Abstract
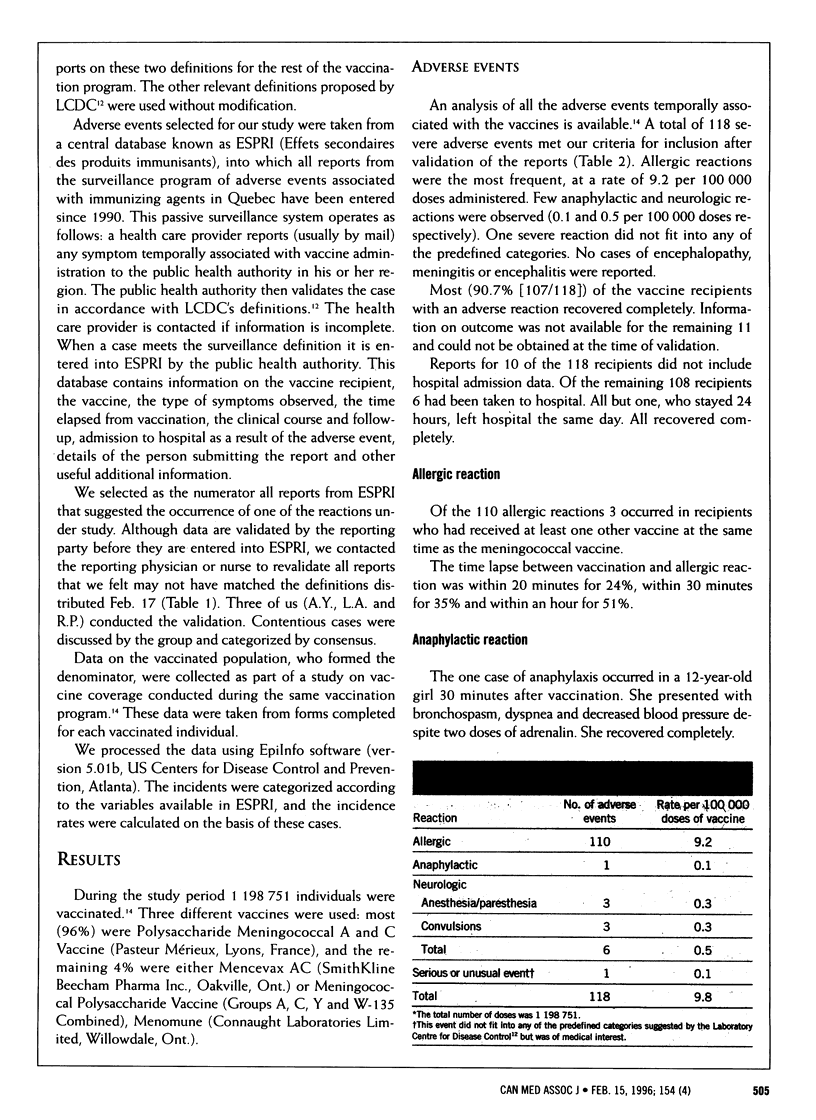
OBJECTIVE: To determine the incidence of severe adverse events temporally associated with meningococcal vaccines administered as part of a mass vaccination program. DESIGN: Retrospective descriptive study of events reported to a passive provincial surveillance system. SETTING: The province of Quebec. PARTICIPANTS: The 1,198,751 individuals aged 6 months to 20 years who were vaccinated against meningococcal disease between Dec. 27, 1992, and Mar. 31, 1993. OUTCOME MEASURES: Total numbers and rates of severe adverse events, including allergic reactions, anaphylactic reactions, neurological events (other than abnormal crying and screaming) and other serious or unusual events. RESULTS: A total of 118 reports of severe adverse events were selected from the surveillance system. The most frequent were allergic reactions (9.2 per 100,000 doses). Few anaphylactic or neurologic reactions were reported (0.1 and 0.5 per 100,000 doses respectively). There were no reports of sequelae or of encephalopathy, meningitis or encephalitis. CONCLUSION: Meningococcal vaccines seem to be associated with fewer adverse events than have previously been reported. Existing surveillance programs are useful for determining the incidence of adverse events temporally associated with vaccines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cadoz M., Armand J., Arminjon F., Gire R., Lafaix C. Tetravalent (A, C, Y, W 135) meningococcal vaccine in children: immunogenicity and safety. Vaccine. 1985 Sep;3(3):340–342. doi: 10.1016/s0264-410x(85)90266-x. [DOI] [PubMed] [Google Scholar]
- Gold R., Lepow M. L., Goldschneider I., Draper T. L., Gotschlich E. C. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J Clin Invest. 1975 Dec;56(6):1536–1547. doi: 10.1172/JCI108235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins W. A., Gwaltney J. M., Jr, Hendley J. O., Farquhar J. D., Samuelson J. S. Clinical and serological evaluation of a meningococcal polysaccharide vaccine groups A, C, Y, and W135. Proc Soc Exp Biol Med. 1982 Jan;169(1):54–57. doi: 10.3181/00379727-169-41306. [DOI] [PubMed] [Google Scholar]
- Hood D. A., Edwards I. R. Meningococcal vaccine--do some children experience side effects? N Z Med J. 1989 Feb 22;102(862):65–67. [PubMed] [Google Scholar]
- Lepow M. L., Beeler J., Randolph M., Samuelson J. S., Hankins W. A. Reactogenicity and immunogenicity of a quadrivalent combined meningococcal polysaccharide vaccine in children. J Infect Dis. 1986 Dec;154(6):1033–1036. doi: 10.1093/infdis/154.6.1033. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Peltola H., Käyhty H., Jousimies H., Pettay O., Ruoslahti E., Sivonen A., Renkonen O. V. Polysaccharide vaccines of group A Neisseria meningtitidis and Haemophilus influenzae type b: a field trial in Finland. J Infect Dis. 1977 Aug;136 (Suppl):S43–S50. doi: 10.1093/infdis/136.supplement.s43. [DOI] [PubMed] [Google Scholar]
- Novelli V. M., Dawod S., Ali M., al-Kuwari A., al-Jaber K. Febrile seizures after immunization with meningococcal A + C vaccine. Pediatr Infect Dis J. 1989 Apr;8(4):250–251. [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Kuronen T., Haque N., Sarna S., Mäkelä P. H. Meningococcus group A vaccine in children three months to five years of age. Adverse reactions and immunogenicity related to endotoxin content and molecular weight of the polysaccharide. J Pediatr. 1978 May;92(5):818–822. doi: 10.1016/s0022-3476(78)80165-6. [DOI] [PubMed] [Google Scholar]
- Peltola H., Mäkelä P. H., ELO O., Pettay O., Renkonen O. V., Sivonen A. Vaccination against meningococcal group A disease in Finland 1974-75. Scand J Infect Dis. 1976;8(3):169–174. doi: 10.3109/inf.1976.8.issue-3.09. [DOI] [PubMed] [Google Scholar]


