Abstract
Hepatocyte growth factor (HGF), the ligand for the Met receptor tyrosine kinase, is a potent modulator of epithelial–mesenchymal transition and dispersal of epithelial cells, processes that play crucial roles in tumor development, invasion, and metastasis. Little is known about the Met-dependent proximal signals that regulate these events. We show that HGF stimulation of epithelial cells leads to activation of the Rho GTPases, Cdc42 and Rac, concomitant with the formation of filopodia and lamellipodia. Notably, HGF-dependent activation of Rac but not Cdc42 is dependent on phosphatidylinositol 3-kinase. Moreover, HGF-induced lamellipodia formation and cell spreading require phosphatidylinositol 3-kinase and are inhibited by dominant negative Cdc42 or Rac. HGF induces activation of the Cdc42/Rac-regulated p21-activated kinase (PAK) and c-Jun N-terminal kinase, and translocation of Rac, PAK, and Rho-dependent Rho-kinase to membrane ruffles. Use of dominant negative and activated mutants reveals an essential role for PAK but not Rho-kinase in HGF-induced epithelial cell spreading, whereas Rho-kinase activity is required for the formation of focal adhesions and stress fibers in response to HGF. We conclude that PAK and Rho-kinase play opposing roles in epithelial–mesenchymal transition induced by HGF, and provide new insight regarding the role of Cdc42 in these events.
INTRODUCTION
Epithelial–mesenchymal transition and cell migration are required during normal embryonic development and during pathological situations such as the dispersal of tumor cells. Hepatocyte growth factor (HGF) is a multifunctional factor that, in addition to promoting epithelial cell growth and survival, has the ability in vitro to stimulate epithelial cell dissociation, motility, invasion, and the endogenous morphogenic program of epithelial cells in three-dimensional matrix or organ culture (Matsumoto and Nakamura, 1996, 1997; Montesano et al., 1997). Genetic studies have shown that HGF/Met signaling is essential for normal murine embryological development (Birchmeier and Gherardi, 1998). In addition, HGF/Met signaling was also shown to be involved in angiogenesis (Bussolino et al., 1992; Grant et al., 1993) and has been implicated in the dissociation and migration of muscle precursor cells of the dermomyotome, in the guidance and survival of motor neurons, and in the development and survival of sensory neurons (Birchmeier and Gherardi, 1998). HGF and Met were also shown to play a role in pathological conditions, including tissue regeneration (Matsumoto and Nakamura, 1997), tumorigenesis, and metastasis (Jeffers et al., 1996; Bardelli et al., 1997).
The conversion from a sessile to a migratory phenotype requires an extensive remodeling of the actin cytoskeleton (Mitchison and Cramer, 1996). Members of the Rho family of small GTP-binding proteins, including Cdc42, Rac, and Rho, are involved in regulating the organization of the actin cytoskeleton and the assembly of associated integrin complexes (Hall, 1998). These proteins cycle between an inactive (GDP-bound) and an active (GTP-bound) conformation in which they interact with specific effector proteins. The conversion from the inactive to the active state is regulated by members of the Dbl family of guanine nucleotide exchange factors (Cerione and Zheng, 1996), whereas inactivation is stimulated by the RhoGAP family of GTPase-activating proteins (Lamarche and Hall, 1994). Initial studies in Swiss 3T3 fibroblasts have demonstrated that activation of Rho promotes the formation of stress fibers and focal adhesion complexes (Ridley and Hall, 1992), that Rac promotes the polymerization of actin at the cell membrane, producing lamellipodia and membrane ruffles (Ridley et al., 1992), and that Cdc42 promotes the formation of filopodia and microspikes at the cell periphery (Kozma et al., 1995; Nobes and Hall, 1995). Rac and Cdc42 also induce the assembly of integrin complexes associated with polymerized actin (Nobes and Hall, 1995). Moreover, in Swiss 3T3 fibroblasts, activation of Cdc42 can lead to activation of Rac, which in turn can lead to activation of Rho, implicating signal cross-talk between these proteins (Ridley and Hall, 1992; Ridley et al., 1992; Nobes and Hall, 1995).
Several extracellular factors have been shown to activate Rho family members and induce the corresponding changes on the actin cytoskeleton. In fibroblasts, lysophosphatidic acid or bombesin stimulate Rho activation (Ridley and Hall, 1992); PDGF, EGF, bombesin, and insulin promote Rac activation (Ridley et al., 1992; Nobes et al., 1995); and bradykinin leads to activation of Cdc42 (Kozma et al., 1995). Thus, the Rho family proteins represent key signal transducers that link membrane receptors to the organization of the actin cytoskeleton. Several downstream target molecules of the Rho family proteins have now been identified (Aspenström, 1999), and among them, the p21-activated kinase (PAK) family and Rho-kinase isoforms have been implicated as mediators of actin and focal adhesion reorganization (Leung et al., 1996; Amano et al., 1997; Ishizaki et al., 1997; Manser et al., 1997; Sells et al., 1997; Frost et al., 1998). PAK kinases bind to and are strongly activated by both GTP-bound Cdc42 and Rac (Manser et al., 1994; Bagrodia et al., 1995b; Martin et al., 1995), whereas Rho-kinase/Rokα and p160ROCK isoforms bind to activated Rho (Leung et al., 1995; Ishizaki et al., 1996; Matsui et al., 1996), although they have also been reported to interact with GTP-bound Rac (Joneson et al., 1996; Lamarche et al., 1996).
To understand the HGF-dependent signaling pathways involved in actin reorganization required for epithelial–mesenchymal transition and cell dispersal, we have used Madin-Darby canine kidney (MDCK) epithelial cells that grow as colonies of tightly associated cells. However, unlike serum-starved quiescent Swiss 3T3 cells that do not contain highly polymerized actin, MDCK cells grown as colonies contain abundant circumferential or peripheral bundles of polymerized actin in lateral membranes in association with junctional complexes involved in cell–cell adhesion. Therefore, the dispersal of epithelial sheets requires the dissolution of these peripheral actin bundles in conjunction with the dissociation of the junctional complexes. We have shown previously that HGF-induced dissociation of MDCK epithelial cells is preceded by the centrifugal spreading of cells within colonies. This is dependent on phosphatidylinositol 3-kinase (PI3K) activity and is concomitant with the loss of insoluble E-cadherin and desmoplakins from sites of cell–cell adhesion (Royal and Park, 1995; Potempa and Ridley, 1998). Moreover, studies by others have revealed that Ras and Rac were also required for HGF-induced MDCK cell spreading and dissociation (Hartmann et al., 1994; Ridley et al., 1995). Epithelial cell spreading is completed 3–5 h after stimulation with HGF; however, nothing was known about the HGF-induced proximal cellular and biochemical events required for these later morphological changes. In this paper, we have characterized the signaling pathways required for the immediate effects of HGF on the actin cytoskeleton and the subsequent consequences of these signals on the initial steps of epithelial–mesenchymal transition, cell spreading, and dissociation.
MATERIALS AND METHODS
Cell Culture and Microinjection
MDCK epithelial cells were cultured in DMEM supplemented with 10% FBS and 50 μg/ml gentamicin (Life Technologies, Burlington, Ontario, Canada). For microinjection, MDCK cells (5 × 103) were seeded on glass coverslips (Bellco Glass, Vineland, NJ) in 24-well dishes (Nunc) and grown for 2–3 d. Cells were injected in serum because prolonged serum starvation promotes a decrease in E-cadherin at cell–cell junctions. Small colonies of 10–50 cells were partially microinjected (Eppendorf Scientific, Westbury, NY). For all experiments, 100–200 cells were microinjected during a 15-min period, and data shown are representative of the results obtained in a minimum of four experiments. When assaying for HGF-induced lamellipodia formation, MDCK cells (5 × 104) were seeded 1 d before injection. In each experiment, ∼100 cells microinjected with the corresponding constructs were evaluated for biological response. Expression vectors (50, 100, and 200 μg/ml) were diluted in PBS and occasionally coinjected with rabbit immunoglobulin G (2 mg/ml; Pierce, Rockford, IL) to mark injected cells. The cells were incubated for 2–5 h to allow for protein expression and were further incubated, as indicated, in the presence of HGF (5 U/ml) before fixation. Human HGF was purified from conditioned medium of COS cells after their transient transfection with a human HGF expression construct (Zhu et al., 1994) or was obtained from Dr. George Vande Woude (National Cancer Institute–Frederick Cancer Research and Development Center, Frederick, MD). One unit is defined as the minimal amount of HGF per milliliter of medium required to induce scatter of MDCK cells and is generally equivalent to 1–2 ng/ml.
Immunofluorescence Microscopy
Injected cells were fixed in 3.7% formaldehyde for 10 min and permeabilized in 0.2% Triton X-100 in PBS for 5 min. Cells were incubated for 30 min with a FITC-labeled rabbit antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) to detect injected cells. Actin filaments were visualized by incubating cells for 45 min with 0.05 μg/ml TRITC-labeled phalloidin (Sigma, Oakville, Ontario, Canada) diluted in 0.2% Triton X-100 (in PBS). Where indicated, cells were incubated for 30 min with the 9E10 Myc antibody to detect the expressed Myc-tagged proteins, followed by a 30-min incubation with a FITC-labeled mouse antibody (Sigma). For vinculin staining, cells were incubated for 10 min with CSK lysis buffer (10 mM piperazine-N,N′-bis[2-ethanesulfonic acid], pH 7.0, 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, 0.5% Triton X-100), fixed in 3.7% formaldehyde for 5 min, and then incubated with a mouse vinculin antibody (Sigma) for 30 min, followed by a 30-min incubation with the Cy3-conjugated mouse antibody (Jackson ImmunoResearch Laboratories). The coverslips were mounted on slides in Immuno-Fluore medium (ICN, Costa Mesa, CA), and cells were viewed on a Nikon (Garden City, NY) Labophot-2 epifluorescence microscope. Images were photographed with the use of Kodak (Rochester, NY) TMZ-3200 film and digitalized with an Agfa Canada (Montreal, Quebec, Canada) T-1200 color scanner.
For immunolocalization of endogenous Rac, MDCK cells were fixed and permeabilized as described above and then incubated for 30 min with a Rac mAb (Transduction Laboratories, San Diego, CA), followed by a 30-min incubation with an anti-mouse mAb coupled to Alexa 488 (Molecular Probes, Eugene, OR) and TRITC-phalloidin to stain for actin. In this case, however, cell washes were performed with PBS containing 5% FBS and 0.1% Triton X-100, which was also used to dilute the antibodies. Immunolocalization of Myc-PAK and Myc-Rho-kinase was performed with the 9E10 antibody as described above. Immunostained cells were examined with a Zeiss (Montreal, Quebec, Canada) laser confocal microscope, and sectioning along the z-axis was performed at intervals of 0.35 μm with a pinhole number of 20 and scanning times of 16 s.
Cell Transfection
Cells were transfected by the standard calcium phosphate technique. MDCK cells (6 × 105/100 mm) were transfected with 5 μg of pEF-BOSMyc-Rho-kinase or pRK5Myc-PAK1 in the presence of 25 μg of carrier DNA (pBluescript, Stratagene, La Jolla, CA) and 250 ng of the neomycin-encoding vector pcDNA3.1 (Invitrogen, Carlsbad, CA). The precipitate was removed the next day, and the selection drug G-418 (400 μg/ml) was added to the medium 24 h later. Clones were obtained after 10–14 d, and the levels of expression were determined by Western blotting. Clones were used for Rho-kinase and PAK localization and for PAK kinase assays in response to HGF. For Cdc42 and Rac activation assays, MDCK cells (1 × 106/100 mm) were transiently transfected with 10 μg of pRK5Myc-Cdc42 or pRK5Myc-Rac cDNAs and 50 μl of Geneporter transfection reagent (Gene Therapy System, San Diego, CA) in 5 ml of DMEM without serum. The next day, cells were stimulated with HGF.
Purification of GST-WASP and GST-PAK
GST-WASP (aa 201–321) (Aspenström et al., 1996) and GST-PAK (aa 56–272 of PAK1; Sander et al., 1998) (a kind gift of Dr. John Collard, The Netherlands Cancer Institute, Division of Cell Biology, Amsterdam) were used to isolate activated Cdc42 and Rac, respectively, from stimulated MDCK cell lysates. Escherichia coli transformed with the GST-WASP and GST-PAK constructs were grown at 37°C to an absorbance of 0.5. Expression of the fusion proteins was induced with isopropyl-β-d-thiogalactopyranoside (0.1 mM) for 3 h at 37°C. Cells were washed once in STE buffer (100 mM Tris-Cl, pH 8.0, 100 mM NaCl, 1 mM EDTA) before sonication in 20 mM HEPES, pH 7.5, 120 mM NaCl, 2 mM EDTA, 10% glycerol, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF. The lysates were cleared by centrifugation, and NP-40 was added to a final concentration of 0.5%. The proteins were stored at −80°C until use. Per sample, 10–15 μg of GST-WASP or GST-PAK (as estimated by Coomassie blue staining) was purified on glutathione–Sepharose beads (Amersham Pharmacia Biotech, Baie d'Urfe, Quebec, Canada) for 30 min at room temperature with gentle rocking. The Sepharose beads were washed twice with lysis buffer, and the protein lysates were added as described below.
Cdc42/Rac Activation Assays
MDCK cells transiently expressing Myc-tagged G12Cdc42 or G12Rac were stimulated 16–20 h after transfection with 100 or 200 U/ml HGF for different times. Cells were lysed in 25 mM HEPES, pH 7.5, 1% NP-40, 10 mM MgCl2, 100 mM NaCl, 5% glycerol, 5 mM sodium fluoride, 1 mM sodium vanadate, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin. Equal protein levels (700 μg) were incubated for 1 h at 4°C with purified GST-PAK or GST-WASP (10–15 μg) purified in a twofold volume of binding buffer (25 mM HEPES, pH 7.5, 30 mM MgCl2, 40 mM NaCl, 0.5% NP-40, 1 mM DTT) as described (Benard et al., 1999). Beads were washed four times in binding buffer containing 1% NP-40 and boiled in Laemmli sample buffer, and the proteins were separated on a 15% SDS-polyacrylamide gel. The levels of Myc-tagged Cdc42 and Myc-tagged Rac bound to the fusion proteins, as well as the levels present in whole cell lysates (10 μg), were evaluated by Western blotting with the 9E10 Myc mAb followed by the ECL detection according to the manufacturer's protocol (Amersham Pharmacia Biotech).
PAK Kinase Assay
MDCK cells expressing Myc-tagged PAK1 (8 × 105) were seeded in 100-mm dishes and serum starved the next day for 24 h in 0.1% FBS. Cells were stimulated with HGF (100 U/ml) at 37°C for the indicated times. Cells were lysed in 10 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.6% Triton X-100, 20 mM β-glycerophosphate, 10% glycerol, 5 mM sodium fluoride, 1 mM sodium vanadate, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin. Proteins (200 μg of cell lysates) were incubated at 4°C with the monoclonal 9E10 Myc antibody for 1 h followed by incubation with 30 μl of protein G–Sepharose (40% suspension; Amersham Pharmacia Biotech) for an additional 1 h. The immune complexes were then washed three times with the lysis buffer and once with the kinase buffer (20 mM HEPES, pH 7.5, 20 mM MgCl2, 10 mM MnCl2, 20 mM β-glycerophosphate, 1 mM DTT, 0.1 mM sodium vanadate, 5 mM sodium fluoride, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin). The kinase reaction was performed for 10 min at 37°C in 25 μl of kinase buffer containing 10 μM ATP, 5 μg of myelin basic protein (MBP), and 20 μCi of [γ-32P]ATP. The reaction was stopped by adding 4× Laemmli sample buffer, and proteins were separated by electrophoresis on a 15% SDS-polyacrylamide gel. Myc-tagged PAK1 expression levels were evaluated by Western blotting with the 9E10 Myc antibody.
c-Jun N-terminal Kinase Assay
MDCK cells (8 × 105/100 mm) were prepared and stimulated as described above for the PAK kinase assay. After stimulation, cells were lysed as described (Adler et al., 1992). Bacteria expressing GST–c-Jun (aa 5–89) were kindly provided by Dr. James Woodgett (Ontario Cancer Institute, Toronto, Canada). Fusion proteins were produced by isopropyl-β-d-thiogalactopyranoside induction, lysed, and purified on glutathione–Sepharose beads. Kinase reactions were performed in the presence of 10 μg of cell lysate from each time point, 3 μg of GST–c-Jun, 10 μM ATP, and 20 μCi of [γ-32P]ATP, as described (Adler et al., 1992).
RESULTS
Microspikes and Lamellipodia Are the Earliest Morphological Changes Induced by HGF in MDCK Cells
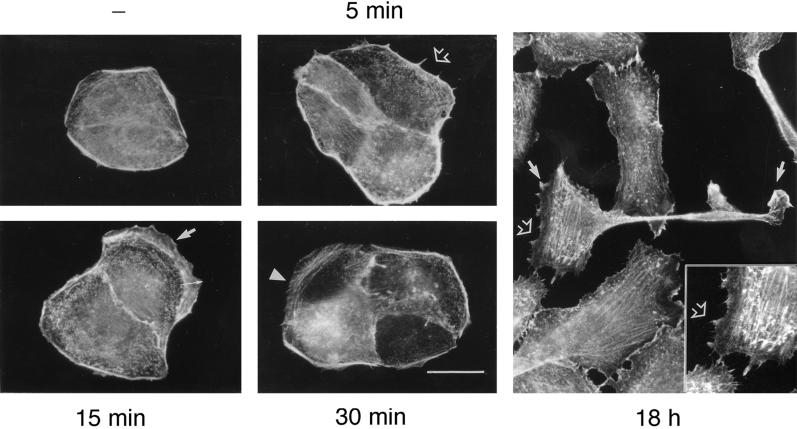
In response to HGF, colonies of MDCK epithelial cells undergo multiple morphological changes leading to epithelial–mesenchymal transition and cell dispersal. This occurs as a series of sequential events in which the centrifugal spreading of all cells within the colony is concomitant with a decrease in cell–cell adhesion molecules by 3–4 h of HGF stimulation and the subsequent loss of cell–cell contacts and the acquisition of a motile mesenchymal cell phenotype by 8–16 h (Royal and Park, 1995). To characterize the earliest morphological and biochemical changes induced by HGF and to evaluate their contribution to the spreading and dissociation of colonies of MDCK cells, the reorganization of the actin cytoskeleton in response to HGF was first investigated. Visualization of F-actin with TRITC-phalloidin revealed that few actin stress fibers were present in small colonies of unstimulated MDCK cells and that actin was mostly concentrated at the edge of the colonies (Figure 1, −) and at cell–cell borders in peripheral bundles associated with the lateral membranes of the cells (Figure 1). The earliest change observed after stimulation was the appearance of actin-containing hair-like structures resembling filopodia or microspikes 5 min after HGF stimulation (Figure 1, 5 min). By 15 min, lamellipodia harboring small membrane ruffles developed on the outer membranes of cells at the edge of the colony (Figure 1, 15 min), and by 30 min, actin stress fibers appeared within these lamellipodia (Figure 1, 30 min). Moreover, by 18 h after HGF stimulation, MDCK cells had adopted a fibroblastic phenotype and exhibited lamellipodia and membrane ruffles typical of motile cells (Figure 1, 18 h, inset). These observations indicate that in the course of events ultimately leading to epithelial–mesenchymal transition and cell dispersal, the formation of filopodia and lamellipodia are the earliest morphological changes observed in response to HGF.
Figure 1.
HGF-induced actin reorganization in MDCK cells. MDCK cells were stimulated with HGF (5 U/ml) for the indicated times and fixed in 3.7% formaldehyde in PBS. After fixation, cells were permeabilized with Triton X-100 (0.2%), and filamentous actin was stained with TRITC-phalloidin. Note the appearance of microspikes at 5 min (open arrow) and lamellipodia at 15 min (thin arrow) after HGF stimulation. Membrane ruffles are found at the edge of lamellipodia (thick arrow), whereas by 30 min, actin fibers are present in the lamellipodia (arrowhead). After overnight stimulation (18 h), cells harbor filopodia-like structures (inset), lamellipodia, and membrane ruffles typical of motile cells. Bar, 25 μm.
Cdc42, Rac, PAK and c-Jun N-terminal Kinase Are Activated by HGF in MDCK Cells
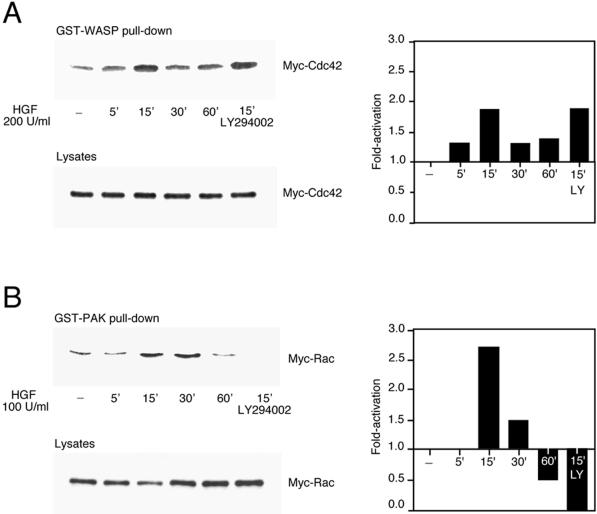
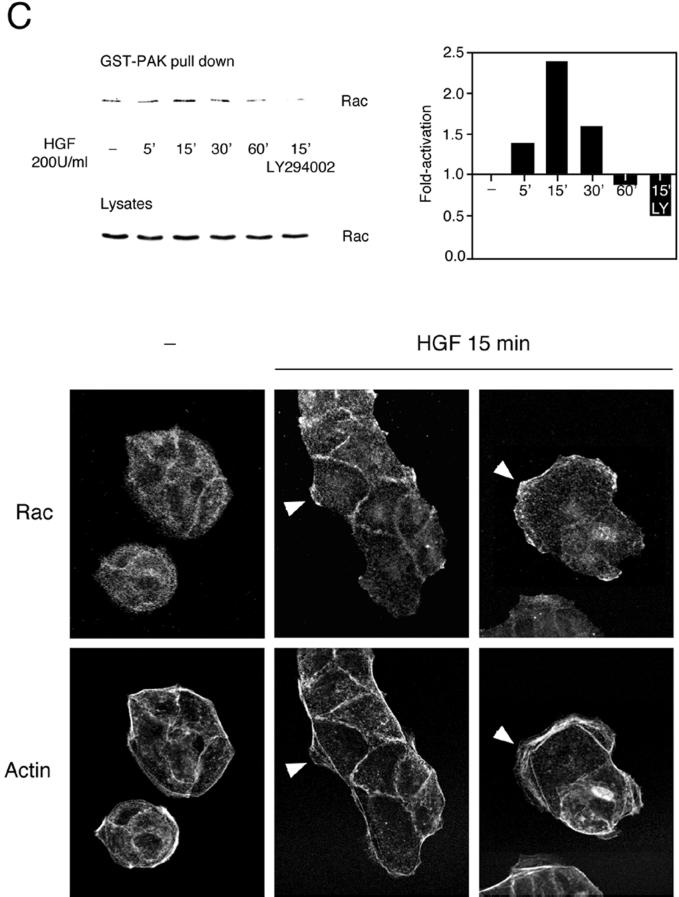
Two members of the Rho family of small GTP-binding proteins, Cdc42 and Rac, control actin polymerization into filopodial and lamellipodial membrane protrusions in Swiss 3T3 cells (Hall, 1998). To determine if Cdc42 and Rac were activated by HGF, an assay based on the ability of activated Cdc42 and activated Rac to bind effector proteins was used (Sander et al., 1998; Benard et al., 1999). Lysates of HGF-stimulated MDCK cells, transiently expressing Myc-tagged Cdc42 or Myc-tagged Rac, were incubated with GST-WASP or GST-PAK fusion proteins coupled to glutathione–Sepharose beads. GST-WASP (aa 201–321) encodes the Cdc42-binding site of the WASP effector protein and was used to isolate activated Cdc42, whereas GST-PAK (aa 56–272) encodes the Cdc42- and Rac-binding domain of the PAK1 effector protein and was used to isolate activated Rac (Figure 2). Upon stimulation of MDCK cells with HGF, activated Cdc42 was detected 5 min after stimulation, reached a maximum at 15 min, and decreased thereafter (Figure 2A). In contrast, after correcting for protein levels, Rac activation was highest at 15 min, decreased at 30 min, and decreased below the basal level at 60 min (Figure 2B). Significantly, the pretreatment of MDCK cells with the PI3K inhibitor LY294002 led to the inhibition of Rac but not Cdc42 activity (Figure 2, A and B). Activation of endogenous Rac occurred with similar kinetics in response to HGF (Figure 2C). Moreover, Rac was relocalized to membrane ruffles after stimulation with HGF for 15 min (Figure 2C). Together, these results confirm the data derived from the actin staining and show for the first time that Cdc42 is activated by HGF in MDCK cells, with distinct kinetics from Rac activation.
Figure 2.
HGF-induced Cdc42 and Rac activation in MDCK cells. MDCK cells transiently expressing Myc-tagged G12Cdc42 (A) or G12Rac (B), or MDCK cells expressing endogenous Rac (C), were stimulated with 100 or 200 U/ml HGF for the indicated times. Cells were lysed, and proteins in equal amounts were incubated with GST-WASP (aa 201–321) (A) or GST-PAK (aa 56–272) (B and C) proteins and purified on glutathione–Sepharose beads. Beads were washed, and bound proteins were separated on a 15% SDS-polyacrylamide gel and transferred to nitrocellulose membrane. The levels of Myc-tagged Cdc42 (A), Myc-tagged Rac (B), and endogenous Rac (C) pulled down by the fusion proteins, as well as the levels present in whole cell lysates, were evaluated by Western blotting with the 9E10 Myc (A and B) or Rac (C) mAbs. The corresponding graphs indicate the activation detected over basal levels present in unstimulated cells. (C, bottom) HGF-induced translocation of Rac in MDCK cells. MDCK cells stimulated with HGF (5 U/ml) for 15 min were fixed in 3.7% formaldehyde in PBS, permeabilized in Triton X-100 (0.2%), and stained with anti-Rac, followed by incubation with anti-mouse Alexa 488 together with TRITC-phalloidin. Fluorescent images were obtained by laser confocal microscopy. Note the presence of Rac in the membrane ruffles found at the edges of lamellipodia (arrowheads).
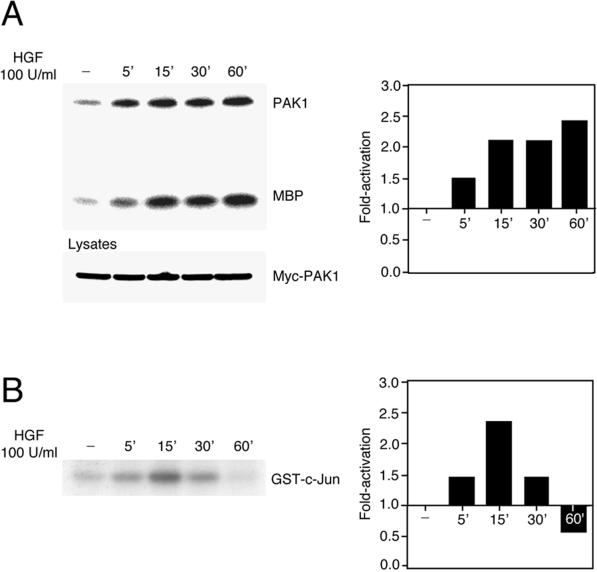
Constitutively activated Cdc42 or Rac mutants lead to stimulation of PAK and c-Jun N-terminal kinase (JNK) activity in transiently transfected COS-1, COS-7, and HeLa cells (Bagrodia et al., 1995a; Coso et al., 1995; Minden et al., 1995; Olson et al., 1995; Brown et al., 1996). As further support for Cdc42 and Rac activation by HGF, we investigated if PAK and JNK were activated after stimulation of MDCK cells (Figure 3). Serum-starved MDCK cells stably expressing Myc-tagged PAK1 were treated with 100 U/ml HGF for various times. Myc-PAK1 was immunoprecipitated with the 9E10 Myc mAb, and its kinase activity was assessed using MBP as a substrate. PAK1 autophosphorylation and MBP phosphorylation were increased at 5 min after HGF stimulation (1.5-fold), were maximal at 15 min (2.1-fold), and remained high up to 1 h after stimulation (Figure 3A). An increase in the phosphorylation of GST-c-Jun, indicative of endogenous JNK activation, was detected 5 min after HGF stimulation (1.4-fold), was maximal at 15 min (2.2-fold), and decreased below basal levels by 1 h (Figure 3B). Thus, HGF induces activation of PAK and JNK in MDCK cells, consistent with the observed Cdc42 and Rac activation (Figure 2).
Figure 3.
HGF-induced PAK1 and JNK activation in MDCK cells. (A) MDCK cells stably expressing Myc-tagged PAK1 were serum starved for 24 h and then stimulated with HGF (100 U/ml) for the indicated times. PAK1 was immunoprecipitated from cell lysates with the Myc 9E10 mAb, and its kinase activity was assayed in the presence of the exogenous substrate MBP and [γ-32P]ATP. The reaction was stopped by adding Laemmli sample buffer, and proteins were separated by electrophoresis on a 15% SDS-polyacrylamide gel. Cell lysates were subjected to Western blotting with the 9E10 Myc mAb to show amounts of PAK1 present. (B) JNK activation was assayed in the presence of 10 μg of cell lysates, GST-c-Jun (aa 5–89), and [γ-32P]ATP, and proteins were resolved by SDS-PAGE (10%). The bar graphs indicate the activation of PAK1 and JNK detected over basal levels present in unstimulated cells.
Cdc42, Rac, and PI3K Are Required for HGF-induced Lamellipodia Formation, Cell Spreading, and Dissociation
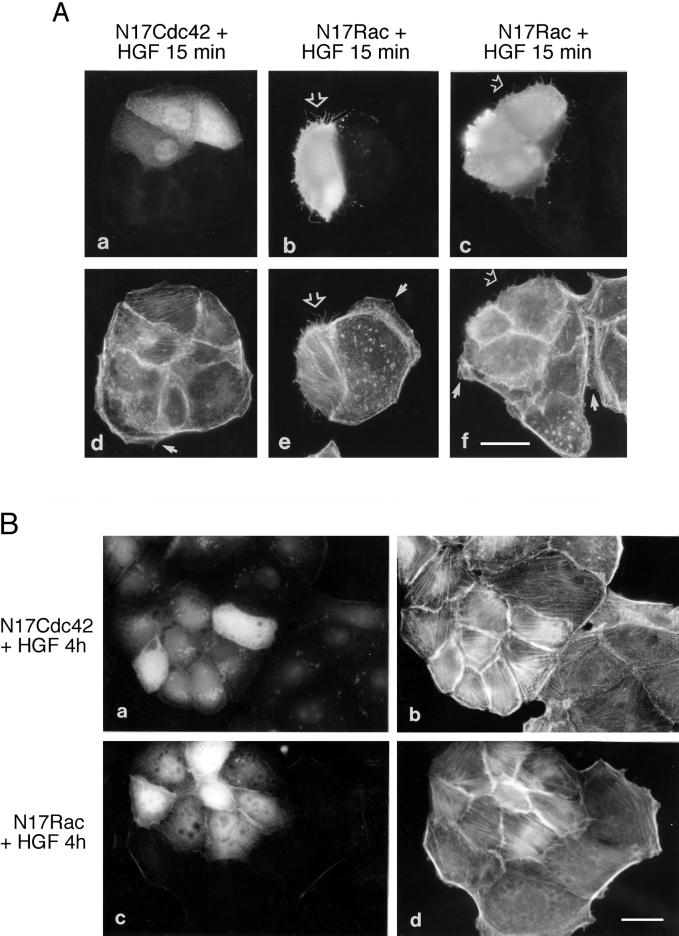
To assess the requirement for Cdc42 and Rac in the actin reorganization observed in MDCK cells at early times after HGF stimulation, expression vectors encoding dominant negative mutants of Cdc42 (N17Cdc42) and Rac (N17Rac) were injected into the nuclei of MDCK cells. The expression of N17Cdc42 (Figure 4A, a and d) or N17Rac (Figure 4A, b, c, e, and f) reduced the number of cells that formed HGF-induced lamellipodia to 55 ± 6% and 34 ± 15%, respectively, that of control cells injected with vector alone (normalized to 100%; Table 1), and instead, an increase in the amount of actin stress fibers was observed. In addition, expression of N17Rac enhanced the presence of microspikes at 15 min after HGF stimulation in 77 ± 8% of the injected cells (Figure 4A, e and f). Microinjection of wild-type Cdc42 into MDCK cells promotes the formation of filopodia and subsequent lamellipodia (I. Royal and M. Park, unpublished observations), whereas microinjection or transfection of activated Rac promotes lamellipodia formation (Jou and Nelson, 1998; I. Royal and M. Park, unpublished observation). Hence, the data showing that dominant negative N17Rac expression increases HGF-induced filopodia formation suggest that in colonies of MDCK epithelial cells, as observed in serum-starved quiescent Swiss 3T3 fibroblasts, Rac acts downstream from Cdc42 in a signaling cascade required for the formation of lamellipodia. Moreover, consistent with a requirement for PI3K activity in lamellipodia formation downstream of PDGF and insulin (Wennstrom et al., 1994; Nobes et al., 1995) and in HGF-dependent Rac activation (Figure 2, B and C), MDCK cells pretreated with the PI3K inhibitor LY294002 were unable to form lamellipodia in response to HGF for 15 min (our unpublished result). However, in agreement with the observation that HGF-induced Cdc42 activation is independent of PI3K activation (Figure 2A), pretreatment of MDCK cells with LY294002 did not inhibit the formation of microspikes in response to HGF (our unpublished result).
Figure 4.
Cdc42 and Rac are required for HGF-induced lamellipodia formation and cell spreading/dissociation. (A) cDNAs of N17Cdc42 and N17Rac (in pRK5Myc vector; 0.1 mg/ml) were injected into the nuclei of MDCK cells. After a 2-h incubation period, cells were stimulated with HGF (5 U/ml) for 15 min. Cells were fixed as described in Figure 1 and double stained with anti-rabbit FITC to identify the cells coinjected with rabbit immunoglobulin G (a) or with anti-Myc and anti-mouse FITC to detect protein expression (b and c), followed by TRITC-phalloidin (d–f) to stain actin. Note the presence of lamellipodia (arrows) and microspikes (open arrows). Bar, 25 μm. (B) MDCK cells were coinjected with N17Cdc42 (a and b) or N17Rac (c and d) together with rabbit immunoglobulin G. After a 2-h incubation period, cells were stimulated with HGF for 4 h, fixed as described, and double stained with anti-rabbit FITC (a and c) and TRITC-phalloidin (b and d). Bar, 25 μm.
Table 1.
Cdc42, Rac, PAK, and Rho-kinase are involved in HGF-induced lamellipodia formation in MDCK cells
| cDNA | Percent of cells with lamellipodiaa | SD (%) | pb |
|---|---|---|---|
| PRK5 | 100 | – | – |
| N17Cdc42 | 55 | ±6 | <0.05 |
| N17Rac | 34 | ±15 | <0.05 |
| RB/PH(TT) | 36 | ±6 | <0.05 |
| PAKR | 19 | ±1 | <0.05 |
Not all injected cells will form lamellipodia. The percentage of cells injected with the control vector pRK5 that formed lamellipodia was normalized to 100%, and the values obtained in cells expressing the dominant negative mutants were modified accordingly (see MATERIALS AND METHODS).
A statistical analysis (analysis of variance) was performed on the results obtained in these experiments and gave p < 0.05, which confirms that they are highly significant.
In addition to inhibiting the formation of lamellipodia in response to HGF, the expression of N17Cdc42 also blocked HGF-induced MDCK cell spreading and dissociation that occur as late events at 3–4 h after stimulation (Figure 4B, a and b). In a similar manner, MDCK cells injected with N17Rac cDNA (Figure 4B, c and d) (Ridley et al., 1995) or treated with LY294002 (our unpublished result) or wortmannin (Royal and Park, 1995) also failed to spread. Together, these data demonstrate that PI3K, Cdc42, and Rac are essential for the initial formation of lamellipodia and the subsequent spreading and dissociation of MDCK colonies in response to HGF.
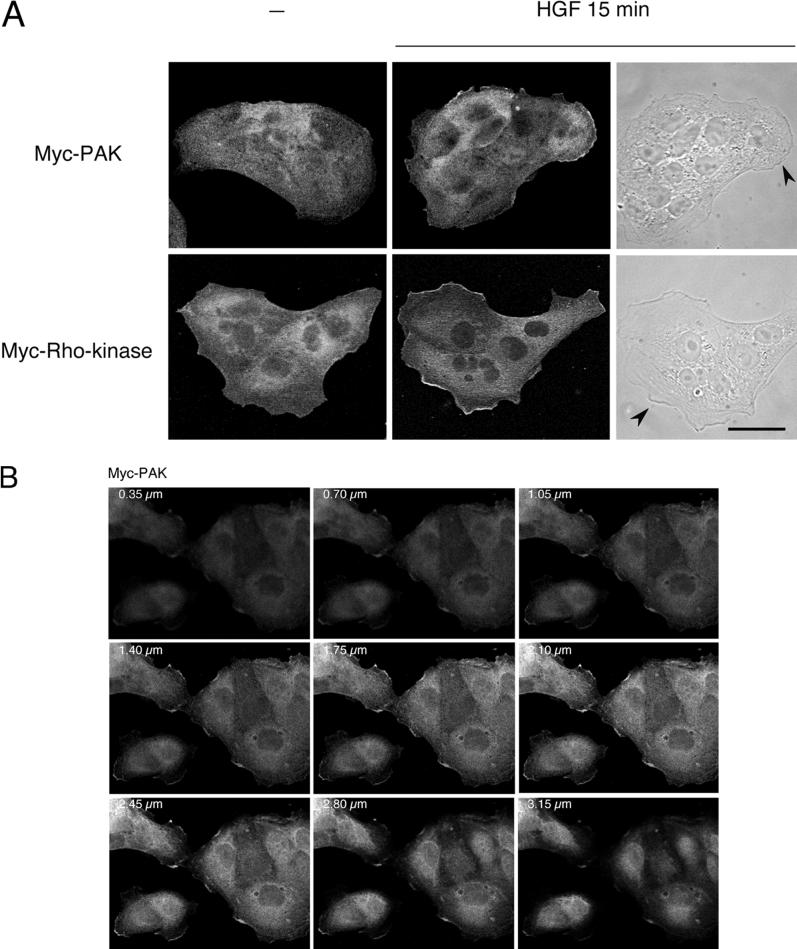
Rho Family Effector Proteins PAK and Rho-Kinase Translocate to Membrane Ruffles in Response to HGF
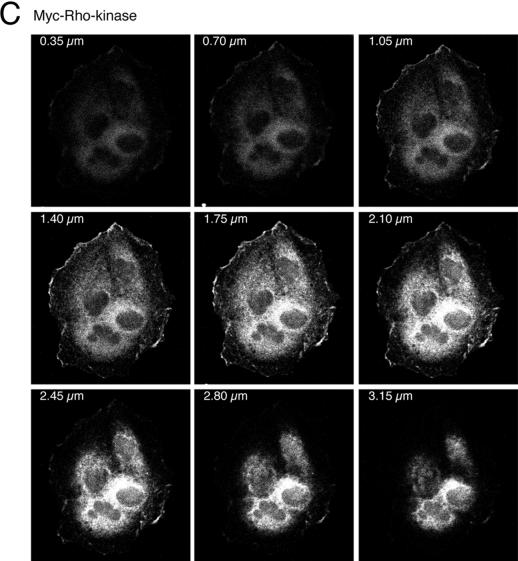
Several proteins binding to Rho family GTPases are involved in actin and focal adhesion reorganization (Aspenström, 1999). Among these, PAK family members and Rho-kinase isoforms exert opposite effects, where PAK leads to the disruption and Rho-kinase leads to the formation of stress fibers and focal adhesions via regulation of the myosin light chain kinase (Amano et al., 1996, 1997; Kimura et al., 1996; Manser et al., 1997; Frost et al., 1998; Sanders et al., 1999). PAK family members, as mentioned above, interact with and are activated by Cdc42 and Rac, whereas Rho-kinase isoforms (Rho-kinase/ROKα, p160ROCK) bind to activated Rho, although they have also been reported to bind to activated Rac (Leung et al., 1995; Ishizaki et al., 1996; Joneson et al., 1996; Lamarche et al., 1996; Matsui et al., 1996). Activation of PAK and Rho-kinase by PDGF and V14Rho, respectively, leads to their translocation from the cytosol to the cell membrane (Leung et al., 1995; Matsui et al., 1996; Dharmawardhane et al., 1997). Consistent with activation of PAK by HGF (Figure 3), translocation of PAK to membrane ruffles was observed 15 min after HGF stimulation of MDCK cells stably expressing myc-tagged PAK1 (Figure 5A). Similarly, Rho-kinase translocated from the cytosol to membrane ruffles 15 min after stimulation of MDCK cells stably expressing myc-tagged Rho-kinase (Figure 5A). Confocal sectioning of the cells in the z-axis in successive sections through the lamellipodia and membrane ruffles demonstrated enrichment of PAK and Rho-kinase at those sites (Figure 5, B and C). Therefore, based on the reported activities of these two proteins on the actin cytoskeleton and on their localization in membrane ruffles at the edge of lamellipodia in response to HGF, PAK and Rho-kinase represent likely modulators of MDCK cell morphology.
Figure 5.
HGF stimulation induces membrane translocation of PAK1 and Rho-kinase. (A) MDCK cells stably expressing Myc-tagged PAK1 or Myc-tagged Rho-kinase were stimulated with HGF (5 U/ml) for 15 min. Cells were fixed in 3.7% formaldehyde in PBS, permeabilized in Triton X-100 (0.2%), and stained with anti-Myc and anti-mouse FITC. Fluorescent images were obtained by laser confocal microscopy. Note the presence of PAK1 and Rho-kinase in the membrane ruffles found at the edges of lamellipodia (arrowheads). Bar, 25 μm. (B and C) Confocal sectioning in the z-axis of cells depicting successive sections through the lamellipodia and membrane ruffles of HGF-stimulated cells expressing Myc-PAK and Myc-Rho-kinase.
HGF-induced Lamellipodia in MDCK Cell Colonies Involves Rho-Kinase and PAK
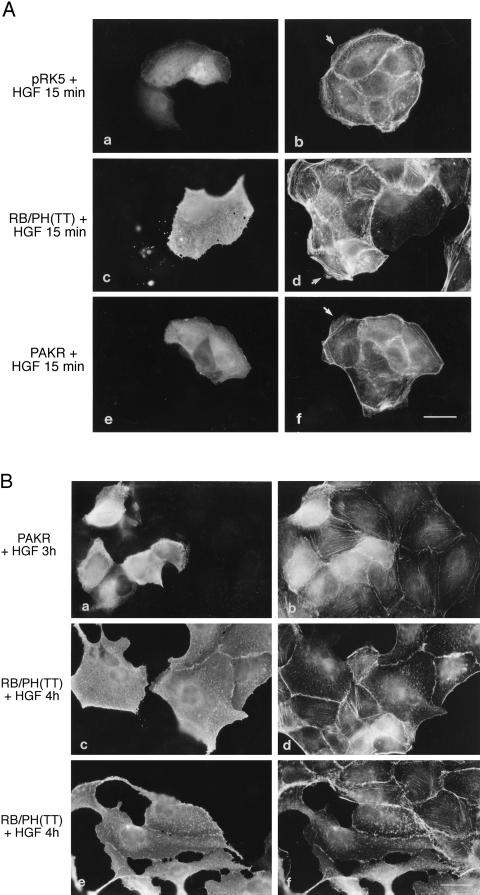
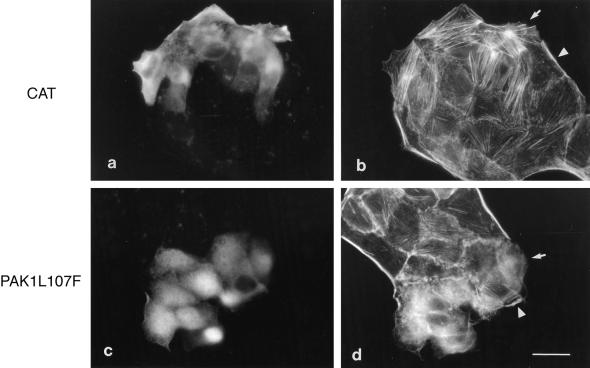
To examine the role of Rho-kinase in the early HGF-induced morphological changes, MDCK cells were injected with a dominant interfering mutant of Rho-kinase, RB/PH(TT) (aa 941-1388). This mutant encompasses the C-terminal pleckstrin homology (PH) domain of Rho-kinase in addition to the Rho-binding domain (RB) that is rendered nonfunctional by the substitution of Asn-1027 and Lys-1028 with threonine (Amano et al., 1998). Consistent with a role for Rho-kinase in stress fiber formation (Leung et al., 1996; Amano et al., 1997), the expression of RB/PH(TT) in unstimulated MDCK cells resulted in the loss of endogenous stress fibers and, in addition, of peripheral actin (our unpublished results). After HGF stimulation for 15 min, RB/PH(TT)-expressing cells failed to develop lamellipodia harboring an organized network of actin, and lamellipodia were present in only 36 ± 6% of the injected cells compared with MDCK cells injected with a control vector (normalized to 100%) (Table 1; Figure 6A, a–d). Similarly, MDCK cells pretreated with HA1077 (20 μM), an inhibitor of Rho-kinase and other kinases (Uehata et al., 1997), lost actin fibers and were unable to induce lamellipodia formation after HGF stimulation (our unpublished results). Thus, Rho-kinase activity and/or a preassembled actin network was required for the ability of HGF to induce lamellipodia in MDCK cells. Consistent with this, the injection of MDCK cells with a construct expressing a constitutively activated Rho-kinase (Rho-kinase catalytic domain [CAT]) induced an increase in actin stress fibers, which, in cells at the edge of the colony, frequently emanated from discrete areas and terminated in membrane extensions (in 34 ± 3% of the injected cells; Figure 7, a and b), suggesting that Rho-kinase activity and/or the resulting actin network may act to potentiate HGF-dependent lamellipodia formation.
Figure 6.
Rho-kinase and PAK are involved in HGF-induced lamellipodia formation, but only PAK is required for MDCK cell spreading. (A) pRK5Myc vector (0.2 mg/ml) together with rabbit immunoglobulin G (a and b) or expression vectors (0.2 mg/ml) encoding Myc-tagged RB/PH(TT) (c and d) or Myc-tagged PAKR (e and f) were injected into the nuclei of MDCK cells. After a 5-h incubation period, cells were stimulated with HGF (5 U/ml) for 15 min. Cells were double stained with anti-rabbit FITC (a) and TRITC-phalloidin (b) or anti-Myc and anti-mouse FITC (c and e) and TRITC-phalloidin (d and f). Arrows point to lamellipodia. Bar, 25 μm. (B) MDCK cells were injected with expression vectors (0.2 mg/ml) encoding Myc-tagged PAKR (a and b) or Myc-tagged RB/PH(TT) (c–f). After a 2-h incubation period, cells were stimulated with HGF for 3 h (a and b) or 4 h (c–f) and then fixed and double stained with anti-Myc and anti-mouse FITC (a, c, and e) and TRITC-phalloidin (b, d, and f). Bar, 25 μm.
Figure 7.
Activated Rho-kinase and PAK1 lead to peripheral actin reorganization. MDCK cells were injected with Myc-tagged expression vectors encoding activated Rho-kinase (CAT; 0.2 mg/ml) (a and b) or coinjected with rabbit immunoglobulin G and activated PAK1L107F (0.05 mg/ml) (c and d). Cells were incubated for 3 h to allow for CAT expression and for 1 h for PAK1L107F expression. Cells were fixed and double stained with anti-Myc and anti-mouse FITC (a) or anti-rabbit FITC (c), followed by TRITC-phalloidin (b and d). Note the reorganized peripheral actin in injected cells (arrows) compared with noninjected cells (arrowheads). Bar, 25 μm.
In contrast to CAT, the injection of a cDNA vector encoding a constitutively activated PAK (PAK1L107F) induced the loss of actin stress fibers and peripheral actin in 100% of the injected cells 1 h after injection (Figure 7, c and d) and cell rounding by 2 h (our unpublished result). Even at low concentrations of DNA (1 μg/ml), loss of stress fibers could be observed 3.5 h after injection. To establish if PAK activity was required for HGF-induced actin remodeling, MDCK cells were injected with an expression vector encoding the N-terminal regulatory domain of PAK2 (PAKR; aa 1–225) (Martin et al., 1995; Minden et al., 1995). In addition to the Cdc42/Rac interaction domain, PAKR encodes an autoinhibitory domain that inhibits PAK kinase activity in vitro and in vivo and contains proline-rich domains with potential to compete for PAK-binding proteins (Frost et al., 1998; Zhao et al., 1998; Tu and Wigler, 1999). Notably, the ability of MDCK cells expressing PAKR for 5 h to remodel peripheral actin and to extend typical lamellipodia in response to HGF for 15 min was decreased to 19 ± 1% compared with control pRK5-injected cells (Figure 6A, e and f; Table 1). Together, our results demonstrate that both PAK and Rho-kinase are required for the early remodeling of peripheral actin leading to lamellipodia formation in response to HGF.
PAK Activity Is Required for HGF-induced Cell Spreading and Dissociation
To determine if PAK and Rho-kinase activity were also required for the late HGF-induced cell spreading and dissociation, MDCK cells injected with vectors expressing the PAKR or RB/PH(TT) dominant negative mutant were stimulated with HGF for 3–4 h. Strikingly, MDCK cells expressing PAKR remained small and tightly associated 3 h after HGF stimulation (Figure 6B, a and b). In contrast, MDCK cells expressing RB/PH(TT) (Figure 6B, c and d) spread equally or more than noninjected cells in response to HGF for 4 h. Notably, expression of RB/PH(TT) could induce cell spreading on its own (our unpublished result). In addition, the vast majority of cells expressing RB/PH(TT) had dissociated and changed shape, whereas noninjected cells were still associated (Figure 6B, e and f). Thus, in contrast to PAK, inhibition of Rho-kinase activation by dominant negative Rho-kinase RB/PH(TT) enhanced HGF-induced MDCK cell spreading and dissociation. Moreover, MDCK cells pretreated for 1.5 h with HA1077 (20 μM) showed a similar phenotype to cells expressing RB/PH(TT) in response to HGF for 4 h and were equally or more spread than DMSO-treated control cells (our unpublished result).
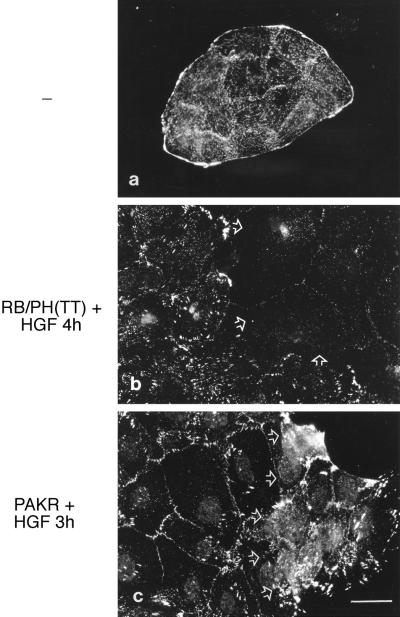
Both Rho-kinase and PAK have been implicated in focal complex and focal adhesion dynamics (Leung et al., 1996; Amano et al., 1997; Ishizaki et al., 1997; Manser et al., 1997). To establish if the alterations observed in cell morphology and the actin cytoskeleton after expression of RB/PH(TT) or PAKR correlated with alterations in focal complexes/focal adhesions, injected cells were subjected to indirect immunofluorescence with the use of vinculin antibodies (Figure 8). Unstimulated MDCK cells contain predominantly small vinculin-containing focal complexes throughout the cells and at cell–cell contacts, whereas larger focal complexes are concentrated at the periphery of the cell colony (Figure 8a). After HGF stimulation for 3–4 h, there is a decrease in the density of small focal complexes, concomitant with the appearance of larger focal complexes throughout the cells (Figure 8, b and c, noninjected cells). The HGF-dependent formation of the large focal complexes was blocked in MDCK cells expressing RB/PH(TT) (Figure 8b, arrows), consistent with the observed decrease in actin stress fibers in these cells (Figure 6). However, few of the smaller vinculin-containing complexes localized at sites of cell–cell junctions and at the cell periphery were still observed in cells expressing RB/PH(TT), indicating that RB/PH(TT) expression at that time did not lead to a complete loss of focal adhesive contacts (Figure 8b). In contrast, MDCK cells expressing PAKR showed an increase in the number of, and larger, vinculin-containing focal complexes after HGF stimulation for 3 h compared with uninjected cells (Figure 8c). These results suggest a role for PAK in the turnover or breakdown of focal complexes critical for cell spreading in response to HGF. In contrast, Rho-kinase is required for the maintenance and generation of new focal complexes associated with HGF-induced MDCK cell spreading, but their presence or formation is not essential for spreading.
Figure 8.
Inhibition of Rho-kinase and PAK alters focal adhesion reorganization in HGF-stimulated MDCK cells. MDCK cells injected with expression vectors (0.2 mg/ml) encoding Myc-tagged RB/PH(TT) (b) or Myc-tagged PAKR (c) were incubated for 2 h and then stimulated with HGF for 4 and 3 h, respectively. Cells were then incubated with Triton X-100–containing CSK buffer and fixed with 3.7% formaldehyde. Vinculin-containing focal complexes/adhesions were revealed with the use of anti-vinculin mAb followed by anti-mouse Cy3. Injected areas are identified by open arrows. Bar, 25 μm.
DISCUSSION
Epithelial–mesenchymal transition, which leads to the dissociation and dispersal of epithelial cells, is a critical event during development, tumor invasion, and metastasis. HGF, the ligand for the Met receptor tyrosine kinase, is a potent inducer of epithelial–mesenchymal transition. Although several signaling pathways have been shown to play a role in epithelial cell dispersal regulated by HGF, little is known about the proximal signals regulating these later events. In this study, we show for the first time that HGF stimulation of epithelial MDCK cells leads within minutes to transient activation of the Rho GTPases, Cdc42 and Rac, which correlates with the induction of filopodia and lamellipodia by HGF. Rac translocates to membrane ruffles, and this is accompanied by activation and translocation of downstream effectors of Rho GTPases, PAK and Rho-kinase. Our results provide evidence that the activation and targeting of Rho GTPases, as well as their effectors PAK and Rho-kinase, are critical for the HGF-induced remodeling of the actin cytoskeleton, cell spreading, and dissociation.
Although Cdc42 has been implicated in cell spreading of fibroblasts and macrophages (Clark et al., 1998; Price et al., 1998), its activation downstream from receptor tyrosine kinases has not been demonstrated. Notably, microinjection of dominant negative N17Cdc42 expression constructs inhibits the formation of HGF-dependent lamellipodia (Figure 4). Moreover, as was shown for Rac (Ridley et al., 1995), N17Cdc42 also inhibits the spreading and dissociation of MDCK cells in response to HGF (Figure 4). These data place Cdc42 as an important modulator of the HGF-dependent biological responses that lead to epithelial–mesenchymal transition. The observation that N17Rac expression enhances the formation of filopodia in response to HGF (Figure 4), which is indicative of sustained Cdc42 activity, suggests that the HGF-dependent activation of Cdc42 is independent of Rac and that Rac activity is essential for lamellipodia formation even in the presence of activated Cdc42. Consistent with this, the PI3K inhibitor LY294002 does not inhibit HGF-dependent activation of Cdc42 or the formation of filopodia downstream of HGF (Figure 2 and our unpublished result), whereas HGF-dependent activation of Rac and lamellipodia formation is blocked in the presence of LY294002 (Figure 2 and our unpublished result). This is in agreement with our previous data that PI3K is required for spreading of MDCK cells in response to HGF (Royal and Park, 1995). Interestingly, LY294002 does not inhibit the formation of lamellipodia downstream from activated L61Rac in MDCK cells (I. Royal and M. Park, unpublished observation), suggesting that PI3K activity is required upstream of HGF-dependent Rac activation, possibly as a consequence of the PI3K product, phosphatidylinositol 3,4,5-trisphosphate, recruiting and activating exchange factors for Rac in MDCK cells (Cerione and Zheng, 1996; Han et al., 1998; Sander et al., 1998) .
MDCK cells expressing N17Cdc42 show a decreased ability to form lamellipodia in response to HGF, suggesting a requirement for Cdc42 activity in the formation of HGF-dependent lamellipodia (Table 1). This may suggest that, in a similar manner to Swiss 3T3 cells (reviewed by Hall, 1998), activation of Cdc42 contributes to activation of Rac. However, the kinetics of activation of Cdc42 and Rac in response to HGF are distinct. HGF-dependent activation of Rac is transient (15–30 min), whereas that of Cdc42 is prolonged (>60 min; Figure 2). This supports the interpretation that Cdc42 and Rac may be activated by HGF independently of one another, although it does not exclude the possibility that Cdc42 activates Rac. Hence, from these studies we show for the first time that activation of Cdc42 is critical for both the formation of HGF-dependent lamellipodia and the spreading and dissociation of colonies of MDCK cells.
In MDCK epithelial cells, activated Rac and Cdc42 were shown previously to promote enhanced E-cadherin–mediated cell–cell adhesion and not spreading (Hordijk et al., 1997; Kuroda et al., 1997; Takaishi et al., 1997; Jou and Nelson, 1998). However, in low-density MDCK cells, macrophages, T lymphocytes, and HeLa cells, activated Rac can promote cell spreading and in some instances enhance cell migration (Allen et al., 1997; Manser et al., 1997; D'Souza-Schorey et al., 1998; Jou and Nelson, 1998; Sander et al., 1998). Thus, at high cell density when cell–cell junctions are favored, other HGF-induced signals are required to break down cell–cell junctions and promote cell spreading. For example, HGF-induced cell dispersal is inhibited in cells expressing dominant negative Ras proteins or after the inhibition of MEK1, an activator of ERK kinases, implicating the Ras pathway (Hartmann et al., 1994; Ridley et al., 1995; Khwaja et al., 1998; Potempa and Ridley, 1998). Moreover, in the context of HGF-stimulated MDCK cells, both the subcellular localization and the cycling of Rho GTPases between active and inactive states may promote a biological response that will be distinct from that induced by the expression of their constitutively activated forms.
Consistent with activation of Cdc42 and Rac and the translocation of Rac to membrane ruffles in HGF-stimulated MDCK cells, we have observed that the PAK1 serine/threonine kinase, an effector protein activated by Cdc42 and Rac, is activated and translocates to membrane ruffles at the edge of lamellipodia by 15 min of HGF stimulation (Figures 3 and 5). PAK1 is involved in actin reorganization (Manser et al., 1997; Sells et al., 1997; Frost et al., 1998) and localizes to PDGF- or Rac-induced membrane ruffles (Dharmawardhane et al., 1997) and focal complexes (Manser et al., 1997). Pak1 activation is sustained for >60 min after HGF stimulation (Figure 3), which correlates with the prolonged activation of Cdc42 and not the transient activation of Rac, suggesting that PAK activation in response to HGF may be mediated by Cdc42.
A role for Pak in HGF-dependent remodeling of the actin cytoskeleton is supported by the finding that a dominant negative PAK mutant (PAKR) inhibits HGF-dependent lamellipodia formation (Figure 6A) and spreading (Figure 6B) of MDCK cells. In response to HGF, cells expressing PAKR showed an increase in poorly organized F-actin and both an increased number of and larger focal complexes (Figure 8). Similarly, expression of the PAK autoinhibitory domain in single HeLa cells, which leads to the inhibition of endogenous PAK kinase activity, increased the size of focal complexes formed downstream of V12Rac or V12Cdc42 (Zhao et al., 1998). Our results show that the increase in focal adhesions in the presence of the PAKR dominant negative mutant cannot be rescued by the activation of Met-dependent signals, indicating that PAKR inhibits HGF-dependent turnover of focal adhesions required for MDCK cell spreading. Conversely, as observed in MDCK cells (Figure 7), activated PAK kinase was shown to inhibit the formation of stress fibers and focal adhesions, to induce cell retraction of adhering cells, and to inhibit cell spreading after plating (Manser et al., 1997; Frost et al., 1998; Zhao et al., 1998; Sanders et al., 1999). Together, these results are consistent with the recent demonstration that PAK activity, through phosphorylation and inhibition of myosin light chain kinase, promotes a decrease in actin-myosin filament assembly (Sanders et al., 1999). Thus, the targeting of an activated PAK kinase to membrane ruffles would be predicted to inhibit actin-myosin assembly and stress fiber formation, thus allowing remodeling of the actin cytoskeleton to form a lamellipodium and allow cell spreading.
PAKR, which encodes the Cdc42/Rac-binding site in addition to the autoinhibitory domain and proline-rich sequences, may compete with other Cdc42/Rac effectors involved in lamellipodia formation. However, as observed in HeLa cells, in which the PAK autoinhibitory domain had no effect on V12Rac-induced lamellipodia (Zhao et al., 1998), PAKR expression failed to inhibit L61Rac-induced lamellipodia in MDCK cells (I. Royal and M. Park, unpublished observation), demonstrating that PAKR does not block or soak up Rac-dependent signaling per se but instead inhibits HGF-dependent signaling pathways acting upstream of and leading to lamellipodia formation. A possible role for PAK upstream of Rac in lamellipodia formation has been suggested through the interaction of the PAK proline-rich N terminus with the Rac exchange factor PIX, a complex that has been proposed to be involved in Cdc42-to-Rac signaling (Manser et al., 1998; Obermeier et al., 1998). Thus, PAKR may both inhibit endogenous PAK kinase activity, via the autoinhibitory domain, and block remodeling of peripheral actin through the ability of the N-terminal proline-rich domain to inhibit Pak localization or interactions with proteins such as PIX.
In addition to Rac and Pak, Rho-kinase, an effector of Rho, is rapidly translocated to membrane ruffles after stimulation of MDCK cells with HGF (Figure 5). Rho-kinase translocation in response to activation of receptor tyrosine kinases has not been documented, but this is similar to the recruitment of Rho-kinase to cell membranes downstream from activated Rho (Leung et al., 1995; Matsui et al., 1996). Rho-kinase isoforms are implicated as regulators of actin organization and focal adhesion dynamics downstream from activated Rho (Leung et al., 1996; Amano et al., 1997; Ishizaki et al., 1997), possibly through the ability of Rho-kinase to increase myosin light chain phosphorylation and activation, promoting bundling of actin stress fibers into filaments (Amano et al., 1996; Kimura et al., 1996). Hence Rho-kinase would act to antagonize the activity of PAK (Burridge, 1999). Consistent with this notion, the expression of a constitutively activated Rho-kinase (CAT) promoted stress fiber bundling (Figure 7), whereas the expression of the dominant negative Rho-kinase mutant RB/PH(TT) in MDCK cells induced the loss of stress fibers and peripheral actin (Figure 6 and our unpublished results). Importantly, in cells expressing RB/PH(TT) or treated with HA1077, an inhibitor of Rho-kinase as well as other kinases (Uehata et al., 1997), HGF was unable to stimulate de novo stress fiber formation, indicating an essential role for Rho-kinase in HGF-dependent actin bundling. Moreover, these cells were unable to form typical lamellipodia containing an obvious actin network emanating from peripheral actin bundles, as was observed in control cells (Figure 6 and our unpublished result). These data are in agreement with the recent observation that Rho-kinase is required for tetradecanoylphorbol-13-acetate (TPA)-induced membrane ruffling in MDCK cells, possibly through its ability to phosphorylate adducin, a membrane protein that promotes the formation of a spectrin-actin meshwork beneath plasma membranes (Fukata et al., 1999). The targeting of Rho-kinase to membrane ruffles after HGF stimulation supports a role for Rho-kinase in the rapid reorganization of the actin cytoskeleton required for lamellipodium formation in MDCK cells, possibly to antagonize the activity of PAK1 and promote actin bundling and stabilization of newly formed lamellipodia.
HGF-dependent cell spreading, dissociation, and acquisition of a spindle cell shape were enhanced after prolonged expression of RB/PH(TT) (Figure 6B) or of a kinase-dead Rho-kinase mutant (our unpublished result), and cell spreading could be observed in the absence of HGF stimulation (our unpublished result). The enhanced cell spreading and dissociation observed in HGF-stimulated RB/PH(TT)-expressing cells were accompanied by the loss of focal complexes present throughout the ventral surface of the cell, whereas some focal complexes around the cell perimeter and at areas of cell–cell contact were retained (Figure 8). Because both Rac and Cdc42 stimulate the assembly of peripheral focal complexes in Swiss 3T3 cells (Nobes and Hall, 1995), the remaining or de novo HGF-induced focal complex formation in spread MDCK cells expressing RB/PH(TT) is likely to be dependent on Cdc42 and possibly Rac (Figure 8). These data are in agreement with a recent report (Nobes and Hall, 1999) showing that inhibition of Rho-kinase enhanced the speed of wound closure by a fibroblast monolayer containing few actin stress fibers, although other authors have reported that Rho-kinase was required for wound-induced migration of NRK49F fibroblasts and transcellular migration of mm1 hepatoma cells (Fukata et al., 1999; Itoh et al., 1999). These opposing observations might be attributed to cell type differences or to different mechanisms involved in the migration of single cells versus cell sheets or colonies. Whether Rho-kinase is required for the migration of single MDCK cells remains to be determined.
In the present study, we show that HGF is a potent activator of Cdc42, Rac, and Pak1 and promotes translocation of these proteins to membrane ruffles. We provide new insight regarding a critical role of Cdc42 in the formation of lamellipodia and in the dispersal of sheets of epithelial cells and show opposing actions of PAK1 and Rho-kinase in HGF-induced cell dispersal. We conclude that the regulation of activation and subcellular localization of different Rho family GTPases and their effectors by HGF is a critical event in the epithelial–mesenchymal transition, a process required for tumor cell dispersal.
Note Added in Proof. In agreement with our data, Kiosses et al. (1999) demonstrated that the amino-terminal proline-rich domain of Pak, and not the Rac/Cdc42 interaction domain, was required for the inhibition of cell migration by a dominant negative Pak protein.
ACKNOWLEDGMENTS
We thank members of the Park laboratory for critical reading of the manuscript and Dr. Svetlana Sadekova for helping with confocal microscopy. We also thank those who generously gave us reagents used in this study: Dr. Alan Hall for Rho family constructs, Dr. Arie Abo for the PAKR construct, Dr. John Chant for the PAKWT and PAKL107F constructs, Dr. James Woodgett for the GST-c-Jun construct, Dr. John Collard for the GST-PAK (aa 56–272) construct, Dr. George Vande Woude for HGF, and Asahi Chemical Industry (Shizuoka, Japan) for the HA1077 inhibitor. This research was supported by operating grants to M.P. from the National Cancer Institute of Canada. I.R. was the recipient of fellowships from the Fonds de la Recherche en Santé du Québec and the Medical Research Council of Canada. N.L.-V. is a junior scholar of the Fonds de la Recherche en Santé du Québec. L.L. is the recipient of a Natural Sciences and Engineering Research Council studentship. M.P. is a Scientist of the Medical Research Council of Canada.
Abbreviations used:
- aa
amino acids
- CAT
Rho-kinase catalytic domain
- HGF
hepatocyte growth factor
- JNK
c-Jun N-terminal kinase
- MBP
myelin basic protein
- MDCK
Madin-Darby canine kidney
- PAK
p21-activated kinase
- PH
pleckstrin homology
- PI3K
phosphatidylinositol 3-kinase
- RB
Rho-binding domain
REFERENCES
- Adler V, Franklin CC, Kraft AS. Phorbol esters stimulate the phosphorylation of c-Jun but not v-Jun: regulation by the N-terminal ∂ domain. Proc Natl Acad Sci USA. 1992;89:5341–5345. doi: 10.1073/pnas.89.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen WE, Jones GE, Pollard JW, Ridley AJ. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110:707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. doi: 10.1046/j.1365-2443.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Aspenström P. Effectors for the Rho GTPases. Curr Opin Cell Biol. 1999;11:95–102. doi: 10.1016/s0955-0674(99)80011-8. [DOI] [PubMed] [Google Scholar]
- Aspenström P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott Aldrich syndrome. Curr Biol. 1996;6:70–75. doi: 10.1016/s0960-9822(02)00423-2. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Derijard B, Davis RJ, Cerione RA. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995a;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Creasy CL, Chernoff J, Cerione RA. Identification of a mouse p21Cdc42/Rac activated kinase. J Biol Chem. 1995b;270:22731–22737. doi: 10.1074/jbc.270.39.22731. [DOI] [PubMed] [Google Scholar]
- Bardelli A, Pugliese L, Comoglio PM. “Invasive-growth” signaling by the Met/HGF receptor: the hereditary renal carcinoma connection. Biochim Biophys Acta. 1997;1333:M41–M51. doi: 10.1016/s0304-419x(97)00026-7. [DOI] [PubMed] [Google Scholar]
- Benard V, Bohl BP, Bokoch GM. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;10:404–410. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- Brown JL, Stowers L, Baer M, Trejo J, Coughlin S, Chant J. Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway. Curr Biol. 1996;6:598–605. doi: 10.1016/s0960-9822(02)00546-8. [DOI] [PubMed] [Google Scholar]
- Burridge K. Crosstalk between Rac and Rho. Science. 1999;283:2028–2029. doi: 10.1126/science.283.5410.2028. [DOI] [PubMed] [Google Scholar]
- Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerione RA, Zheng Y. The Dbl family of oncogenes. Curr Opin Cell Biol. 1996;8:216–222. doi: 10.1016/s0955-0674(96)80068-8. [DOI] [PubMed] [Google Scholar]
- Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the Rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Yu J-C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding protein Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J Cell Biol. 1997;138:1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza-Schorey C, Boettner B, Van Aelst L. Rac regulates integrin-mediated spreading and increased adhesion of T lymphocytes. Mol Cell Biol. 1998;18:3936–3946. doi: 10.1128/mcb.18.7.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JA, Khokhlatchev A, Stippec S, White MA, Cobb MH. Differential effects of Pak1-activating mutations reveal activity-dependent and -independent effects on cytoskeletal regulation. J Biol Chem. 1998;273:28191–28198. doi: 10.1074/jbc.273.43.28191. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Oshiro N, Kinoshita N, Kawano Y, Matsuoka Y, Bennett V, Matsuura Y, Kaibuchi K. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J Cell Biol. 1999;145:347–361. doi: 10.1083/jcb.145.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P, Rosen EM. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci USA. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Weidner KM, Schwarz H, Birchmeier W. The motility signal of scatter factor/hepatocyte growth factor mediated through the receptor tyrosine kinase Met requires intracellular action of Ras. J Biol Chem. 1994;269:21936–21939. [PubMed] [Google Scholar]
- Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LCJM, Collard JG. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Itoh K, Yoshioka K, Akedo H, Uehata M, Ishizaki T, Narumiya S. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nat Med. 1999;5:221–225. doi: 10.1038/5587. [DOI] [PubMed] [Google Scholar]
- Jeffers M, Rong S, Vande Woude GF. Hepatocyte growth factor/scatter factor-Met signaling in tumorigenicity and invasion/metastasis. Mol Med. 1996;74:505–513. doi: 10.1007/BF00204976. [DOI] [PubMed] [Google Scholar]
- Joneson T, McDonough M, Bar-Sagi D, Van Aelst L. Rac regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science. 1996;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- Jou T-S, Nelson WJ. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol. 1998;142:85–100. doi: 10.1083/jcb.142.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja A, Lehmann K, Marte B, Downward J. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J Biol Chem. 1998;273:18793–18801. doi: 10.1074/jbc.273.30.18793. [DOI] [PubMed] [Google Scholar]
- Kimura K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kiosses WB, Daniels RH, Otey C, Bokoch GM, Schwartz MA. A role for p21-activated kinase in endothelial cell migration. J Cell Biol. 1999;147:831–843. doi: 10.1083/jcb.147.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Fujii K, Nakamura T, Izawa I, Kaibuchi K. Regulation of cell-cell adhesion of MDCK cells by Cdc42 and Rac1 small GTPases. Biochem Biophys Res Commun. 1997;240:430–435. doi: 10.1006/bbrc.1997.7675. [DOI] [PubMed] [Google Scholar]
- Lamarche N, Hall A. GAPs for rho-related GTPases. Trends Genet. 1994;10:436–440. doi: 10.1016/0168-9525(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenström P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen X-Q, Manser E, Lim L. The p160 RhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganisation of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- Manser E, Huang H-Y, Loo T-H, Chen X-Q, Dong J-M, Leung T, Lim L. Expression of constitutively active α-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Manser E, Loo T-H, Koh C-G, Zhao Z-S, Chen X-Q, Tan L, Tan I, Leung T, Lim L. Pak kinases are directly coupled to the Pix family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- Martin GA, Bollag G, McCormick F, Abo A. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 1995;14:1970–1978. doi: 10.1002/j.1460-2075.1995.tb07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for the small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119:591–600. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Hepatocyte growth factor (HGF) as a tissue organizer for organogenesis and regeneration. Biochem Biophys Res Commun. 1997;239:639–644. doi: 10.1006/bbrc.1997.7517. [DOI] [PubMed] [Google Scholar]
- Minden A, Lin A, Claret F-X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Montesano R, Soriano JV, Pepper MS, Orci L. Induction of epithelial branching tubulogenesis in vitro. J Cell Physiol. 1997;173:152–161. doi: 10.1002/(SICI)1097-4652(199711)173:2<152::AID-JCP14>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- Obermeier A, Ahmed S, Manser E, Yen SC, Hall C, Lim L. PAK promotes morphological changes by acting upstream of Rac. EMBO J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Royal I, Park M. Hepatocyte growth factor-induced scatter of MDCK cells requires phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:27780–27787. doi: 10.1074/jbc.270.46.27780. [DOI] [PubMed] [Google Scholar]
- Sander EE, vanDelft S, ten Klooster JP, Reid T, van der Kammen RA, Michiels F, Collard JG. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell–cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by Rac and Rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Wigler M. Genetic evidence for Pak1 autoinhibition and its release by Cdc42. Mol Cell Biol. 1999;19:602–611. doi: 10.1128/mcb.19.1.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Wennstrom S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol. 1994;4:385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Zhao Z-S, Manser E, Chen X-Q, Chong C, Leung T, Lim L. A conserved negative regulatory region in αPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Naujokas MA, Park M. Receptor chimeras indicate that the Met tyrosine kinase mediates the motility and morphogenic responses of hepatocyte growth factor/scatter factor. Cell Growth Differ. 1994;5:359–366. [PubMed] [Google Scholar]