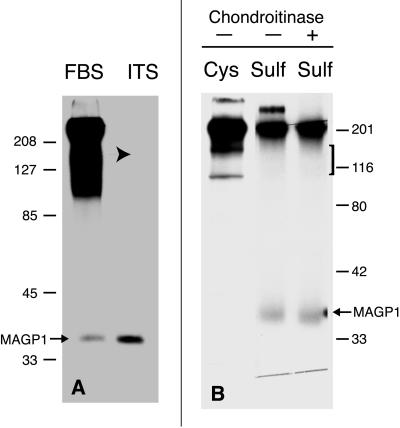
MAGP-1 and Fibrillin Coprecipitate in the Absence of CSPGs
As shown above, fibrillin and MAGP-1 both interact with a similar CSPG in chondrocyte medium. Although a ternary complex is suggested, it is not known whether MAGP-1 is capable of binding directly to fibrillin or if the interaction is mediated by the CSPG. Through a chance observation, we found that production of the CSPG, but not synthesis of fibrillin or MAGP-1, is inhibited when FBCs are cultured in serum-free medium supplemented with ITS (Figure 6A). Northern blotting for decorin mRNA confirmed its reduced expression (our unpublished results). When fibrillin is immunoprecipitated from the medium of sulfate-labeled FBCs cultured in ITS-supplemented medium, no coprecipitating CSPG is detected. However, sulfated MAGP-1 is still evident, suggesting that interaction between fibrillin and MAGP-1 can occur in the absence of proteoglycan (Figure 6B).
Figure 6.
MAGP-1 and fibrillin coprecipitate in the absence of decorin. (A) Immunoprecipitation for MAGP-1 from [35S]sulfate-labeled FBCs cultured in 10% FBS or ITS-supplemented medium. The absence of high-molecular-mass sulfated proteins (arrow) indicates that secretion of CSPGs is inhibited when cells are grown in ITS-containing medium. The secretion of other sulfated proteins is not affected, as indicated by the immunoprecipitation of MAGP-1. (B) Immunoprecipitation of fibrillin from the medium of sulfate-labeled chondrocytes cultured in medium supplemented with ITS shows a decrease in the amount of coprecipitating proteoglycan (bracket). A sulfate-labeled band that migrates at the molecular mass of MAGP-1 still coprecipitates (arrow). Molecular mass markers are in kilodaltons.
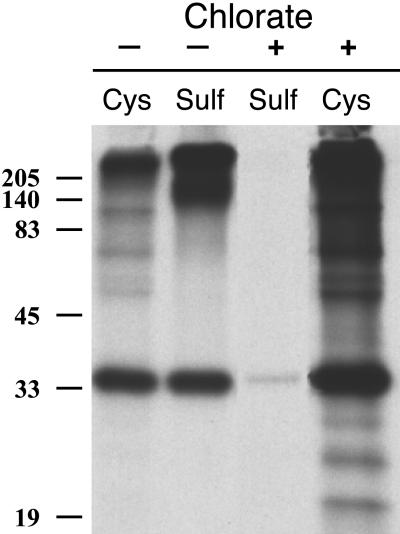
Inhibition of Sulfation Prevents Elastic Matrix Assembly In Vitro
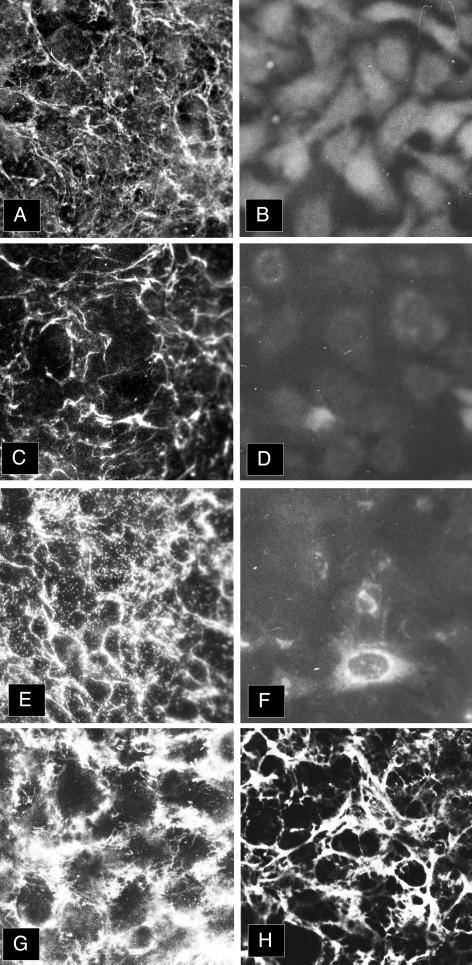
To understand the role of sulfation in the process of elastic fiber assembly, we compared elastic fiber formation by FBCs cultured in the presence and absence of chlorate. Figure 7 shows that treatment of chondrocytes with chlorate inhibits the sulfation of secreted proteins by documenting the absence of sulfate incorporation into both MAGP-1 and the coprecipitating decorin proteoglycan. Figure 8 shows that under normal culture conditions, both microfibrils, as assessed by MAGP-1 (A), fibrillin staining (C), and tropoelastin (E), are assembled into the extracellular matrix of FBCs. When cells are cultured in the presence of 10 mM KClO3 to inhibit the sulfation of proteins and proteoglycans, both microfibrils (B and D) and tropoelastin (F) are absent from the matrix. Interestingly, chlorate treatment did not inhibit the incorporation of another sulfated molecule, fibronectin, into the matrix (compare G and H), although some qualitative differences are observed in sulfate-inhibited cultures. Whether it is sulfation of a microfibrillar protein or modification of another matrix component that is critical to elastic matrix assembly is not yet clear.
Figure 7.
Chlorate treatment of FBCs inhibits sulfation of secreted proteins. Immunoprecipitation of [35S]cysteine- and [35S]sulfate-labeled MAGP-1 from the medium of FBCs shows that chlorate treatment inhibits both MAGP-1 and proteoglycan sulfation but has no effect on the secretion of MAGP-1 or its detection with the anti-MAGP-1 antibody. Molecular mass markers are in kilodaltons.
Figure 8.
Sulfation is required for elastic fiber formation. Indirect immunofluorescence for matrix proteins deposited by untreated (A, C, E, and G) and chlorate-treated (B, D, F, and H) FBCs demonstrates that sulfation is required for the assembly of elastic fibers by these cells. (A and B) MAGP-1; (C and D) fibrillin; (E and F) tropoelastin; (G and H) fibronectin.
DISCUSSION
Sulfated proteoglycans have a wide distribution in the extracellular matrix, where they serve multiple functions relating to maintenance of matrix structure and integrity, regulation of hydration of extracellular spaces, and modulation of matrix assembly (Iozzo and Murdoch, 1996). Their presence in elastin-rich tissues has been appreciated for many years, although the molecules with which the proteoglycans interact or their precise functions have been elusive. Our study demonstrates that the CSPG decorin interacts with the microfibril-associated proteins fibrillin-1 and MAGP-1. Although the significance of these interactions is still unknown, an association between these two proteins helps explain reports of proteoglycan/microfibril colocalization.
Evidence for a direct interaction between proteoglycans and elastic fiber proteins was provided by Kielty et al. (1996), who showed that a CSPG the size of decorin coprecipitates with fibrillin from smooth muscle cell conditioned medium. Interestingly, a coprecipitating 35-kDa sulfated protein was also observed. Although the identity of this protein was not established, its size and sulfation is inconsistent with it being decorin or biglycan core protein, because neither bears this modification. Although fibromodulin is sulfated and is a member of the same small, leucine-rich family of proteoglycans, its GAG chains are composed of keratan sulfate rather than chondroitin sulfate and, therefore, would be insensitive to chondroitinase digestion. Although the coprecipitation of a novel proteoglycan cannot be discounted, the molecular mass and sulfation of the coprecipitating protein are in agreement with it being MAGP-1. Thus, the results of Kielty et al. (1996) are consistent with our finding of a ternary complex between fibrillin, MAGP-1, and a CSPG.
Because decorin binds to both fibrillin-1 and MAGP-1, one obvious question is whether the interaction between fibrillin and MAGP-1 is dependent on the presence of decorin. Coprecipitation of MAGP-1 with fibrillin from FBCs cultured under conditions in which decorin synthesis is suppressed (Figure 6) provides evidence that these two proteins can interact independent of decorin. Because a decorin-like proteoglycan binds to the short fibrillin-1 PET construct, the proteoglycan-binding site must lie between the beginning of the proline-rich domain and the end of the second eight-cysteine domain of fibrillin-1. MAGP-1, in contrast, does not bind to PET (our unpublished results), indicating that its binding site on fibrillin is outside of the region encoded by this construct.
Ultrastructural studies have provided a clue to how CSPGs and MAGP-1 are oriented within the microfibril. Cuprolinic blue staining in conjunction with GAG-degrading enzymes have localized the CSPG to the bead structure (Chan and Choi, 1995; Kielty et al., 1996; Sherratt et al., 1997), where immunolocalization studies have also placed MAGP-1 (Henderson et al., 1996). This localization is consistent with our finding that a CSPG binds to a small fragment of fibrillin-1 and suggests that the sequence encoded by PET is in or close to the bead. This is consistent with previously published epitope immunolocalization studies (Reinhardt et al., 1996). Interestingly, only the fibrillin-1 proline-rich construct was capable of binding decorin; the spatially analogous region of fibrillin-2 was not. It remains to be determined whether a decorin-binding site exists in fibrillin-1 or fibrillin-2 outside of the region contained in our expressed constructs.
The importance of sulfation to elastic fiber assembly is suggested by the absence of elastin and microfibrils in FBC cultures in which sulfation had been inhibited with chlorate. Whether the aberrant assembly results from the absence of a proteoglycan or the undersulfation of a component protein (such as MAGP-1) remains unresolved. Although the MAGP-1–decorin interaction is not dependent on decorin's sulfated GAG chain, the association between these two proteins may be dependent on MAGP-1 sulfation. Similarly, sulfation of either MAGP-1 or decorin may be required for their interactions with fibrillin.
The significance of fibrillin and MAGP-1 interactions with decorin in elastic fiber assembly or function is not known. Decorin has been shown to influence collagen fibril assembly both in vitro and in vivo (Scott, 1988; Danielson et al., 1997) by regulating fiber diameter and interfibrillar spacing. The proteoglycan may play a similar role in regulating the assembly of microfibrils. Kielty et al. (1996) have suggested that the CSPG stabilizes the organization of individual microfibrils, because treatment with chondroitinase results in both a disruption of the microfibril beaded architecture and an increased interbead periodicity. Another possibility suggested by Chan and Choi (1995) is that the CSPG coordinates the collection of individual microfibrils into bundles. The importance of decorin to microfibril assembly or stability, however, is unclear, because mice harboring a targeted disruption of the decorin gene are viable and seem to have functional elastic tissues (Danielson et al., 1997). It remains to be determined whether other CSPGs substitute for decorin in its absence or if decorin has no essential function in elastin assembly.
ACKNOWLEDGMENTS
We thank Clarina Tisdale and Terese Hall for excellent technical and secretarial assistance, respectively. This work was supported by grants HL53325, HL61006, and HL62295 from the National Institutes of Health to R.P.M.
REFERENCES
- Baccarani Contri M, Fornieri C, Pasquali Ronchetti I. Elastin-proteoglycans association revealed by cytochemical methods. Connect Tissue Res. 1985;13:237–249. doi: 10.3109/03008208509152403. [DOI] [PubMed] [Google Scholar]
- Baccarani Contri M, Vincenzi D, Cicchetti F, Mori G, Pasquali Ronchetti I. Immunocytochemical localization of proteoglycans within normal elastin fibers. Eur J Cell Biol. 1990;53:305–312. [PubMed] [Google Scholar]
- Bressan GM, Pasquali-Ronchetti I, Fornieri C, Mattioli F, Castellani I, Volpin D. Relevance of aggregation properties of tropoelastin to the assembly and structure of elastic fibers. J Ultrastruct Mol Struct Res. 1986;94:209–216. doi: 10.1016/0889-1605(86)90068-6. [DOI] [PubMed] [Google Scholar]
- Brown-Augsburger P, Broekelmann T, Mecham L, Mercer R, Gibson MA, Cleary EG, Abrams WR, Rosenbloom J, Mecham RP. Microfibril-associated glycoprotein binds to the carboxyl-terminal domain of tropoelastin and is a substrate for transglutaminase. J Biol Chem. 1994;269:28443–28449. [PubMed] [Google Scholar]
- Brown-Augsburger P, Broekelmann T, Rosenbloom J, Mecham RP. Functional domains on elastin and microfibril-associated glycoprotein involved in elastic fiber assembly. Biochem J. 1996;318:149–155. doi: 10.1042/bj3180149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan FL, Choi HL. Proteoglycans associated with the ciliary zonule of the rat eye: a histochemical and immunocytochemical study. Histochem Cell Biol. 1995;104:369–381. doi: 10.1007/BF01458131. [DOI] [PubMed] [Google Scholar]
- Crouch E, Chang D, Rust K, Persson A, Heuser J. Recombinant pulmonary surfactant protein D. J Biol Chem. 1994;269:15808–15813. [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LW, Stubbs JT, 3rd, Young MF. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand Suppl. 1995;66:61–65. [PubMed] [Google Scholar]
- Gibson MA, Kumaratilake JS, Cleary EG. The protein components of the 12-nanometer microfibrils of elastic and nonelastic tissues. J Biol Chem. 1989;264:4590–4598. [PubMed] [Google Scholar]
- Gibson MA, Sandberg LB, Grosso LE, Cleary EG. Complementary DNA cloning establishes microfibril-associated glycoprotein (MAGP) to be a discrete component of the elastin-associated microfibrils. J Biol Chem. 1991;266:7596–7601. [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- Henderson M, Polewski R, Fanning JC, Gibson MA. Microfibril-associated glycoprotein-1 (MAGP-1) is specifically located on the beads of the beaded-filament structure for fibrillin-containing microfibrils as visualized by the rotary shadowing technique. J Histochem Cytochem. 1996;44:1389–1397. doi: 10.1177/44.12.8985131. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10:598–614. [PubMed] [Google Scholar]
- Kadar A, Gardner DL, Bush V. Alterations of the connective tissue components induced by β-aminopropionitrile. J Pathol. 1972;108:42–56. [Google Scholar]
- Kielty CM, Cummings C, Whittaker SP, Shuttleworth CA, Grant ME. Isolation and ultrastructural analysis of microfibrillar structures from fetal bovine elastic tissues. J Cell Sci. 1991;99:797–807. doi: 10.1242/jcs.99.4.797. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Shuttleworth CA. Fibrillin-containing microfibrils: structure and function in health and disease. Int J Biochem Cell Biol. 1995;27:747–760. doi: 10.1016/1357-2725(95)00028-n. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Whittaker SP, Shuttleworth CA. Fibrillin: evidence that chondroitin sulfate proteoglycans are components of microfibrils and associate with newly synthesized monomers. FEBS Lett. 1996;386:169–173. doi: 10.1016/0014-5793(96)00423-1. [DOI] [PubMed] [Google Scholar]
- Kumaratilake JS, Gibson MA, Fanning JC, Cleary EC. The tissue distribution of microfibrils reacting with a monospecific antibody to MAGP, the major glycoprotein antigen of elastin-associated microfibrils. Eur J Cell Biol. 1989;50:117–127. [PubMed] [Google Scholar]
- Mecham RP. Modulation of elastin synthesis: in vitro models. Methods Enzymol. 1987;144:232–246. doi: 10.1016/0076-6879(87)44181-5. [DOI] [PubMed] [Google Scholar]
- Mecham RP, Davis EC. Elastic fiber structure and assembly. In: Urchenco PD, Birk DE, Mecham RP, editors. Extracellular Matrix Assembly and Structure. San Diego, CA: Academic Press; 1994. pp. 281–310. [Google Scholar]
- Pasquali-Ronchetti I, Bressan GM, Fornieri C, Baccarani-Contri M, Castellani I, Volpin D. Elastin fiber-associated glycosaminoglycans in β-aminopropionitrile-induced lathyrism. Exp Mol Pathol. 1984;40:235–245. doi: 10.1016/0014-4800(84)90080-7. [DOI] [PubMed] [Google Scholar]
- Pereira L, D'Alessio M, Ramirez F, Lynch JR, Sykes B, Pangilinan T, Bonadio J. Genomic organization of the sequence coding for fibrillin, the defective gene product in Marfan syndrome. Hum Mol Genet. 1993;2:961–968. doi: 10.1093/hmg/2.7.961. [DOI] [PubMed] [Google Scholar]
- Reinhardt DP, Keene DR, Corson GM, Poschl E, Bachinger HP, Gambee JE, Sakai LY. Fibrillin-1: organization in microfibrils and structural properties. J Mol Biol. 1996;256:104–116. doi: 10.1006/jmbi.1996.0237. [DOI] [PubMed] [Google Scholar]
- Ritty TM, Milewicz DS, Mecham RP. Processing of the fibrillin-1 carboxy-terminal domain. J Biol Chem. 1999;274:8933–8940. doi: 10.1074/jbc.274.13.8933. [DOI] [PubMed] [Google Scholar]
- Ross R, Bornstein P. The elastic fiber. J Cell Biol. 1969;40:366–381. doi: 10.1083/jcb.40.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986;103:2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Scott JE. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988;252:313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt MJ, Holmes DF, Shuttleworth CA, Kielty CM. Scanning transmission electron microscopy mass analysis of fibrillin-containing microfibrils from foetal elastic tissues. Int J Biochem Cell Biol. 1997;29:1063–1070. doi: 10.1016/s1357-2725(97)00028-9. [DOI] [PubMed] [Google Scholar]
- Trask TM, Ritty TM, Broekelmann T, Tisdale C, Mecham RP. N-terminal domains of fibrillin-1 and fibrillin-2 direct the formation of homodimers: a possible first step in microfibril assembly. Biochem J. 1999;340:693–701. [PMC free article] [PubMed] [Google Scholar]
- Vrhovski B, Jensen S, Weiss AS. Coacervation characteristics of recombinant human tropoelastin. Eur J Biochem. 1997;250:92–98. doi: 10.1111/j.1432-1033.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- Wrenn DS, Griffin GL, Senior RM, Mecham RP. Characterization of biologically active domains on elastin: identification of a monoclonal antibody to a cell recognition site. Biochemistry. 1986;25:5172–5176. doi: 10.1021/bi00366a028. [DOI] [PubMed] [Google Scholar]
- Zhang H, Apfelroth SD, Hu W, Davis EC, Sanguineti C, Bonadio J, Mecham RP, Ramirez F. Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J Cell Biol. 1994;124:855–863. doi: 10.1083/jcb.124.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann DR, Dours-Zimmernann MT, Schubert M, Bruckner-Tuderman L. Versican is expressed in the proliferating zone in the epidermis and in association with the elastic network of the dermis. J Cell Biol. 1994;124:817–825. doi: 10.1083/jcb.124.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]