Abstract
Tyrosine kinases of the Src family are synthesized as cytosolic proteins that subsequently translocate to membranes. Little is known of the mechanisms responsible for targeting these proteins to membranes, although a role for the cytosolic chaperone Hsp90 has been proposed. Here, we have studied the involvement of Hsp90 in the synthesis, membrane binding, and maintenance of the Src-kinase Lck. Using specific inhibitors of Hsp90, geldanamycin and radicicol, we found that functional Hsp90 is essential for the stability of newly synthesized, but not mature, Lck. Similar results were obtained for two other Src-kinases, c-Src and Lyn. In contrast, LckY505F and LckΔSH2, constitutively active Lck mutants lacking the C-terminal regulatory tyrosine or the entire Src homology 2 domain, respectively, required Hsp90 activity to stabilize the mature proteins. Lck synthesized in the absence of Hsp90 activity was degraded within 30–45 min. This unstable Lck was myristoylated normally but did not associate with membranes or CD4, interactions that normally start within minutes of the completion of Lck synthesis. A construct composed of the N-terminal unique domain of Lck fused to green fluorescent protein did not require Hsp90 activity during synthesis. In addition, this protein associated with membranes efficiently in the absence of Hsp90 activity. Together these data suggest that interaction with Hsp90 is necessary for the correct synthesis and subsequent membrane binding of Lck. However, Hsp90 does not appear to play a direct role in Lck membrane, or CD4, association.
INTRODUCTION
Lck is a lymphocyte-specific member of the Src family of nonreceptor tyrosine kinases that is essential for T-cell development and function. In mature T-cells, the majority of Lck is associated with cell surface CD4 or CD8 (Rudd et al., 1988; Veillette et al., 1988), the coreceptors of the T-cell receptor complex, but other membrane-binding partners may be used with low stoichiometry or during the early phases of T cell ontogeny (Rudd et al., 1993). However, in all cases the functional activity of Lck is dependent on its ability to associate with the cytoplasmic face of cellular membranes (Yurchak and Sefton, 1995; Yurchak et al., 1996; Kabouridis et al., 1997). This association is dependent on cotranslational and posttranslational acylation reactions. Like other members of the Src family, Lck is cotranslationally myristoylated (Resh, 1994). Subsequently, the completed protein becomes membrane associated and is palmitoylated on either one or both of two cysteine residues close to its N terminus (Paige et al., 1993; Shenoy-Scaria et al., 1993). Failure to undergo either of these acylations generates a cytosolic protein that cannot couple to CD4 or CD8 (Turner et al., 1990; Kwong and Lublin, 1995; Yurchak and Sefton, 1995; Bijlmakers et al., 1997; Zlatkine et al., 1997).
The mechanisms via which the Src-kinases, and related acylated proteins, are targeted to specific membrane compartments are not well understood. We have demonstrated recently that newly synthesized myristoylated Lck is initially targeted to intracellular membranes, where it is palmitoylated and, when present, combined with newly synthesized CD4 (Bijlmakers and Marsh, 1999). Membrane association starts soon after synthesis but can take 30–45 min to complete. The membrane-associated Lck is then transported to the cell surface (Bijlmakers and Marsh, 1999). However, other modes of membrane association have been proposed for related Src-kinases. Fyn, which gains acyl modifications similar to those of Lck, appears to associate rapidly with the plasma membrane directly after synthesis (van't Hof and Resh, 1997). In contrast c-Src, which uses a polybasic region instead of palmitoylation for stable membrane association (Silverman and Resh, 1992), becomes membrane bound with kinetics similar to that seen for Lck (van't Hof and Resh, 1997).
Previous studies have implicated a role for the cytosolic chaperone Hsp90 in the synthesis, membrane association, and maintenance of Src-kinases. Hsp90 is an abundant cytosolic chaperone that, together with cochaperones and cofactors, is required for the folding and conformational regulation of a group of substrates that include steroid hormone receptors, the signaling kinase Raf-1, p53, and nNos (Buchner, 1999). Hsp90 has also been found to interact with the products of several viral oncogenes including the Src-kinases v-Src (Brugge et al., 1981; Oppermann et al., 1981) and v-Fgr (Ziemiecki et al., 1986). The cellular forms of both Lck and Fgr have been reported to interact with Hsp90 during and after in vitro translation (Hartson and Matts, 1994). In addition, the catalytic activity of a constitutively active form of Lck appears to require Hsp90 when the protein is expressed in fibroblasts (Hartson et al., 1996). Thus, Hsp90 may assist in folding and stabilizing Src-kinases. Hsp90 has also been implicated in regulating the intracellular trafficking of its substrates. For example, steroid receptors remain in the cytosol or translocate to the nucleus depending on their association with Hsp90 (Pratt, 1993; Czar et al., 1997), and Raf-1 appears to require association with Hsp90 for its interaction with Ras and, therefore, with membranes (Schulte et al., 1995). Moreover, the observation that newly synthesized cytosolic v-Src, but not the membrane-associated protein, is associated with Hsp90 (Courtneidge and Bishop, 1982; Brugge et al., 1983) has suggested that Hsp90 might play a role in escorting v-Src to membranes.
Here we have investigated the role of Hsp90 in the synthesis, membrane targeting, and maintenance of Lck in cells. These studies have been facilitated by the use of two specific inhibitors of Hsp90 activity, geldanamycin (Whitesell et al., 1994) and radicicol (Schulte et al., 1998; Sharma et al., 1998). These naturally occurring drugs were initially identified as antitumor agents and inhibitors of tyrosine kinase activity. However, both were found recently to bind specifically, and with high affinity, to the ATP-binding sites of Hsp90 (Prodromou et al., 1997; Roe et al., 1999) and the related endoplasmic reticulum chaperone GRP94 (Chavany et al., 1996). Using these drugs, we found that Hsp90 activity is required for the stable synthesis of Lck and two other Src-kinases but not for the maintenance of these proteins. Although Hsp90 activity is required for Lck synthesis, it is not directly involved in the membrane, and CD4, association of newly synthesized Lck.
MATERIALS AND METHODS
Reagents
Tissue culture reagents and plastics were from Life Technologies (Paisley, United Kingdom). Other chemicals were from Sigma (Poole, United Kingdom), unless indicated otherwise. Geldanamycin was provided by Laurence Pearl (Institute of Cancer Research, London, United Kingdom), and lactacystin was purchased from Calbiochem-Novabiochem (Nottingham, United Kingdom).
Cell Lines and Antibodies
The human T-cell line SupT1 and B-cell line Daudi were cultured in RPMI-1640 supplemented with 10% FCS and with 100 U/ml penicillin and 0.1 mg/ml streptomycin (pen/strep). The murine T-cell line LSTRA was grown in this medium supplemented with 50 μM 2-mercaptoethanol. HeLa cells stably transfected with Lck or the unique domain of Lck attached to green fluorescent protein (UD-GFP)1 (Bijlmakers et al., 1997) were grown in DMEM, 4% FCS, pen/strep, and 1 mg/ml G418. NIH-3T3 cells stably transfected with Lck (Bijlmakers et al., 1997), LckY505F, LckΔSH2, or c-Src (Pelchen-Matthews et al., 1992) were grown in DMEM, 10% FCS, and pen/strep. The LckY505F cDNA was provided by D. Littman (Skirball Institute, New York, NY). LckΔSH2 cDNA, with a precise deletion of the Src homology 2 (SH2) domain (aa 120–226), was generated by PCR in three steps. In the first step the N-terminal and SH3 domains were amplified, and in the second step the kinase domain was amplified. The primers were designed so that the 3′ end of PCR product 1 and the 5′ end of PCR product 2 were complementary. In the third PCR, products 1 and 2 were combined, and primers for the 5′ end of product 1 and the 3′ end of product 2 were used. The final PCR product was checked by sequencing. LckY505F and LckΔSH2 were transfected into NIH-3T3 fibroblasts by electroporation as described previously (Bijlmakers et al., 1997).
For Lck immunoprecipitation, a polyclonal rabbit antiserum against an N-terminal Lck peptide (aa 39–58) was provided by Jannie Borst (The Netherlands Cancer Institute, Amsterdam, The Netherlands) (Brouns et al., 1993). For detection of Lck by Western blotting, mouse mAb 3A5 was used (Santa Cruz Biotechnology, Santa Cruz, CA). Lyn was immunoprecipitated and detected on Western blots with a polyclonal rabbit antiserum raised against a Lyn peptide (aa 39–58) provided by Jannie Borst (Brouns et al., 1993). For detection of c-Src, the mouse mAb 327 (Lipsich et al., 1983) was used (Oncogene Science, Manhasset, NY). CD4 was immunoprecipitated with a mixture of two mouse mAbs, 4 and 19 (Bijlmakers and Marsh, 1999), provided by J. Hoxie (University of Pennsylvania, Philadelphia, PA).
Metabolic Labeling
Cells were washed once in methionine/cysteine-free DME medium (ICN Biomedicals, Thame, United Kingdom). For continuous labeling, SupT1 and Daudi cells were resuspended in this medium supplemented with 10% FCS and [35S]methionine/cysteine (Express [1175 Ci/mmol]; DuPont, Stevenage, United Kingdom) and incubated for 3.5 h at 37°C. Typically, 200 μCi of [35S]methionine/cysteine was used for 2 × 107 cells resuspended in 1 ml. NIH-3T3 fibroblasts were labeled in 3 ml of medium containing 2% FCS and 500 μCi of [35S]methionine/cysteine per 10-cm dish.
For pulse labeling, cells were preincubated for 45 min in methionine/cysteine-free DMEM, supplemented with 10% FCS. Labeling was performed in methionine/cysteine-free DMEM containing 2% FCS and [35S]methionine/cysteine at 1 mCi/ml. SupT1 and LSTRA cells were labeled at 1 mCi per 108 cells, and typically, 1.5 × 107 cells were used per time point. HeLa cells were detached from dishes with 5 mM EDTA in PBS and labeled in suspension at 1 mCi per 1.5 × 107 cells, and 2 × 106 cells were used for each time point. After pulse labeling for 5 min, cells were transferred to 10 ml of 37°C DMEM containing 1 mM each of unlabeled methionine and cysteine. Samples were taken at the indicated chase times and kept in 10 ml of ice-cold DMEM until the final time point. The cells were then pelleted by centrifugation (5 min at 1500 rpm, 4°C) and further processed for membrane separation and/or immunoprecipitation.
Myristic Acid Labeling
LSTRA cells (3 × 107 per labeling) were preincubated in DMEM, 5% FCS, 5 mM sodium pyruvate, and 4× nonessential amino acids for 60 min at 37°C, 5% CO2. For labeling, cells were subsequently resuspended in 500 μl of medium. [9,10-3H]myristic acid in ethanol (10–60 Ci/mmol; DuPont) was dried under nitrogen to a final volume of ∼35 μl, taken up in 200 μl of medium, and added to cells. Typically for each labeling, 110 μl of medium containing 250 μCi of [3H]myristic acid was used. After a 15-min incubation at 37°C, 5% CO2, cells were washed once in ice-cold DMEM (10 ml) and subsequently taken up in 5 ml of warm (37°C) DMEM containing cycloheximide (100 μg/ml) to prevent further incorporation of label. One-half (2.5 ml) of the cell suspension was immediately put on ice (0-min chase); the other 2.5 ml was added to 5 ml of warm medium and incubated at 37°C, 5% CO2, for another 45 min (45-min chase). The cells were then pelleted by centrifugation (5 min at 1500 rpm, 4°C) and further processed for immunoprecipitation.
Treatment with Hsp90 and Protease Inhibitors
When labeling was performed in the presence of the Hsp90 inhibitors geldanamycin and radicicol, cells were pretreated for 30 min, and the drugs were present during both the pulse and the chase, unless indicated otherwise. Geldanamycin (5 μM final concentration) was added from a 25 mM stock in DMSO, and radicicol (10 μM final concentration) was added from a 10 mM stock in methanol. In experiments using the protease inhibitors acetyl-leu-leu-norleucinal (LLnL) and lactacystin, cells were pretreated with the drugs for 90 min, and the drugs were included during both the pulse and chase. LLnL (50 μM final concentration) and lactacystin (10 μM final concentration) were added from 50 and 10 mM stocks in DMSO, respectively.
Cells analyzed by immunoblotting were treated for 3.5 h with geldanamycin or radicicol at the concentrations indicated above. Cycloheximide was used at 100 μg/ml and added from a stock of 100 mg/ml in DMSO.
Immunoprecipitation and Gel Electrophoresis
Cells were lysed in Nonidet P-40 lysis buffer (2% NP-40 [Pierce and Warriner, Chester, United Kingdom], 20 mM Tris, pH 7.8, 150 mM NaCl, 2 mM MgCl2, 1 mM EDTA) containing the protease inhibitors phenylmethylsulfonyl fluoride (PMSF, at 1 mM) and chymostatin, pepstatin A, and antipain hydrochloride (each at 5 μg/ml) and leupeptin hemisulfate (at 10 μg/ml) (CLAP). After removal of nuclei and cell debris by centrifugation at 13,000 × g for 5 min at 4°C, the lysates were cleared of nonspecifically binding proteins by three incubations with 15 μl of packed protein A–Sepharose beads (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) for 30 min at 4°C. The samples were then incubated on ice for 45 min with the following specific antibodies: 1 μl of the polyclonal rabbit sera for Lck or Lyn, 2 μl of mAb 327 (0.4 μg) for Src, or a mixture of mAbs 4 (1.7 μg) and 19 (0.5 μg) for CD4. Immune complexes were recovered by incubation with protein A–Sepharose (15 μl of packed beads) or with protein A–Sepharose preincubated with rabbit anti-mouse antibodies (for CD4 and Src immunoprecipitation) for 45 min at 4°C. After immunoprecipitation, beads were washed five times in NP-40 lysis buffer. Immune complexes were eluted by addition of nonreducing SDS-sample buffer, incubated for 5 min at 95°C, and loaded onto 8% SDS-polyacrylamide gels. After electrophoresis, gels were enhanced in salicylic acid (16% wt/vol in 30% methanol), dried, and exposed to XOmat-AR film (Eastman Kodak, Rochester, NY) for 1–9 d.
Immunoblotting
After gel electrophoresis, proteins were transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). The blots were incubated in blocking buffer (10% skimmed milk, 0.1% Tween-20 in PBS) for 1 h at room temperature. Incubations with primary and secondary antibodies were in blocking buffer for 1 h each, at room temperature. Blots were developed using enhanced chemiluminescence (Pierce and Warriner) and visualized with autoradiography film (Fuji Photo Film, Tokyo, Japan).
In Vitro Kinase Assay
Lck was immunoprecipitated as described above from 1.5 × 106 SupT1 cells. Immunoprecipitates were resuspended in 20 μl of kinase buffer (10 mM Tris, pH 7.8, 150 mM NaCl, 10 mM MnCl2, 0.1% NP-40) containing 2.5 μCi of [32P-γ]ATP (3000 Ci/mmol; Amersham Pharmacia Biotech) and incubated at room temperature for 20 min. The reaction was stopped by the addition of SDS-sample buffer, and samples were heated for 5 min to 95°C. One-quarter of each sample was loaded onto a 10% SDS-polyacrylamide gel. Gels were fixed, dried, and exposed to XOmat-AR film.
Membrane Separation
After pulse labeling and chase, cells were incubated in 1 ml of hypotonic buffer (20 mM Tris, pH 7.8, 2 mM MgCl2, 1 mM EDTA, 1 mM PMSF, CLAP as above) on ice for 12 min and homogenized using a Dounce homogenizer (Wheaton Scientific, Millville, NJ; 15 strokes for T-cells or 50 strokes for HeLa cells). The homogenates were centrifuged for 5 min at 1500 rpm, at 4°C, to remove the nuclei. The postnuclear supernatant was centrifuged in an Optima TL Ultracentrifuge (Beckman, High Wycombe, United Kingdom) for 45 min at 100,000 × g, at 4°C, to recover the membranes and cytosol. The pellets (membrane fractions) were resuspended in hypotonic buffer, Dounce homogenized (20 strokes), and adjusted to 2% NP-40, 150 mM NaCl, 1 mM PMSF, and CLAP. Similarly, the cytosol fractions were adjusted to 2% NP-40 and 150 mM NaCl. Both fractions were adjusted to the same final volume and analyzed by immunoprecipitation.
RESULTS
Hsp90 Activity Is Required for Synthesis, But Not for Maintenance, of Lck
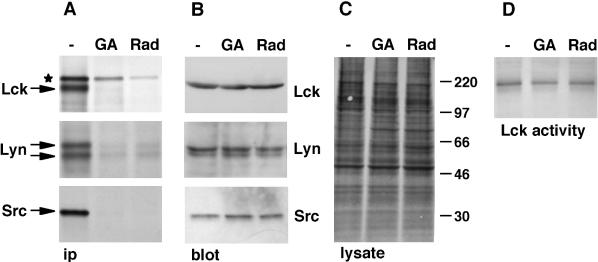
Previous studies have reported that prolonged (4–16 h) treatment of cells with inhibitors of Hsp90 reduces the levels of Lck protein and kinase activity (June et al., 1990; Hartson et al., 1996). To determine whether Hsp90 activity is required for the synthesis of Lck or for maintenance of the mature protein, we examined the effect of Hsp90 inhibition on newly synthesized and mature Lck separately. SupT1 cells were metabolically labeled with [35S]methionine/cysteine for 3.5 h in the presence or absence of the Hsp90 inhibitors geldanamycin and radicicol. Both of these agents readily cross cellular membranes and bind with high affinity to Hsp90, thereby blocking the activity of this protein (Whitesell et al., 1994; Schulte et al., 1998; Sharma et al., 1998). After labeling, cells were lysed and analyzed by immunoprecipitation for newly synthesized Lck and by Western blotting for total Lck. Virtually no newly synthesized Lck was recovered from cells treated with geldanamycin or radicicol (Figure 1A). In contrast, the level of total Lck, predominantly consisting of mature membrane-bound protein, was not affected by the presence of these inhibitors (Figure 1B).
Figure 1.
Hsp90 inhibition affects newly synthesized, but not mature, Src-kinases. (A—C) Cells were labeled with [35S]methionine/cysteine for 3.5 h in the absence (−) or presence of 5 μM geldanamycin (GA) or of 10 μM radicicol (Rad). Cell lysates were analyzed by immunoprecipitation (ip; A), immunoblotting (B), or autoradiography (C). SupT1 cells were used for the detection of Lck, Daudi cells were used for Lyn, and transfected NIH-3T3 fibroblasts were used for Src. (A) Eighty percent of the volume of each lysate was used for immunoprecipitations. The anti-Lck serum recognizes in addition to Lck a nonrelated, slower-migrating protein (★) (Bijlmakers and Marsh, 1999). Lyn exists as two splice variants of 53 and 56 kDa (Yi et al., 1991). (B) Ten percent of the volume of each lysate was used for immunoblotting. (C) The blot shown in B for Daudi lysates was exposed to film to detect total labeled proteins. Molecular weight markers are indicated in kilodaltons on the right. (D) Lck was immunoprecipitated from unlabeled SupT1 cells treated with or without Hsp90 inhibitors as described above. Immunoprecipitates were analyzed for kinase activity. Autophosphorylated Lck is shown.
To determine whether these effects were specific for Lck, we studied two other members of the Src family: Lyn in the B-cell line Daudi and c-Src in transfected NIH-3T3 fibroblasts. v-Src, the virally encoded counterpart of c-Src, associates with Hsp90 during synthesis (Oppermann et al., 1981; Courtneidge and Bishop, 1982; Brugge et al., 1983). A similar interaction has not been observed for c-Src (Iba et al., 1984; Brugge, 1986); however, c-Src binds Hsp90 in vitro (Hutchison et al., 1992), and Hsp90 was shown recently to be essential for the activity of c-Src expressed in yeast (Xu et al., 1999). We found that Hsp90 is also essential for normal cellular synthesis of c-Src. Similar to Lck, newly synthesized c-Src could not be detected after treatment with Hsp90 inhibitors (Figure 1A). On the other hand, total levels of c-Src protein were not affected by these drugs (Figure 1B). Similar results were also obtained for Lyn (Figure 1, A and B). The lack of detectable newly synthesized Src-kinases was not caused by inhibition of protein synthesis by the Hsp90 inhibitors, because similar levels of [35S]methionine/cysteine were incorporated into both treated and untreated cells (in Figure 1C shown for Daudi cells and quantitated for other lysates by scintillation counting of trichloroacetic acid–precipitated samples). Both Hsp90 inhibitors reduced protein synthesis in SupT1 cells to some extent, but also in these cells the absence of newly synthesized Lck in the presence of these inhibitors is not caused by reduced protein synthesis (see below).
To investigate whether inhibition of Hsp90 activity affected the conformation of Lck, we determined Lck kinase activity after a 3.5-h treatment with the drugs. No significant effect on kinase activity, measured by Lck autophosphorylation, was observed (Figure 1D). Together, these results suggest that Hsp90 activity is required for the synthesis of at least three Src-kinases but is not required for the stability or conformation of the mature proteins.
Hsp90 Is Required to Stabilize Mature LckY505F and LckΔSH2
Src-kinases are composed of four domains; an N-terminal unique domain is followed by an SH3, an SH2, and a tyrosine kinase domain (Brown and Cooper, 1996). The activity of the kinase domain is influenced by intramolecular interactions, most importantly between the SH2 domain and a C-terminal phosphorylated tyrosine (Cooper and Howell, 1993; Brown and Cooper, 1996) and between the SH2/kinase linker and the SH3 and kinase domains (Sicheri et al., 1997; Xu et al., 1997). These interactions render the kinase domain inactive, but they can be released by alternative substrates of the SH2 or SH3 domains or by dephosphorylation of the C-terminal tyrosine, leading to activation of the kinase domain.
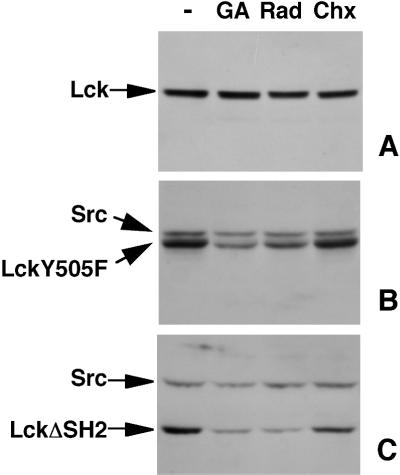
In contrast to our result with wild-type (wt) Lck, it was reported previously that Hsp90 is required for the stability and activity of LckY505F (Hartson et al., 1996), a constitutively active Lck mutant with the C-terminal regulatory tyrosine 505 substituted by a phenylalanine. We compared the effects of Hsp90 inhibitors on the steady-state levels of LckY505F and wt Lck in transfected fibroblasts. Similar to the situation in T-cells (Figure 1), no significant change in the levels of wt Lck was detected after a 3.5-h treatment with the drugs (Figure 2A). In contrast, LckY505F levels were significantly reduced after incubation with either geldanamycin or radicicol (Figure 2B). This reduction was not caused by a lack of LckY505F synthesis during drug treatment, because inhibition of protein synthesis by cycloheximide for the same time did not lead to a loss of LckY505F (Figure 2B). Therefore, the reduced steady-state level of LckY505F after drug treatment is most likely caused by the disappearance of mature LckY505F. Similar to wt Lck, the steady-state levels of endogenous wt c-Src in the LckY505F-transfected cells were not affected by geldanamycin or radicicol (Figure 2B). We next examined the stability of LckΔSH2, a Lck mutant with a deletion of the SH2 domain. Similar to LckY505F, steady-state levels of LckΔSH2 were significantly reduced after a 3.5-h incubation with Hsp90 inhibitors, whereas again endogenous c-Src levels were not affected (Figure 2C). These data show that mutant forms of Lck, which lack an interaction between the SH2 domain and the regulatory tyrosine, require Hsp90 activity for continued stability of the mature protein.
Figure 2.
Stable expression of LckY505F and LckΔSH2 requires Hsp90. NIH-3T3 cells transfected with wt Lck (A), LckY505F (B), or LckΔSH2 (C) were treated without (−) or with 5 μM geldanamycin (GA), 10 μM radicicol (Rad), or 100 μg/ml cycloheximide (Chx) for 3.5 h. Cell lysates, containing equal amounts of protein, were immunoblotted with antibodies against Lck (A) or against both Lck and Src (B and C).
Lack of Hsp90 Activity Leads to Rapid Degradation of Newly Synthesized Lck
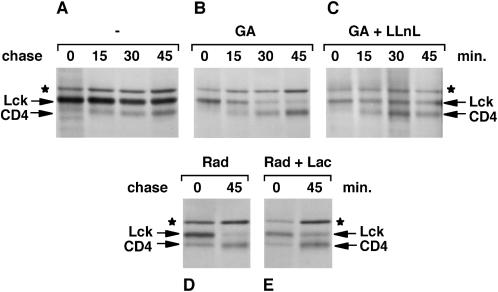
Treatment of cells with Hsp90 inhibitors resulted in an almost complete absence of newly synthesized Lck, Lyn, or Src (Figure 1A). Because these inhibitors have minimal effects on protein synthesis in general (Figure 1C) and Lck translation rates in reticulocyte lysates are normal in their presence (Hartson et al., 1998), this loss of the Src-kinases is most likely caused by rapid degradation of newly synthesized proteins. To investigate whether this is the case, we followed newly synthesized Lck in SupT1 cells by pulse–chase analysis. Cells were labeled for 5 min with [35S]methionine/cysteine and chased for various times in medium containing an excess of unlabeled methionine and cysteine. In the presence of geldanamycin, newly synthesized Lck could be detected directly after the 5-min pulse (Figure 3B). However, this Lck disappeared during the chase, and very little was detectable 45 min later (Figure 3B). Similar results were seen with radicicol-treated cells (Figure 3D), whereas no reduction of Lck was observed in untreated cells (Figure 3A). The normal half-life of Lck in T-cells has been reported to be 20–30 h (Hurley and Sefton, 1989).
Figure 3.
Lck synthesized in the absence of Hsp90 activity is rapidly degraded. SupT1 cells were labeled with [35S]methionine/cysteine for 5 min and chased in an excess of unlabeled amino acids for the indicated times. Cells were either not treated (−; A) or treated as described in MATERIALS AND METHODS with 5 μM geldanamycin only (GA; B), with both 5 μM geldanamycin and 50 μM LLnL (GA + LLnL; C), with 10 μM radicicol only (Rad; D), or with both 10 μM radicicol and 10 μM lactacystin (Rad + Lac; E). Lck was immunoprecipitated with anti-Lck serum. In addition to Lck, coimmunoprecipitated CD4 and a background band (★) were detected.
To examine whether the disappearance of newly synthesized Lck was caused by degradation, cells were treated with both geldanamycin and LLnL. LLnL is a peptide aldehyde that reversibly inhibits proteasomes as well as lysosomal proteases and calpains (Lee and Goldberg, 1998). In the presence of both LLnL and geldanamycin, the disappearance of Lck was reduced (Figure 3C). A similar result was obtained with lactacystin, a more specific irreversible inhibitor of proteasomes (Lee and Goldberg, 1998) (Figure 3E). Together, these results show that Lck synthesized in the absence of Hsp90 activity is unstable and rapidly degraded, at least in part by proteasomes.
Association of newly synthesized CD4 with Lck can be detected by coimmunoprecipitation of CD4 with anti-Lck antibodies (Bijlmakers and Marsh, 1999). This association occurred normally in geldanamycin-treated cells (Figure 3B), indicating that synthesis and maturation of CD4 were not affected. The newly synthesized CD4 apparently associated with premade unlabeled Lck. Moreover, the amount of labeled CD4 did not decrease with time in the presence of Hsp90 inhibitors. Similarly, the background band did not disappear during the chase (Figure 3, B and D). This unidentified protein is recognized by the anti-Lck serum but is not associated with, or related to, Lck (Bijlmakers and Marsh, 1999). These observations show that the degradation of newly synthesized proteins is not general in cells treated with Hsp90 inhibitors.
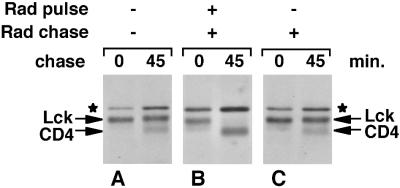
To examine the timing of the Hsp90-dependent step, we added radicicol either at the beginning or at the end of a 5-min labeling. When radicicol was added at the beginning of the pulse together with [35S]methionine/cysteine, newly synthesized Lck was completely degraded within the 45-min chase period (Figure 4B), indicating that radicicol inhibits Hsp90 very rapidly. However, when radicicol was added at the end of 5-min pulse labeling, no loss of newly synthesized Lck was observed (Figure 4C). This implies that Hsp90 is only required for a short time. After synthesis has occurred in the presence of Hsp90 activity, the newly synthesized Lck is stable and insensitive to subsequent Hsp90 inhibition.
Figure 4.
Hsp90 activity is required only during the synthesis of Lck. SupT1 cells were labeled for 5 min with [35S]methionine/cysteine and chased as indicated. Radicicol (Rad) was either absent (A), added at 10 μM together with [35S]methionine/cysteine at the beginning of the pulse and present during the chase (B), or present during the chase only (C). Lck was immunoprecipitated as indicated in MATERIALS AND METHODS. Lck, coimmunoprecipitated CD4, and the background band (★) are indicated.
Lck Synthesized in the Absence of Hsp90 Activity Does Not Associate with Membranes
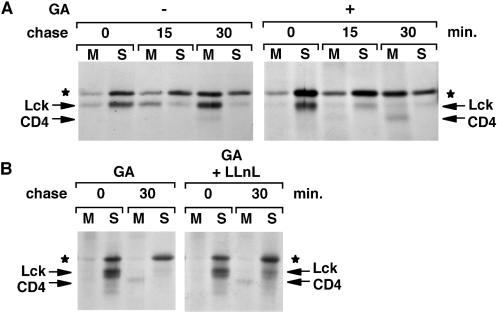
In addition to its role in protein folding, Hsp90 has been suggested to play a role in the translocation of Src-kinases to membranes (Courtneidge and Bishop, 1982; Brugge, 1986). We determined previously the membrane-binding kinetics of newly synthesized Lck in SupT1 cells. The association with membranes starts soon after synthesis but is not complete until 30–45 min later (Bijlmakers and Marsh, 1999). We compared Lck membrane-binding kinetics in the presence or absence of Hsp90 activity. SupT1 cells were pulse labeled and chased as described above. For each time point, total cellular membranes were separated from the cytosol, and Lck was immunoprecipitated from both fractions (see MATERIALS AND METHODS). We found that in geldanamycin-treated cells, virtually no membrane binding of Lck occurred. At the 15-min chase, for instance, the majority of Lck is recovered from the membrane fraction of untreated cells but from the soluble fraction of geldanamycin-treated cells (Figure 5A). At the 30-min chase, most newly synthesized Lck is degraded in geldanamycin-treated cells.
Figure 5.
In the absence of Hsp90 activity, newly synthesized Lck does not associate with membranes. (A) SupT1 cells were labeled for 5 min with [35S]methionine/cysteine and chased as indicated, either in the absence (−) or presence (+) of 5 μM geldanamycin (GA) as described in MATERIALS AND METHODS. Cells were homogenized, nuclei were removed, and cellular membranes were recovered by centrifugation at 100,000 × g. Lck was immunoprecipitated from both membrane (M) and soluble (S) fractions. Lck, coimmunoprecipitated CD4, and the background band (★) are indicated. (B) SupT1 cells were pulse labeled with [35S]methionine/cysteine for 5 min and chased as indicated, in the presence of either 5 μM geldanamycin only (GA) or both 5 μM geldanamycin and 50 μM LLnL (GA + LLnL) as described in MATERIALS AND METHODS. Lck was immunoprecipitated from membrane (M) and soluble (S) fractions, prepared as described above.
To examine whether the membrane association of Lck could be enhanced by inhibiting its degradation, cells were treated simultaneously with geldanamycin and the protease inhibitor LLnL. In these dually treated cells, newly synthesized Lck could still be detected after a 30-min chase. However, the membrane association of Lck was again significantly reduced compared with that of untreated cells (Figure 5B). Thus, blocking protein degradation did not restore the membrane association of Lck synthesized in the absence of Hsp90 activity.
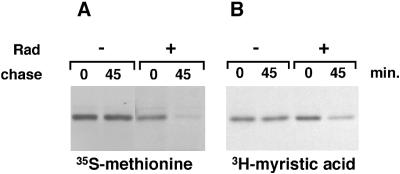
Posttranslational palmitoylation of Lck is required for its membrane association. The lack of stable Lck membrane binding in the presence of Hsp90 inhibitor indicates that, under these conditions, palmitoylation of Lck might not take place. Cotranslational myristoylation of Lck is required before palmitoylation. To investigate whether myristoylation is affected by the inhibition of Hsp90, we studied the incorporation of [3H]myristic acid in the presence and absence of radicicol. LSTRA cells, a murine leukemia T-cell line that overexpresses Lck 40-fold, were used for these experiments. The high level of Lck expression enabled us to use short [3H]myristic acid labeling times. In LSTRA cells, Lck behaved similarly to that in SupT1 cells; [35S]methionine labeling showed complete degradation of Lck synthesized in the presence of radicicol after the 45-min chase, whereas no degradation occurred in the absence of inhibitor (Figure 6A). Labeling with myristic acid for 15 min showed myristoylation of Lck, both in the absence and presence of radicicol (Figure 6B, 0-min chase). However, in the absence of radicicol the myristoylated protein could still be detected at the 45-min chase, whereas in the presence of inhibitor the labeled protein almost completely disappeared during the chase (Figure 6B). This result indicates that myristoylation of Lck does occur in the presence of Hsp90 inhibitors and that the myristoylated protein is degraded. Thus, the absence of membrane binding of Lck in the presence of Hsp90 inhibitors is not caused by a failure to myristoylate Lck.
Figure 6.
In the absence of Hsp90 activity, newly synthesized Lck is myristoylated. (A) LSTRA cells were labeled for 15 min in the absence (−) or presence (+) of 10 μM radicicol (Rad) with [35S]methionine/cysteine and chased as indicated. Lck was immunoprecipitated. (B) LSTRA cells were labeled for 15 min in the absence (−) or presence (+) of 10 μM radicicol with [3H]myristic acid and chased as indicated. Lck was immunoprecipitated.
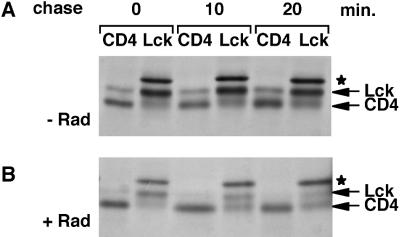
Lck Synthesized in the Absence of Hsp90 Activity Does Not Associate with CD4
In SupT1 cells, a large proportion of Lck associates with the cell surface receptor CD4 at steady state (Bijlmakers and Marsh, 1999). This noncovalent interaction requires a pair of cysteines in the unique domain of Lck and a pair of cysteines in the cytoplasmic domain of CD4 (Shaw et al., 1990; Turner et al., 1990). In addition, the interaction depends on the membrane binding of Lck (Turner et al., 1990). We found previously that the kinetics of newly synthesized Lck binding to CD4 is similar to its membrane-binding kinetics (Bijlmakers and Marsh, 1999). Thus, CD4 association starts soon after synthesis and continues for 30–45 min. We now compared the CD4 association for Lck synthesized in the absence or presence of Hsp90 inhibitors. Cells were pulse labeled for 5 min and chased for various times, and cell lysates were subjected to parallel immunoprecipitations with anti-CD4 or anti-Lck antibodies. Association of Lck with CD4 was detected by coimmunoprecipitation with anti-CD4 antibodies, whereas the anti-Lck immunoprecipitation detected the total amount of newly synthesized Lck. In the absence of Hsp90 inhibitors, CD4 binding of Lck was obvious from the earliest chase point (Figure 7A). However, association with CD4 was not observed in the presence of radicicol (Figure 7B) or geldanamycin at any chase point. Thus, in the absence of Hsp90 activity, newly synthesized Lck does not associate with CD4, most likely as a consequence of its failure to bind to membranes under these conditions. Geldanamycin and radicicol do not inhibit CD4–Lck association per se, because newly synthesized CD4 could be detected on Lck synthesized before pulse labeling (Figure 3).
Figure 7.
In the absence of Hsp90 activity, newly synthesized Lck does not associate with CD4. SupT1 cells were labeled for 5 min with [35S]methionine/cysteine and chased as indicated in the absence (−; A) or presence (+; B) of 10 μM radicicol (Rad) as described in MATERIALS AND METHODS. For each time point, cell lysates were split into two equal parts. One part was subjected to immunoprecipitation with anti-CD4 antibodies (CD4); the other was subjected to immunoprecipitation with anti-Lck antibodies (Lck). (★) indicates the background band.
UD-GFP Folding and Membrane Binding Are Independent of Hsp90
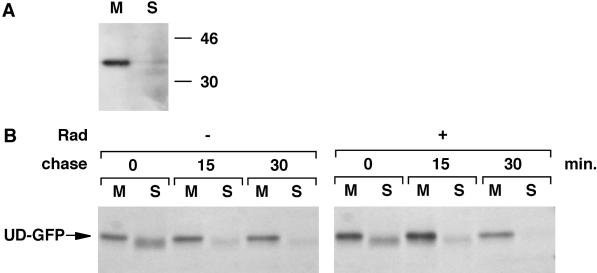
The lack of membrane association of Lck in the presence of Hsp90 inhibitors suggests that Hsp90 is important for the translocation of newly synthesized Lck to membranes. Hsp90 could play a direct role in this process by escorting Lck from cytosol to membranes. Alternatively, Hsp90 could play an indirect role by assisting the folding of newly synthesized Lck. In the latter case, the absence of Hsp90 activity could result in misfolded Lck that is unable to associate with membranes and is targeted for degradation. To discriminate between these two possibilities, we investigated the effect of Hsp90 inhibitors on the biosynthesis and membrane binding of UD-GFP, a construct composed of the unique domain of Lck fused to the N terminus of GFP (Bijlmakers et al., 1997). The N-terminal unique domain is important for the membrane binding and subcellular distribution of Lck (Kwong and Lublin, 1995; Yurchak and Sefton, 1995; Bijlmakers et al., 1997; Zlatkine et al., 1997). At the extreme N terminus, myristic and palmitic acids are attached, modifications that are crucial for the interaction with membranes (Resh, 1994). UD-GFP is completely membrane associated at steady state (see Figure 9A), and its subcellular distribution is very similar to that of wt Lck (Bijlmakers et al., 1997). The pair of cysteines (C20 and C23) required for the interaction with CD4 are also located in this domain, and interaction of UD-GFP with CD4 could be demonstrated (our unpublished result). Thus, the unique domain in UD-GFP behaves similarly to that in wt Lck.
Figure 9.
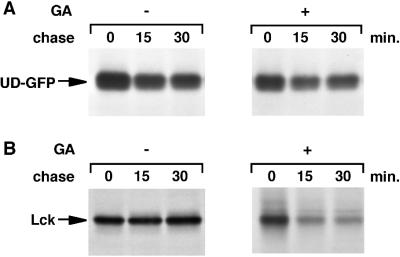
UD-GFP does not require Hsp90 for membrane binding. (A) HeLa cells transfected with UD-GFP were homogenized, and total cellular membranes were recovered by centrifugation at 100,000 × g. Membrane (M) and soluble (S) fractions were analyzed for UD-GFP by immunoblotting with anti-Lck serum. Molecular weight markers are indicated in kilodaltons on the right. (B) HeLa cells transfected with UD-GFP were labeled for 5 min with [35S]methionine/cysteine and chased as indicated in the absence (−) or presence (+) of 10 μM radicicol (Rad) as described in MATERIALS AND METHODS. UD-GFP was immunoprecipitated from both membrane (M) and soluble (S) fractions.
We first studied the role of Hsp90 in the biosynthesis of UD-GFP. Stably transfected HeLa cells were pulse labeled and chased in the presence or absence of geldanamycin, and UD-GFP was immunoprecipitated with anti-Lck antiserum. The half-life of UD-GFP was much shorter than that of Lck (Figure 8A). However, geldanamycin had little effect on the half-life of this protein (Figure 8A). As seen in T-cells (Figure 3), newly synthesized Lck rapidly disappeared from stably transfected HeLa cells treated with geldanamycin (Figure 8B), whereas Lck was stable in untreated HeLa cells (Figure 8B). We conclude that Hsp90 is not required for the stability of newly synthesized UD-GFP.
Figure 8.
UD-GFP does not require Hsp90 for synthesis. HeLa cells transfected with UD-GFP (A) or Lck (B) were labeled for 5 min with [35S]methionine/cysteine and chased as indicated in either the absence (−) or presence (+) of 5 μM geldanamycin (GA) as described in MATERIALS AND METHODS. UD-GFP and Lck were immunoprecipitated with anti-Lck serum from detergent lysates.
The lack of an Hsp90 requirement for the synthesis of UD-GFP allowed us to study the involvement of Hsp90 in membrane association independent of its role in protein folding. We determined the membrane-binding kinetics of UD-GFP in the absence or presence of radicicol. For every chase point, the relative amount of membrane-associated UD-GFP was similar in the absence or presence of Hsp90 inhibition (Figure 9B). Thus, membrane binding of UD-GFP does not require Hsp90 activity. We therefore conclude that Hsp90 does not play a direct role in UD-GFP or Lck membrane binding and that the lack of Lck membrane binding in the absence of Hsp90 activity is caused by an effect on domains outside the unique domain. Hsp90 activity is required for correct folding of Lck, and in the absence of a stable conformation, the unique domain does not bind to membranes.
DISCUSSION
Acylated cytosolic proteins comprise a large and heterogeneous group that include Src family kinases, the α subunits of heterotrimeric GTP-binding proteins, and many others. The common feature of these proteins is that they reside on, and function in association with, cellular membranes. Their association with membranes is dependent on the cotranslational and posttranslational covalent attachment of fatty acids (Milligan et al., 1995; Wedegaertner et al., 1995; Bhatnagar and Gordon, 1997). Although membrane association is crucial for their function, little is known of the mechanisms by which these proteins are targeted to specific membrane compartments (Magee and Marshall, 1999). To obtain insight into this, we followed previously the fate of newly synthesized Lck, a Src family protein, by pulse–chase labeling experiments. We determined membrane- and CD4-binding kinetics and showed that Lck is targeted first to a compartment of the secretory pathway and then transported to the plasma membrane (Bijlmakers and Marsh, 1999). However, it remains unclear how the initial membrane association of newly synthesized Lck and other acylated proteins occurs. In this study, we focused on the cytosolic chaperone Hsp90 that has been suggested to play a role in the translocation of v-Src to membranes (Courtneidge and Bishop, 1982; Brugge, 1986). In addition, Hsp90 is thought to be important for the folding of Src-kinases (Brugge, 1986; Xu and Lindquist, 1993; Xu et al., 1999; Hartson et al., 1996, 1998; Buchner, 1999). We studied the requirement of Hsp90 activity in the cellular biosynthesis of Lck and distinguished between its postulated roles. We found that Hsp90 is essential for the initial synthesis of Lck but is not required for the maintenance of mature Lck. In contrast, Hsp90 is required for the stability of the Lck mutants LckY505F and LckΔSH2. Although Hsp90 is important for Lck synthesis, it is not directly involved in the membrane association of newly synthesized Lck.
Various studies have suggested a role for Hsp90 in the de novo synthesis of Src-kinases. The first indication came from experiments on virally encoded oncogenic v-Src, which was found to associate during synthesis with two proteins (Oppermann et al., 1981; Courtneidge and Bishop, 1982; Brugge et al., 1983), later identified as Hsp90 and the cochaperone cdc37 (Ziemiecki et al., 1986; Whitelaw et al., 1991; Perdew et al., 1997). However, these interactions could not be detected for c-Src in cells (Iba et al., 1984; Brugge, 1986), and it remained unclear whether Hsp90 was required for the synthesis of Src-kinases in general or of virally encoded Src-kinases exclusively. The role of Hsp90 was further studied in rabbit reticulocyte lysates, in which the Src-kinases Fgr and Lck were found to interact with Hsp90 during in vitro translation (Hartson and Matts, 1994). This interaction was transient, and inhibition of Hsp90 activity resulted in enhanced protease sensitivity and loss of Lck kinase activity (Hartson et al., 1996). These experiments suggested that Hsp90 is important for the folding of newly synthesized Lck in reticulocyte lysates. However, this could be particular to in vitro synthesis, in which high levels of nascent protein and the absence of membranes might promote misfolding.
We therefore examined the involvement of Hsp90 in the synthesis of Src-kinases in cells. For these studies, two inhibitors of Hsp90 activity, geldanamycin and radicicol, were used. These chemically unrelated compounds bind with high affinity to the ATP-binding site of Hsp90 (Prodromou et al., 1997; Roe et al., 1999). Using these drugs we found that Hsp90 is essential for the synthesis of Lck in T-cells (Figures 1 and 6), as well as for Lck transfected into HeLa cells (Figure 8). Similar results were obtained for Lyn constitutively expressed in B-cells and c-Src transfected into fibroblasts (Figure 1). This suggests that Hsp90 might be required during the synthesis of all Src-kinases, regardless of the cellular background. The absence of Hsp90 activity resulted in rapid degradation of newly synthesized Lck that occurred, at least in part, via proteasomes (Figure 3). Geldanamycin-induced degradation by the proteasome has been reported for several proteins including Raf-1, p53, c-erbB-2, and cystic fibrosis transmembrane conductance regulator (Mimnaugh et al., 1996; Schulte et al., 1997; Whitesell et al., 1997; Loo et al., 1998). However, with the exception of cystic fibrosis transmembrane conductance regulator, these studies did not discriminate between proteolysis of newly synthesized or mature proteins.
The requirement for Hsp90 activity is apparently restricted to a short time during synthesis. When radicicol was added to cells at the beginning of a 5-min pulse, the newly synthesized radiolabeled Lck was rapidly degraded (Figure 4). In contrast, when radicicol was added at the end of a 5-min pulse, newly synthesized Lck was stable for at least 45 min (Figure 4). Although Hsp90 clearly plays a role in the synthesis of Lck, we have not yet been able to detect a direct association between Lck and Hsp90, either by analysis of radiolabeled Lck immunoprecipitates or by immunoblotting Lck immunoprecipitates with anti-Hsp90 antibodies. This could be explained by a transient interaction of Hsp90 with Lck during synthesis, in which case only a small amount of the total Lck is Hsp90 associated at steady state. Interaction with Hsp90 was demonstrated previously for Lck in reticulocyte lysates (Hartson and Matts, 1994) and in LSTRA cells (Hartson et al., 1996), a murine T-cell line that overexpresses Lck 40-fold. In both situations, the large amount of newly synthesized Lck might facilitate the detection of Hsp90 interaction.
In addition to its role during synthesis, Hsp90 is thought to be required for the maintenance of mature Src-kinases. This role of Hsp90 was suggested by experiments on Src-kinases in reticulocyte lysates (Hartson et al., 1996, 1998) and yeast (Xu and Lindquist, 1993; Xu et al., 1999) and on mutant kinases in cells (Whitesell et al., 1994; Hartson et al., 1996). We show here that in cells, three wt Src-kinases do not require Hsp90 for maintenance, whereas Lck mutants do. The protein levels of Lck, Lyn, and Src were unchanged after Hsp90 inhibition for 3.5 h, suggesting that the mature wild-type proteins are stable in the absence of Hsp90 activity (Figure 1). In contrast, the Lck mutants LckY505F and LckΔSH2 showed reduced protein levels when Hsp90 function was inhibited (Figure 2). The point mutation in LckY505F prevents the normal intramolecular interaction between the regulatory phosphotyrosine 505 and the SH2 domain. As a result, the kinase domain can adopt an active conformation (Cooper and Howell, 1993; Brown and Cooper, 1996). Similarly, in LckΔSH2 this intramolecular interaction cannot occur. The reduction in steady-state levels of the Lck mutants in the absence of Hsp90 activity suggests that these activated kinases need constant monitoring by Hsp90. It is possible that the activated kinase domain is more susceptible to degradation in the absence of Hsp90 activity. Several other studies have reported differences between wild-type and mutant Src-kinases in their requirement for Hsp90 (Hartson et al., 1998; Xu et al., 1999). The kinase domain might be the main, or only, substrate of Hsp90. In reticulocyte lysates, folding of the kinase domain of Lck requires Hsp90 activity during synthesis, whereas folding of the SH2 domain does not (Hartson et al., 1998). Hsp90 might play a role in regulating Src-proteins by directing the folding of the kinase domains into the inactive conformation. Failure to do this would lead to degradation of the protein.
Hsp90 differs from other chaperones by its apparent limited set of substrates and by interacting with these substrates at relatively late stages of their folding (Buchner, 1999). Moreover, in some cases only a particular fraction of substrate interacts with Hsp90. Thus, Hsp90 might play a role in the trafficking of substrates in addition to its chaperone function (Pratt, 1993). In the case of Src, the interaction with Hsp90 is restricted to the cytosolic fraction. It was therefore postulated that Hsp90 might assist in the translocation of Src-kinases from cytosol to membranes (Courtneidge and Bishop, 1982; Brugge, 1986; Pratt, 1993). Indeed, we observed that Lck synthesized in the absence of Hsp90 activity was unable to associate with membranes or CD4 (Figures 5 and 7). However, this could be interpreted in two ways. Hsp90 could play a direct role in membrane binding. Alternatively, Hsp90 could be required for proper folding, and proper folding could be required for Lck membrane association. The construct UD-GFP allowed us to discriminate between these two possibilities. UD-GFP is a GFP hybrid protein that contains the unique domain of Lck at its N terminus (Bijlmakers et al., 1997). This unique domain determines the subcellular distribution of Lck as well as membrane and CD4 binding. We found that in contrast to Lck, Lyn, and Src, UD-GFP does not require Hsp90 activity for stable synthesis (Figure 8). Moreover, membrane binding of newly synthesized UD-GFP was also unaffected by Hsp90 inhibitors (Figure 9). Because of the similar properties of the unique domain in UD-GFP and Lck, we conclude that Hsp90 is unlikely to be involved directly in the membrane association of Lck. The lack of Lck membrane binding in the presence of geldanamycin must therefore be regarded as an indirect result of Hsp90 inhibition. We show that cotranslational myristoylation occurs normally in the absence of Hsp90 activity. Possibly, the conformation of Lck synthesized in the absence of Hsp90 activity is not compatible with palmitoylation and/or membrane insertion. Alternatively, tagging Lck for degradation might interfere with membrane association or direct the protein away from the site where membrane association occurs.
The data presented here show that functional Hsp90 is essential during the early steps of Lck synthesis. Hsp90 may exert quality control surveillance over Lck and other Src-kinases to ensure that only correctly folded proteins become membrane bound. However, the identification of proteins involved in the membrane binding of Src-kinases remains a challenge for further experiments.
ACKNOWLEDGMENTS
We thank members of the Marsh laboratory for constructive comments and support, Robert Kypta for critically reading this manuscript, and Jannie Borst, Laurence Pearl, Dan Littman, and Jim Hoxie for reagents. This work was supported by the United Kingdom Medical Research Council.
Abbreviations used:
- CLAP
chymostatin, pepstatin A, and antipain hydrochloride (each at 5 μg/ml) and leupeptin hemisulfate (at 10 μg/ml)
- LLnL
acetyl-leu-leu-norleucinal
- NP-40
Nonidet P-40
- pen/strep
100 U/ml penicillin and 0.1 mg/ml streptomycin
- PMSF
phenylmethylsulfonyl fluoride
- SH
Src homology
- UD-GFP
the unique domain of Lck attached to green fluorescent protein
- wt
wild-type
REFERENCES
- Bhatnagar RS, Gordon JI. Understanding covalent modifications of proteins by lipids: where cell biology and biophysics mingle. Trends Cell Biol. 1997;7:14–21. doi: 10.1016/S0962-8924(97)10044-7. [DOI] [PubMed] [Google Scholar]
- Bijlmakers MJ, Isobe-Nakamura M, Ruddock LJ, Marsh M. Intrinsic signals in the unique domain target p56(lck) to the plasma membrane independently of CD4. J Cell Biol. 1997;137:1029–1040. doi: 10.1083/jcb.137.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlmakers MJ, Marsh M. Trafficking of an acylated cytosolic protein: newly synthesized p56(lck) travels to the plasma membrane via the exocytic pathway. J Cell Biol. 1999;145:457–468. doi: 10.1083/jcb.145.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouns GS, de Vries E, van Noesel CJ, Mason DY, van Lier RA, Borst J. The structure of the mu/pseudo light chain complex on human pre-B cells is consistent with a function in signal transduction. Eur J Immunol. 1993;23:1088–1097. doi: 10.1002/eji.1830230517. [DOI] [PubMed] [Google Scholar]
- Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- Brugge J, Yonemoto W, Darrow D. Interaction between the Rous sarcoma virus transforming protein and two cellular phosphoproteins: analysis of the turnover and distribution of this complex. Mol Cell Biol. 1983;3:9–19. doi: 10.1128/mcb.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge JS. Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–22. doi: 10.1007/978-3-642-70810-7_1. [DOI] [PubMed] [Google Scholar]
- Brugge JS, Erikson E, Erikson RL. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981;25:363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Buchner J. Hsp90 & Co.—a holding for folding. Trends Biochem Sci. 1999;24:136–141. doi: 10.1016/s0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- Chavany C, Mimnaugh E, Miller P, Bitton R, Nguyen P, Trepel J, Whitesell L, Schnur R, Moyer J, Neckers L. p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J Biol Chem. 1996;271:4974–4977. doi: 10.1074/jbc.271.9.4974. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Howell B. The when and how of Src regulation. Cell. 1993;73:1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- Courtneidge SA, Bishop JM. Transit of pp60v-src to the plasma membrane. Proc Natl Acad Sci USA. 1982;79:7117–7121. doi: 10.1073/pnas.79.23.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czar MJ, Galigniana MD, Silverstein AM, Pratt WB. Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid-dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. Biochemistry. 1997;36:7776–7785. doi: 10.1021/bi970648x. [DOI] [PubMed] [Google Scholar]
- Hartson SD, Barrett DJ, Burn P, Matts RL. Hsp90-mediated folding of the lymphoid cell kinase p56lck. Biochemistry. 1996;35:13451–13459. doi: 10.1021/bi961332c. [DOI] [PubMed] [Google Scholar]
- Hartson SD, Matts RL. Association of Hsp90 with cellular Src-family kinases in a cell-free system correlates with altered kinase structure and function. Biochemistry. 1994;33:8912–8920. doi: 10.1021/bi00196a008. [DOI] [PubMed] [Google Scholar]
- Hartson SD, Ottinger EA, Huang W, Barany G, Burn P, Matts RL. Modular folding and evidence for phosphorylation-induced stabilization of an hsp90-dependent kinase. J Biol Chem. 1998;273:8475–8482. doi: 10.1074/jbc.273.14.8475. [DOI] [PubMed] [Google Scholar]
- Hurley TR, Sefton BM. Analysis of the activity and phosphorylation of the lck protein in lymphoid cells. Oncogene. 1989;4:265–272. [PubMed] [Google Scholar]
- Hutchison KA, Brott BK, De Leon JH, Perdew GH, Jove R, Pratt WB. Reconstitution of the multiprotein complex of pp60src, hsp90, and p50 in a cell-free system. J Biol Chem. 1992;267:2902–2908. [PubMed] [Google Scholar]
- Iba H, Takeya T, Cross FR, Hanafusa T, Hanafusa H. Rous sarcoma virus variants that carry the cellular src gene instead of the viral src gene cannot transform chicken embryo fibroblasts. Proc Natl Acad Sci USA. 1984;81:4424–4428. doi: 10.1073/pnas.81.14.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, Fletcher MC, Ledbetter JA, Schieven GL, Siegel JN, Phillips AF, Samelson LE. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc Natl Acad Sci USA. 1990;87:7722–7726. doi: 10.1073/pnas.87.19.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabouridis PS, Magee AI, Ley SC. S-acylation of LCK protein tyrosine kinase is essential for its signaling function in T lymphocytes. EMBO J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong J, Lublin DM. Amino-terminal palmitate or polybasic domain can provide required second signal to myristate for membrane binding of p56lck. Biochem Biophys Res Commun. 1995;207:868–876. doi: 10.1006/bbrc.1995.1266. [DOI] [PubMed] [Google Scholar]
- Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- Lipsich LA, Lewis AJ, Brugge JS. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983;48:352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo MA, Jensen TJ, Cui L, Hou Y, Chang XB, Riordan JR. Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J. 1998;17:6879–6887. doi: 10.1093/emboj/17.23.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee T, Marshall C. New insights into the interaction of Ras with the plasma membrane. Cell. 1999;98:9–12. doi: 10.1016/S0092-8674(00)80601-7. [DOI] [PubMed] [Google Scholar]
- Milligan G, Parenti M, Magee AI. The dynamic role of palmitoylation in signal transduction. Trends Biochem Sci. 1995;20:181–186. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem. 1996;271:22796–22801. doi: 10.1074/jbc.271.37.22796. [DOI] [PubMed] [Google Scholar]
- Oppermann H, Levinson AD, Levintow L, Varmus HE, Bishop JM, Kawai S. Two cellular proteins that immunoprecipitate with the transforming protein of Rous sarcoma virus. Virology. 1981;113:736–751. doi: 10.1016/0042-6822(81)90202-6. [DOI] [PubMed] [Google Scholar]
- Paige LA, Nadler MJ, Harrison ML, Cassady JM, Geahlen RL. Reversible palmitoylation of the protein-tyrosine kinase p56lck. J Biol Chem. 1993;268:8669–8674. [PubMed] [Google Scholar]
- Pelchen-Matthews A, Boulet I, Littman DR, Fagard R, Marsh M. The protein tyrosine kinase p56lck inhibits CD4 endocytosis by preventing entry of CD4 into coated pits. J Cell Biol. 1992;117:279–290. doi: 10.1083/jcb.117.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew GH, Wiegand H, Van den Heuvel JP, Mitchell C, Singh SS. A 50 kilodalton protein associated with raf and pp60(v-src) protein kinases is a mammalian homolog of the cell cycle control protein cdc37. Biochemistry. 1997;36:3600–3607. doi: 10.1021/bi9612529. [DOI] [PubMed] [Google Scholar]
- Pratt WB. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993;268:21455–21458. [PubMed] [Google Scholar]
- Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Resh MD. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- Rudd CE, Janssen O, Prasad KVS, Raab M, da Silva A, Telfer JC, Yamamoto M. Src-related protein tyrosine kinases and their surface receptors. Biochem Biophys Acta. 1993;1155:239–266. doi: 10.1016/0304-419x(93)90007-y. [DOI] [PubMed] [Google Scholar]
- Rudd CE, Trevillyan JM, Dasgupta JD, Wong LL, Schlossman SF. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci USA. 1988;85:5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, Neckers LM. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998;3:100–108. doi: 10.1379/1466-1268(1998)003<0100:arbttn>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte TW, An WG, Neckers LM. Geldanamycin-induced destabilization of Raf-1 involves the proteasome. Biochem Biophys Res Commun. 1997;239:655–659. doi: 10.1006/bbrc.1997.7527. [DOI] [PubMed] [Google Scholar]
- Schulte TW, Blagosklonny MV, Ingui C, Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J Biol Chem. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- Sharma SV, Agatsuma T, Nakano H. Targeting of the protein chaperone, HSP90, by the transformation suppressing agent, radicicol. Oncogene. 1998;16:2639–2645. doi: 10.1038/sj.onc.1201790. [DOI] [PubMed] [Google Scholar]
- Shaw AS, Chalupny J, Whitney JA, Hammond C, Amrein KE, Kavathas P, Sefton BM, Rose JK. Short related sequences in the cytoplasmic domains of CD4 and CD8 mediate binding to the amino-terminal domain of the p56lck tyrosine protein kinase. Mol Cell Biol. 1990;10:1853–1862. doi: 10.1128/mcb.10.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Gauen LK, Kwong J, Shaw AS, Lublin DM. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol. 1993;13:6385–6392. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- Silverman L, Resh MD. Lysine residues form an integral component of a novel NH2-terminal membrane targeting motif for myristylated pp60v-src. J Cell Biol. 1992;119:415–425. doi: 10.1083/jcb.119.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JM, Brodsky MH, Irving BA, Levin SD, Perlmutter RM, Littman DR. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990;60:755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- van't Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997;136:1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Wilson PT, Bourne HR. Lipid modifications of trimeric G proteins. J Biol Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- Whitelaw ML, Hutchison K, Perdew GH. A 50-kDa cytosolic protein complexed with the 90-kDa heat shock protein (hsp90) is the same protein complexed with pp60v-src hsp90 in cells transformed by the Rous sarcoma virus. J Biol Chem. 1991;266:16436–16440. [PubMed] [Google Scholar]
- Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L, Sutphin P, An WG, Schulte T, Blagosklonny MV, Neckers L. Geldanamycin-stimulated destabilization of mutated p53 is mediated by the proteasome in vivo. Oncogene. 1997;14:2809–2816. doi: 10.1038/sj.onc.1201120. [DOI] [PubMed] [Google Scholar]
- Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- Xu Y, Lindquist S. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc Natl Acad Sci USA. 1993;90:7074–7078. doi: 10.1073/pnas.90.15.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Singer MA, Lindquist S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc Natl Acad Sci USA. 1999;96:109–114. doi: 10.1073/pnas.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi TL, Bolen JB, Ihle JN. Hematopoietic cells express two forms of lyn kinase differing by 21 amino acids in the amino terminus. Mol Cell Biol. 1991;11:2391–2398. doi: 10.1128/mcb.11.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchak LK, Hardwick JS, Amrein K, Pierno K, Sefton BM. Stimulation of phosphorylation of Tyr394 by hydrogen peroxide reactivates biologically inactive, non-membrane-bound forms of Lck. J Biol Chem. 1996;271:12549–12554. doi: 10.1074/jbc.271.21.12549. [DOI] [PubMed] [Google Scholar]
- Yurchak LK, Sefton BM. Palmitoylation of either Cys-3 or Cys-5 is required for the biological activity of the Lck tyrosine protein kinase. Mol Cell Biol. 1995;15:6914–6922. doi: 10.1128/mcb.15.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A, Catelli MG, Joab I, Moncharmont B. Association of the heat shock protein hsp90 with steroid hormone receptors and tyrosine kinase oncogene products. Biochem Biophys Res Commun. 1986;138:1298–1307. doi: 10.1016/s0006-291x(86)80424-7. [DOI] [PubMed] [Google Scholar]
- Zlatkine P, Mehul B, Magee AI. Retargeting of cytosolic proteins to the plasma membrane by the Lck protein tyrosine kinase dual acylation motif. J Cell Sci. 1997;110:673–679. doi: 10.1242/jcs.110.5.673. [DOI] [PubMed] [Google Scholar]