Abstract
We examined the role of the actin cytoskeleton in secretion in Saccharomyces cerevisiae with the use of several quantitative assays, including time-lapse video microscopy of cell surface growth in individual living cells. In latrunculin, which depolymerizes filamentous actin, cell surface growth was completely depolarized but still occurred, albeit at a reduced level. Thus, filamentous actin is necessary for polarized secretion but not for secretion per se. Consistent with this conclusion, latrunculin caused vesicles to accumulate at random positions throughout the cell. Cortical actin patches cluster at locations that correlate with sites of polarized secretion. However, we found that actin patch polarization is not necessary for polarized secretion because a mutant, bee1Δ(las17Δ), which completely lacks actin patch polarization, displayed polarized growth. In contrast, a mutant lacking actin cables, tpm1-2 tpm2Δ, had a severe defect in polarized growth. The yeast class V myosin Myo2p is hypothesized to mediate polarized secretion. A mutation in the motor domain of Myo2p, myo2-66, caused growth to be depolarized but with only a partial decrease in the level of overall growth. This effect is similar to that of latrunculin, suggesting that Myo2p interacts with filamentous actin. However, inhibition of Myo2p function by expression of its tail domain completely abolished growth.
INTRODUCTION
Secretion is polarized in many types of eukaryotic cells. Targeting of secretory vesicles to specific membrane domains proceeds by conserved pathways (reviewed by Keller and Simons, 1997). Saccharomyces cerevisiae is an excellent model system for studies of polarized secretion because many components of the secretory machinery have homologues in yeast (reviewed by Finger and Novick, 1998). In yeast, polarized secretion results in local expansion of the cell wall and thereby cell growth (reviewed by Kaiser et al., 1997). Insertion of the new components into the cell wall and the plasma membrane occurs by targeting of secretion to specific regions of the cell surface (Tkacz and Lampen, 1972, 1973; Farkašet al., 1974; Field and Schekman, 1980; Waddle et al., 1996).
At the beginning of the cell cycle, local surface area expansion leads to the emergence of a new bud and then to growth of the bud. While the bud is growing, there is almost no increase in the surface area of the mother cell (Mitchison, 1958; Waddle et al., 1996). Later, the bud stops growing, and secretion is directed to the neck between the mother and the bud, causing formation of the septum and cell separation (Tkacz and Lampen, 1972).
Actin has been implicated in secretion in yeast (Adams and Pringle, 1984; Kilmartin and Adams, 1984; Novick and Botstein, 1985). The exact role of the actin cytoskeleton in secretion is not well understood. In particular, it is not clear whether actin is necessary for the polarization of secretion or for the process of secretion itself. Previous experimental approaches have not been able to examine polarization of secretion with direct and quantitative assays. Biochemical studies have found that secretion is partially inhibited in actin and actin cytoskeleton mutants (Novick and Botstein, 1985; Johnston et al., 1991; Govindan et al., 1995). In addition, microscopy of fixed cells has revealed that membranous vesicles accumulate in certain actin cytoskeleton mutants (Novick and Botstein, 1985; Liu and Bretscher, 1992; Govindan et al., 1995; Li, 1997), suggesting that secretion is impaired. Studies by Pruyne et al. (1998) have implicated actin cables in polarized secretion. In this work, rapid disruption of actin cables was induced by temperature shift of cells with a conditional tropomyosin mutation. Loss of actin cables resulted in the rapid loss of Myo2p, a class V myosin, and Sec4p, a rab, at the bud tip.
However, in those previous studies, polarized growth could not be measured directly but only with assays that reflect some aspect of the process or outcome of polarized secretion. In addition, the viable actin and actin cytoskeleton mutants studied retained some level of filamentous actin (reviewed by Kaiser et al., 1997). Therefore, actin function has never been inhibited completely in studies of secretion.
Given these limitations of previous approaches, we do not know how directly actin might be involved in the polarization of secretion. Also, we do not know whether actin is absolutely necessary for the process of secretion, i.e., whether complete loss of filamentous actin would lead to complete abolition of secretion. To address these questions, we used several novel and powerful experimental approaches. First, we directly observed polarized cell growth with the use of digital video microscopy of individual living cells. Second, we treated cells with latrunculin, which completely depolymerizes filamentous actin and does not require a temperature shift.
Next, we used these same novel approaches to examine whether polarization of cortical actin patches is necessary for polarized secretion. This widely held hypothesis is based on the observation that actin patches cluster at sites that correlate with sites of polarized secretion. We examined a mutant, bee1Δ(las17Δ), in which actin patches have no polarization. The orientation of actin cables also correlates with the location of polarized secretion. Therefore, we examined a mutant lacking cables, tpm1-2 tpm2Δ. Finally, we investigated the role of Myo2p, a yeast class V myosin, in polarized secretion with the use of a conditional mutant and expression of the tail domain.
MATERIALS AND METHODS
Strain Construction and Origin
Strains used in this work are listed in Table 1. All strains expressing GFP fusion proteins carried no wild-type copies of the genes for the proteins that had been GFP labeled.
Table 1.
Strains used in this study
| Strain | Relevant genotype | Source |
|---|---|---|
| YJC1193 | MATα leu2 ura3 his3-Δ200 | Karpova et al. (1998a) |
| YJC1411 | MATa leu2 ura3 his3-Δ200 | This study |
| MATα leu2 ura3 his3-Δ200 | ||
| YJC1423 | MATα CAP1-GFP-HIS3 | This study |
| YJC1443 | MATα CAP1-GFP-HIS3 lys2 | This study |
| YJC1453 | MATa CAP1-GFP-HIS3 | This study |
| MATα CAP1-GFP-HIS3 | ||
| YJC1454 | MATa MYO2-GFP-HIS3 | This study |
| MATα MYO2-GFP-HIS3 | ||
| YJC1682 | MATa bee1Δ∷LEU2 CAP1-GFP-HIS3 | This study |
| YJC1691 | MATa bee1Δ∷LEU2 CAP1-GFP-HIS3 | This study |
| MATα bee1Δ∷LEU2 CAP1-GFP-HIS3 | ||
| YJC1779 | MATa leu2∷GAL-MYO2DN-LEU2 MYO2-GFP-HIS3 | This study |
| MATα leu2 MYO2-GFP-HIS3 | ||
| RLY157 | MATa bee1Δ∷LEU2 | Li (1997) |
| GR663-13 | MATα his6 ura1 myo2-66 | Johnston et al. (1991) |
| NY13 | MATa ura3-52 | Walch-Solimena et al. (1997) |
| NY17 | MATa ura3-52 sec6-4 | Walch-Solimena et al. (1997) |
| ABY971 | MATa tpm1-2∷LEU2 tpm2Δ∷HIS3 | Pruyne et al. (1998) |
| MATα tpm1-2∷LEU2 tpm2Δ∷HIS3 | ||
| ABY973 | MATa tpm2Δ∷HIS3 | Pruyne et al. (1998) |
| MATα tpm2Δ∷HIS3 |
The haploid YJC1423, containing a C-terminal Cap1p-GFP fusion, was produced by integration of a GFP-HIS3 cassette into the genome of YJC1193 by means of previously described methods (Karpova et al., 1998b). To obtain the haploid YJC1443, a spontaneous lys2 mutation was selected in YJC1423 by plating on medium containing α-aminoadipic acid.
The diploid YJC1411 was produced by diploidization of the wild-type haploid YJC1193 with the use of an HO-induced mating type switch. Heterozygotes for another C-terminal Cap1p-GFP fusion and a C-terminal Myo2p-GFP fusion were created by insertion of a GFP-HIS3 cassette into the genome of the diploid YJC1411 by means of previously described methods (Karpova et al., 1998b). From these diploid transformants, haploid segregants carrying the GFP fusions were obtained and then diploidized by an HO-induced mating type switch to create YJC1453 (CAP1-GFP/CAP1-GFP) and YJC1454 (MYO2-GFP/MYO2-GFP).
The diploid YJC1779 (leu2::GAL1-MYO2DN-LEU2/leu2 MYO2-GFP/MYO2-GFP) was constructed by integrative transformation of YJC1454 with plasmid RPB38, which expresses the Myo2p tail domain (MYO2DN) from the GAL1 promoter. The bee1Δ(las17Δ) haploid mutant RLY157 was provided by Dr. Rong Li (Harvard Medical School, Boston, MA). We crossed RLY157 to YJC1443 to obtain YJC1682 (las17Δ CAP1-GFP). YJC1682 was diploidized by an HO-induced mating type switch to create YJC1691. The myo2-66 mutant GR663-13 was provided by Dr. R. Singer (Dalhousie University, Halifax, Nova Scotia, Canada).
Media and Growth Conditions
Strains were grown at 25°C unless noted otherwise. Liquid YPD and synthetic dextrose minimal media were prepared from dry stock (BIO101, La Jolla, CA). Nonfluorescent (NF) medium was prepared as described (Waddle et al., 1996) with the addition of β-alanine (500 μg/L), thiamine (200 μg/L), biotin (2 μg/L), Ca2+-pantothenate (400 μg/L), and inositol (2 mg/L). For Myo2p tail induction, cells were grown to mid-log phase in YP with raffinose (20 g/l) and transferred either to YP with raffinose and galactose (20 g/l) or to YPD. Latrunculin A was obtained from Dr. Philip Crews (University of California, Santa Cruz). Latrunculin was stored as a 10 or 100 mM stock in DMSO.
Depolymerization of Actin with Latrunculin
A culture was grown in YPD to mid-log phase and then washed into NF medium. For movies of the cell cycle, 50-μl samples containing 106 cells were mixed with an equal volume of 2× latrunculin solution (1 mM latrunculin A and 2% DMSO in NF medium). The cell mixture was incubated for 10 min at room temperature before observation. This treatment induced the loss of cables and patches in 95% of the cells, documented by rhodamine-phalloidin staining of fixed cells.
To monitor the effectiveness of latrunculin treatment in living cells, Cap1p of cortical actin patches and Myo2p were labeled with GFP. To determine the time course of disassembly of GFP-tagged bud clusters and neck clusters of Cap1p-GFP and Myo2p-GFP in living cells, 5-μl samples of 105 cells were mixed with 2× latrunculin solution directly on a slide and observed immediately. To confirm that the disappearance of GFP patches reflected filamentous actin disassembly, the cells were mixed with 2× latrunculin solution in test tubes, fixed at different time intervals in 3.7% formaldehyde added directly to the medium from a 37% stock, and stained with rhodamine-phalloidin (Molecular Probes, Eugene, OR) (Pringle et al., 1989; Karpova et al., 1998a).
For electron microscopy experiments, latrunculin as a 10 mM stock in DMSO was added to early-log-phase cells growing in YPD to obtain a 200 μM final concentration. DMSO alone was added to control cells. Cells were incubated at 25°C for various times. In an epistasis experiment, sec6-4 cells were shifted to the restrictive temperature of 37°C when latrunculin was added. After 30 min, cells were fixed for electron microscopy.
Video Microscopy
Time-lapse imaging was performed with an ISIT-68 video camera, an RC68 controller, a DSP-2000 processor (DAGE-MTI, Michigan City, IN), a stage and shutter controller (MAC2000, Ludl Electronic Products, Hawthorne, NY), and an epifluorescence upright microscope (Bmax-60F, Olympus, Tokyo, Japan) with a 100× UPlanApo objective (numerical aperture, 1.35) and a U-MWIBA filter set. Photobleaching was reduced by placing two U-ND25 neutral density filters in the excitation light path. The video system was controlled by custom macros in NIH Image 1.62 software (NIH Image was written by Wayne Rasband at the National Institutes of Health and is available by anonymous ftp at zippy.nimh.nih.gov). Single-frame images (see Figure 5) were collected with a cooled charge-coupled device video camera (RC300, Dage-MTI) on an inverted epifluorescence microscope (IX70, Olympus) with a 100× UPlanApo objective (numerical aperture, 1.35) and U-MNG (rhodamine-phalloidin) and U-MWIBA (GFP) filter sets. Both movies and single images were collected with a framegrabber LG-3 (Scion, Frederick, MD) on a Power Macintosh computer (Cupertino, CA).
Figure 5.
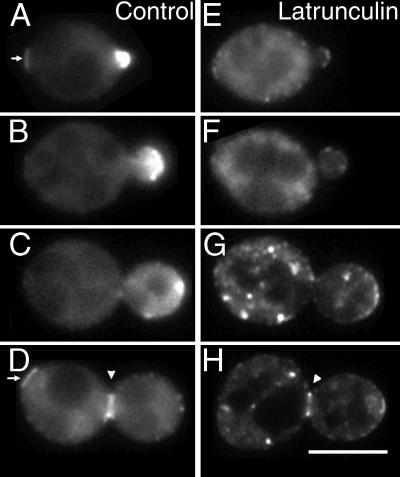
Myo2p-GFP localization in latrunculin-treated cells. Myo2p-GFP cells (YJC1454) were grown in medium with DMSO (A–D) or 500 μM latrunculin (E–H) for 20 min. Representative cells at different stages of the cell cycle are shown. Arrowheads point to the Myo2p neck ring. Arrows point to the remnants of the Myo2p neck ring from the previous cell cycle. Bar, 5 μm. Movies from this experiment are available online.
Cell Imaging
For time-lapse imaging, cells were spread on the surface of an agarose pad as described (Waddle et al., 1996). The pad contained NF medium with 2% agarose with different carbon sources, with or without 500 μM latrunculin and 1% DMSO. At each time point, Z-scans consisting of 12–20 focal planes 0.5 μm apart were made. Both bright-field and fluorescence images were collected. To create time-lapse movies such as those shown in Figure 4, the focal planes of each Z-scan were projected onto a single two-dimensional image. For single-focal-plane images, living cells with a GFP label or fixed cells stained with rhodamine-phalloidin were mounted on glass slides.
Figure 4.
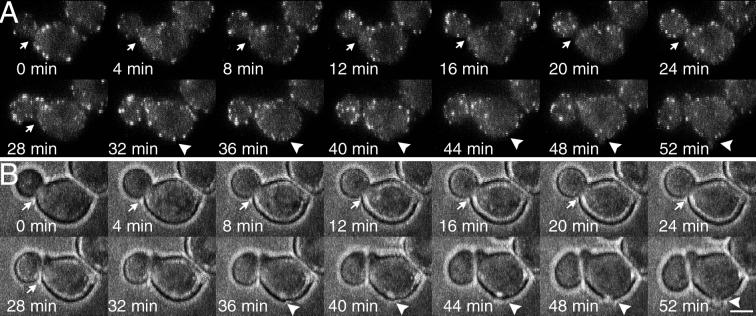
Bud formation and cell separation in the absence of patch polarization in the bee1Δ(las17Δ) mutant (YJC1691). Observations were made at 4-min intervals over a 2-h period spanning the whole cell cycle. Sequential frames show fluorescence (A) and bright-field (B) images of the same cell. The arrows point to the site of cell separation, and the arrowheads point to the site of bud formation. Cell separation is indicated by a slight change in the position of the bud relative to the mother between 24 and 28 min. This movement is more obvious in the video sequence available online. Cortical actin patches were tagged with Cap1-GFP. Bar, 2.5 μm.
For experiments not at room temperature, the slide, stage, objective, and condenser of the microscope were thermoisolated with a system of plastic tenting and maintained at a constant temperature by the flow of air from a thermostat-controlled heater (AirTherm, World Precision Instruments, Sarasota, FL).
Surface Area Growth of Individual Living Cells
For each time point, several transmitted-light images from different focal planes of one cell were collected. The image corresponding to the focal plane at the middle of the cell was selected. The diameters of the mother and bud along their long (a) and short (b) axes were measured with the use of NIH Image. The surface area (S) was then calculated as S = 2πb (b + a/ε arc sin ε), where ε = (√(a2 − b2))/a.
Small-budded cells, defined as cells for which the volume of the bud was <30% the volume of the mother, were chosen for analysis. In a typical wild-type cell with a small bud, the bud volume increases in a relatively linear manner for ∼60 min (Waddle et al., 1996). Each cell was observed at 8-min time intervals for 64 min. The surface areas for the bud, mother, and total (bud + mother) were calculated and plotted versus time. Slopes were determined by linear regression with Kaleidagraph (Synergy Software, Reading, PA) to give surface area growth rate.
Myo2p Experiments
In the myo2-66 experiment shown in Figure 6, cells were grown at 37°C for 2 h before observation. Growth was then measured during the course of 64 min at 37°C. A MYO2DN construct expressing the Myo2p tail from the GAL1 promoter was integrated into the genome of diploid MYO2-GFP cells (Reck-Peterson et al., 1999). Galactose caused rapid induction of Myo2p tail expression. Expression of the tail, on a molar basis, was 9 times that of endogenous Myo2p at 30 min, reaching a maximum level of 13 times at 2 h. Expression levels were measured by quantitative immunoblotting as described (Reck-Peterson et al., 1999). In the MYO2DN experiment shown in Figure 6, cells were shifted to galactose-containing medium for 10 min. Growth was then measured during the course of 64 min in galactose-containing medium.
Figure 6.
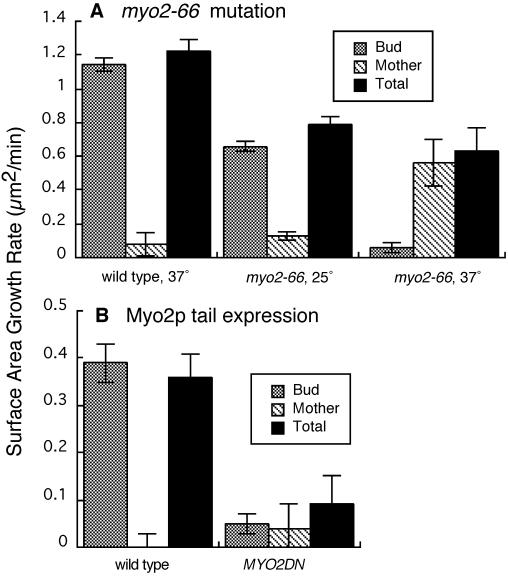
Effect of inhibition of Myo2p on cell growth. Mean values for surface area growth rate are plotted. Error bars represent SEM. (A) Wild-type and myo2-66 cells at 25 or 37°C. (B) Wild-type and MYO2DN cells in galactose. Number of cells: (A) wild-type 37°C, 11; myo2-66 25°C, 8; myo2-66 37°C, 9; (B) wild-type, 6; MYO2DN, 13. Strains were as follows: wild type, YJC1454; myo2-66, GR663-13; MYO2DN, YJC1779.
Tropomyosin Mutant Experiments
A tpm1-2 tpm2Δ diploid strain (ABY971; kindly provided by Dr. Anthony Bretscher, Cornell University, Ithaca, NY) grown at 25°C was transferred to an agarose pad on a microslide. The microslide was placed on a microscope stage preheated to 35°C. After 10 or 70 min, collection of images for movies was begun. Individual cells with small buds were selected and observed for 64 min at 8-min time intervals. Plots of surface area versus time for mother and bud were linear over this time course.
To confirm that this temperature shift rapidly affects the cables in the manner described previously (Pruyne et al., 1998), 3-ml cultures of TPM1 tpm2Δ (ABY973) and tpm1-2 tpm2Δ (ABY971) grown at 25°C were transferred to 35°C for 5 min, fixed, and stained with rhodamine-phalloidin. Cables were lost in the double mutant, but not in the single mutant, as described (Pruyne et al., 1998).
Electron Microscopy
Cells were prepared as described previously (Salminen and Novick, 1987). Thin sections were viewed with a Philips 301 electron microscope (Philips Electronic Instruments, Mahwah, NJ) at 80 kV.
RESULTS
The Role of Filamentous Actin in Polarized Secretion
Latrunculin-induced Disassembly of F-Actin Blocks Polarization of Secretion but Does Not Abolish Secretion.
To assess the role of filamentous actin in secretion, we depolymerized filamentous actin by treating cells with latrunculin. Cell surface area was measured for mothers and buds over time with the use of digital video analysis of individual living cells. The increase in surface area over time, or growth rate, is a manifestation of secretion. Representative examples of data from single cells are shown in Figure 1. The compiled data for all cells are presented in Figure 3.
Figure 1.
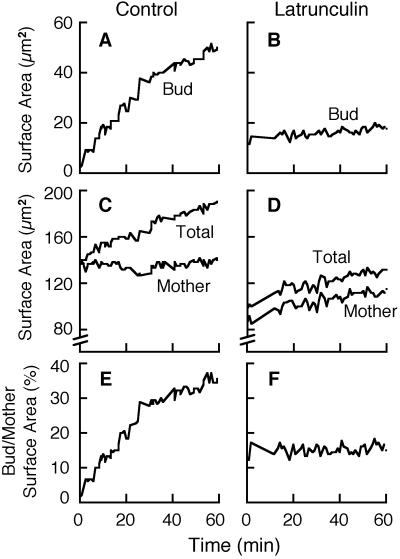
Effect of latrunculin-induced disassembly of filamentous actin on polarized cell growth. Representative results are shown for one control cell (A, C, and E) and one cell treated with latrunculin (B, D, and F). The surface area of the bud (A and B), the surface area of the mother (C and D), the total surface area of the bud plus the mother (C and D), and the ratio of bud/mother surface areas (E and F) are plotted versus time. The strain was YJC1411.
Figure 3.
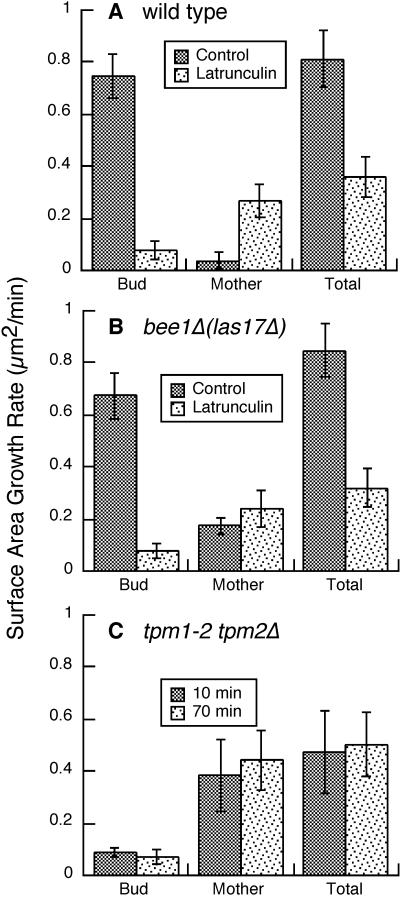
Effect of depolarization of cortical actin patches, latrunculin treatment, and loss of actin cables on cell growth. Surface area growth rates are plotted for wild-type cells with and without latrunculin (A), bee1Δ(las17Δ) mutant cells with and without latrunculin (B), and tpm1-2 tpm2Δ cells incubated at the restrictive temperature for 10 or 70 min (C). The values are the mean growth rates for small-budded cells. Error bars represent SEM. Number of cells: wild-type control, 10; wild-type latrunculin, 9; bee1Δ(las17Δ) control, 19; bee1Δ(las17Δ) latrunculin, 4; tpm1-2 tpm2Δ 10 min, 6; tpm1-2 tpm2Δ 70 min, 5. Strains were as follows: wild type, YJC1411; bee1Δ(las17Δ), RLY157, YJC1682, and YJC1691; tpm1-2 tpm2Δ, ABY971.
First, we examined the total growth rate by combining the data for mother and bud. Latrunculin caused a decrease, but only a partial one, in the total growth rate (see Figure 3A). Because growth was inhibited but not abolished, filamentous actin was not essential for the process of secretion per se.
Next, we determined how latrunculin affected the polarization of secretion by examining the data for growth of the bud and mother separately (see Figure 3A). In control cells, growth was restricted almost exclusively to the bud, and the mean surface area growth rate was much higher for buds than it was for mothers. In contrast, in latrunculin-treated cells mothers and buds both grew, and the mean surface area growth rate was lower for buds than for mothers.
We asked whether the rate of growth in latrunculin was simply proportional to the surface area, as predicted if secretion occurred uniformly over the cell surface. First, we calculated the ratio of bud surface area to mother surface area over time. In control cells, the bud/mother surface area ratio increased over time. In contrast, in latrunculin-treated cells, the bud/mother surface area ratio remained constant over time. Figure 1 shows results from a representative control cell and a representative latrunculin-treated cell. A total of 10 control cells and 9 latrunculin-treated cells gave similar results. These results are consistent with uniform growth over the cell surface proportional to the area.
Second, for all the individual buds and mothers, we normalized the rate of growth to the mean surface area. If growth is proportional to surface area, i.e., not polarized at all, then the predicted ratio of normalized bud growth to normalized mother growth is 1:1. In 10 control cells, the ratio varied from 10:1 to 270:1. In 8 latrunculin-treated cells, the ratio of normalized bud growth to normalized mother growth was between 1:1 and 2:1. In one latrunculin-treated cell the ratio was 8:1. This higher ratio in one cell might be explained by the fact that the bud in this cell was relatively small and therefore difficult to measure. Therefore, growth in latrunculin-treated cells was nearly proportional to the cell surface area.
Bud formation and cell separation also depend on polarized secretion (reviewed by Govindan and Novick, 1995). Previous studies have found that latrunculin inhibited bud formation and cell separation (Ayscough et al., 1997; Bi et al., 1998). We quantified the frequency of bud formation and cell separation as additional parameters reflecting polarized secretion (Table 2). In our assays, latrunculin treatment quantitatively abolished bud formation and cell separation.
Table 2.
Frequency of bud formation and cell separation
| Relevant genotype | Growth conditions | Bud formation | Cell separation |
|---|---|---|---|
| Wild type | No Lat A | 2/2 (100%) | 8/8 (100%) |
| Wild type | Lat A | 0/8 (0%) | 0/14 (0%) |
| bee1Δ | No Lat A | 17/19 (89%) | 15/17 (88%) |
| bee1Δ | Lat A | 0/3 (0%) | 0/9 (0%) |
| Wild type | Galactose | 1/3 (33%) | 5/13 (38%) |
| MYO2DN | Galactose | 0/13 (0%) | 4/37 (11%) |
Bud formation was scored in cells without a bud, and cell separation was scored in budded cells. In both cases, cells were observed for 2 h. Lat A, latrunculin A.
Vesicles Accumulate in Cells Treated with Latrunculin.
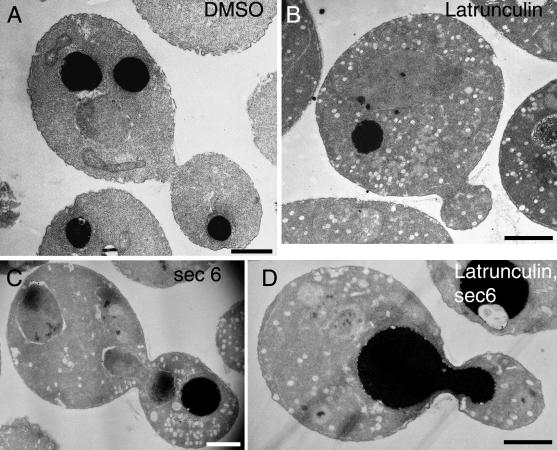
Temperature-sensitive actin mutants accumulate post-Golgi vesicles at the restrictive temperature (Novick and Botstein, 1985). We asked whether cells treated with latrunculin would accumulate similar vesicles. In latrunculin, an increased number of vesicles was observed after 30 min (Figure 2B). Vesicles were present in both buds and mothers, dispersed at random about the cell. In contrast, in temperature-sensitive sec6-4 cells, which are defective in the docking and fusing of vesicles with the plasma membrane, vesicles accumulated primarily in the bud (Figure 2C) (Govindan et al., 1995). In an epistasis experiment, sec6-4 cells were shifted to the restrictive temperature concurrent with the addition of latrunculin. Vesicles accumulated in both buds and mothers (Figure 2D). This result suggests that filamentous actin functions upstream of Sec6p and is required for the polarized transport of post-Golgi vesicles.
Figure 2.
Accumulation of vesicles in latrunculin-treated cells observed by thin-section electron microscopy. Wild-type cells (NY13) were grown at 25°C for 120 min in the presence of DMSO (A) or 200 μM latrunculin (B). sec6-4 cells (NY17) were grown at 37°C for 30 min in the presence of DMSO (C) or 200 μM latrunculin (D). Bars, 1 μm.
The Role of Polarization of Actin Patches in Polarized Secretion
The experiments described above show that filamentous actin is necessary for polarized secretion. We asked what structural element of the actin cytoskeleton might perform this function. Cortical actin patches cluster at sites that correlate with sites of polarized secretion (Adams and Pringle, 1984; Kilmartin and Adams, 1984), suggesting that actin patches mediate some aspect of secretion, such as the fusion of vesicles with the plasma membrane or the tethering of vesicles at the site of secretion. This hypothesis predicts that polarized actin patches are necessary for polarized secretion.
We tested this hypothesis by measuring polarized secretion in a mutant, bee1Δ(las17Δ) (Li, 1997), in which the actin patches are completely depolarized (Karpova et al., 1998b). We confirmed that actin patches are completely delocalized in this mutant by monitoring the localization of Cap1p-GFP, a marker for actin patches.
We measured cell surface growth in bee1Δ(las17Δ) mutant cells. The total surface area growth rate was normal in bee1Δ(las17Δ) cells (Figure 3B). In terms of polarization of growth, buds of bee1Δ(las17Δ) cells grew at a greater rate than did mothers, although the degree of polarization was slightly less than that seen in wild-type cells (Figure 3B). In bee1Δ(las17Δ) cells, the ratio of normalized bud growth to normalized mother growth varied from 6:1 to 98:1. In control cells, the ratio varied from 10:1 to 270:1. Overall, growth in bee1Δ(las17Δ) cells was polarized to a high degree despite the absence of polarization of cortical actin patches.
Previously, Li (1997) observed a greater effect of the bee1Δ(las17Δ) mutation on bud growth. This difference may be due to the fact that in Li's experiments the bee1Δ(las17Δ) cells had a more abnormal actin cytoskeleton. The cells contained abnormal actin structures and few or no patches; in our experiments, bee1Δ(las17Δ) cells contained a normal complement of patches and no abnormal structures. We suspect that these differences resulted from different methods of culturing and passing the mutant strains. These differences are not relevant to the goal of our analysis, which was to observe cells with a specific defect in polarization of patches to determine whether patch polarization is important for polarized growth. If the bee1Δ(las17Δ) mutant had a more severe actin phenotype in our hands, it would not have been useful for our analysis.
We also assessed polarized secretion in bee1Δ(las17Δ) cells by quantitating bud formation and cell separation, both of which occurred at levels near normal (Table 2). We asked whether transient clustering of actin patches might be sufficient to provide enough polarized secretion for bud formation or cell separation. To address this question, we monitored the localization of actin patches labeled with Cap1p-GFP by time-lapse fluorescence microscopy. A representative example is shown in Figure 4. In 13 unbudded cells and 9 budded cells, we observed no case of transient clustering of actin patches before bud formation or cell separation, respectively.
Thus, quantitative assays of cell growth, bud formation, and cell separation all show that polarized secretion occurs in the bee1Δ(las17Δ) mutant and does not require polarization of actin patches.
We considered the possibility that polarized secretion in bee1Δ(las17Δ) mutant cells might occur via some unusual and abnormal process independent of filamentous actin. We asked whether polarized secretion would occur in bee1Δ(las17Δ) mutant cells treated with latrunculin. In assays of cell growth, latrunculin-treated bee1Δ(las17Δ) cells resembled latrunculin-treated wild-type cells (Figure 3B). Bud formation and cell separation were abolished by latrunculin treatment of bee1Δ(las17Δ) cells (Table 2). Therefore, the mechanism of polarized secretion of bee1Δ(las17Δ) cells does require filamentous actin, resembling the mechanism in wild-type cells in this respect.
The Role of Actin Cables in Polarized Secretion
These experiments showed that polarization of actin patches is not necessary for polarized secretion. We then asked whether polarization of actin cables was necessary for polarized secretion. Unfortunately, no mutation or drug treatment results in a stable loss of cable polarization without a concomitant loss of patch polarization. However, Pruyne and colleagues (1998) developed a conditional double tropomyosin mutant, tpm1-2 tpm2Δ, which shows a selective loss of cables at early times after the shift to restrictive temperature. During the initial 5 min, cables disappear; patches are present and polarized at this time. Later, at 15 min, patches also become depolarized.
Pruyne and colleagues (1998) examined the effect of this selective loss of cables on polarized secretion. Some, but not all, markers of polarized secretion in the bud became depolarized promptly. Myo2p and Sec4p became depolarized rapidly, but Sec8p became depolarized only at longer times, when actin patches were also depolarized. These authors also found that double tropomyosin mutant cells became round and large when fixed cells from an asynchronous culture at a single time point, 4 h, well beyond the time for selective loss of cables, were examined.
We examined the double tropomyosin mutant in our assay for cell surface growth. Ideally, to examine the role of cables specifically, the assay should be performed within the first 5 min after the shift to restrictive temperature, because at 15 min patches are also depolarized. Unfortunately, this was not possible because cells grew too little during this short time for us to measure increases in surface area reliably. We were able to measure cell surface growth over a more extended time course of 64 min. Double tropomyosin mutants were shifted to the restrictive temperature for 10 or 70 min and then observed for 64 min. Bud growth was markedly inhibited, mother growth was markedly increased, and total growth was inhibited by about half (Figure 3C). These effects were similar to the effects of latrunculin. Thus, the loss of cables may account for the effect of the loss of filamentous actin on polarized growth.
The Role of Myo2p in Polarized Secretion
The class V myosin Myo2p appears to function in polarized secretion and to depend on filamentous actin. Myo2p is hypothesized to move secretory vesicles along actin cables to sites of exocytosis (Johnston et al., 1991; Govindan et al., 1995; Pruyne et al., 1998). We tested this hypothesis in several ways.
Dynamics of Myo2p-GFP Localization.
To study the relationships between Myo2p, actin, and polarized secretion in living cells, we tagged Myo2p with GFP at its C terminus by integration, which converted endogenous Myo2p to Myo2p-GFP. The GFP tag did not appear to affect the function of Myo2p. Myo2p-GFP cells grew as well as wild-type cells, and the localization of Myo2p-GFP was similar to the localization of Myo2p in wild-type cells by antibody staining (Lillie and Brown, 1994) (Figure 5, A–D).
Myo2p-GFP formed caps at incipient bud sites and at the tips of small buds, which are sites of polarized secretion (Figure 5, A and B). Movies of living cells showed that these caps were highly dynamic. The caps were not a single static structure; instead, they consisted of multiple moving particles of various sizes. Even though the particles moved constantly, their movement was confined to a small region, creating the image of a cap at any given moment. The movements of the particles appeared to be random with respect to each other, although it was difficult to track individual particles because they were so close to each other. The large sizes of the particles makes it unlikely that one particle corresponds to one vesicle transported by Myo2p. Later in the cell cycle, in medium-sized buds, the particles moved greater distances and assumed a more dispersed distribution. In large-budded cells, the particles disappeared entirely (Figure 5C). Before cell separation, Myo2p-GFP formed first a single ring and later a double ring at the bud neck (Figure 5D, arrowhead). After cell separation, remnants of this ring were observed in the daughter cell for extended periods of time, occasionally persisting until the end of the next cell cycle (Figure 5, A and D, arrows).
Dependence of Myo2p Localization on Filamentous Actin.
To investigate the role of filamentous actin in Myo2p localization, we observed the effects of latrunculin treatment on an asynchronously growing population of living MYO2-GFP cells. This analysis revealed that maintenance of Myo2p localization at the bud tip requires filamentous actin, whereas maintenance of Myo2p localization at the neck ring depends only partially on filamentous actin.
In buds, latrunculin induced the delocalization of Myo2p caps within 5–10 min (Figure 5, E–H). As the cap was lost, numerous GFP fluorescent dots appeared throughout the cytoplasm of the bud and mother (Figure 5, G and H). These dots moved randomly about the cytoplasm. The dots appeared to be brighter in cells with medium to large buds than in cells with small buds.
At the mother–bud neck, rings of Myo2p-GFP became thinner and less bright, but they could be observed even after 20–60 min of latrunculin treatment (Figure 5H, arrowhead). In control experiments, we documented that actin patches labeled with Cap1-GFP were completely lost from the neck in response to latrunculin.
Effect of Loss of Myo2p Function on Polarized Secretion.
To investigate the role of Myo2p in polarized secretion, we inhibited Myo2p in two ways and measured cell surface growth. First, we used the temperature-sensitive myo2-66 mutant, which bears a point mutation that maps to the predicted actin-binding face of the motor domain of Myo2p (Prendergast et al., 1990; Lillie and Brown, 1994). At the permissive temperature of 25°C, growth occurred and was polarized in myo2-66 cells, similar to that in wild-type cells (Figure 6A). In myo2-66 cells at the restrictive temperature of 37°C, growth was highly depolarized and total growth was decreased (Figure 6A). In myo2-66 cells at the restrictive temperature, the ratio of normalized bud growth to normalized mother growth varied from 0.3:1 to 2:1 for eight cells. The ratio was 56:1 for one cell. In control cells, the ratio varied from 8:1 to 840:1. Therefore, inhibition of Myo2p via the myo2-66 mutation caused growth to be depolarized and partially inhibited. The effect was very similar to the effect of latrunculin.
The myo2-66 mutation has certain disadvantages in this analysis. First, the mutation requires a temperature shift, which alone is sufficient to depolarize the actin cytoskeleton. Second, the mutation also induces depolarization of the actin cytoskeleton. Therefore, we used an alternative approach to inhibit Myo2p by inducing expression of the tail domain of Myo2p (MYO2DN). This approach does not require a temperature shift. In addition, the polarity of the actin cytoskeleton is not affected, unlike in myo2-66. The tail domain displaces the endogenous full-length Myo2p from its normal location (Reck-Peterson et al., 1999).
Observation of endogenous Myo2p-GFP localization in living cells revealed that localization of Myo2p in the bud was prevented or rapidly lost upon tail induction in galactose-containing medium. During 2 h of induction with galactose, no caps of Myo2p formed in prebudded and small-budded cells. In addition, during galactose induction, preexisting Myo2p-GFP caps were lost in 11 of 12 cells. In 6 cells, the Myo2p-GFP cap disappeared in 4–8 min, and in 5 cells, the cap disappeared in 24–28 min. In wild-type cells under the same conditions, the lifetime of Myo2p-GFP caps was 30–40 min.
To determine the effect of Myo2p tail expression on polarized secretion in the bud, we induced tail expression and measured cell surface growth in cells that lost Myo2p-GFP localization. Growth was nearly completely inhibited, as well as being completely depolarized, in these cells (Figure 6B). We also assessed polarized secretion in cells expressing the Myo2p tail with a quantitative assay of bud formation. Myo2p tail expression abolished bud formation (Table 2), consistent with the absence of Myo2p-GFP caps in prebudded cells.
In contrast, Myo2p tail expression had only a partial effect on localization of Myo2p at the mother–bud neck ring and on cell separation. Neck rings of Myo2p-GFP that were present when galactose was added did not disappear. None of four large-budded MYO2DN cells that had formed a Myo2p-GFP neck ring before induction lost their neck rings during 1 h of induction. On the other hand, none of these four cells underwent cell separation. In contrast, three of the large-budded control cells that had formed Myo2p-GFP neck rings before induction lost their neck rings and proceeded through cell separation within 30 min. Therefore, tail expression inhibited cell separation even though existing Myo2p was not displaced from neck rings.
On the other hand, the formation of new Myo2p-GFP neck rings was inhibited to a much greater extent by tail expression. Only 2 of 29 large-budded MYO2DN cells formed neck rings in 2 h of induction. In contrast, five of the large-budded control cells formed neck rings in 2 h.
We also quantified the effect of Myo2p tail expression on cell separation in a larger number of large-budded cells, but without simultaneous observation of the Myo2p-GFP ring. Cell separation was inhibited, but not completely, by tail expression. In galactose medium, cell separation occurred in 11% of budded MYO2DN cells and 38% of wild-type cells (Table 2). In this experiment, all large-budded cells were examined for cell separation, whereas the experiments described above examined only cells that had Myo2p-GFP neck rings. This difference explains why a greater effect on cell separation was observed in the first set of experiments. Overall, we observed a partial inhibition of cell separation with tail expression, which is consistent with the partial effects of tail expression on the formation and maintenance of Myo2p-GFP rings at the neck.
DISCUSSION
The actin cytoskeleton is considered to play a role in polarized secretion in yeast. We investigated its role by quantifying several manifestations of polarized secretion in living yeast cells. In particular, we measured growth of the cell surface in buds and mothers of individual cells over time.
First, we found that filamentous actin is necessary for polarization of secretion but not for secretion itself. We used latrunculin to depolymerize filamentous actin, which provides a significant improvement over approaches that use conditional or null mutants. In latrunculin-treated cells, growth was depolarized, vesicles were distributed randomly about the mother and bud, and bud formation and cell separation, which also require polarized secretion, did not occur. Therefore, the actin cytoskeleton is necessary for polarization of secretion.
On the other hand, our results argue against a direct role for filamentous actin in the fusion of vesicles with the plasma membrane, because overall growth persisted in latrunculin. This hypothesis had been suggested to us based on the correlation of the location of cortical actin patches with the location of polarized secretion (Adams and Pringle, 1984) and the inhibition of secretion in conditional actin mutants with the use of biochemical assays (Novick and Botstein, 1985). Instead, filamentous actin appears to be involved in the targeting of secretory vesicles to the sites of polarized secretion. Filamentous actin might be involved in either the transport of these vesicles or their retention at the sites of secretion.
Second, we asked what morphological component of the actin cytoskeleton was responsible for the role of actin in polarized secretion. Structures containing filamentous actin in yeast include cortical actin patches, cytoplasmic cables, and a ring at the mother–bud neck. The correlation between sites of polarized secretion and the locations of clusters of patches suggested the hypothesis that clustered patches may mediate polarized secretion (Adams and Pringle, 1984).
To test this hypothesis, we studied a mutant, bee1Δ(las17Δ), which showed complete depolarization of actin patches under our conditions. Secretion was rather well polarized in these cells based on several quantitative assays, especially direct measurements of cell surface growth. These results show that actin patch polarization is not necessary for polarized secretion and argue against a direct role for patches in secretion.
Next, we considered whether the cytoplasmic cables of actin filaments mediate polarized secretion. Pruyne and colleagues (1998) found that a specific and rapid loss of cables, induced in conditional tropomyosin mutants by a shift to restrictive temperature, caused the rapid loss of polarization of two proteins involved in polarized secretion, Sec4p and Myo2p. The loss of cables was also associated with a defect in polarized growth; buds failed to grow, whereas mothers grew isotropically. However, the latter observations were made on cells that were at the restrictive temperature for a relatively long time, so that patches were also depolarized. We also studied polarized growth in this conditional tropomyosin mutant; polarized growth was severely inhibited, similar to the effect of latrunculin. However, our experiments also required relatively long observation times, so that patches were also depolarized during the experiment. The results are consistent with the hypothesis that cables mediate polarized secretion, especially when coupled with our finding that polarization of patches is not necessary for polarized growth in bee1Δ(las17Δ) cells.
Cables, therefore, may serve as tracks along which secretory vesicles or the Golgi move to sites of polarized secretion (Pruyne et al., 1998). Myosin motors are obvious candidates to provide the power for such movement of vesicles. The unconventional class V myosin, Myo2p, is a likely candidate to target secretory vesicles to sites of fusion (Johnston et al., 1991; Govindan et al., 1995; Pruyne et al., 1998). In early work on Myo2, Johnston et al. (1991) observed that myo2-66 cultures accumulated large cells at the restrictive temperature, which was consistent with the hypothesis that mothers continued to grow and buds grew poorly. Here we tested this hypothesis in a more direct manner and found that inhibiting Myo2p function inhibited polarized secretion, again based on several quantitative assays that reflect different aspects of polarized secretion.
Because Myo2p contains a myosin motor domain, Myo2p may depend on actin filaments for function. Consistent with this hypothesis, we observed that the effect of the myo2-66 mutation on polarized secretion is similar to that of depolymerization of filamentous actin. Also, we found that filamentous actin is necessary to maintain Myo2p at the bud tip. In a previous publication, as discussed above, Myo2p maintenance at the bud tip was shown to depend on cytoplasmic actin cables (Pruyne et al., 1998). In another study with latrunculin, the appearance of Myo2p at the incipient bud site was partially dependent on but also partially independent of filamentous actin (Ayscough et al., 1997). In our results, the maintenance of Myo2p at the bud tip was completely dependent on filamentous actin. We feel that our results do not contradict the previous findings. Differences in experimental conditions or in the mechanisms of appearance versus maintenance may account for the somewhat different observations. First, we assayed the maintenance of Myo2p-GFP in the bud tip, whereas the previous work examined the initial localization, or appearance. Second, in the previous work, the initial localization of Myo2p-GFP was compromised, although not completely abolished, in response to latrunculin treatment (Ayscough et al., 1997).
Myo2p may transport vesicles along actin cables, capture vesicles at sites of polarized secretion, or both. The results of Pruyne et al. (1998) provide evidence that this myosin is involved in the polarized transport of vesicles along cables. Moreover, vertebrate myosin Va has recently been shown to be a processive motor, a property consistent with involvement in organelle transport (Mehta et al., 1999). However, analyses of pigment granule organelle movement in dilute melanocytes lacking myosin Va indicate that although myosin Va may affect organelle movements, it is most critical for retaining organelles within dendritic processes at the cell periphery, a role consistent with organelle capture (Wu et al., 1998).
In yeast, little evidence exists to allow one to independently assess transport and capture, mainly because one cannot yet visualize or identify secretory vesicles or vesicles that carry Myo2p in living cells. Another isoform of myosin V in yeast, Myo4p, colocalizes with mRNA transported from the mother to the bud (Bertrand et al., 1998; Munchow et al., 1999). In living cells, a large particle of reporter mRNA is capable of directed movement, and this movement depends on Myo4p and actin (Bertrand et al., 1998). Cables may be the tracks for this movement.
However, regarding the function of Myo2p, directed movement of secretory vesicles or Myo2p has not yet been described. Myo2p is clustered at sites of polarized secretion, which may occur because Myo2p moves there with vesicles or because it captures vesicles at the site. A capture mechanism is supported by the observations that localization of Myo2p can be independent of actin at the incipient bud site (Ayscough et al., 1997) and at the mother–bud neck (this work). On the other hand, a transport mechanism is supported by the observations that maintenance of Myo2p in the bud depends on filamentous actin (this work) and specifically on actin cables (Pruyne et al., 1998). Capture and transport mechanisms are not mutually exclusive. Both may exist, as appears to be the case for pigment granule organelles in mouse melanocytes (Wu et al., 1998).
Observation of directed movement of discrete Myo2p particles might indicate that these particles are vesicles bound to Myo2p and support the “transport” hypothesis. In our experiments, we observed that the cluster of Myo2p at the bud tip indeed consists of several particles. However, these particles appear to be too large to represent individual vesicles. Also, their movement appears to be random, not directed. This movement of Myo2p particles at the tip of the bud is similar to the movement observed for the Myo4p-mRNA particle after this particle was transported to the bud (Bertrand et al., 1998). Movement of Myo2p particles indicates that they are not anchored in the bud tip.
In addition, we did not observe any cases of directed movement of Myo2p particles from the mother into the bud. Of course, this lack of observation does not imply that directed movement of vesicles propelled by Myo2p does not exist. First, the amount of Myo2p-GFP associated with one vesicle may be too small to produce observable fluorescence. Second, because of the presence of Golgi in the bud (Preuss et al., 1992), the distances traveled by vesicles might be quite short compared with the distance traveled by Myo4p-mRNA particles from the mother to the bud. Short movements of Myo2p-powered vesicles may be obscured by the relatively bright fluorescence from the Myo2p cluster in the bud.
The dependence of Myo2p localization on the actin cytoskeleton and the similarity of the myo2-66 mutant phenotype and the latrunculin-induced phenotype suggest that Myo2p binds to and requires actin for its function. On the other hand, expression of the MYO2DN construct led to a more severe phenotype, in which the overall level of growth was reduced to near zero. One potential explanation for the discrepancy between the phenotypes of the MYO2DN and myo2-66 mutants is that the myo2-66 mutant may cause only a partial loss of function at the restrictive temperature, whereas the tail expression causes a more complete loss of function. If so, then Myo2p must have some other function in secretion independent of filamentous actin, perhaps at the site of secretion. A second potential explanation for the discrepancy is that latrunculin may not disassemble all filamentous actin in the cell. A small fraction of filamentous actin may be resistant to the action of latrunculin. Therefore, the ability of the cells treated with latrunculin to secrete may be due to the presence of some remnants of filamentous actin. Finally, the expressed tail may interact with a molecule with which Myo2p normally does not interact because the tail is present at higher molar concentrations than Myo2p.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants GM47337 to J.A.C., DK35387 to M.S.M., GM35370 and CA46128 to P.J.N., and CA47135 to P. Crews for the preparation of latrunculin A. J.A.C. was an Established Investigator of the American Heart Association. N.B.E. was supported by a fellowship from the Human Frontiers Program.
Footnotes
REFERENCES
- Adams AEM, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ASH1 mRNA particles in living yeast. Mol Cell. 1998;2:437–445. doi: 10.1016/s1097-2765(00)80143-4. [DOI] [PubMed] [Google Scholar]
- Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E, Pringle JR. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J Cell Biol. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkaš V, Kovařiḱ J, Košinová A, Bauer Š. Autoradiographic study of mannan incorporation into the growing cell walls of Saccharomyces cerevisiae. J Bacteriol. 1974;117:265–269. doi: 10.1128/jb.117.1.265-269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field C, Schekman R. Localized secretion of acid phosphatase reflects the pattern of cell surface growth in Saccharomyces cerevisiae. J Cell Biol. 1980;86:123–128. doi: 10.1083/jcb.86.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger FP, Novick P. Spatial regulation of exocytosis: lessons from yeast. J Cell Biol. 1998;142:609–612. doi: 10.1083/jcb.142.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan B, Novick P. Development of cell polarity in budding yeast. J Exp Zool. 1995;273:401–424. doi: 10.1002/jez.1402730505. [DOI] [PubMed] [Google Scholar]
- Johnston GC, Prendergast JA, Singer RA. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser CA, Gimeno RE, Shaywitz DA. Protein secretion, membrane biogenesis, and endocytosis. In: Pringle JR, Broach JR, Jones EW, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces: Cell Cycle and Cell Biology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1997. pp. 91–228. [Google Scholar]
- Karpova TS, McNally JG, Moltz SL, Cooper JA. Assembly and function of the actin cytoskeleton of yeast: relationships between cables and patches. J Cell Biol. 1998a;142:1501–1517. doi: 10.1083/jcb.142.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova TS, Moltz SL, Riles LE, Gueldener U, Hegemann JH, Veronneau S, Bussey H, Cooper JA. Depolarization of the actin cytoskeleton is a specific phenotype in Saccharomyces cerevisiae. J Cell Sci. 1998b;111:2689–2696. doi: 10.1242/jcs.111.17.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Simons K. Post-Golgi biosynthetic trafficking. J Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- Kilmartin JV, Adams AEM. Structural rearrangements of tubulin and actin during the cell cycle of the yeast. Saccharomyces. J Cell Biol. 1984;98:922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. Bee1, a yeast protein with homology to Wiskott-Aldrich syndrome protein, is critical for the assembly of cortical actin cytoskeleton. J Cell Biol. 1997;136:649–658. doi: 10.1083/jcb.136.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie SH, Brown SS. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J Cell Biol. 1994;125:825–842. doi: 10.1083/jcb.125.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Bretscher A. Characterization of TPM1 disrupted yeast cells indicates an involvement of tropomyosin in directed vesicular transport. J Cell Biol. 1992;118:285–299. doi: 10.1083/jcb.118.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AD, Rock RS, Rief M, Spudich JA, Mooseker MS, Cheney RE. Myosin-V is a processive actin-based motor. Nature. 1999;400:590–593. doi: 10.1038/23072. [DOI] [PubMed] [Google Scholar]
- Mitchison JM. The growth of single cells. II. Saccharomyces cerevisiae. Exp Cell Res. 1958;15:214–221. doi: 10.1016/0014-4827(58)90077-6. [DOI] [PubMed] [Google Scholar]
- Munchow S, Sauter C, Jansen RP. Association of the class V myosin Myo4p with a localized messenger RNA in budding yeast depends on She proteins. J Cell Sci. 1999;112:1511–1518. doi: 10.1242/jcs.112.10.1511. [DOI] [PubMed] [Google Scholar]
- Novick P, Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985;40:405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Prendergast JA, Murray LE, Rowley A, Carruthers DR, Singer RA, Johnston GC. Size selection identifies new genes that regulate Saccharomyces cerevisiae cell proliferation. Genetics. 1990;124:81–90. doi: 10.1093/genetics/124.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss D, Mulholland J, Franzusoff A, Segev N, Botstein D. Characterization of the Saccharomyces Golgi complex through the cell cycle by immunoelectron microscopy. Mol Biol Cell. 1992;3:789–803. doi: 10.1091/mbc.3.7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle JR, Preston RA, Adams AEM, Stearns T, Drubin DG, Haarer BK, Jones EW. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- Pruyne DW, Schott DH, Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J Cell Biol. 1998;143:1931–1945. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- Reck-Peterson SL, Novick PJ, Mooseker MS. The tail of a yeast class V myosin, Myo2p, functions as a localization domain. Mol Biol Cell. 1999;10:1001–1017. doi: 10.1091/mbc.10.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Novick PJ. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987;49:527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Tkacz JS, Lampen JO. Wall replication in Saccharomyces species: use of fluorescein-conjugated concanavalin A to reveal the site of mannan insertion. J Gen Microbiol. 1972;72:243–247. doi: 10.1099/00221287-72-2-243. [DOI] [PubMed] [Google Scholar]
- Tkacz JS, Lampen JO. Surface distribution of invertase in growing Saccharomyces cells. J Bacteriol. 1973;113:1073–1075. doi: 10.1128/jb.113.2.1073-1075.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddle JA, Karpova TS, Waterston RH, Cooper JA. Movement of cortical actin patches in yeast. J Cell Biol. 1996;132:861–870. doi: 10.1083/jcb.132.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Collins RN, Novick P. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Bowers B, Rao K, Wei Q, Hammer JA. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. J Cell Biol. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.