Abstract
The ordered activation of the ubiquitin protein ligase anaphase-promoting complex (APC) or cyclosome by CDC20 in metaphase and by CDH1 in telophase is essential for anaphase and for exit from mitosis, respectively. Here, we show that CDC20 can only bind to and activate the mitotically phosphorylated form of the Xenopus and the human APC in vitro. In contrast, the analysis of phosphorylated and nonphosphorylated forms of CDC20 suggests that CDC20 phosphorylation is neither sufficient nor required for APC activation. On the basis of these results and the observation that APC phosphorylation correlates with APC activation in vivo, we propose that mitotic APC phosphorylation is an important mechanism that controls the proper timing of APCCDC20 activation. We further show that CDH1 is phosphorylated in vivo during S, G2, and M phase and that CDH1 levels fluctuate during the cell cycle. In vitro, phosphorylated CDH1 neither binds to nor activates the APC as efficiently as does nonphosphorylated CDH1. Nonphosphorylatable CDH1 mutants constitutively activate APC in vitro and in vivo, whereas mutants mimicking the phosphorylated form of CDH1 are constitutively inactive. These results suggest that mitotic kinases have antagonistic roles in regulating APCCDC20 and APCCDH1; the phosphorylation of APC subunits is required to allow APC activation by CDC20, whereas the phosphorylation of CDH1 prevents activation of the APC by CDH1. These mechanisms can explain the temporal order of APC activation by CDC20 and CDH1 and may help to ensure that exit from mitosis is not initiated before anaphase has occurred.
INTRODUCTION
The initiation of anaphase and exit from mitosis depend on activation of the anaphase-promoting complex (APC) or cyclosome, a multisubunit complex that ubiquitinates mitotic regulators such as securin and cyclin B and thus targets them for destruction by the 26S proteasome (Peters, 1998; Morgan, 1999; Zachariae and Nasmyth, 1999). Because the proper timing of APC activation is important for the correct timing of anaphase and other late mitotic events, APC has to be tightly regulated. Several mechanisms have been implicated in APC regulation, but how APC is activated in mitosis is not well understood.
Several APC subunits are phosphorylated during mitosis (King et al., 1995; Peters et al., 1996; Yamada et al., 1997). These modifications appear to be required for high levels of APC activity because dephosphorylation of mitotic APC in vitro reduces its activity (Lahav-Baratz et al., 1995; Peters et al., 1996; Fang et al., 1998) and because phosphorylation of interphase APC in vitro partially stimulates its activity (Lahav-Baratz et al., 1995; Kotani et al., 1998). Consistent with these results, mitotic kinases are required for cyclin B degradation in Xenopus extracts (Felix et al., 1990; Grieco et al., 1996; Descombes and Nigg, 1998; Patra and Dunphy, 1998), but the identity of the kinases involved remains controversial, and the precise mechanism of phosphorylation-mediated APC activation is unknown.
It is furthermore clear that phosphorylation alone cannot be responsible for mitotic activation of the APC because in vivo and in vitro this event also depends on a protein called CDC20, Fizzy, p55CDC, or Slp1 (Dawson et al., 1995; Sigrist et al., 1995; Visintin et al., 1997; Fang et al., 1998; Kramer et al., 1998; Lorca et al., 1998; Shirayama et al., 1998). CDC20 is one of two related WD40 repeat proteins that are thought to activate the APC by physical association. CDC20 is a mitosis-specific activator, whereas the related protein CDH1 (also called Cdh1p/Hct1p, Fizzy-related, Ste9, or Srw1) appears to maintain APC active during the G1 phase of proliferating cells (Schwab et al., 1997; Sigrist and Lehner, 1997; Visintin et al., 1997; Fang et al., 1998; Kramer et al., 1998; Zachariae et al., 1998; Jaspersen et al., 1999) and during G0 in differentiated cells (Gieffers et al., 1999). In budding yeast, Cdc20p is specifically required for degradation of the securin Pds1p, whereas Cdh1p/Hct1p is required for proteolysis of the mitotic cyclin Clb2p (Schwab et al., 1997; Visintin et al., 1997; Shirayama et al., 1998). Because Pds1p and Clb2p degradation is important for anaphase and for exit from mitosis, respectively, the temporal order of APC activation by Cdc20p and Cdh1p/Hct1p is probably essential to ensure that exit from mitosis does not occur before sister chromatid separation has been initiated. How the temporal order of APC activation is achieved is only partially understood. In yeast, only nonphosphorylated forms of Cdh1p/Hct1p can bind to and activate the APC (Zachariae et al., 1998; Jaspersen et al., 1999), indicating that low-kinase levels allow Cdh1p/Hct1p to activate the APC during G1. Recently, the phosphatase Cdc14p has been shown to dephosphorylate Hct1p/Cdh1p at the end of mitosis and thus to activate it (Visintin et al., 1998; Jaspersen et al., 1999). Whether CDH1 is regulated by similar mechanisms in other organisms has not been investigated yet.
It is also not well understood, in any organism, how the ability of CDC20 to activate the APC is temporally regulated. Several mechanisms may contribute to this. First, in somatic animal cells and in yeast, CDC20 levels are controlled by transcriptional and proteolytic mechanisms that result in CDC20 synthesis during S and G2 phase and in its degradation at the end of mitosis (Weinstein, 1997; Fang et al., 1998; Kramer et al., 1998; Prinz et al., 1998; Shirayama et al., 1998). However, this phenomenon is not sufficient to explain the mitotic specificity of CDC20 because CDC20 accumulates before APC becomes active (Fang et al., 1998; Kramer et al., 1998; Prinz et al., 1998; Shirayama et al., 1998) and because the levels of this protein are constant in embryonic cell cycles (Lorca et al., 1998). Second, in cells in which spindle assembly has not been completed, a surveillance mechanism known as the spindle assembly checkpoint inhibits the ability of CDC20–APC complexes (APCCDC20) to initiate anaphase (reviewed by Sorger et al., 1997; Amon, 1999). The checkpoint mechanism depends on MAD2, a protein that is believed to inhibit CDC20 function by directly binding to it. It is therefore possible that derepression of the MAD2 signal has a role in mitotic activation of APCCDC20. However, this mechanism is also not sufficient to explain the mitotic regulation of CDC20 because the spindle assembly checkpoint is not functional in embryonic cell cycles (Minshull et al., 1994). The idea that MAD2 alone cannot account for CDC20 regulation is further supported by the observation that the degradation of A-type cyclins, which depends on APCCDC20 (Dawson et al., 1995; Sigrist et al., 1995; Sudakin et al., 1995) (Geley and Hunt, personal communication), is not inhibited by activation of the spindle assembly checkpoint (Whitfield et al., 1990; Hunt et al., 1992; Edgar et al., 1994; Minshull et al., 1994). Finally, it has also been observed that CDC20 is phosphorylated during mitosis (Weinstein, 1997; Kramer et al., 1998; Lorca et al., 1998), raising the possibility that this modification contributes to CDC20 regulation.
Here we show that the phosphorylation of APC core subunits is required to allow CDC20 to bind to and to activate the vertebrate APC, whereas the phosphorylation of CDH1 prevents binding to and activation of the APC by CDH1.
These mechanisms may explain the temporal order of APC activation by CDC20 and CDH1. During preparation of this manuscript, two articles were published that also address the activation of APC by CDC20; in agreement with our results, Shteinberg et al. (1999) found that in vitro–translated human CDC20/Fizzy can only activate the mitotically phosphorylated form of the clam APC or cyclosome, whereas Kotani et al. (1999) reported that phosphorylation of CDC20, but not of the APC, is required for APC activation. We compare these results with our data in the DISCUSSION.
MATERIALS AND METHODS
Recombinant Protein Expression
[35S]methionine- and [35S]cysteine-labeled human CDC20 and CDH1 and Xenopus CDC25 proteins were prepared by coupled transcription–translation reactions in rabbit reticulocyte lysate (Promega, Madison, WI). For baculovirus expression, human CDC20 and CDH1 cDNAs were cloned with an N-terminal 6-His-hemagglutinin tag into pFASTBAC (Life Technologies, Gaithersburg, MD). Virus was generated with the BAC-TO-BAC system (Life Technologies). Sf9 cells were used for virus amplification and protein expression. Protein extracts were prepared 45–48 h after infection in buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40 (NP-40), 10% glycerol, 2 mM EDTA, 50 mM NaF, 0.25 mM Na3VO4, 1 mM PMSF, 1 mM DTT, 1 μM okadaic acid (OA; Calbiochem, San Diego, CA), and 10 μg/ml each of chymostatin, leupeptin, and pepstatin (Sigma, St. Louis, MO). After 20 min on ice with occasional douncing, the lysates were centrifuged at 15,000 rpm in a microcentrifuge, shock frozen in liquid nitrogen, and stored at −70°C. In some experiments Sf9 cells were treated with 0.1 μM OA for 3 h before harvesting. Where indicated, extracts were used directly for APC binding and activation assays. For native purification of CDC20 and CDH1 on nickel-nitrilotriacetic acid agarose (Qiagen, Hilden, Germany), cells were lysed in an EmulsiFlex-C5 homogenator (Avestin, Ottawa, Ontario, Canada) in buffer containing 50 mM sodium phosphate, pH 8.0, 450 mM NaCl, 10 mM imidazole, 1% NP-40, 10% glycerol, 50 mM NaF, 0.25 mM Na3VO4, 2 mM β-mercaptoethanol, 0.5 μM OA, 20 mM β-glycerophosphate, and 10 μg/ml each of chymostatin, leupeptin, and pepstatin. After washing with 20 and 50 mM imidazole, the protein was eluted with 250 mM imidazole. Lysates from Sf9 cells triply infected with baculoviruses encoding human cyclin B and CDK1 and Xenopus p9 (Patra and Dunphy, 1998) were used directly for phosphorylation experiments.
Antibodies
Antibodies to human cyclin A and B were from Santa Cruz Biotechnology (Santa Cruz, CA), monoclonal anti-6-His-tag antibodies were from Clontech (Cambridge, UK), and monoclonal CDC27 antibodies were from Transduction Laboratories (Lexington, KY). Monoclonal antibodies against Xenopus cyclin A and B were kindly provided by T. Hunt (Imperial Cancer Research Fund, South Mimms, United Kingdom), and polyclonal CDC27 antibodies were kindly provided by C. Gieffers (Research Institute of Molecular Pathology, Vienna, Austria). CDC20 and CDH1 antibodies have been described (Kramer et al., 1998; Gieffers et al., 1999).
Cell Culture and Cell Synchronization
HeLa cells were synchronized by a thymidin block as described (Fang et al., 1998). Xenopus XL177 cells were grown at 25°C in 70% L-15 Leibovitz media (Sigma), 10% fetal bovine serum, 20% PBS, 0.3 mg/ml l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. HeLa and Xenopus XL177 cell extracts and Xenopus interphase egg extracts were prepared as described (Kramer et al., 1998; Vorlaufer and Peters, 1998). Lipofectamine Plus (Life Technologies) was used to transfect HeLa cells with the different pEGFP-N (Clontech) constructs. Green fluorescent protein (GFP)-positive and -negative cells were sorted 24 and 48 h after transfection in a Becton Dickinson fluorescence-activated cell sorting (FACS) vantage (Rutherford, NJ).
cDNA Mutagenesis
Point mutations were constructed by the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The constructs pET-CDC20 and pET-CDH1 were used as templates.
In Vitro Kinase and Phosphatase Assays
Kinase assays were performed in a volume of 15 μl using 2 μl of in vitro translation mixture and 2 μl of lysate from Sf9 cells containing cyclin B, CDK1, and p9 (see above). The kinase buffer contained 80 mM β-glycerophosphate, 15 mM MgCl2, 20 mM EGTA, 1 mM DTT, 1 mM ATP, 0.1% NP-40, 1 μM OA, and 10 mg/ml each of chymostatin, leupeptin, and pepstatin. Kinase assays were stopped by the addition of 10 μM staurosporin (Sigma). λ-Protein phosphatase treatment was done in a volume of 50 μl using 25 μl of APC beads, 4 μl of λ-protein phosphatase (1600 U; New England Biolabs, Beverly, MA), and 2 mM MnCl2 in the reaction buffer provided by the manufacturer. The reaction was stopped by the addition of 50 mM EDTA.
Immunoprecipitation, Binding, and Ubiquitination Assays
Immunoprecipitations, in vitro binding, and ubiquitination assays were done as described (Kramer et al., 1998) with the following modified washing conditions: immunoprecipitates were washed once with buffer A (buffer QA [Kramer et al., 1998] plus 2 mM EDTA, pH 8.0, 50 mM NaF, 0.25 mM Na3VO4, 20 mM β-glycerophosphate, and 10 μg/ml each of chymostatin, leupeptin, and pepstatin), twice with buffer A plus 400 mM KCl and 0.5% NP-40, twice with buffer A, and once with QA. Ubiquitination activities were quantified with the ImageQuant version 1.11 program from Molecular Dynamics (Sunnyvale, CA) and CA-Cricket Graph III version 1.5.1 from Computer Associates International (Islandia, NY).
Mass Spectrometry
Phosphorylated and nonphosphorylated CDC20s were treated as described previously, resulting in tryptic peptide mixtures (Shevchenko et al., 1996). They were purified on homemade microcolumns using POROS R2 and R3 (Perseptive, Framingham, MA.). To localize phosphopeptides, parent ion scans of the reporter ion m/z 79 were performed in the negative ion mode on a triple quadrupole mass spectrometer (API 300; Sciex, Toronto, Ontario, Canada). These parent ion scans specifically recognize peptides that lose phosphate ions. Multistep elutions were used to improve sequence coverage (Neubauer and Mann, 1999). A quadrupole time-of-flight mass spectrometer (Q-STAR; Sciex) equipped with a nanoelectrospray source (MDS Protana, Odense, Denmark) was used to sequence selected phosphopeptides (Shevchenko et al., 1997).
RESULTS
The Kinetics of Mitotic APC Phosphorylation Correlates with the Onset of Cyclin Degradation in Somatic and Embryonic Cell Cycles
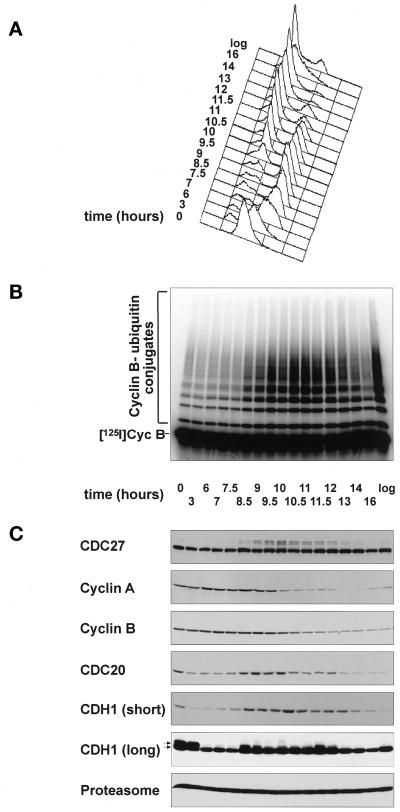
Vertebrate APC is composed of 10 or more subunits (Grossberger et al., 1999) at least 4 of which are phosphorylated during mitosis in Xenopus eggs (Peters et al., 1996) and in human cells (Gieffers and Peters, unpublished results). To test whether the temporal occurrence of these modifications is consistent with them having a role in APC activation, we first analyzed the kinetics of mitotic APC phosphorylation in somatic cell cycles in vivo and in embryonic cell cycles in vitro and compared them with the kinetics of mitotic cyclin ubiquitination and degradation. To analyze somatic cell cycles, we synchronized human HeLa cells by a double-thymidine arrest-and-release protocol (Figure 1A–C). The cyclin B ubiquitination activity of the APC isolated at different time points increased at 8.5 h (Figure 1B), at the same time at which a portion of the APC subunit CDC27 underwent a phosphorylation-mediated electrophoretic mobility shift (Figure 1C). The levels of cyclin A began to decrease between 9 and 9.5 h when CDC27 phosphorylation reached maximal levels (Figure 1C). Cyclin B and CDC20 began to disappear after 10.5 h (Figure 1C), consistent with previous reports of cyclin A being degraded before cyclin B (Whitfield et al., 1990; Hunt et al., 1992; Edgar et al., 1994; Minshull et al., 1994). We conclude that the ability of immunopurified APC to ubiquitinate cyclin B in vitro (Figure 1B) temporally correlates with CDC27 phosphorylation (Figure 1C). Because cyclin A is believed to be a substrate of APCCDC20, maximal CDC27 phosphorylation also appears to correlate with the onset of APC activity in vivo (Figure 1C). We did not investigate further why the cyclin B ubiquitination activity of immunopurified APC (Figure 1B) increased significantly before the levels of endogenous cyclin B decreased (Figure 1C), but this observation would be consistent with the possibility that MAD2 delays cyclin B proteolysis in vivo (Gorbsky et al., 1998) but may be lost during our APC immunoprecipitation procedure. The CDH1 data obtained in this experiment (Figure 1C) will be described below.
Figure 1.
Mitotic APC activation correlates with CDC27 phosphorylation in somatic and in embryonic cell cycles. a–c, Analysis of human HeLa cells progressing through mitosis after a double-thymidine arrest-and-release protocol. (A) Analysis of the cell cycle stage by measurement of the cellular DNA content by FACS. (B) Analysis of the cyclin B ubiquitination activity of APC immunopurified from cell extracts that were prepared at the indicated time points. Reactions were stopped after 15 min. (C) Immunoblot analysis of the same cell extracts as in b using antibodies to the proteins indicated. For CDH1, one immunoblot using 100 μg of protein/lane and short exposure times [CDH1 (short)] and one using 200 μg of protein/lane and long exposure times [CDH1 (long)] are shown. The arrows indicate phosphorylated and nonphosphorylated forms of CDH1. (D and E) Analysis of Xenopus eggextract entering a mitotic state in vitro. Entry into mitosis was triggered by supplementing an interphase extract with a nondegradable cyclin B fragment (Δ90) at time zero. (D) Analysis of the cyclin B ubiquitination activity of the extract at the indicated time points. (E) Analysis of the indicated proteins by phosphorimager analysis (CDC25) and by immunoblotting (all other proteins). In vitro–translated 35S-labeled CDC25 and [125I]Cyc B were added to a portion of the extract before Δ90 addition. All other proteins analyzed were endogenous Xenopus proteins. [125I]Cyc B, 125I-labeled recombinant cyclin B fragment 13–110.
Similar, but not identical, results were obtained in Xenopus egg extracts that entered a mitotic state in vitro (Figure 1, D and E). Addition of recombinant nondegradable cyclin B to interphase extracts resulted in entry into mitosis after 15 min, as judged by a phosphorylation-dependent electrophoretic mobility shift of the phosphatase CDC25 (Figure 1E). CDC27 phosphorylation could first be observed at 20 min and became maximal between 27 and 30 min (Figure 1E). The levels of cyclin A began to decrease at 25 min, i.e., shortly after CDC27 phosphorylation was initiated (Figure 1E). The levels of endogenous cyclin B began to decrease at 30 min when CDC27 phosphorylation was maximal (Figure 1E), and the assembly of polyubiquitin chains on radiolabeled cyclin B added to the extracts began at the same time (Figure 1D). CDC20 is not degraded in Xenopus extracts (Lorca et al., 1998), but CDC20 phosphorylation could be observed at the same time as CDC27 phosphorylation (Figure 1E). We conclude that in embryonic cell cycles both CDC27 and CDC20 phosphorylation correlates with the onset of APC activity. CDH1 was not analyzed in this experiment because Xenopus embryos do not contain detectable amounts of this protein (Lorca et al., 1998) (Figure 2). Our data from somatic and from embryonic cell cycles would thus be consistent with mitotic APC phosphorylation having a role in APC activation.
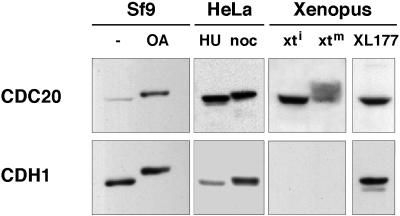
Figure 2.
Ectopic expression of phosphorylated and nonphosphorylated forms of CDC20 and CDH1 in insect cells. Immunoblot analysis of soluble fractions from Sf9 cells infected with baculoviruses encoding human CDC20 and CDH1. Before lysis, the Sf9 cells were treated either with (OA) or without (−) OA. For comparison, extracts from HeLa cells enriched in S phase with hydroxyurea (HU) or in mitosis with nocodazole (noc) and from logarithmically growing Xenopus XL177 cells (XL177) and Xenopus interphase (xti) and mitotic (xtm) extracts were analyzed side by side. CDC20 and CDH1 expressed in Sf9 cells were visualized with a monoclonal anti-6-His-tag antibody; HeLa and Xenopus proteins were visualized with specific CDC20 and CDH1 antibodies.
Mitotic Phosphorylation of the APC Is Required to Allow Activation by CDC20 But Has No Influence on Activation by CDH1
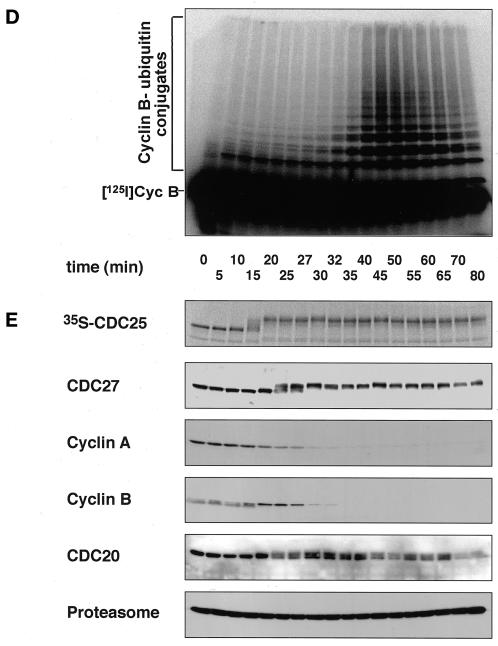
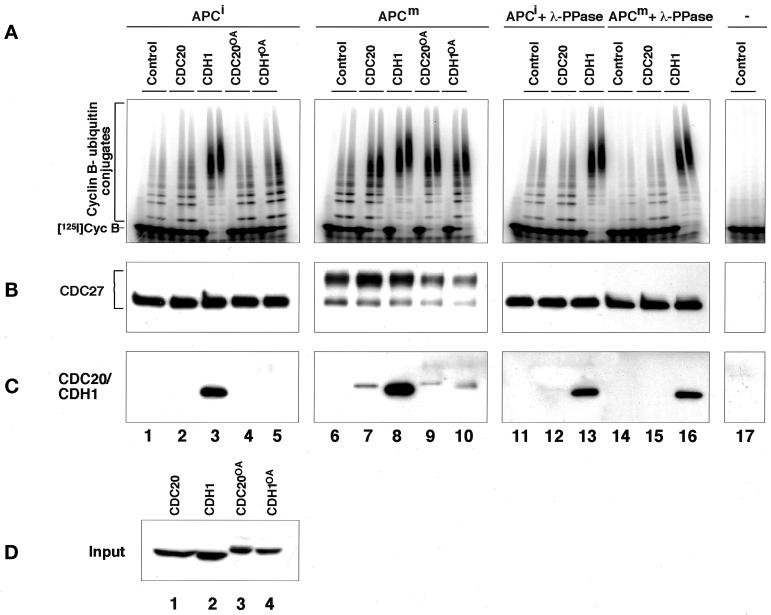
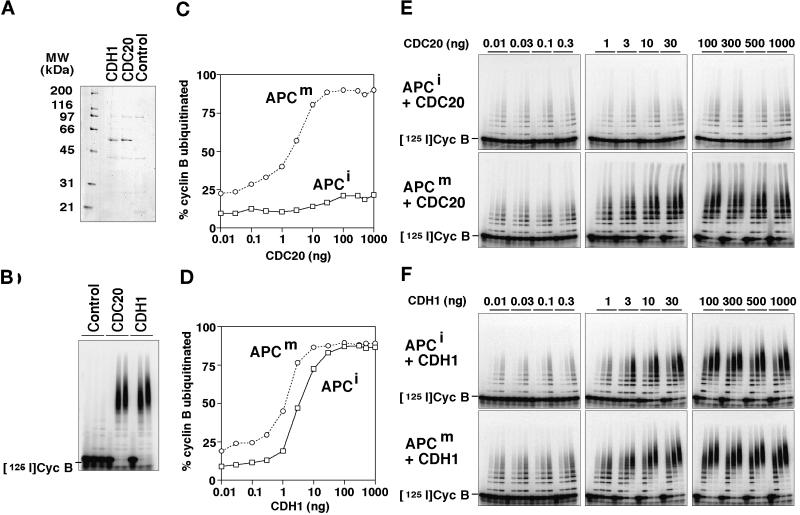
To obtain insight into the functional relevance of mitotic APC phosphorylation, we next analyzed the ability of recombinant CDC20 and CDH1 to associate with and to activate phosphorylated and nonphosphorylated forms of the APC. We generated baculoviruses encoding tagged versions of human CDC20 and CDH1 and expressed these proteins in Sf9 insect cells. Figure 2 shows that these proteins are present as soluble proteins in insect cell lysates and that their phosphorylation in vivo results in an electrophoretic mobility shift similar to the one seen in mitotic human cells and in mitotic Xenopus extracts. We tested the effects of these proteins by incubating immunopurified Xenopus APC in fractions from Sf9 cell lysates containing either CDC20, CDH1, or no ectopically expressed protein. APC was subsequently removed from the lysates, washed, and analyzed for its ability to support the ubiquitination of a radiolabeled cyclin B fragment in a reconstituted reaction containing purified E1 and E2 enzymes (Figure 3A). In these experiments, the CDC20-containing fraction was able to stimulate the cyclin B ubiquitination activity of mitotic APC (Figure 3A, reaction 7), whereas CDC20 had a smaller effect on APC isolated from interphase extracts (Figure 3A, reaction 2). This difference was not caused by a general inability of the interphase APC to be activated because fractions containing CDH1 stimulated both interphase and mitotic forms of APC equally well (Figure 3A, reactions 3 and 8; note that the activation of mitotic APC by CDH1 can occur in vitro but presumably not in vivo because CDH1 phosphorylation would prevent APC activation under these conditions [see below]). Similar results were obtained when we used CDC20 and CDH1 that were highly enriched from the Sf9 cell lysates by affinity chromatography (Figure 4, A and B). Fractions prepared by the same technique from noninfected Sf9 cell lysates had no effect on APC activity (Figure 4, A and B), suggesting that the stimulatory effects observed were caused by CDC20 and CDH1 and not by contaminating proteins from the Sf9 cells.
Figure 3.
The role of mitotic APC, CDC20, and CDH1 phosphorylation in regulating the association of CDC20 and CDH1 with the APC and APC activation. (A–C) Immunopurified Xenopus interphase APC (APCi), mitotic APC (APCm), or antibody beads without APC (−) were incubated directly or after λ-protein-phosphatase treatment (+ λ-PPase) with soluble fractions from Sf9 cells. The Sf9 cells had either been infected with baculoviruses encoding CDC20 or CDH1 or remained uninfected (Control). In some cases the Sf9 cells were treated with OA before lysis, yielding phosphorylated CDC20 (CDC20OA) and CDH1 (CDH1OA). Subsequently, the APC and control beads were stringently washed, and the beads were analyzed one-half for their associated cyclin B ubiquitination activity (A) and the other half for the amount and phosphorylation state of CDC27 (B) and CDC20 and CDH1 (C) bound to the beads. Samples from the ubiquitination assay were taken at 0, 15, and 30 min. CDC27 was detected in immunoblots using CDC27 antibodies, and recombinant CDC20 and CDH1 were detected by His-tag antibodies. (D) Immunoblot analysis of the Sf9 cell fractions added to the APC beads (corresponding to 1/8 of the total input) is shown. [125I]Cyc B, 125I-labeled recombinant cyclin B fragment 13–110.
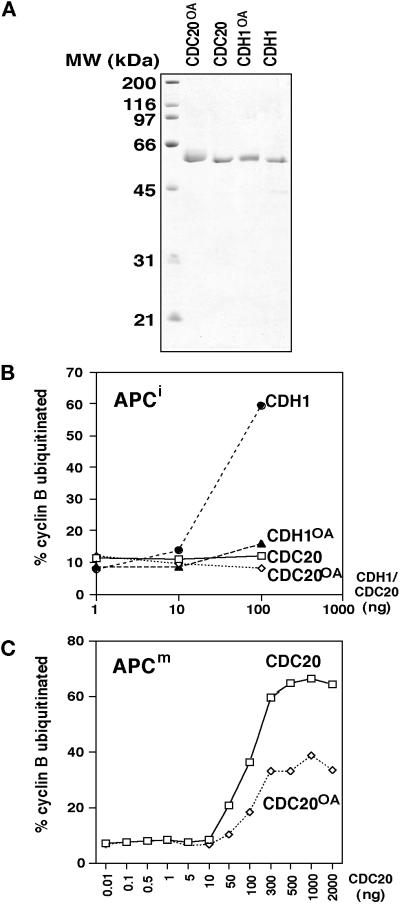
Figure 4.
Activation of mitotic and interphase APC by purified CDC20 and CDH1. (A and B) CDC20 and CDH1 were purified from baculovirus-infected Sf9 cells and analyzed by SDS-PAGE and Coomassie staining (A) and tested for their ability to stimulate the cyclin B ubiquitination activity of purified mitotic Xenopus APC (B). Extracts from noninfected Sf9 cells were subjected to the same purification scheme, and the resulting fraction (Control) was analyzed in the same way. Samples from the ubiquitination assay were taken at 0, 15, and 30 min. (C–F) Immunopurified Xenopus interphase APC (APCi) and mitotic APC (APCm) were incubated with increasing amounts (0.01–1000 ng) of purified CDC20 (C and E) and CDH1 (D and F), stringently washed, and subsequently analyzed in cyclin B ubiquitination assays. Samples were taken at 0, 5, 10, and 20 min. The data from the 10-min time point were quantitated and are shown as the percentage of 125I-labeled cyclin B that has been ubiquitinated. [125I]Cyc B, 125I-labeled recombinant cyclin B fragment 13–110.
To investigate whether activation of mitotic APC by CDC20 and by CDH1 correlated with the ability of these proteins to associate with the APC, we analyzed the amounts of recombinant CDC20 and CDH1 that bound to APC (Figure 3C). We were unable to detect any recombinant CDC20 in association with interphase APC, but a small amount of CDC20 could be detected in association with mitotic APC (Figure 3C, reactions 2 and 7). The CDC20 bound to mitotic APC could not be removed by washing in buffers containing high-salt concentrations and detergents, and no CDC20 could be recovered on antibody beads without bound APC (our unpublished results), suggesting that CDC20 binds specifically to mitotic APC. In contrast to CDC20, CDH1 bound equally well to mitotic and interphase APC (Figure 3C, reactions 3 and 8). We observed reproducibly in these experiments that much less CDC20 than CDH1 was bound to mitotic APC (Figure 3C, compare reactions 7 and 8), although similar amounts of these proteins were added to the reactions (Figure 3D, lanes 1 and 2). We presently do not know the reason for this difference.
To test whether differences in the phosphorylation status of APC were responsible for the different results obtained with interphase and mitotic APC, we dephosphorylated mitotic APC with λ-protein phosphatase (λ-PPase) before incubation with CDC20. The λ-PPase treatment resulted in the dephosphorylation of APC subunits as judged by CDC27 electrophoretic mobility shifts (Figure 3B, reactions 14–16) and abolished the ability of CDC20 to bind to and to activate APC strongly (Figure 3, A and C, reaction 15). This effect was not caused by general damage of the APC that could have occurred during the λ-PPase treatment because both dephosphorylated mitotic and interphase APCs associated with CDH1 and were activated by it equally as well as APC that had not been treated with λ-PPase (Figure 3, A and C, reactions 13 and 16).
Our data so far suggested that mitotic phosphorylation of the APC is important for activation by CDC20 but not for activation by CDH1. To quantitate this difference we determined the amounts of purified CDC20 and CDH1 that are required to activate interphase and mitotic APC (Figure 4, C–F). These experiments revealed that similar doses of CDH1 were required to activate interphase and mitotic APC (Figure 4D). Similar amounts of CDC20 were able to activate mitotic APC, but the activity of interphase APC could only be stimulated weakly, even when very high doses of CDC20 were used (Figure 4C). These results further support the conclusion that only the mitotic form of the APC can be activated by CDC20. On the basis of the observation that CDC20 can only bind to mitotic APC (Figure 3C), we suspect that the different susceptibilities of interphase and mitotic APC to be activated by CDC20 reflect different affinities of mitotic and interphase APC for CDC20, although direct affinity measurements would be required to confirm this.
To ensure that the differences that we observed between mitotic and interphase APC were not restricted to embryonic forms of the APC, we also analyzed the effects of CDC20 on APC isolated from human cells enriched in S phase or metaphase. In agreement with the results obtained with Xenopus APC, we found that CDC20 could activate mitotic human APC but not APC from S phase cells or mitotic APC treated with λ-PPase, whereas CDH1 activated all forms of the APC equally well (our unpublished results).
Mitotic CDC20 Phosphorylation Is Not Required for APC Activation
To analyze whether mitotic CDC20 phosphorylation influences CDC20 binding to the APC or APC activation, we generated phosphorylated forms of CDC20. We observed that electrophoretic mobility shifts resembling the phosphorylation-dependent shifts in human cells and in Xenopus extracts could be obtained by treating Sf9 cells expressing CDC20 with the phosphatase inhibitor OA (Figure 2), a drug known to induce a subset of mitotic events. In APC binding and activation assays, phosphorylated CDC20 from lysates of OA-treated Sf9 cells was able to associate with and to activate mitotic APC but had little effect on interphase APC (Figure 3, A and C, reactions 4 and 9). The amounts of phosphorylated CDC20 that remained bound to mitotic APC and the degree of APC activation were similar to the behavior and effects of nonphosphorylated CDC20 (Figure 3, A and C, reactions 7 and 9). To confirm these results further, we purified CDC20 from OA-treated and nontreated Sf9 cells (Figure 5A) and compared the ability of these proteins to activate mitotic and interphase APC in dose–response experiments. As seen before, phosphorylated CDC20 was unable to stimulate interphase APC (Figure 5B), indicating that CDC20 phosphorylation is not sufficient for its interaction with the APC. In contrast, mitotic APC was stimulated by both phosphorylated and nonphosphorylated CDC20 (Figure 5C), with the phosphorylated form of CDC20 being approximately twofold less active than nonphosphorylated CDC20. We presently do not know whether the apparent reduction of CDC20 activity by phosphorylation is physiologically relevant or not, but in either case the data clearly suggest that CDC20 phosphorylation does not stimulate APC activation in vitro.
Figure 5.
Activation of mitotic and interphase APC by purified phosphorylated CDC20 and CDH1. (A) CDC20 and CDH1 were purified from baculovirus-infected Sf9 cells that had been treated (CDC20OA and CDH1OA) or not treated (CDC20 and CDH1) with OA and analyzed by SDS-PAGE and Coomassie staining. (B and C) Different amounts of the purified proteins were tested for their ability to stimulate the cyclin B ubiquitination activity of interphase APC (APCi; B) or mitotic APC (APCm; C) purified from Xenopus eggs. The ubiquitination assays were analyzed as described in Figure 4.
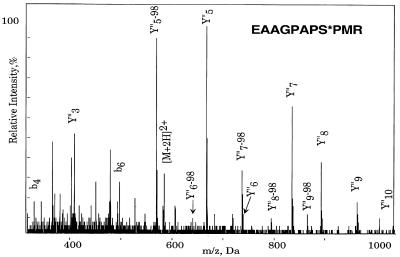
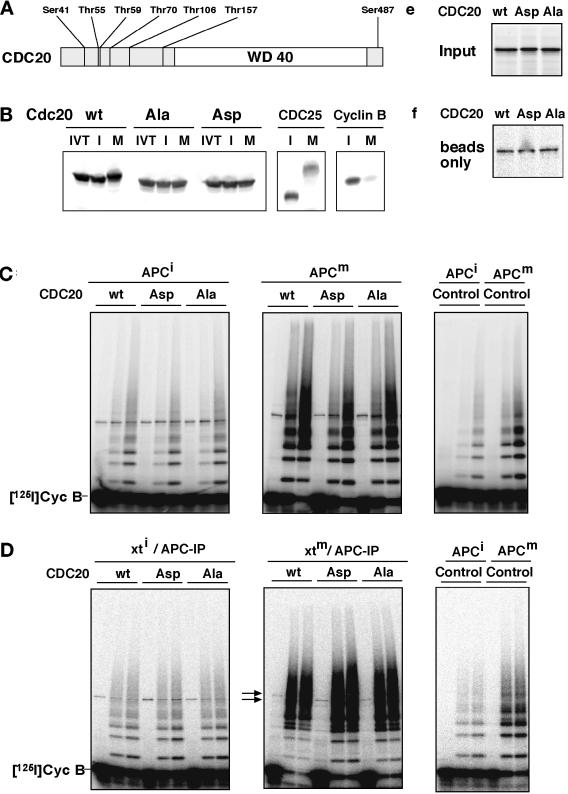
To test this conclusion further, we generated nonphosphorylatable CDC20 mutants by site-directed mutagenesis. Because it is not known on which amino acid residues CDC20 is phosphorylated, we performed a phosphopeptide analysis by using a combination of mass spectrometry techniques. The bands representing the nonphosphorylated and the phosphorylated forms of CDC20 were excised from a polyacrylamide gel (Figure 5A) and trypsinized. Resulting peptides were measured by matrix-assisted laser desorption ionization mass spectrometry but did not reveal significant differences (our unpublished results). Next, nanoelectrospray parent ion scans, which specifically measure phosphopeptides, were performed on a triple quadrupole mass spectrometer. Only one candidate for a phosphopeptide was detected. This peptide was then sequenced on a quadrupole time-of-flight instrument, revealing serine 41 to be phosphorylated (Figure 6). By both nanoelectrospray and matrix-assisted laser desorption ionization mass spectrometry, the nonphosphorylated form of the peptide was also found in the band corresponding to the phosphoprotein indicating partial phosphorylation on this site.
Figure 6.
Mass spectrometric identification of serine 41 as a phosphorylation site in CDC20. Phosphorylated CDC20 was purified as described in Figure 5A, excised from the gel, and trypsinated. After determination of a candidate phosphopeptide mass (m/z 580.3) by parent ion scans (see MATERIALS AND METHODS), the peptide corresponding to this mass was sequenced on a quadrupole time-of -flight instrument. The MS/MS spectrum of observed phosphopeptide with m/z 582.3 in positive mode is shown. It resulted in the sequence EAAGPAPS*PMR and localizes the modification site to serine 41 because of the losses of H3PO4 (98 Da) in the Y“ ion series.
To test whether phosphorylation on serine 41 is responsible for the electrophoretic mobility shift of mitotic CDC20, we generated a mutant in which this residue was changed to alanine (CDC20S41A). When 35S-labeled wild-type and mutant proteins were generated by in vitro translation and incubated in Xenopus egg extracts, CDC20S41A was still subject to a mitosis-specific mobility shift that was only slightly reduced compared with that of the wild-type protein (our unpublished results), suggesting that sites in addition to serine 41 can be phosphorylated in CDC20. However, in further mass spectrometry experiments using Lys-C digestion, which produces larger peptide fragments, and immobilized metal affinity columns, we were not able to retrieve other phosphopeptides.
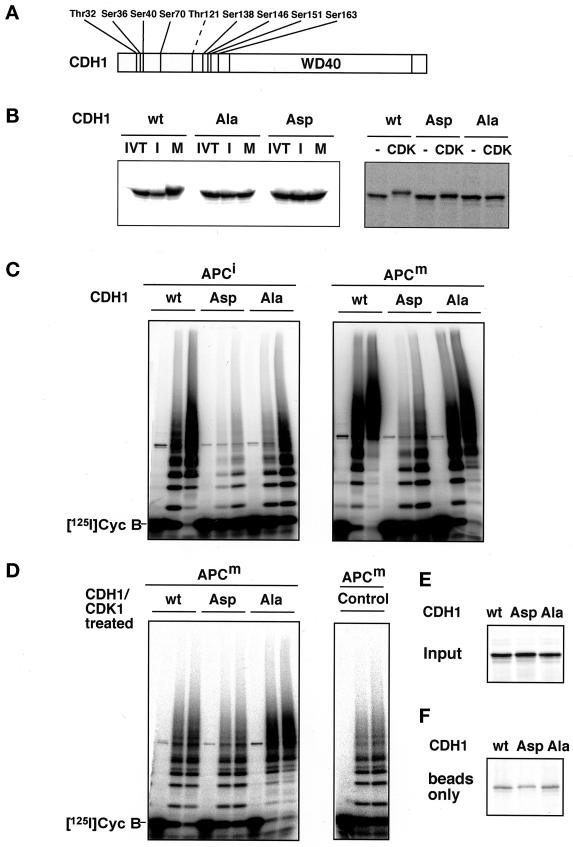
Serine 41 is part of a sequence that matches the consensus sequence S/T-P-X-K/R for phosphorylation by CDK1 (Figure 6), and we found that in vitro Sf9 cell fractions containing vertebrate cyclin B/CDK1/p9 complexes were able to phosphorylate CDC20 and CDH1 (see Figure 8B) (our unpublished results). Together, these observations implicate CDK1 in mitotic CDC20 phosphorylation. We therefore mutated seven serine and threonine residues in CDC20 that are potential CDK1 phosphorylation sites to alanine residues, thereby creating the mutant CDC20Ala (Figure 7A). In another mutant, CDC20Asp, we changed the same sites to aspartate residues with a view to creating a mutant that mimics the phosphorylated state of CDC20. When in vitro translation products of these proteins were incubated in Xenopus extracts, neither CDC20Ala nor CDC20Asp was subject to mitosis-specific electrophoretic mobility shifts (Figure 7B), indicating that they cannot be phosphorylated to the same degree as wild-type CDC20. When we tested the abilities of the three forms of CDC20 to activate interphase or mitotic APC, we found that none of the CDC20 proteins had significant effects on the activity of interphase APC, whereas both CDC20 wild-type and mutants could activate mitotic APC (Figure 7C). Similar results were obtained when all three forms of CDC20 were first incubated in mitotic Xenopus extracts before APC activation assays were performed (Figure 7D), i.e., under conditions in which CDC20wt is phosphorylated but CDC20Ala is not (Figure 7B). These results further suggest that mitotic CDC20 phosphorylation is not required for APC activation. We noticed that a portion of the in vitro–translated CDC20 proteins bound to antibody beads even in the absence of APC (Figure 7F), i.e., nonspecifically, and therefore did not attempt to quantitate the binding of the CDC20 proteins to APC in these experiments.
Figure 8.
Analysis of CDH1 phosphorylation mutants. (A) Schematic representation of the amino acid sequence of CDH1 and of the threonine and serine residues that were mutated to either alanine (all residues indicated; CDH1Ala) or aspartate (all residues indicated were mutated except Thr121 [dashed line]; CDH1Asp) residues is shown. The position of the WD40 repeat domain is indicated. (B) 35S-labeled in vitro translation products (IVT) of wild-type CDH1 (wt), CDH1Ala (Ala), and CDH1Asp (Asp) were incubated in interphase (I) or mitotic (M) Xenopus extracts (left) or in fractions from Sf9 cells infected with cyclin B/CDK1/p9 baculoviruses (CDK1) or not infected (−; right). All samples were subsequently analyzed by SDS-PAGE and phosphorimaging. (C) Immunopurified Xenopus interphase APC (APCi) or mitotic APC (APCm) were incubated with 35S-labeled proteins as described in B, washed stringently, and subsequently analyzed for cyclin B ubiquitination activity. Samples from the ubiquitination assay were taken at 0, 15, and 30 min. (D) In vitro–translated proteins as described in b were first incubated for 30 min in Sf9 cell fractions containing ectopically expressed cyclin B/CDK1/p9 complexes and then subjected to staurosporine treatment before they were added to immunopurified APC. In a control reaction (Control) mitotic APC was incubated with an in vitro translation mixture without DNA and analyzed as in C. After stringent washes, APC was analyzed for its cyclin B ubiquitination activity. (E) One-eightieth of the amount of 35S-labeled proteins used in C and D is shown. (F) The amount of 35S-labeled proteins that bind nonspecifically to the antibody-coupled beads is shown. [125I]Cyc B, 125I-labeled recombinant cyclin B fragment 13–110.
Figure 7.
Analysis of CDC20 phosphorylation mutants. (A) Schematic representation of the amino acid sequence of CDC20 and of the threonine and serine residues that were mutated to either alanine (CDC20Ala) or aspartate (CDC20Asp) residues is shown. The position of the WD40 repeat domain is indicated. (B) 35S-labeled in vitro translation products (IVT) of wild-type CDC20 (wt), CDC20Ala (Ala), and CDC20Asp (Asp) were incubated in Xenopus interphase (I) or mitotic (M) extracts and subsequently analyzed by SDS-PAGE and phosphorimaging. To control the interphase and mitotic state of the extracts, 35S-labeled CDC25 and cyclin B were added and analyzed side by side. (C) Immunopurified Xenopus interphase APC (APCi) or mitotic APC (APCm) were incubated with 35S-labeled proteins as described in b or with an in vitro translation mixture without DNA (Control), washed stringently, and subsequently analyzed for cyclin B ubiquitination activity. Samples from the ubiquitination assay were taken at 0, 15, and 30 min. (D) In vitro–translated proteins as in b were incubated in Xenopus interphase (xti) and mitotic (xtm) extracts for 30 min before APC was immunoprecipitated from these extracts, washed, and analyzed for ubiquitination activity. (E) One-eightieth of the amount of 35S-labeled proteins used in C and D is shown. (F) The amount of 35S-labeled proteins that bind nonspecifically to the antibody-coupled beads is shown. IP, immunoprecipitate. [125I]Cyc B, 125I-labeled recombinant cyclin B fragment 13–110.
Mitotic CDH1 Phosphorylation Prevents APC Activation
To test whether CDH1 phosphorylation inhibits its interaction with the APC, as shown previously in yeast (Zachariae et al., 1998; Jaspersen et al., 1999), we first reinvestigated the behavior of CDH1 in HeLa cells progressing through mitosis after double-thymidine arrest and release. Previous experiments had shown that the abundance of CDH1 transcripts increases as human cells enter mitosis (Fang et al., 1998), but no corresponding changes in protein levels and no changes in protein mobility were detected (Fang et al., 1998; Kramer et al., 1998). In contrast, we observed that CDH1 levels first decreased as cells were released from the thymidine arrest and then increased significantly as cells entered mitosis [Figure 1C, CDH1 (short)]. The CDH1 levels began to decrease again after 10.5 h, i.e., somewhat after cyclin B and CDC20 proteolysis was initiated. CDH1 activity may therefore also be regulated by changes in its abundance. Our CDH1 antibodies do not recognize recombinant CDC20 (our unpublished results), and we can therefore exclude that the variation in protein levels can be attributed to CDC20 cross-reactivity. Instead, we suspect that changes in CDH1 abundance may not have been observed before because of the inferior quality of the peptide antibodies used in previous experiments (Fang et al., 1998; Kramer et al., 1998). In addition, it is possible that in some experiments CDH1 levels were influenced by drug treatments as, for example, the G1 arrest induced by lovastatin has been attributed recently in part to inhibition of the proteasome (Rao et al., 1999).
When larger amounts of proteins were analyzed by long immunoblot exposures, an additional slower-migrating phosphorylated form of CDH1 was reproducibly observed in extracts from cells in S, G2, and M phase but not in G1 extracts [Figure 1C, CDH1 (long)]. To test the functional relevance of this modification, we phosphorylated ectopically expressed CDH1 in Sf9 cells in vivo by treating cells with OA (Figure 2). The resulting phosphorylated CDH1 bound only poorly to interphase and mitotic APC, and its ability to stimulate APC activity was reduced compared with the effects of nonphosphorylated CDH1 (Figure 3, A and C, reactions 5 and 10), suggesting that phosphorylation decreases binding of CDH1 to the APC. This conclusion was supported by dose–response experiments that confirmed that phosphorylated CDH1 showed little activity in APC activation assays (Figure 5B).
To test the role of CDH1 phosphorylation further, we generated mutants of CDH1 in which potential CDK1 phosphorylation sites were lacking. Nine serine and threonine residues were either changed to alanine, or a subset of eight of these residues was mutated to aspartate residues (Figure 8A). As in their CDC20 counterparts, these mutations abolished the electrophoretic mobility shifts that are observed when wild-type CDH1 is incubated in mitotic Xenopus extracts (Figure 8B, left). In APC activation assays, in vitro–translated wild-type CDH1 (CDH1wt) and CDH1Ala were able to stimulate the activity of interphase and mitotic APC (Figure 8C). In contrast, CDH1Asp could activate neither form of the APC (Figure 8C), consistent with the possibility that this mutant mimicks the phosphorylated form of CDH1. To test whether the CDH1Ala mutant had lost the ability to be inhibited by mitotic phosphorylation, we phosphorylated all three forms of CDH1 by incubating them in fractions from Sf9 cells containing ectopically expressed vertebrate cyclin B/CDK1/p9. This treatment resulted in a mobility shift of CDH1wt that was reduced in CDH1Asp and abolished in CDH1Ala (Figure 8B, right). The kinase was subsequently inhibited by addition of the inhibitor staurosporine, and the CDH1 proteins were tested for their ability to activate interphase or mitotic APC. Whereas cyclin B/CDK1/p9 inhibited the ability of CDH1wt to activate either form of the APC, the CDH1Ala mutant was resistant to this treatment (Figure 8D). Similar results were obtained when the CDH1 proteins were incubated in mitotic Xenopus extracts instead of being treated with cyclin B/CDK1/p9 before their effects on APC were tested (our unpublished results). These results suggest that mitotic CDH1 phosphorylation inhibits the ability of this protein to activate the APC.
Analysis of CDC20 and CDH1 Mutants In Vivo
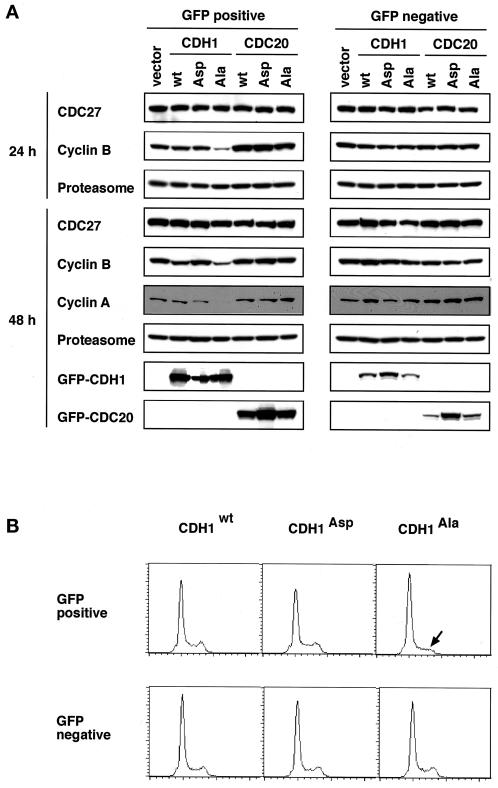
Our results from in vitro assays so far suggested that mitotic APC phosphorylation is required for assembly of APCCDC20, whereas the formation of APCCDH1 is inhibited by CDH1 phosphorylation. To test these conclusions in vivo, we transfected logarithmically growing HeLa cells with cDNAs encoding wild-type and mutant forms of CDC20 and CDH1 that are N-terminally tagged with GFP. At different times after the transfection, we isolated GFP-positive cells by FACS and analyzed the effects of CDH1/CDC20 expression on known APC substrates by immunoblotting.
Ectopic expression of CDH1Ala resulted in significantly reduced levels of the mitotic cyclins A and B (Figure 9A) but not of cyclin E (our unpublished results) that is not known to be an APC substrate. CDH1Asp did not detectably influence cyclin A and B levels, whereas CDH1wt expression resulted in a small reduction of cyclin B at late time points (Figure 9A, 48 h after transfection). These data are consistent with our in vitro data showing that nonphosphorylatable CDH1 (CDH1Ala) can constitutively activate the APC. To test whether the reduction of cyclin levels by CDH1Ala and CDH1wt expression was caused directly or indirectly by ectopically formed APCCDH1 complexes, we analyzed the DNA contents of the transfected cells by FACS (Figure 9B). This revealed a reduction in G2/M phase cells in the population of cells transfected with CDH1Ala and a corresponding increase in G1 cells. This result suggests that constitutively active APCCDH1 may delay entry into the cell cycle at the G1–S phase transition. Because cyclins are unstable in the G1 phase, the effects of CDH1Ala expression on cyclin levels may be both direct and indirect.
Figure 9.
Analysis of HeLa cells transiently transfected with wild-type and mutant CDH1 and CDC20. (A) Cells were transfected with GFP-expressing vector (vector) and N-terminal GFP fusions of wild-type CDH1 (CDH1 wt), CDH1Ala (CDH1 Ala), CDH1Asp (CDH1 Asp), wild-type CDC20 (CDC20 wt), CDC20Ala (CDC20 Ala), and CDC20Asp (CDC20 Asp). Twenty-four and 48 h after transfection GFP-positive and -negative cells were isolated by FACS and analyzed by immunoblotting using antibodies to the proteins indicated. (B) FACS analysis of the DNA content of HeLa cells 24 h after transfection with the GFP–CDH1 constructs CDH1wt, CDH1Asp, and CDH1Ala is shown. The arrow indicates a decrease in the G2/M FACS signal in cells transfected with CDH1Ala.
In contrast to the results obtained with CDH1, we could not detect any effects on either cyclin levels or cell cycle distribution of the transfected cells after the ectopic expression of wild-type and mutant CDC20 (Figure 9A) (our unpublished results). This result is consistent with our observation that CDC20 can only bind to mitotically phosphorylated APC that would only be present in a very small percentage of the cells analyzed in these experiments.
DISCUSSION
The separation of sister chromatids at the metaphase–anaphase transition depends on activation of the APC by the protein CDC20 (Dawson et al., 1995; Sigrist et al., 1995; Visintin et al., 1997; Shirayama et al., 1998). Because premature initiation of anaphase could lead to the formation of aneuploid daughter cells, the activation of APC by CDC20 has to be tightly controlled. Both CDC20 turnover and the spindle assembly checkpoint contribute to the regulation of this event, but neither of these mechanisms is sufficient to explain the mitotic specificity of APCCDC20 activation. Our results suggest that the mitotic phosphorylation of APC core subunits is required to allow CDC20 to bind to the APC and to activate its ubiquitination activity in vertebrate cells. Because ectopic overexpression of CDC20 in logarithmically cycling human cells is not sufficient to induce cyclin B proteolysis (Figure 9A), APC phosphorylation may be a rate-limiting step for the assembly of active APCCDC20 complexes. The proper timing of anaphase may therefore not only depend on the previously observed accumulation of CDC20 between S phase and mitosis and on completion of spindle assembly but also on the activation of mitotic kinases that phosphorylate APC subunits. These results may explain previous observations that mitotic phosphorylation is required for high APC activity (Lahav-Baratz et al., 1995; Peters et al., 1996; Kotani et al., 1998), although it is unclear whether CDC20 was present in the previous experiments. Alternatively, it remains possible that the phosphorylation of APC subunits has some allosteric effects on APC activity that are independent of CDC20.
Previous work has implicated both Polo-like kinase 1 (PLK1) and cyclin B/CDK1/p9 in APC phosphorylation (Lahav-Baratz et al., 1995; Kotani et al., 1998; Patra and Dunphy, 1998). In vertebrates both of these kinases are activated early during entry into mitosis, at the same time or even slightly before the phosphatase CDC25 is activated (Gautier et al., 1991; Kumagai and Dunphy, 1996). Our results show, however, that APC core phosphorylation occurs significantly after CDC25 and CDK1 activation, presumably in prometaphase or metaphase (Figure 1E) (Vorlaufer and Peters, 1998). It will therefore be interesting to investigate whether other kinases have a role in APC phosphorylation in vivo or whether a special lag phase mechanism delays the ability of PLK1 and cyclin B/CDK1/p9 to recognize APC subunits in mitosis. It will also be important to identify which of the vertebrate APC subunits needs to be phosphorylated to allow CDC20 binding and whether this mechanism is conserved in other species, such as yeast (to date, APC phosphorylation has been observed in fission yeast [Yamada et al., 1997] but not in budding yeast [Jaspersen et al., 1999]).
Three other articles have recently reported experiments that address the activation of APC by CDC20, although with different conclusions. Fang et al. (1998) proposed that in vitro–translated CDC20 can activate Xenopus APC independent of the phosphorylation state of the APC. In contrast, Kotani et al. (1999) found that phosphorylation of CDC20, but not of the APC, is required for APC activation. Again in contrast with these results, Shteinberg et al. (1999) reported recently (during preparation of this manuscript) that in vitro–translated human Fizzy/CDC20 can only activate the mitotically phosphorylated form of the clam cyclosome or APC. Our data agree with the results of Shteinberg et al. (1999), but we can only speculate why different conclusions were reached by the other authors. One possibility is that some APC phosphorylation occurred during the experiments of Fang et al. (1998) and of Kotani et al. (1999); for example, the mitotic kinases used by Kotani et al. (1999) to treat CDC20 could have had direct effects on APC. Alternatively, it is conceivable that the different sources of CDC20 used may have contributed to the different results (Kotani et al. [1999] used bacterially expressed CDC20 that presumably is devoid of any phosphorylation, whereas we used CDC20 expressed in insect cells in which noncell cycle–regulated modifications could occur).
We specifically tested whether mitotic CDC20 phosphorylation has a role in APC activation, but even in careful dose–response experiments we could not detect a stimulatory effect of CDC20 phosphorylation on APC activation; CDC20 phosphorylation was neither sufficient to activate interphase APC (Figure 5B) nor required to activate mitotic APC (Figure 5C). We confirmed these results by generating CDC20 mutants lacking seven CDK1 consensus sites that we found to be as potent as wild-type CDC20 in activating mitotic APC. Mass spectrometric analyses showed that at least one of these sites is phosphorylated in vivo (Figure 6), and we found that CDK1 can phosphorylate CDC20 in vitro (our unpublished results), suggesting that the sites mutated by us are physiologically relevant. We can nevertheless not exclude that CDC20 may in addition be phosphorylated on other residues that may be of relevance, but we note that mutation of a similar set of CDK1 sites in CDH1 did have strong effects on its regulation (see below). It therefore remains to be seen whether CDC20 phosphorylation is required for full APC activation under physiologic conditions or whether this modification may influence CDC20's ability to interact with other proteins, such as the checkpoint protein MAD2 that has been found to interact specifically with the mitotic form of APCCDC20 (Li et al., 1997).
Previous work in yeast has shown that the late mitotic/G1-specific activator of the APC, Hct1p/Cdh1p, is negatively regulated by phosphorylation (Zachariae et al., 1998; Jaspersen et al., 1999). Our results indicate that vertebrate CDH1 is regulated at least in part by the same mechanism, i.e., that phosphorylation of CDH1 inhibits its ability to associate with the APC and to activate it. In addition, our work suggests that CDH1 function may also be cell cycle regulated by controlling the rates of CDH1 synthesis and/or destruction. Together with the observation that human CDH1 is phosphorylated in vivo from S phase until the end of mitosis but not in G1 phase (Figure 1C), our results suggest that the ability of CDH1 to activate the APC is inhibited from the G1–S transition until the end of mitosis by CDH1 phosphorylation. Previous experiments in Drosophila have shown that ectopic expression of cyclin E is sufficient to stabilize APC substrates prematurely in G1, consistent with the idea that cyclin E-dependent kinases may directly inhibit CDH1 by phosphorylation (Knoblich et al., 1994). More recent experiments suggest that cyclin A–dependent kinases have a similar role in human cells (Lukas et al., 1999). Interestingly, ectopic expression of nonphosphorylatable CDH1 in logarithmically cycling human cells caused a decrease in G2/M phase cells and a corresponding increase in cells in G1 (Figure 9B). This observation suggests that inactivation of APCCDH1 may be required to allow entry into the cell cycle. According to this hypothesis, APCCDH1 activity would contribute to the length of the G1 phase by keeping the levels of S phase–activating proteins low, a model that is consistent with the previous observation that the expression of CDH1 correlates with the establishment of a G1 phase during early development of flies and frogs (Sigrist and Lehner, 1997; Lorca et al., 1998).
In budding yeast, APCCDC20 has been shown to be specifically required for the initiation of anaphase by allowing the destruction of the securin Pds1p, whereas APCCDH1 has an important role in the exit from mitosis by ubiquitinating the mitotic cyclin Clb2p (Schwab et al., 1997; Visintin et al., 1997; Shirayama et al., 1998). Although it is not known yet whether APCCDC20 and APCCDH1 display similar substrate specificities in other organisms, the budding yeast data have led to the idea that the temporal order of APC activation by CDC20 and CDH1 is an important mechanism that prevents exit from mitosis before anaphase has occurred. Genetic experiments in yeast suggest that activation of APCCDH1 depends on the previous elimination of an APCCDH1 inhibitor by APCCDC20 (Yamamoto et al., 1996; Lim et al., 1998). Good candidates for an APCCDC20 substrate with this function are the cyclin subunits of mitotic CDKs that could inactivate Hct1p/Cdh1p directly as well as indirectly by inhibiting the phosphatase Cdc14p (Shirayama et al., 1999). Our results expand this model by showing that mitotic kinases have directly antagonistic roles in regulating CDC20 and CDH1; whereas mitotic APC phosphorylation is required for activation of APCCDC20, stimulation of the APC by CDH1 is simultaneously inhibited by the phosphorylation of CDH1. This mechanism ensures that APCCDC20 can only be activated under conditions (high-kinase levels) in which APCCDH1 is inactive and may thus help to guarantee the proper order of anaphase and exit from mitosis.
ACKNOWLEDGMENTS
We are particularly grateful to C. Gieffers for a generous supply of CDC27 antibodies, to K. Paiha and P. Steinlein for help with the FACS, to E. Vorlaufer and I. Sumara for Xenopus extracts, to T. Hunt for Xenopus cyclin antibodies, and to B. Dunphy and D. Patra for CDK1, cyclin B, and p9 baculoviruses. We also thank J. Bartek, D. Bohmann, M. Brandeis, C. and. J. Lukas, K. Todokoro, W. Wunderlich, and members of the Peters lab for helpful discussions; K. Kaplan, L. Kahr, T. Bajari, and D. Solka for advice on baculovirus expression; K. Mechtler, S. Aigner, H. Auer, and G. Schaffner for technical help; A. Marchler-Bauer for secondary structure predictions; M. Glotzer for comments on this manuscript; and H. Tkadletz and S. Hauf for graphics. This work was supported by grant FFF 3/12801 from the Austrian Industrial Research Promotion Fund to J.-M.P.
REFERENCES
- Amon A. The spindle checkpoint. Curr Opin Genet Dev. 1999;9:69–75. doi: 10.1016/s0959-437x(99)80010-0. [DOI] [PubMed] [Google Scholar]
- Dawson IA, Roth S, Artavanis-Tsakonas S. The Drosophila cell cycle gene fizzy is required for normal degradation of cyclins A and B during mitosis and has homology to the CDC20 gene of Saccharomyces cerevisiae. J Cell Biol. 1995;129:725–737. doi: 10.1083/jcb.129.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P, Nigg EA. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J. 1998;17:1328–1335. doi: 10.1093/emboj/17.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA, Sprenger F, Duronio RJ, Leopold P, O'Farrell PH. Distinct molecular mechanisms regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes Dev. 1994;8:440–452. doi: 10.1101/gad.8.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G, Yu H, Kirschner MW. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell. 1998;2:163–171. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]
- Felix MA, Cohen P, Karsenti E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J. 1990;9:675–683. doi: 10.1002/j.1460-2075.1990.tb08159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Gieffers C, Peters BH, Kramer ER, Dotti CG, Peters JM. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc Natl Acad Sci USA. 1999;96:11317–11322. doi: 10.1073/pnas.96.20.11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky GJ, Chen RH, Murray AW. Microinjection of antibody to Mad2 protein into mammalian cells in mitosis induces premature anaphase. J Cell Biol. 1998;141:1193–1205. doi: 10.1083/jcb.141.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco D, Porcellini A, Avvedimento EV, Gottesman ME. Requirement for cAMP-PKA pathway activation by M phase-promoting factor in the transition from mitosis to interphase. Science. 1996;271:1718–1723. doi: 10.1126/science.271.5256.1718. [DOI] [PubMed] [Google Scholar]
- Grossberger R, Gieffers C, Zachariae W, Podtelejnikov AV, Schleiffer A, Nasmyth K, Mann M, Peters JM. Characterization of the DOC1/APC10 subunit of the yeast and the human anaphase-promoting complex. J Biol Chem. 1999;274:14500–14507. doi: 10.1074/jbc.274.20.14500. [DOI] [PubMed] [Google Scholar]
- Hunt T, Luca FC, Ruderman JV. The requirements for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J Cell Biol. 1992;116:707–724. doi: 10.1083/jcb.116.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspersen SL, Charles JF, Morgan DO. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr Biol. 1999;9:227–236. doi: 10.1016/s0960-9822(99)80111-0. [DOI] [PubMed] [Google Scholar]
- King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- Knoblich JA, Sauer K, Jones L, Richardson H, Saint R, Lehner CF. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 1994;77:107–120. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Kotani S, Tanaka H, Yasuda H, Todokoro K. Regulation of APC activity by phosphorylation and regulatory factors. J Cell Biol. 1999;146:791–800. doi: 10.1083/jcb.146.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kotani S, Tugendreich S, Fujii M, Jorgensen PM, Watanabe N, Hoog C, Hieter P, Todokoro K. PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol Cell. 1998;1:371–380. doi: 10.1016/s1097-2765(00)80037-4. [DOI] [PubMed] [Google Scholar]
- Kramer ER, Gieffers C, Holzl G, Hengstschlager M, Peters JM. Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family. Curr Biol. 1998;8:1207–1210. doi: 10.1016/s0960-9822(07)00510-6. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- Lahav-Baratz S, Sudakin V, Ruderman JV, Hershko A. Reversible phosphorylation controls the activity of cyclosome-associated cyclin-ubiquitin ligase. Proc Natl Acad Sci USA. 1995;92:9303–9307. doi: 10.1073/pnas.92.20.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gorbea C, Mahaffey D, Rechsteiner M, Benezra R. MAD2 associates with the cyclosome/anaphase-promoting complex and inhibits its activity. Proc Natl Acad Sci USA. 1997;94:12431–12436. doi: 10.1073/pnas.94.23.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HH, Goh PY, Surana U. Cdc20 is essential for the cyclosome-mediated proteolysis of both Pds1 and Clb2 during M phase in budding yeast. Curr Biol. 1998;8:231–234. doi: 10.1016/s0960-9822(98)70088-0. [DOI] [PubMed] [Google Scholar]
- Lorca T, Castro A, Martinez AM, Vigneron S, Morin N, Sigrist S, Lehner C, Doree M, Labbe JC. Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J. 1998;17:3565–3575. doi: 10.1093/emboj/17.13.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas C, Sørensen CS, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters J-M, Bartek J, Lukas J. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature. 1999;401:815–818. doi: 10.1038/44611. [DOI] [PubMed] [Google Scholar]
- Minshull J, Sun H, Tonks NK, Murray AW. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Regulation of the APC and the exit from mitosis. Nat Cell Biol. 1999;1:E47–E53. doi: 10.1038/10039. [DOI] [PubMed] [Google Scholar]
- Neubauer G, Mann M. Mapping of phosphorylation sites of gel-isolated proteins by nanoelectrospray tandem mass spectrometry: potentials and limitations. Anal Chem. 1999;71:235–242. doi: 10.1021/ac9804902. [DOI] [PubMed] [Google Scholar]
- Patra D, Dunphy WG. Xe-p9, a Xenopus Suc1/Cks protein, is essential for the Cdc2-dependent phosphorylation of the anaphase-promoting complex at mitosis. Genes Dev. 1998;12:2549–2559. doi: 10.1101/gad.12.16.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM. SCF and APC: the yin and yang of cell cycle regulated proteolysis. Curr Opin Cell Biol. 1998;10:759–768. doi: 10.1016/s0955-0674(98)80119-1. [DOI] [PubMed] [Google Scholar]
- Peters JM, King RW, Hoog C, Kirschner MW. Identification of BIME as a subunit of the anaphase-promoting complex. Science. 1996;274:1199–1201. doi: 10.1126/science.274.5290.1199. [DOI] [PubMed] [Google Scholar]
- Prinz S, Hwang ES, Visintin R, Amon A. The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis. Curr Biol. 1998;8:750–760. doi: 10.1016/s0960-9822(98)70298-2. [DOI] [PubMed] [Google Scholar]
- Rao S, Porter DC, Chen X, Herliczek T, Lowe M, Keyomarsi K. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc Natl Acad Sci USA. 1999;96:7797–7802. doi: 10.1073/pnas.96.14.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M, Lutum AS, Seufert W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell. 1997;90:683–693. doi: 10.1016/s0092-8674(00)80529-2. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Chernushevich I, Ens W, Standing KG, Thomson B, Wilm M, Mann M. Rapid “de novo” peptide sequencing by a combination of nanoelectrospray, isotopic labeling and a quadrupole/time-of-flight mass spectrometer. Rapid Commun Mass Spectrom. 1997;11:1015–1024. doi: 10.1002/(SICI)1097-0231(19970615)11:9<1015::AID-RCM958>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Shirayama M, Toth A, Galova M, Nasmyth K. APCCDC20 promotes exit from mitosis by destroying the anaphase inhibitor Pds1 and cyclin Clb5. Nature. 1999;402:203–207. doi: 10.1038/46080. [DOI] [PubMed] [Google Scholar]
- Shirayama M, Zachariae W, Ciosk R, Nasmyth K. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 1998;17:1336–1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shteinberg M, Protopopov Y, Listovsky T, Brandeis M, Hershko A. Phosphorylation of the cyclosome is required for its stimulation by Fizzy/cdc20. Biochem Biophys Res Commun. 1999;260:193–198. doi: 10.1006/bbrc.1999.0884. [DOI] [PubMed] [Google Scholar]
- Sigrist S, Jacobs H, Stratmann R, Lehner CF. Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B and B3. EMBO J. 1995;14:4827–4838. doi: 10.1002/j.1460-2075.1995.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Lehner CF. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell. 1997;90:671–681. doi: 10.1016/s0092-8674(00)80528-0. [DOI] [PubMed] [Google Scholar]
- Sorger PK, Dobles M, Tournebize R, Hyman AA. Coupling cell division and cell death to microtubule dynamics. Curr Opin Cell Biol. 1997;9:807–814. doi: 10.1016/s0955-0674(97)80081-6. [DOI] [PubMed] [Google Scholar]
- Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca FC, Ruderman JV, Hershko A. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol Biol Cell. 1995;6:185–197. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin R, Craig K, Hwang ES, Prinz S, Tyers M, Amon A. The phosphates Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol Cell. 1998;2:1–20. doi: 10.1016/s1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- Visintin R, Prinz S, Amon A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- Vorlaufer E, Peters JM. Regulation of the cyclin B degradation system by an inhibitor of mitotic proteolysis. Mol Biol Cell. 1998;9:1817–1831. doi: 10.1091/mbc.9.7.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. Cell cycle-regulated expression, phosphorylation, and degradation of p55Cdc. A mammalian homolog of CDC20/Fizzy/slp1. J Biol Chem. 1997;272:28501–28511. doi: 10.1074/jbc.272.45.28501. [DOI] [PubMed] [Google Scholar]
- Whitfield WG, Gonzalez C, Maldonado-Codina G, Glover DM. The A- and B-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2-M transition. EMBO J. 1990;9:2563–2572. doi: 10.1002/j.1460-2075.1990.tb07437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Kumada K, Yanagida M. Distinct subunit functions and cell cycle regulated phosphorylation of 20S APC/cyclosome required for anaphase in fission yeast. J Cell Sci. 1997;110:1793–1804. doi: 10.1242/jcs.110.15.1793. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Guacci V, Koshland D. Pds1p, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s) J Cell Biol. 1996;133:99–110. doi: 10.1083/jcb.133.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae W, Nasmyth K. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- Zachariae W, Schwab M, Nasmyth K, Seufert W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science. 1998;282:1721–1724. doi: 10.1126/science.282.5394.1721. [DOI] [PubMed] [Google Scholar]