Abstract
The airway sensations stimulated by smoking are an important source of hedonic impact (pleasure) for dependent smokers. The learning process by which these sensations become pleasurable is not well understood. The classical conditioning model predicts that airway sensory stimulation will elicit sympathetic arousal that is positively correlated with the hedonic impact that is elicited by airway sensory stimulation. To test this prediction, we measured skin conductance responses (SCRs) and subjective hedonic impact elicited by a series of individual puffs from nicotinized, denicotinized and unlit cigarettes. Nicotinized puffs elicited more subjective hedonic impact than denicotinized and unlit puffs partly as a result of the fact that they provided a greater level of airway sensory stimulation. We found that SCRs were not larger for nicotinized puffs than for denicotinized puffs, but that they were larger for both nicotinized and denicotinized puffs than for unlit puffs. We also found that the average SCR of a subject to denicotinized puffs was positively correlated with the average hedonic impact that a subject obtained from denicotinized puffs. Together, this suggests that SCR magnitude does not reflect within-subject variations in hedonic impact that are due to variations in the level of airway sensory stimulation, but that it does reflect individual differences in the amount of hedonic impact that is derived from a given level of airway sensory stimulation. The results of a post hoc correlation analysis suggest that these individual differences may have been due to variations in the prevailing urge to smoke. The implications of these findings for the classical conditioning model, as well as for other learning models, are discussed.
Keywords: Skin conductance response (SCR), Airway sensory effects, Nicotinized puffs, Denicotinized puffs, Unlit puffs, Cigarette
1. Introduction
Cigarette smoking is the most common preventable cause of morbidity and mortality in the developed world (Peto et al., 1992). Cigarette smoking is an addictive behavior, characterized by a compulsion to smoke despite awareness of its negative consequences (American Psychiatric Association, 2001). An accumulating body of evidence from both human and animal models (reviewed in Caggiula et al., 2001) suggests that the sensory context of smoking is important for maintaining dependence. Through learning that depends upon the pharmacologic effects of nicotine, the myriad sensory stimuli that are associated with the act of smoking acquire powerful motivational properties, and so come to drive smoking behavior.
Particularly important for the subjective pleasure, or hedonic impact, obtained from smoking are the airway sensations stimulated by each puff from a cigarette. For example, blocking airway sensation reduces smoking desirability and satisfaction and also the reduction in urge that is obtained from smoking by dependent smokers (Rose et al., 1985; Rose et al., 1999, 1984). Conversely, stimulating airway sensations through inhalation of irritant substances is experienced as desirable and reduces smoking urges in dependent smokers (Behm et al., 1990; Rose and Behm, 1994; Rose and Hickman, 1987). In addition, smoking of cigarettes from which nicotine has been nearly completely removed (denicotinized cigarettes), elicits a number of subjective positive hedonic effects that are actually greater than those elicited by intravenous nicotine administration in dependent smokers (Rose et al., 2000; Westman et al., 1996). In a clinical trial, it has been shown that “replacement” of the airway sensory effects of smoking can more than double the efficacy of nicotine replacement therapy for smoking cessation (Westman et al., 1995). Together, this evidence suggests that airway sensory stimulation is not only pleasurable, but that it is one of the primary goals that dependent smokers achieve by smoking. For this reason, understanding the psychological and neural processes by which airway sensory stimulation gives rise to pleasure may aid the development of more effective treatments for smoking dependence.
It has been proposed that the airway sensory effects of smoking, which are aversive for non-smokers, become pleasurable through repeated association with the pharmacologic effects of nicotine (Rose and Levin, 1991). An extensive literature (reviewed in Childress et al., 1993) has examined how drug-associated cues sensory cues acquire the capacity to elicit motivational states through classical conditioning. In classical conditioning, an initially neutral sensory cue (conditioned stimulus) acquires motivational value through repeated pairing with the pharmacologic effect of a drug (unconditioned stimulus). Through this process, the sensory cue comes to elicit behavioral, subjective and autonomic responses (conditioned responses) that resemble the behavioral, subjective and autonomic responses elicited by the pharmacologic effect of the drug (unconditioned responses). The existence of conditioned responses that are similar the unconditioned responses to the drug provides indirect evidence that the drug cue activates a central state that is similar to the central state elicited by the pharmacologic effects of the drug. Further evidence for this comes from showing that the behavioral, subjective and autonomic responses to drug cues are correlated with each other (Carter and Tiffany, 1999; Glautier and Remington, 1995).
The classical conditioning model has been used primarily as a means to understand the learning process by which previously neutral environmental smoking cues, such as the sight of another person smoking, come to elicit a subjective urge to smoke. The classical conditioning model may also be used to understand how airway sensory effects of smoking can come to elicit subjective pleasure during smoking. According to the classical conditioning model, airway sensory stimulation is a conditioned stimulus that, through repeated association with the pharmacologic effects of nicotine, comes to elicit subjective and autonomic responses that are similar to the pharmacologic effects of nicotine. A large body of literature (reviewed in Kalman, 2002) points to nicotine being a source of subjective pleasure, or positive hedonic impact. Nicotine also elicits an increase in sympathetic arousal (Niedermaier et al., 1993). The classical conditioning model therefore predicts that airway sensory stimulation will elicit both positive hedonic impact and sympathetic arousal. Since these are proposed to arise from the same central state, the classical conditioning model also predicts that the hedonic impact and sympathetic arousal elicited by airway sensory stimulation will be positively correlated with each other.
In this study, we sought to determine whether the airway sensory effects of smoking elicit skin conductance responses (SCRs) and whether the magnitude of these responses is related to the amount of positive hedonic impact that is elicited by the airway sensory effects of smoking. Skin conductance response was chosen because it is a relatively pure measure of sympathetic arousal (Boucsein, 1992). We measured subjective hedonic impact and SCRs elicited by individual puffs from nicotinized, denicotinized and unlit cigarettes. Previous findings have shown that the airway sensory effects of nicotine give rise to hedonic impact (Pritchard et al., 1996; Rose et al., 1999) and that this contributes significantly to the difference in hedonic impact between nicotinized and denicotinized puffs (Naqvi and Bechara, 2005). We therefore predicted that SCR would be larger for nicotinized puffs than for denicotinized and unlit puffs, since more hedonic impact is derived from the airway sensory effects of nicotinized puffs than from the airway sensory effects of denicotinized puffs. We also examined the relationship between individual differences in SCR and individual differences in subjective hedonic impact for nicotinized and denicotinized puffs. Since hedonic impact is derived from airway sensory stimulation for both nicotinized and denicotinized puffs, we predicted that subjects who obtain more hedonic impact from nicotinized and denicotinized puffs would have a higher SCR to nicotinized and denicotinized puffs, respectively.
2. Method
2.1. Subjects
All procedures were approved by the University of Iowa Institutional Review Board for Human Subjects Research. Eighteen cigarette smokers were recruited through advertisements in the university and local community. All reported smoking more than 20 cigarettes per day for at least 1 year. Subjects were screened by self-report to exclude any current medical, neurological or psychiatric disorders, including a history of dependence upon substances other than tobacco. Three subjects were excluded due to low skin conductance responses (<1 μS) to a series of deep breaths, measured as described below. The characteristics of the remaining subjects are described in Table 1.
Table 1.
Subject characteristics
| N | Mean | Range | S.D. | |
|---|---|---|---|---|
| Females | 6 | |||
| Males | 9 | |||
| Menthol smokers | 1 | |||
| Hand-rolled smokers | 2 | |||
| Age | 27.33 | 18–54 | 10.66 | |
| Cigarettes/day | 23.6 | 20–40 | 5.99 | |
| Years smoking this much | 6.47 | 1 – 36 | 8.73 | |
| Hours since last cigarette | 0.55 | 0.33– 1.0 | 0.21 | |
| FTND score | 5.2 | 1 – 8 | 2.11 | |
| Usual brand nicotinea | 0.9 | 0.7–1.2 | 0.2 | |
| Usual brand tara | 12.8 | 9 – 15 | 3.1 |
Calculation of nicotine and tar content excludes hand-rolled cigarettes.
All subjects smoked one of their own cigarettes before entering the facility. This was done in order to reduce the contribution of withdrawal relief to smoking hedonic impact. For all subjects, less than 1 h elapsed between the time of smoking this cigarette and the beginning of the procedure. Recent smoking may also have acted to reduce the pharmacologic effects of nicotine from individual puffs as a result of acute tolerance, which lasts for up to 2 h following nicotine exposure (Perkins et al., 1995). Previous results (Naqvi and Bechara, 2005) have shown that, despite a diminished pharmacologic effect of nicotine due to recent smoking, nicotine still exerts airway sensory effects.
2.2. Pre-procedure assessments
Upon arriving at the facility, subjects provided informed consent, completed the Fagerström Test for Nicotine Dependence (FTND) (Heatherton et al., 1991) and reported the time of smoking the last cigarette and their usual brand of cigarette. Following this, skin conductance responses were measured in response to 5 sharp, deep breaths, separated by 15 s. Smoking urges were assessed just before the beginning of the controlled smoking procedure using the Brief Questionnaire of Smoking Urges (Cox et al., 2001).
2.3. Stimuli
Subjects took puffs from nicotinized, denicotinized and unlit puffs. All cigarettes were Quest cigarettes (Vector Tobacco, Inc., New York, NY). Nicotinized cigarettes were Quest 1: Low Nicotine (0.6 mg nicotine; 10 mg tar; FTC method). Denicotinized cigarettes were Quest 3: Nicotine Free (less than 0.06 mg nicotine; 10 mg tar; FTC method). Unlit cigarettes were either Quest 1 or Quest 3. Quest denicotinized cigarettes are made using tobacco from which nicotine has been removed by inactivation of a gene that is necessary for tobacco biosynthesis. All identifying marks were covered so that the cigarettes appeared identical. Cigarettes were handed to the subject by the experimenter, who sat directly across from the subject, separated by a small table. A screen obscured the view of the lit cigarettes, ashtrays and lighter.
2.4. Controlled smoking procedure
Before the procedure, subjects were told that they would receive a series of puffs from several different cigarettes, including some unlit cigarettes. They were not informed that there would be two different kinds of lit cigarettes or that the cigarettes would differ in terms of their hedonic impact or sensory qualities. They were instructed to inhale and exhale each of the puffs as if smoking one of their own cigarettes and to puff on the lit and unlit cigarettes in the same way, inhaling and exhaling them to the same extent. Puffs were presented in six blocks. Each block consisted of three trials, with each puff type (nicotinized, denicotinized and unlit) being presented once per block in pseudo-random order.
On each trial, the subject was handed a cigarette and instructed to hold the cigarette, without touching it to the lips. After 5 s, the subject was then instructed to take a single puff from the cigarette. Ten seconds after the onset of inhalation, the subject was prompted to rate the puff for pleasantness, strength and desirability, in that order. A 10-s interval was chosen so that the SCRs would not be contaminated by the ratings. Subjects were instructed before the procedure that pleasantness referred to how much they liked the puff, or found it pleasurable, that strength referred to the strength of the sensations in the throat and chest, and desirability referred to the desire for another puff from this particular cigarette and not a general desire to smoke more. During the procedure itself, subjects were prompted to provide ratings using brief instructions: “Rate pleasure [rating]. . .Rate strength [rating]. . .Rate desire [rating].” Ratings were made verbally using 7-point (1–7), Likert-type scales, which were displayed before the subject throughout the procedure. Fifteen seconds after the desirability rating, the cigarette for the next trial was handed to the subject.
Subjects took two practice puffs from unlit cigarettes before the beginning of data collection, in order to gain familiarity with the procedure. New cigarettes were lit after every 6 trials, such that no more than two puffs were taken from any one cigarette and the char lines never reached more than 2/3 of the way to the filter. While the experimenter lit the new cigarettes, subjects were allowed to sip water and to communicate with the experimenter.
2.5. Physiological responses
Skin conductance was recorded continuously during the controlled smoking procedure. Disposable Ag/AgCl EKG electrodes were attached to the thenar and hypothenar eminences of the non-smoking hand. Skin conductance was measured using the constant voltage (0.5 V) technique. Skin conductance output voltage was amplified with a gain of 5 μS/V and low-pass filtered at 10 Hz. No high-pass filter was set. Respiratory effort was measured using a strain-gauge belt fitted 5 cm below the axilla. Respiratory output voltage was amplified with a gain of 10 and low-pass filtered at 10 Hz. No high-pass filter was set. A marker channel recorded along with the physiological signals marked the point in each trial at which the subject was instructed to puff. All signals were acquired at 200 Hz. The transducers, amplifiers, analog to digital converter (MP100) and data acquisition and analysis software (Acknowledge, v. 2.2) were all from Biopac, Inc. (Santa Barbara, CA).
Skin conductance responses were defined as increases in skin conductance that were greater than 0.05 μS. The SCR to a puff was defined as the largest SCR with an onset occurring more than 1 s and less than 5 s after the onset of inhalation. Inhalation onset was determined subjectively from the respiration trace. SCR amplitude (SCR.amp) was measured as the difference in skin conductance level between the peak and trough value of the skin conductance trace. As long as the onset of the SCR (the trough) occurred within the 1–5 s post-inhalation window, the peak was allowed to occur after this window. For trials in which multiple responses were initiated within the measurement interval, the amplitude of the largest response was recorded. For trials on which there was no SCR by these criteria, the SCR amplitude was entered as zero. The measurement of SCR is illustrated in Fig. 1.
Fig. 1.
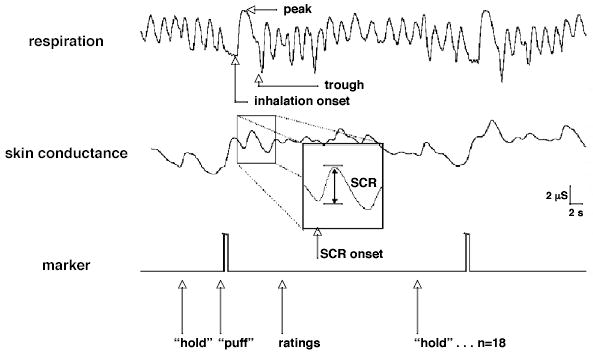
The measurement of the skin conductance response to a puff from a cigarette. The box shows the response to a puff from a nicotinized cigarette. The next trial is a puff from a denicotinized cigarette.
In order to address differences in SCR due to differences in the depth of respiration between puff types, the peak and peak-to-trough amplitude of the respiration trace were measured for each puff-related inhalation–exhalation cycle. The measurement of these respiratory parameters is illustrated in Fig. 1.
2.6. Data analysis
Responses from the first block were excluded in order to reduce novelty effects. The remaining puff-related responses were averaged within each subject for each puff type. The dependent measures for each puff type were thus: mean pleasantness rating (pleasantness), mean desirability rating (desirability), mean strength rating (strength), mean SCR amplitude (SCR.amp), mean respiration peak amplitude (RESP.pk) and mean respiration peak-to-trough amplitude (RESP.pt).
Repeated measures ANOVAs were used to examine the effects of puff-type on SCR.amp, RESP.pk and RESP.pt, comparing unlit, denicotinized and nicotinized puffs. Post hoc paired t-tests were used to compare puff types pair-wise. The significance levels of the t-tests were adjusted for multiple comparisons using Bonferroni correction. Paired t-tests were used to compare self-report responses to nicotinized and denicotinized puffs. All t-tests were 2-tailed.
Each of the self-report measures was correlated with mean SCR.amp to examine how individual differences in hedonic response from the puffs were related to individual differences in autonomic response to the puffs. This was done separately for nicotinized and denicotinized puffs. Partial correlation was used, removing correlation that was due to differences in the mean SCR to 5 deep breaths. This was done to control for individual differences in SCR that were due to “non-psychological” or “peripheral” factors, such as baseline sympathetic tone and skin hydration.
In order to determine potential subject factors that may have driven individual differences in both hedonic impact and SCR from nicotinized and denicotinized puffs, we performed post hoc correlations between the puffs related measures and both the FTND and the QSU-B administered at the beginning of the procedure. This was done for both nicotinized and denicotinized puffs. Pearson product moment correlation was used for correlations that did not involve SCR. For correlations involving SCR, partial correlation was used, removing correlation that was due to the mean SCR to 5 deep breaths.
3. Results
3.1. Within-subject differences between nicotinized, denicotinized and unlit puffs
Fig. 2 shows the self-report and skin conductance responses for the different puff types. Puffs from nicotinized cigarettes were reported as stronger [t(14) = 5.85, p < 0.001], more pleasurable [t(14) = 3.84, p < 0.01] and more desirable [t(14) = 4.41, p <0.01] than puffs from denicotinized cigarettes.
Fig. 2.
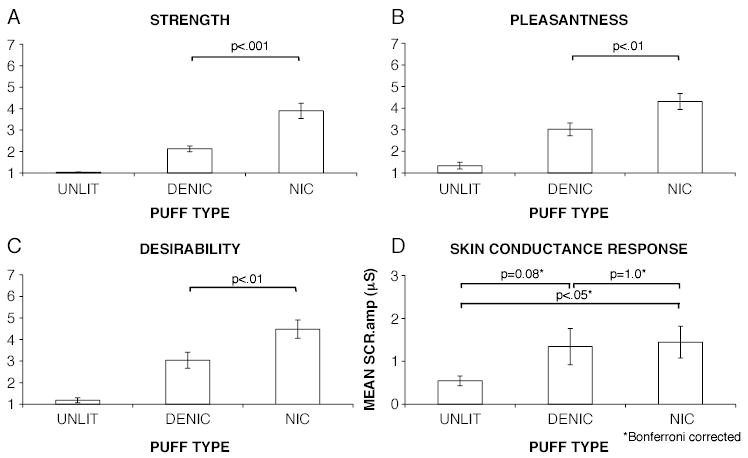
Comparison of self-report and skin conductance responses to different puff types.
ANOVA revealed a main effect of puff-type on SCR.amp [ F(1.13,15.78) = 6.43, Huynh-Feldt corrected (epsilon = 0.56), p < 0.02)]. Post hoc t-tests revealed that SCR.amp was significantly greater for nicotinized puffs than for unlit puffs ( p <0.05, Bonferroni corrected), and was greater for denicotinized puffs than for unlit puffs, though the significance level for the difference between denicotinized puffs and unlit puffs did not survive the correction for multiple comparisons ( p <0.08, Bonferroni corrected). There was no significant difference in SCR.amp between nicotinized and denicotinized puffs ( p > 0.9, Bonferroni corrected).
ANOVA revealed no overall effect of puff type on either RESP.pk [ F(1.35, 18.89) = 0.03, Huynh-Feldt corrected (epsilon = 0.68), p >0.9] or RESP.pt [ F(1.25, 17.49) = 0.73, Huynh-Feldt corrected (epsilon = 0.63), p >0.4], indicating that the depth of respiration did not differ between the puff types. For this reason, these variables were not entered as covariates in the comparison of SCR.amp between puff types.
3.2. Correlations between individual differences in self-report measures and individual differences in SCR
Tables 2 and 3 show the correlations between SCR.amp and self-report measures for nicotinized and denicotinized puffs, respectively. These are between-subjects correlations, with each data point representing the responses of one subject. SCR.amp for nicotinized puffs was not significantly correlated with any self-report measures for nicotinized puffs (|r|<0.1, p >0.8 for all correlations). SCR.amp for denicotinized puffs was significantly positively correlated with pleasantness for denicotinized puffs (r = 0.86, p <0.001) and desirability for denicotinized puffs (r = 0.79, p <0.005), but was not significantly correlated with strength for denicotinized puffs (r = 0.01, p >0.9).
Table 2.
Correlations between self-report and skin conductance responses for nicotinized puffs
| SCR-nic | |
|---|---|
| Ple-nic | 0.05 |
| Des-nic | 0.07 |
| Str-nic | 0.07 |
Correlation coefficients are reported.
p <0.05;
p <0.005.
Table 3.
Correlations between self-report and skin conductance responses for denicotinized puffs
Correlation coefficients are reported.
p <0.005;
p <0.05.
3.3. Post hoc correlations between puff-related variables, FTND scores and QSU-B scores
Table 4 shows the correlations between the FTND score and the mean self-report and skin conductance responses to puffs for each subject. The FTND score was significantly negatively correlated with the strength for nicotinized puffs (r = −0.78, p <0.05). The FTND score was negatively correlated with the pleasantness for nicotinized puffs (r = −0.48, p <0.08) and the desirability for nicotinized puffs (r = −0.24, p >0.1), however, these correlations did not reach the threshold for statistical significance. The FTND score was not correlated with the SCR to nicotinized puffs (r = 0.04, p >0.1). The FTND scores were not correlated with any measure for denicotinized puffs (|r|<0.2, p >0.1 for all correlations).
Table 4.
Correlations between self-report and skin conductance responses and the severity of nicotine dependence (FTND score)
| FTND score | |
|---|---|
| Str-nic | −0.78* |
| Ple-nic | −0.48** |
| Des-nic | −0.24 |
| SCR-nic | 0.04 |
| Str-denic | −0.13 |
| Ple-denic | 0.16 |
| Des-denic | 0.07 |
| SCR-denic | 0.06 |
Correlation coefficients are reported.
p <0.05;
p <0.08.
Table 5 shows the correlations between subjects’ urge preceding the controlled smoking procedure and the self-report and skin conductance responses to puffs. The pre-procedure urge was not significantly correlated with any self-report measure for nicotinized puffs (|r|<0.4, p >0.1 for all correlations). The pre-procedure urge was positively correlated with the SCR to nicotinized puffs, though this correlation was just below the threshold for statistical significance (r = 0.49, p <0.08). The pre-procedure urge was significantly positively correlated with the pleasantness for denicotinized puffs (r = 0.55, p <0.05) and the desirability for denicotinized puffs (r = 0.65, p < 0.02), but was not significantly correlated with the strength for denicotinized puffs (r = −0.22, p = 0.44). The pre-procedure urge was positively correlated with both the SCR.amp for nicotinized puffs, though this correlation was just below the threshold for statistical significance (r = 0.49, p = 0.07).
Table 5.
Correlations between self-report and skin conductance responses and smoking urge preceding the procedure (QSU-B score)
| QSU-B score | |
|---|---|
| Str-nic | −0.01 |
| Ple-nic | 0.33 |
| Des-nic | 0.4 |
| SCR-nic | 0.49* |
| Str-denic | −0.21 |
| Ple-denic | 0.48* |
| Des-denic | 0.63** |
| SCR-denic | 0.49 |
Correlation coefficients are reported.
p <0.08;
p <0.05.
4. Discussion
This the first study to examine the autonomic responses to the airway sensory effects of smoking and their relationship to the positive hedonic impact (pleasure) that is obtained from the airway sensory effects of smoking. Here we show that individual puffs from cigarettes give rise to skin conductance responses, which are indicative of an increase in sympathetic nervous system activity. We further show that the SCRs to the puffs are quantitatively related to the amount of hedonic impact derived from the airway sensory effects of smoking, though this relationship is more complex than initially predicted. Specifically, we found that SCR magnitude does not track within-subject variations in hedonic impact that are due to variations in the level of airway sensory stimulation, but that SCR does track between-subject variations in how much hedonic impact is derived from a given level of airway sensory stimulation. We also found that different subject factors may underlie individual differences in hedonic impact from nicotinized and denicotinized puffs, respectively. As we discuss below, the differences between nicotinized and denicotinized puffs provide a window into how the hedonic impact that is derived from the airway sensory effects of smoking may be modulated by both peripheral and central factors.
4.1. Within-subject differences in SCR between nicotinized, denicotinized and unlit puffs
Skin conductance responses were measured within 5 s of inhalation of the puffs. This is less than the time required for nicotine and non-nicotine constituents to reach the central nervous system following inhalation of cigarette smoke, the minimum estimate of which is 7 s (Benowitz, 1990; Henningfield and Keenan, 1993; Russell and Feyerabend, 1978). Even though the procedure resulted in a steady rise in plasma nicotine, this increase would have affected all of the puff types to the same degree since the puffs were presented in pseudo-random sequence. This means that differences in SCR magnitude between puff types cannot be attributed to the pharmacologic (i.e. direct central nervous system) effects of nicotine and non-nicotine tobacco constituents. All of the puffs shared the same motor action. In addition, we found that the puffs did not differ from each other in terms of the depth of inhalation. This means that differences in SCR between the puff types cannot be attributed to differences in motor demands. This assumes that the puffs did not differ with respect to some subtler motor parameter that was not measured here.
We found that both nicotinized and denicotinized puffs elicited larger SCRs than unlit puffs. Nicotinized and denicotinized puffs both elicited a significant level of airway sensation while unlit puffs elicited very little or no airway sensation. This suggests that the larger SCRs to nicotinized and denicotinized puffs compared to unlit puffs were due to the stimulation of airway sensation. A major limitation of this interpretation is that the puffs from lit (nicotinized and denicotinized) cigarettes and the puffs from unlit cigarettes differed not only in terms of airway sensory effects that followed inhalation, but also in terms of visual and olfactory cues that preceded inhalation. These exteroceptive cues, rather than airway sensory effects of the puffs, may have led to differences in SCR between the lit and unlit puffs. Similarly, since an experimenter controlled the delivery of the puffs, factors such as omission of an expected reinforcer or response inhibition, which may have differed between lit and unlit puffs, may have influenced the SCRs. Notwithstanding these limitations, the close temporal coupling of inhalation onset and SCR onset makes it likely that the SCRs were elicited by airway sensory stimulation, as opposed to some event preceding inhalation.
We found that nicotinized puffs did not elicit larger SCRs than denicotinized puffs, despite eliciting higher levels of airway sensation and hedonic impact. This suggests that SCR does not reflect within-subject differences in hedonic impact that are due to variations in the level of airway sensory stimulation. This interpretation is based upon the assumption that differences in hedonic impact between nicotinized and denicotinized puffs are due at least in part to the airway sensory effects of nicotine. Work by others (Pritchard et al., 1996; Rose et al., 1999) has shown that nicotine’s airway sensory effects are a source of hedonic impact from smoking. An earlier study by our laboratory (Naqvi and Bechara, 2005) in which hedonic impact was assessed before nicotine reached the brain showed that nicotinized puffs elicit more hedonic impact than denicotinized puffs in large measure because nicotinized puffs stimulate more intense airway sensations than denicotinized puffs. Though one cannot exclude the contribution of nicotine’s pharmacologic (i.e. central nervous system) effects to the difference in hedonic impact between nicotinized and denicotinized puffs, it is highly likely that a significant proportion of this difference is due to the airway sensory effects of nicotine.
4.2. Correlations between individual differences in self-report measures and individual differences in SCR
We found that, for a given subject, the mean SCR for denicotinized puffs was correlated with the mean pleasantness and desirability for denicotinized puffs, but not with the mean strength for denicotinized puffs. Since, for denicotinized puffs, hedonic impact is derived almost entirely from airway sensory stimulation, this shows that subjects who derive more hedonic impact from airway sensory stimulation have higher SCRs to airway sensory stimulation. This also shows that SCR is specifically related to the hedonic impact that is derived from airway sensory stimulation, and not to the perceptual appreciation of airway sensory stimulation, which is reflected in self-reported strength. These findings validate our initial prediction for denicotinized puffs.
For nicotinized puffs, we found no self-report measure that was correlated with SCR. We initially predicted that the SCR to nicotinized puffs would be correlated with the hedonic impact from nicotinized puffs because a significant proportion of the hedonic impact from nicotinized puffs was presumed to be due to the airway sensory effects of nicotine. Thus, we expected that nicotinized puffs would behave like denicotinized puffs in this regard. One explanation for this finding is that, even though the airway sensory effects of nicotine contributed to the hedonic impact of nicotinized puffs, a larger proportion of their hedonic impact may have been due to the pharmacologic (i.e. direct central nervous system) effects of nicotine. Thus, individual differences in the hedonic impact from the nicotinized puffs may have been due to variations in the sensitivity to the pharmacologic effects of nicotine. Since SCRs were measured before nicotine had a chance to reach the brain, individual differences that were due to variations in sensitivity to the pharmacologic effects of nicotine would not have been reflected in SCR.
4.3. Potential factors underlying individual differences in hedonic impact from nicotinized and denicotinized puffs
To address potential subject factors that may have led to individual differences in hedonic impact from nicotinized and denicotinized puffs, we performed a post hoc analysis in which we examined the relationship between each of the puff-related measures and both the FTND score and the urge to smoke at the outset of the procedure. We found that the FTND score was negatively correlated with the mean strength for nicotinized puffs and, to a lesser extent, with the mean pleasantness and desirability for nicotinized puffs. These results are consistent with previous findings (Brauer et al., 2001) showing that smokers with higher FTND scores give more similar hedonic ratings to denicotinized and nicotinized cigarettes, compared to smokers with lower FTND scores. The FTND score provides an assessment of the heaviness of smoking and the level of chronic nicotine tolerance to the pharmacologic effects of nicotine (Fagerstrom and Schneider, 1989). This suggests that smokers with higher FTND scores derived less hedonic impact from nicotinized puffs because they were more chronically tolerant to the pharmacologic effects of nicotine. The FTND was not correlated with the SCR to nicotinized puffs or with any measure obtained from denicotinized puffs. This is likely to be due to the fact that all of these measures are tied to airway sensory stimulation and are therefore unlikely to have been affected by chronic tolerance to the pharmacologic effects of nicotine.
The fact that FTND scores were most strongly negatively correlated with the strength for nicotinized puffs suggests that subjects may also have differed from one another with respect to their sensitivity to the airway sensory effects of nicotine. This may have occurred because the airway sensory effects of nicotine, like the pharmacologic effects of nicotine, are mediated by nicotinic acetylcholine receptors (Lee et al., 1993; Rose et al., 1999). Airway nicotinic acetylcholine receptors may therefore be susceptible to long-term desensitization that leads to chronic tolerance. This suggests that individual differences in hedonic impact from nicotinized puffs may have been due in part to individual differences in sensitivity to the airway sensory effects of nicotine. Such individual differences would not have been reflected by individual differences in SCR to nicotinized puffs because SCR does not index variations in hedonic impact that are due to the level of airway sensory stimulation, as shown by the lack of SCR discrimination between nicotinized and denicotinized puffs.
We found that the urge to smoke at the outset of the procedure was positively correlated with the mean pleasantness and desirability for denicotinized puffs and with the mean SCR for both nicotinized and denicotinized puffs. All of these measures are potential indices of the amount of hedonic impact that is derived from the airway sensory effects of smoking. This suggests that the same factor that controls the prevailing urge to smoke also controls the amount of hedonic impact that a subject obtains from the airway sensory effects of smoking. The pre-procedure urge to smoke was not correlated with any of the self-report measures from nicotinized puffs. This could be explained by the fact that chronic tolerance to pharmacologic and/or airway sensory effects of nicotine acts orthogonally to the factor that controls the prevailing urge to smoke. In other words, it would be predicted if all of the subjects had the same FTND score (and thus the same level of chronic tolerance to nicotine), then the pleasantness and desirability for nicotinized puffs would also be correlated with the prevailing urge to smoke. It would also be predicted that in such a case, the pleasantness and desirability for nicotinized puffs would be correlated with the SCR for nicotinized puffs.
An intriguing possibility suggested by these results is that the prevailing urge to smoke somehow modulates amount of hedonic impact that is obtained from the airway sensory effects of smoking. This would be analogous to the way in which physiological drive states, such as hunger and thirst, modulate the hedonic impact of “endogenous” consummatory behaviors, such as eating and drinking. Cabanac (1971) has termed this phenomenon alliesthesia. A further characteristic of alliesthesia is that it specifically modulates the hedonic impact of consummatory behaviors, but does not change the non-hedonic sensory perception of consummatory behaviors (Berridge, 2004). In other words, hunger makes a sweet taste more pleasant, but does not make it seem sweeter. This dissociation can be seen in our data: while the pleasantness and desirability for denicotinized puffs were both correlated with the urge to smoke, the strength for denicotinized puffs was not. In other words, the prevailing drive to smoke may modulate the amount of hedonic impact derived from the airway sensory effects of smoking, but may not modulate the perceived strength of the airway sensory effects of smoking. This modulation of hedonic impact may be reflected in SCR magnitude since SCR is an index of hedonic impact. This interpretation requires caution, however, since the correlations we show do not establish a causal relationship between the pre-existing urge to smoke and the hedonic impact from airway sensory stimulation. Also, these correlations are obtained from a relatively small sample of subjects. These limitations could be overcome by manipulating the urge to smoke as an independent variable, for example by having subjects abstain from smoking, and examining how this manipulation affects the hedonic impact and SCRs elicited by puffs from nicotinized and denicotinized puffs. If alliesthesia operates upon the hedonic impact and SCRs elicited by the airway sensory effects of smoking, then it would be predicted that smoking deprivation would increase both the hedonic impact and SCRs elicited by both nicotinized and denicotinized puffs.
4.4. Implications for the classical conditioning model
The findings of this study provide support for the classical conditioning model, which holds that airway sensory stimulation, through repeated pairing with the pharmacologic effect of nicotine, comes to elicit a central state that is similar to the central state elicited by the pharmacologic effects of nicotine. This central state is evidenced by subjective pleasure and an increase in sympathetic arousal, both of which are also elicited by nicotine’s pharmacologic effects. According to this model, since the subjective pleasure and hedonic impact elicited by airway sensory stimulation arise from the same central state, they should be correlated with each other. We found this to be true when we examined between-subject correlations between SCR and hedonic impact for denicotinized puffs.
A number of studies (e.g. Field and Duka, 2001; Mucha et al., 1998; Niaura et al., 1989; Niaura et al., 1992; Payne et al., 1996; Tiffany, 1991; Tiffany et al., 2000) have used autonomic responses to examine how environmental smoking cues, such as the sight of another person smoking, come to elicit smoking urge through classical conditioning processes. By and large, these studies show that smoking cues elicit both a subjective urge to smoke and an increase in sympathetic arousal, which supports the classical conditioning view. However, unlike the results of the present study, there is a general lack of correlation between the magnitude of the autonomic responses and level of urges elicited by smoking cues, which has been taken as evidence against the role of classical conditioning processes in cue-induced urge (Carter and Tiffany, 1999). This discrepancy may arise from the fact that previous studies have examined urges (wanting, incentive motivation) induced by exteroceptive smoking cues, while the present study examined pleasure (liking, hedonic impact) elicited by airway sensory stimulation. It may be that central state elicited by the pharmacologic effects of nicotine resembles pleasure more than it resembles urge.
Further evidence for the classical conditioning view may be provided by the finding that smokers with a higher urge to smoke at the outset of the procedure had larger SCRs and obtained more hedonic impact from the airway sensory effects of smoking. Incentive motivation theory holds that a conditioned reward activates a representation of the hedonic value of the specific unconditioned reward with which it is associated, such that factors that modulate the hedonic value of the unconditioned reward will also modulate the hedonic value of its associated conditioned reward (Bindra, 1974). This model predicts that the conditioned responses elicited by airway sensory stimulation (e.g. subjective hedonic impact, SCR) should be increased by factors that increase the hedonic value of nicotine’s pharmacologic effects. We found that smokers with a higher prevailing urge to smoke obtained more subjective pleasure and had larger SCRs to the airway sensory effects of smoking. This may have occurred because a higher drive to smoke increased the hedonic value of nicotine’s pharmacologic effects. This interpretation requires caution, however, for the same reasons discussed above (correlative evidence, small sample size). Also, we cannot be sure whether the urges experienced at the outset of the procedure would actually have affected the hedonic value of nicotine’s pharmacologic effects. Indeed, if this were the case, then it would have been expected that the urge to smoke would also have modulated the hedonic impact of nicotinized puffs. However, this assumes that most of the hedonic impact obtained from nicotinized puffs was actually due to the pharmacologic effects of nicotine.
A hypothesis that extends from the classical conditioning model is that the afferent feedback of the autonomic responses elicited by the airway sensory effects of smoking somehow modulates the subjective pleasure elicited by the airway sensory effects of smoking. This hypothesis is related to the James–Lange theory of emotion (James, 1884; Lange, 1885), which states that subjective emotional experience arises from the sensory feedback of autonomic states that are elicited by emotional stimuli. According to this hypothesis, subjective pleasure should be correlated with the magnitude of autonomic responses. We show that autonomic responses do not track variations in hedonic impact that are due to the level of sensory input. However, we do provide correlative evidence that autonomic responses do track variations in hedonic impact that are due to variations in the prevailing motivational state (alliesthesia). This would suggest that sensory feedback of autonomic responses may not be necessary for modulating pleasure according variations in the level of airway sensation, but may be necessary for the modulation of pleasure by alliesthesia.
4.5. Alternatives to the conditioning model
While the existence of SCRs that are positively correlated with the hedonic impact of the airway sensory effects of smoking supports the classical conditioning model, this evidence is largely indirect and correlative. To definitively show that classical conditioning processes are at play would require examining the autonomic and hedonic responses to the airway sensory stimulation in non-smokers and observing how these responses change as a function of repeated pairing of airway sensory stimulation with the pharmacologic effects of nicotine. This is not possible for ethical reasons. This is why drug conditioning studies in human subjects frequently examine autonomic responses to drug associated sensory stimuli. The problem with examining autonomic responses, however, is that they are not elicited specifically by stimuli that acquire motivational significance by association with drugs of abuse.
Skin conductance responses are elicited by a number of sensory stimuli that acquire hedonic value through learning. For example, studies by Lang et al. (Bradley et al., 2001; Lang et al., 1993) have shown that SCRs are elicited by both positively and negatively valenced emotional pictures. Studies by Bechara et al. (1999, 2002, 1996) have shown that SCRs are elicited by both winning and losing money, as well as during the anticipation of winning and losing money. In general, these studies show that SCR does not index variations in hedonic impact that are driven by stimulus properties, similar to the results of the present study. Rather, SCR seems to be most strongly related to the level of subjective “arousal” elicited by emotional stimuli (Lang et al., 1993). Thus, it may be that SCR to the airway sensory effects of smoking are not conditioned responses, but instead reflect the level of subjective arousal level elicited by airway sensory stimulation.
Another possibility is that the airway sensory effects of smoking become pleasurable by co-opting brain areas that normally play a role in deriving pleasure from the consummation of endogenous rewards, such as eating, drinking and sex. These may be areas that also govern the elicitation of autonomic responses, since consummation of endogenous rewards requires changes in the visceral state in order to extract maximum physiological benefit to the organism. These regions may also modulate autonomic responses as a function of drive states, such as hunger and thirst (alliesthesia), since such drive states alter the physiological significance of consummatory behaviors. These regions may be co-opted by the airway sensory effects of smoking because of neural plasticity that is induced through the release of dopamine, which occurs via nicotine’s pharmacologic effects. This model, like the classical conditioning model, predicts that airway sensory stimulation will elicit subjective pleasure and autonomic responses. However, according to this model, the subjective and autonomic responses elicited by airway stimulation are related more to the responses elicited by endogenous rewards than to the responses elicited by the pharmacological effects of nicotine.
Footnotes
Supported by NIDA grant # 1R21 DA16708 (AB), 5F30 DA016847 (NHN).
References
- American Psychiatric Association, A.P., 2001. Diagnostic and Statistical Manual of Mental Disorders. Edition IV.
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6:215– 225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19 (13):5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40 (10):1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Behm FM, Levin ED, Lee YK, Rose JE. Low-nicotine regenerated smoke aerosol reduces desire for cigarettes. J Subst Abuse. 1990;2 (2):237–247. doi: 10.1016/s0899-3289(05)80058-6. [DOI] [PubMed] [Google Scholar]
- Benowitz, N.L., 1990. Clinical pharmacology of inhaled drugs of abuse: implications in understanding nicotine dependence. In: Chiang, L.C., Hawks, R.L. (Eds.), Research Findings on Smoking of Abused Substances. U.S. Department of Health and Human Services, Rockville, pp. 12– 29. [PubMed]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81 (2):179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bindra D. A motivational view of learning, performance, and behavior modification. Psychol Rev. 1974;81 (3):199–213. doi: 10.1037/h0036330. [DOI] [PubMed] [Google Scholar]
- Boucsein, W., 1992. Electrodermal Activity. Plenum, New York.
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation: I. Defensive and appetitive reactions in picture processing. Emotion. 2001;1 (3):276– 298. [PubMed] [Google Scholar]
- Brauer LH, Behm FM, Lane JD, Westman EC, Perkins C, Rose JE. Individual differences in smoking reward from denicotinized cigarettes. Nicotine Tob Res. 2001;3 (2):101–109. doi: 10.1080/14622200123249. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Physiological role of pleasure. Science. 1971;173 (2):1103– 1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70 (4):515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94 (3):327– 340. [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3 (1):7– 16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12 (2):159– 182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T. Smoking expectancy mediates the conditioned responses to arbitrary smoking cues. Behav Pharmacol. 2001;12 (3):183– 194. doi: 10.1097/00008877-200105000-00004. [DOI] [PubMed] [Google Scholar]
- Glautier, S., Remington, B., 1995. The form of responses to drug cues. In: Drummond, D.C., Tiffany, S.T., Glautier, S., Remington, B. (Eds.), Addictive Behavior: Cue Exposure Theory and Practice. John Wiley & Sons, West Sussex, UK, pp. 21– 46.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86 (9):1119– 1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61 (5):743– 750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188– 205. [Google Scholar]
- Kalman D. The subjective effects of nicotine: methodological issues, a review of experimental studies, and recommendations for future research. Nicotine Tob Res. 2002;4 (1):25– 70. doi: 10.1080/14622200110098437. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30 (3):261– 273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lange, K.G., 1885. Om Sindsbevagelser, et Psyko-Fysiologisk Studie. Kjobenhavn, Lund.
- Lee LY, Gerhardstein DC, Wang AL, Burki NK. Nicotine is responsible for airway irritation evoked by cigarette smoke inhalation in men. J Appl Physiol. 1993;75 (5):1955– 1961. doi: 10.1152/jappl.1993.75.5.1955. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Pauli P, Angrilli A. Conditioned responses elicited by experimentally produced cues for smoking. Can J Physiol Pharm. 1998;76 (3):259–268. [PubMed] [Google Scholar]
- Naqvi, N.H., Bechara, A., 2005. The airway sensory impact of nicotine contributes to the conditioned reinforcing effects of individual puffs from cigarettes. Pharmacol. Biochem. Behav. [DOI] [PMC free article] [PubMed]
- Niaura R, Abrams D, Demuth B, Pinto R, Monti P. Responses to smoking-related stimuli and early relapse to smoking. Addict Behav. 1989;14 (4):419– 428. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams DB, Pedraza M, Monti PM, Rohsenow DJ. Smokers’ reactions to interpersonal interaction and presentation of smoking cues. Addict Behav. 1992;17 (6):557– 566. doi: 10.1016/0306-4603(92)90065-4. [DOI] [PubMed] [Google Scholar]
- Niedermaier ON, Smith ML, Beightol LA, Zukowska-Grojec Z, Goldstein DS, Eckberg DL. Influence of cigarette smoking on human autonomic function. Circulation. 1993;88 (2):562–571. doi: 10.1161/01.cir.88.2.562. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, Sturges LV, Holleran SA. Reactivity to smoking cues: mediating roles of nicotine dependence and duration of deprivation. Addict Behav. 1996;21 (2):139–154. doi: 10.1016/0306-4603(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Mitchell SL, Goettler J, Caggiula A, Stiller RL, Scierka A. Acute tolerance to nicotine in smokers: lack of dissipation within 2 hours. Psychopharmacology (Berl) 1995;118 (2):164– 170. doi: 10.1007/BF02245835. [DOI] [PubMed] [Google Scholar]
- Peto R, Lopez AD, Boreham J, Thun M, Heath C., Jr Mortality from tobacco in developed countries: indirect estimation from national vital statistics. Lancet. 1992;339(8804):1268–1278. doi: 10.1016/0140-6736(92)91600-d. (see comments). [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Robinson JH, Guy TD, Davis RA, Stiles MF. Assessing the sensory role of nicotine in cigarette smoking. Psychopharmacology (Berl) 1996;127 (1):55– 62. doi: 10.1007/BF02805975. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Inhalation of vapor from black pepper extract reduces smoking withdrawal symptoms. Drug Alcohol Depend. 1994;34 (3):225– 229. doi: 10.1016/0376-8716(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Rose JE, Hickman CS. Citric acid aerosol as a potential smoking cessation aid. Chest. 1987;92 (6):1005–1008. doi: 10.1378/chest.92.6.1005. [DOI] [PubMed] [Google Scholar]
- Rose JE, Levin ED. Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. Br J Addict. 1991;86 (5):605– 609. doi: 10.1111/j.1360-0443.1991.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Zinser MC, Tashkin DP, Newcomb R, Ertle A. Subjective response to cigarette smoking following airway anesthetization. Addict Behav. 1984;9 (2):211 – 215. doi: 10.1016/0306-4603(84)90060-1. [DOI] [PubMed] [Google Scholar]
- Rose JE, Tashkin DP, Ertle A, Zinser MC, Lafer R. Sensory blockade of smoking satisfaction. Pharmacol Biochem Behav. 1985;23 (2):289– 293. doi: 10.1016/0091-3057(85)90572-6. [DOI] [PubMed] [Google Scholar]
- Rose JE, Westman EC, Behm FM, Johnson MP, Goldberg JS. Blockade of smoking satisfaction using the peripheral nicotinic antagonist trimethaphan. Pharmacol Biochem Behav. 1999;62 (1):165– 172. doi: 10.1016/s0091-3057(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav. 2000;67 (1):71– 81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Russell MA, Feyerabend C. Cigarette smoking: a dependence on high-nicotine boli. Drug Metab Rev. 1978;8 (1):29–57. doi: 10.3109/03602537808993776. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. The production of smoking urges through an imagery manipulation: psychophysiological and verbal manifestations. Addict Behav. 1991;16 (6):1991. doi: 10.1016/0306-4603(91)90047-l. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J Consult Clin Psychol. 2000;68 (2):233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Rose JE. Airway sensory replacement combined with nicotine replacement for smoking cessation. A randomized, placebo-controlled trial using a citric acid inhaler. Chest. 1995;107 (5):1358– 1364. doi: 10.1378/chest.107.5.1358. [DOI] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacol Biochem Behav. 1996;53 (2):309– 315. doi: 10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]


