Abstract
Four unique diastereomers of 3-hydroxy-2,4,6-trimethylheptanoic acid–(2R,3R,4R), (2S,3R,4R), (2S,3R,4S), and (2R,3R,4S)–the fatty acid component of callipeltin A and D, have been synthesized from commercially available (+)- and (−)-pseudoephedrine propionamide in 6 steps and 59% average overall yield. Comparison of the 1H and 13C NMR and optical rotation data of the resulting isomers with the natural fragment unambiguously verifies the configurational assignment of the natural isomer as (2R,3R,4R).
Introduction
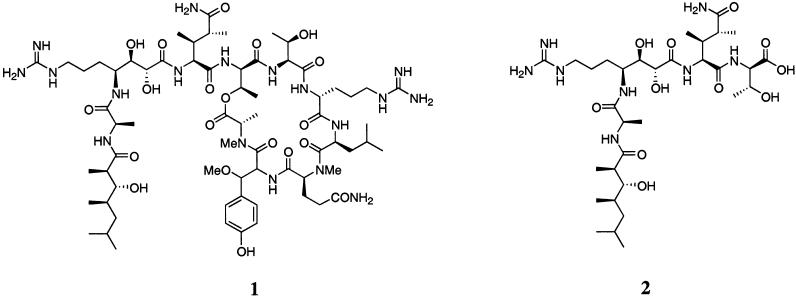
The structure of the novel marine cyclic depsipeptide callipeltin A (1), isolated from the lithistid sponge Callipelta sp. collected off the East coast of New Caledonia, was originally published by Minale and co-workers, and recently revised by D'Auria.1,2 Callipeltin A displays potent cytotoxicity (10 μg/mL, ca. 80% inhibition) against a broad range of human carcinoma cell lines,3 and displays both antiviral activity against HIV-1 (Lai strain)-infected CEM4 lymphocytes and antifungal activity against Fusarium oxysporum, Helminthosporium sativum, Phytophtora hevea, and Candida albicans.1 Additionally, callipeltin A was found to be a selective and powerful inhibitor of the Na+/Ca2+ exchanger and a positive inotropic agent in guinea pig atria.4 Callipeltin A has recently also been isolated from the marine sponge Latrunculia sp. in the Vanuatu Islands along with a new, truncated derivative named callipeltin D (2) (Figure 1).5
FIGURE 1.
Structures of callipeltin A (1) and D (2).
Two asymmetric syntheses of (2R,3R,4S)-3-hydroxy-2,4,6-trimethylheptanoic acid (TMHEA, (R,R,S)-3), the originally identified diastereomer of the fatty acid component of callipeltin A and D, have been published.6 Comparison of the 1H NMR spectrum of (R,R,S)-3 to that of the corresponding fragment obtained from the acid hydrosylate of callipeltin A, however, revealed that the original assignment of configuration was incorrect. D'Auria et al. revised the configuration as (2R,3R,4R), and supported their revision by correlation of the natural fragment with synthetic (R,R,R)-3.2 Comparison of the natural fragment to all diastereomeric possibilities, however, would provide a complete and unambiguous verification of the configurational assignment of TMHEA. The availability of multiple configurational isomers may also expedite future analogue studies. We herein report the efficient synthesis of four unique diastereoisomers of TMHEA, thereby confirming the configurational assignment of D'Auria.
Results and Discussion
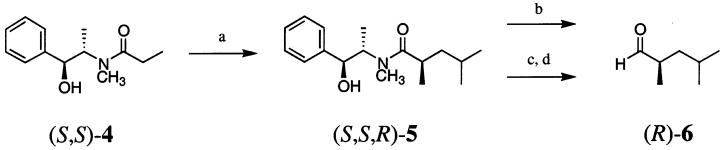
Our approach to the synthesis of (R,R,R)-3 and its diastereomers uses asymmetric crotylboration to stereoselectively form the key C-2/C-3 bond. The substrate aldehyde and its enantiomer could in turn be made in high enantiomeric purity using the diastereoselective alkylation chemistry of Myers.7 Accordingly, commercially available pseudoephedrine propionamide (S,S)-4 was alkylated with 1-iodo-2-methylpropane, using Myers's conditions,7 to give (S,S,R)-5 as a highly viscous oil in 98% yield and 99% de (Scheme 1). Reduction of the resulting tertiary amide to the aldehyde, (R)-6, was expected to proceed smoothly with lithium triethoxyaluminum hydride.8 The reaction initially gave inconsistent results, with yields ranging from 50% to 70%, but when all traces of residual ethyl acetate were removed from (S,S,R)-5 by coevaporation with toluene, aldehyde (R)-6 was obtained reproducibly in 86% yield. A convenient alternative, albeit one that affords lower yields, involves conversion of the tertiary amide to the primary alcohol with lithium amidotrihydroborate9 (LAB) and subsequent reoxidation with TPAP,10 giving (R)-6 in 77% yield over two steps. In the latter case, the enantiomeric purity of the intermediate alcohol was determined to be 98% by employing Mosher's method.11
SCHEME 1 a.
a Reaction conditions: (a) LDA, LiCl, THF, −78 °C; 1-iodo-2-methylpropane, −78 °C, 98%; (b) LiAlH(OEt)3, hexanes–THF, 0 °C; TFA, 1 M HCl, 86%; (c) LAB, THF; (d) TPAP, NMO, 4 Å MS, CH2Cl2, 77% (two steps).
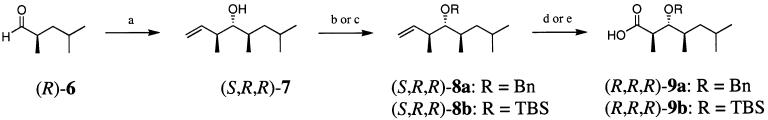
Treatment of (R)-6 with the crotylborane derived from (−)-B-methoxydiisopinocampheylborane and trans-2-butene12 provided alcohol (S,R,R)-7 in 72% yield and 96% de (Scheme 2). Following chromatography, the diastereomeric excess of (S,R,R)-7 was determined to be greater than 98%. Protection of (S,R,R)-7 as the benzyl ether, by reaction with benzyl-2,2,2-trichloroacetimidate and catalytic triflic acid,13 gave (S,R,R)-8a in 96% yield. Alternatively, (S,R,R)-7 could be protected as its silyl ether by reaction with tert-butyldimethylsilyl chloride and imidazole,14 giving (S,R,R)-8b.
SCHEME 2a.
a Reaction conditions: (a) t-BuOK, trans-2-butene, n-BuLi, −78 to −57 °C; (−)-B-methoxydiisopinocampheylborane; BF3·Et2O, (R)-6, −78 °C, 75%; (b) benzyl 2,2,2-trichloroacetimidate, TfOH (cat.), cyclohexane–CH2Cl2, 96%; (c) TBSCl, imidazole, CH2Cl2, 98%; (d) OsO4, NaIO4, NMO, dioxane–H2O; NaClO2, H2NSO3H, 95%; (e) NaIO4/RuCl3·H2O (cat.), CCl4–CH3CN–H2O, 98%.
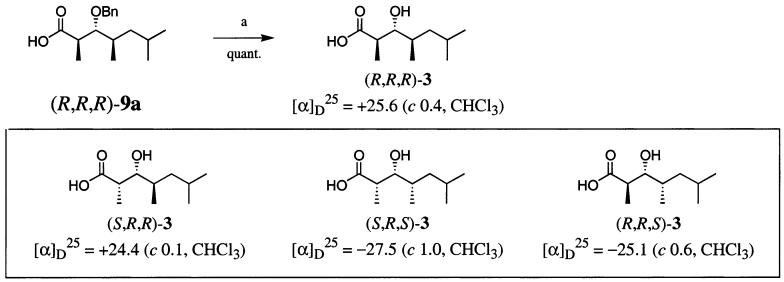
Treatment of (S,R,R)-8b with ruthenium chloride and sodium periodate15 produced carboxylic acid (R,R,R)-9b in nearly quantitative yield, but partial deprotection of the benzyl protecting group of (S,R,R)-8a during this reaction necessitated the use of an alternative method. Instead, treatment of (S,R,R)-8a with catalytic osmium tetraoxide and stoichiometric sodium periodate followed by in situ oxidation of the resultant aldehyde with sodium chlorite produced (R,R,R)-9a in excellent yield (95%). Finally, hydrogenolysis of (R,R,R)-9a proceeded in quantitative yield, giving (R,R,R)-3 in 58% overall yield from (S,S)-4 (Scheme 3).
SCHEME 3a.
a Reaction conditions: (a) H2/Pd(OH)2–C, formic acid, MeOH.
The remaining diastereomers were synthesized with similar stereoselectivities and chemical yields. Accordingly, (S,R,R)-3 was synthesized from (S,S)-4 and B-(Z)-2-butenyl-(−)-diisopinocampheylborane in 56% overall yield. (R,R,S)-3 (56%) and (S,R,S)-3 (62%) were obtained in a similar fashion from (R,R)-4 and the (E)- or (Z)-crotylboranes, respectively, derived from (−)-B-methoxyldiisopinocampheylborane.
Proof of Configurational Assignment
The configuration of TMHEA at C-3 was determined by the modified Mosher method, and unambiguously assigned as R.1,11 Accordingly, (R,R,R)-3,(S,R,R)-3, (S,R,S)-3, and (R,R,S)-3 were synthesized for our study, as they represent the four possible diastereomers of 3-hydroxy-2,4,6-trimethylheptanoic acid (TMHEA) that have the R configuration at C-3.
Comparison of the 1H NMR spectrum of the TMHEA obtained from the acid hydrolysis of callipeltin A with those of the four synthetic analogues unambiguously indicates the configurational assignment of the natural fragment as either R,R,R or S,S,S (Table 1). Aside from differences in the chemical shift values of the methyl signals of (R,R,S)-3 and the natural fragment, the 1H NMR spectrum of (R,R,S)-3 displays two 1H signals (1.16 and 1.24 ppm) where the natural fragment displays a single broad 2H signal (1.12–1.18 ppm). The 1H NMR spectrum of (S,R,R)-3 displays similar deviations in the methylene region, and the presence of two overlapping methyl signals at 0.90 ppm distinguishes this diastereomer as distinct from the natural isomer. (S,R,S)-3 differs from that of the natural fragment by the presence of a 2H signal at 1.66 ppm instead of the two 1H signals (1.64 and 1.70 ppm) observed for the natural fragment. The most compelling evidence for the 1H NMR assignment of the natural fragment is the chemical shift and coupling constant (J) differences observed for the C-2 methine proton. Despite minor differences in the vicinal coupling constants, the spectrum of (R,R,R)-3 matches that of the natural material far better than those of the other isomers.
TABLE 1.
Comparison of the 1H NMR Chemical Shiftsa (δ) of Synthetic 3-Hydroxy-(2,4,6)-trimethylheptanoic Acid Diastereomers with the Natural Fragment
| natural fragmentb δ, multiplicity (J), H | (R,R,R)-3 δ, multiplicity (J), H | (S,R,R)-3 δ, multiplicity (J), H | (S,R,S)-3 δ, multiplicity (J), H | (R,R,S)-3 δ, multiplicity (J), H |
|---|---|---|---|---|
| 0.84, d (6.8), 3H | 0.84, d (6.5), 3H | 0.85, d (6.4), 3H | 0.84, d (6.5), 3H | 0.85, d (6.6), 3H |
| 0.92, d (6.8), 3H | 0.92, d (6.6), 3H | 0.90, d (6.5), 6H | 0.89, d (6.5), 3H | 0.86, d (6.5), 3H |
| 0.96, d (5.8), 3H | 0.95, d (6.8), 3H | 0.95, d (6.5), 3H | 0.89, d (6.6), 3H | |
| 1.12–1.18, m, 2H | 1.12–1.18, m, 2H | 1.04, m, 1H | 1.07, m, 2H | 1.16, m, 1H |
| 1.26, d (7.7), 3H | 1.23, d (7.2), 3H | 1.22, d (7.1), 3H | 1.22, d (7.0), 3H | 1.18, d (6.7), 3H |
| 1.49, m, 1H | 1.24, m, 1H | |||
| 1.64, m, 1H | 1.64, m, 1H | 1.62, m, 1H | 1.66, m, 2H | 1.66, m, 1H |
| 1.70, m, 1H | 1.69, m, 1H | 1.66, m, 1H | 1.70, m, 1H | |
| 2.73, quintet (7.7), 1H | 2.74, dq (7.1,7.1), 1H | 2.74, dq (3.5,3.5), 1H | 2.72, dq (6.0,6.0), 1H | 2.66, dq (7.5,7.5), 1H |
| 3.48, dd (4.1, 7.7), 1H | 3.48, dd (6.4, 6.4), 1H | 3.67, dd (3.4, 7.7), 1H | 3.68, dd (5.5, 5.5), 1H | 3.61, dd (3.3, 8.3), 1H |
All chemical shifts are reported in ppm and were measured in CDCl3. Coupling constants (J) are expressed in Hz.
Spectral data for natural fragment are reproduced from ref 1.
Although 13C NMR spectroscopy should also confirm the configurational assignment, this comparison is not straightforward: the carbon chemical shift values for the natural TMHEA fragment were not available from the acid hydrolysis product, so instead were obtained from the 13C NMR spectrum of intact callipeltin A.1 Because none of the spectra of the four isomers of 3 unambiguously match the chemical shifts of the natural fragment, it is impossible to make any conclusions regarding stereochemical assignment based on the 13C NMR data available.
Comparison of the specific rotation data of the four synthetic isomers of TMHEA (Scheme 3) with that of the natural fragments, [α]25D +14.9 (c 0.4, CHCl3), supports the configurational assignment of R,R,R or S,R,R.16 Combining this fact with the conclusions drawn from the 1H NMR spectra unambiguously defines the assignment of the 3-hydroxy-2,4,6-trimethylheptanoyl residue of callipeltin A as R,R,R.
Conclusion
In summary, we have synthesized four diastereomers of TMHEA (6 steps, 59% average yield), the fatty acid fragment of callipeltin A and D, using an efficient and flexible route. Comparison of the 1H NMR, 13C NMR, and optical rotation data of the natural fragment with all its diastereomers unambiguously verifies the configurational assignment of the natural isomer as R,R,R.
Experimental Section
(R)-2,4-Dimethylpentanal (6)
A 50-mL round-bottomed flask was charged with lithium aluminum hydride (95%, 262 mg, 6.55 mmol) and hexanes (6 mL), and the resulting suspension was cooled to 0 °C. Ethyl acetate (960 μL, 9.83 mmol) was added dropwise over 1.5 h and the reaction mixture was cooled to −78 °C. A solution of (S,S,R)-5 (790 mg, 2.85 mmol) in THF (3 mL) was added over 5 min, and the reaction mixture was stirred at 0 °C for 1 h and transferred to a vigorously stirring solution of 1 M HCl (50 mL) and TFA (2.5 mL). After the mixture was stirred for 10 min at room temperature, 1 M HCl (65 mL) was added, and the solution was extracted with diethyl ether. The combined organic layers were neutralized by the slow addition of saturated aqueous NaHCO3. The aqueous layer was separated and extracted with diethyl ether. The combined organic layers were dried (MgSO4), passed through a short plug of silica, and concentrated to give (R)-6 (280 mg, 86%) as a volatile, colorless liquid that was used in the following step without further purification: 1H NMR (500 MHz, CDCl3) δ 9.60 (d, J = 1.9 Hz, 1H), 2.41 (ddq, J = 4.2, 4.2, 4.2 Hz), 1.63 (m, 1H), 1.59 (m, 1H), 1.20 (m, 1H), 1.08 (d, J = 6.9 Hz, 3H), 0.93 (d, J = 6.5 Hz, 3H), 0.90 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 205.4, 44.4, 39.7, 25.5, 22.9, 22.2, 20.6, 13.7.
(3S,4R,5R)-3,5,7-Trimethylocten-4-ol (7)
A solution of potassium tert-butoxide in THF (1.0 M, 4.89 mL, 4.89 mmol) and THF (5 mL) was cooled to −78 °C, and trans-2-butene (0.60 mL, 6.3 mmol) was added, followed by n-butyllithium (2.50 M, 1.96 mL, 4.73 mmol). The resulting bright yellow solution was stirred at −78 °C for 2 min and −57 °C for 10 min. The reaction was re-cooled to −78 °C, and a solution of (−)-B-methoxydiisopinocampheylborane (1.55 g, 4.89 mmol) in THF (3 mL) was added. The reaction was stirred for 1 h, and BF3·OEt2 (0.614 mL, 4.89 mmol) was added followed by a pre-cooled (−78 °C) solution of (R)-6 (360 mg, 3.15 mmol) in THF (3 mL). The resulting solution was stirred at −78 °C for 4 h, and the reaction was quenched by the slow addition of aqueous sodium hydroxide (3 M, 2 mL) and hydrogen peroxide (30%, 1 mL). The partially frozen mixture was removed from the cold bath and stirred for 18 h. The reaction was diluted with H2O and extracted with diethyl ether. The combined organic extracts were dried and concentrated to give a colorless oil that was purified by flash chromatography to yield (S,R,R)-7 as a colorless oil (400 mg, 75%, 96% de): 1H NMR (500 MHz, CDCl3) δ 5.76 (m, 1H), 5.12 (m, 2H), 3.13 (t, J = 6.0 Hz, 1H), 2.37 (ddq, J = 7.0, 7.0, 7.0 Hz, 1H), 1.65 (m, 2H), 1.26 (m, 1H), 1.13 (m, 1H), 1.00 (d, J = 6.9 Hz, 3H), 0.92 (d, J = 6.7 Hz, 3H), 0.91 (d, J = 6.5 Hz, 3H), 0.86 (d, J = 6.6 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 140.7, 116.2, 79.5, 41.2, 39.5, 33.0, 25.3, 24.4, 21.3, 16.9, 16.8; IR (CHCl3) 3048, 2959, 1705, 1462 cm−1; [α] +21.8 (c 0.7, CHCl3). Anal. Calcd for C11H22O· 0.25H2O: C, 75.58; H, 12.98. Found: C, 75.32; H, 13.35.
(2R,3R,4R)-2,4,6-Trimethyl-3-benzyloxyheptanoic Acid (9a)
To a stirring solution of (S,R,R)-8a (617 mg, 2.37 mmol) in dioxane (3.5 mL) and H2O (3.5 mL) was added 4-methylmorpholine N-oxide (416 mg, 3.55 mmol) and osmium tetraoxide (4% in H2O, 302 μL, 95 μmol). After 1 h, NaIO4 (759 mg, 3.55 mmol) was added, and the suspension was stirred at room temperature for 1.5 h. The reaction mixture was cooled to 0 °C, and sodium chlorite (857 mg, 9.48 mmol) and sulfamic acid (920 mg, 9.48 mmol) were added, and the resulting bright yellow mixture was removed from the cold bath and stirred for 2 h. To the mixture was added 5% aqueous HCl, and the resulting solution was extracted with CH2Cl2. The combined organic extracts were washed with 5% aqueous HCl, dried over MgSO4, concentrated, and purified by flash chromatography to give (R,R,R)-9a as a colorless oil (634 mg, 96%): 1H NMR (500 MHz, CDCl3) δ 7.31–7.26 (m, 5H), 4.58 (d, J = 11.2 Hz, 1H), 4.55 (d, J = 11.5 Hz, 1H), 3.48 (dd, J = 4.1, 6.8 Hz, 1H), 2.77 (dq, J = 7.1, 7.1 Hz, 1H), 1.81 (m, 1H), 1.59 (m, 1H), 1.17 (m, 2H), 1.17 (d, J = 7.1 Hz, 3H), 0.94 (d, J = 6.9 Hz, 3H), 0.87 (d, J = 6.5 Hz, 3H), 0.80 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 179.9, 138.3, 128.4, 128.3, 127.7, 127.7, 127.6, 86.1, 74.5, 42.3, 40.6, 32.9, 25.2, 24.0, 21.4, 16.3, 14.7; IR (CHCl3) 3055, 2960, 1752, 1709, 1457 cm−1; [α]25D +14.9 (c 0.2, CHCl3). Anal. Calcd for C17H26O3·0.5H2O: C, 71.49; H, 9.46. Found: C, 71.27, H, 9.71.
Supplementary Material
Acknowledgment
We thank Prof. Maria D'Auria for helpful discussions and spectra of the natural product. We also gratefully acknowledge the National Institute of Allergy and Infectious Diseases (NIH AI-50888) for financial support.
Footnotes
Supporting Information Available: Complete experimental descriptions of transformations not included in the Experimental Section and characterization for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Zampella A, D'Auria MV, Paloma LG, Casapullo A, Minale L, Debitus C, Henin Y. J. Am. Chem. Soc. 1996;118:6202–6209. [Google Scholar]
- 2.D'Auria MV, Zampella A. Tetrahedron: Asymmetry. 2002;13:1237–1239. [Google Scholar]
- 3.D'Auria MV, Zampella A, Paloma LG, Minale L, Debitus C, Roussakis C, Le Bert V. Tetrahedron. 1996;52:9589–9596. [Google Scholar]
- 4.Trevisi L, Bova S, Cargnelli G, Danieli-Betto D, Floreani M, Germinario E, D'Auria MV, Lucianni S. Biochem. Biophys. Res. Commun. 2000;279:219–222. doi: 10.1006/bbrc.2000.3906. [DOI] [PubMed] [Google Scholar]
- 5.Zampella A, Randazzo A, Borbone N, Luciani S, Trevisi L, Debitus C, D'Auria MV. Tetrahedron Lett. 2002;43:6163–6166. [Google Scholar]
- 6.Joullie MM, Carroll PJ, Guerlavais V. Tetrahedron: Asymmetry. 2002;13:675–680. [Google Scholar]; D'Auria MV, Zampella A, Sorgente M. Tetrahedron: Asymmetry. 2002;13:681–685. [Google Scholar]
- 7.Myers AG, Yang BH, Chen H, McKinstry L, Kopecky DJ, Gleason JL. J. Am. Chem. Soc. 1997;119:6496–6511. [Google Scholar]
- 8.Brown HC, Tsukamoto A. J. Am. Chem. Soc. 1964;86:1089. [Google Scholar]
- 9.Myers AG, Yang BH, Kopecky DJ. Tetrahedron Lett. 1996;37:3623–3626. [Google Scholar]
- 10.Ley SV, Norman J, Griffith WD, Marsden SD. Synthesis. 1994;6:639–666. [Google Scholar]
- 11.Dale JA, Mosher HS. J. Am. Chem. Soc. 1973;95:512–519. [Google Scholar]; Ohtani I, Kusumi T, Kashman Y, Kakisawa H. J. Am. Chem. Soc. 1991;113:4092–4096. [Google Scholar]; Kusumi T, Fukushima T, Ohtani I, Kakisawa H. Tetrahedron Lett. 1991;32:2939–2942. [Google Scholar]
- 12.Brown HC, Bhat KS, Randad RS. J. Org. Chem. 1989;54:1570–1576. [Google Scholar]
- 13.Iversen T, Bundle DR. J. Chem. Soc., Chem. Commun. 1981;23:1240–1241. [Google Scholar]
- 14.Corey EJ, Cho H, Rucker C, Hua DH. Tetrahedron Lett. 1981;22:3455–3458. [Google Scholar]
- 15.Carlsen PHJ, Katsuki T, Martin VS, Sharpless B. J. Org. Chem. 1981;46:3936–3938. [Google Scholar]
- 16. The difference in the optical rotation of the natural fragment, [α]D25 +14.9 (c 0.4, CHCl3), and the synthetic analogue, [α]25D +25.6 (c 0.4, CHCl3), indicated that (R,R,R)-3 was of a higher chemical purity than the crude acid hydrosylate, yet supporting 1H NMR data verifies the assignment as (R,R,R)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.