Abstract
Cryptococcal phospholipase (PLB1) is a secreted enzyme with lysophospholipase hydrolase and lysophospholipase transacylase activities. To investigate the role of PLB1 in the evasion of host immune responses, we characterized pulmonary immune responses to the parental (H99), the plb1 mutant, and the plb1rec reconstituted mutant strains of Cryptococcus neoformans in mice. PLB1 was required for virulence during infection acquired via the respiratory tract. Mice infected with either H99 or the plb1rec strain generated a nonprotective inflammatory response with subsequent eosinophilia, while mice infected with the plb1 mutant generated a protective immune response that controlled the infection. Because PLB1 is believed to facilitate virulence through host cell lysis, we examined the interaction of these strains with macrophages. The plb1rec mutant exhibited decreased survival during coculture with macrophages. One factor which may be involved in the survival of yeast in the presence of macrophages is fungal eicosanoid production. Host eicosanoids have been shown to down-modulate macrophage functions. plb1 exhibited a defect in eicosanoid production derived from exogenous arachidonoyl-phosphatidylcholine, suggesting that PLB1 is required for the release of arachidonic acid from phospholipids. These data suggest that cryptococcal PLB1 may act as a virulence factor by enhancing the ability to survive macrophage antifungal defenses, possibly by facilitating fungal eicosanoid production.
Secreted phospholipases have been implicated in virulence in a number of fungal species, presumably via destruction of host cell membranes and subsequent lysis (11). Cryptococcus neoformans is an opportunistic pathogenic yeast acquired via the respiratory tract. C. neoformans possesses a phospholipase, phospholipase B1 (PLB1), with PLB, lysophospholipase hydrolase, and lysophospholipase transacylase activities (4). This phospholipase can degrade phospholipid components of cellular membranes and lung surfactant (4). Furthermore, phospholipase activity among different strains of C. neoformans correlates with virulence in mice (3). Phospholipases have been proposed to play a role in the virulence of fungal pathogens; however, the underlying mechanism has yet to be elucidated.
Host phospholipases possess many functions. An important function related to the regulation of immune responses is in the liberation of fatty acid precursors (arachidonic acid [AA], dihomo-γ-linolenic acid, or eicosanopentaenoic acid) for host eicosanoid synthesis (23). Eicosanoids are potent regulators of host immune responses and include the prostaglandins (PGs) and leukotrienes (LTs). PGs can inhibit Th1-type immune responses, chemokine production, phagocytosis, and lymphocyte proliferation (1, 13, 18, 23, 27, 29, 31). PGs can promote Th2-type responses and tissue eosinophilia (8, 18, 23, 28). LTs are most notable for their involvement in the recruitment of leukocytes (neutrophils and eosinophils) (10, 12). Members of our laboratory recently reported the production of bioactive eicosanoids by C. neoformans (21). However, the enzymes involved in fungal eicosanoid synthesis have yet to be identified.
Clearance of a pulmonary C. neoformans infection requires the development of protective cell-mediated immune responses. Chronic or disseminating infections will result if the T1-T2 balance of immunity is shifted away from T1 toward T2-type responses. Our objective was to determine whether a C. neoformans phospholipase is involved in the evasion of host immune responses and fungal eicosanoid production. The cloning and site-directed disruption of the PLB1 (phospholipase) gene was previously reported, with the resulting null mutant being significantly less virulent than the parent strain in both mice and rabbits (6). However, the null mutant (the plb1mutant) exhibited no apparent phenotypic defects in known cryptococcal virulence factors such as laccase activity, urease activity, growth at 37°C, and capsule production. Virulence was restored in a reconstituted plb1 mutant (the plb1rec mutant), satisfying molecular Koch's postulates for virulence factors (9). Using the plb1 mutant and the plb1rec mutant strain, we investigated the role of this gene in virulence and in modulation of the murine pulmonary immune responses after infection via the respiratory tract. Further, we identified a novel role for PLB1 in fungal eicosanoid synthesis.
MATERIALS AND METHODS
C. neoformans.
The H99, plb1, and plb1rec isogenic strains were generated as previously described (6). Briefly, a ura5 auxotroph of H99 was transformed by using biolistic DNA delivery with a knockout construct containing URA5 inserted into PLB1. A strain with a single insertion that also exhibited a growth rate, melanin production, and capsule size similar to those of H99 was chosen for analysis (the plb1 mutant). Reconstitution of the plb1 strain was performed by transforming the mutant strain with a construct containing the entire PLB1 gene and the selectable antibiotic resistance gene HygB (7). The plb1rec strain was chosen because it exhibited a growth rate, melanin production, capsule size, urease activity, and PLB production similar to those of strain H99. Reconstitution of PLB1 also resulted in the complete restoration of all extracellular phospholipase activities as measured by radiometric assays (6). For infection, yeast cells were grown to stationary phase (48 h) at 37°C in Sabouraud dextrose broth (SDB; 1% neopeptone, 2% dextrose; Difco, Detroit, Mich.) while being shaken. The cultures were then washed in nonpyrogenic saline (Abbott Laboratories, Chicago, Ill.), and cells were counted on a hemocytometer and diluted to 3.3 × 105 CFU/ml in sterile nonpyrogenic saline. The inoculum was plated out to monitor the number of CFU delivered.
Mice.
Female CBA/J mice (weight, 18 ± 2 g) were purchased from Jackson Laboratories (Bar Harbor, Maine) and housed under specific-pathogen-free conditions in enclosed filter-top cages. Sterile food and water were given ad libitum. The mice were maintained by the Unit for Laboratory Animal Medicine at the University of Michigan (Ann Arbor), and protocols were approved by an animal institutional review board.
Intratracheal inoculation.
Infection was established by intratracheal inoculation with 104 CFU of C. neoformans. Four animals per group were infected during two independent experiments. Mice were anesthetized with ketamine-xylazine solution (consisting of 2.5 mg of ketamine [Fort Dodge Animal Health, Fort Dodge, Iowa] per mouse and 0.1 g of xylazine [Lloyd Laboratories, Shenandoah, Iowa] per mouse) and restrained on a small board. A small incision was made in the skin over the trachea, and the underlying tissue was separated. A tuberculin syringe (Monoject, St. Louis, Mo.) was filled with a diluted C. neoformans culture, and a 30-gauge needle (Becton Dickinson, Rutherford, N.J.) was attached and bent. The needle was inserted into the trachea, and a 30-μl inoculum (containing 104 CFU) was delivered. The skin was sutured with a cyanoacrylate adhesive, and the mice recovered with no visible trauma. Aliquots of the inoculum were analyzed to monitor the number of CFU delivered.
Lung leukocyte isolation.
Mice were euthanized by the administration of CO2. The lungs were excised, minced, and enzymatically digested for 30 min at 37°C with 15 ml of digestion buffer (RPMI 1640, 10% fetal calf serum, antibiotics, a 1-mg/ml concentration of collagenase [Boehringer Mannheim Biochemicals, Chicago, Ill.] per lung, and a 30-μg/ml concentration of DNase [Sigma Chemical Co., St. Louis, Mo.]) per lung. Cells were further dispersed by drawing them up and down through the bore of a 10-ml syringe. A 100-μl aliquot was removed for CFU assay. The cell suspension was pelleted, and erythrocytes were lysed by incubation in an ice-cold NH4Cl buffer (0.829% NH4Cl, 0.1% KHCO3, 0.0372% Na2EDTA [pH 7.4]; Sigma). Excess RPMI 1640 was added to make the solution isotonic, and the cells were pelleted and resuspended in complete medium (RPMI 1640, 10% fetal calf serum [Life Technologies], 5 × 10−5 M 2-mercaptoethanol, sodium pyruvate, nonessential amino acids, glutamine, and antibiotics [Sigma]). Cell concentrations were determined by counting the cells after trypan blue staining.
Whole-lung homogenates.
Mice were euthanized with CO2. The lungs were excised and placed in 1 ml of homogenization buffer (consisting of distilled water and a 1:50 protease inhibitor tablet; Boehringer Mannheim Biochemicals). The lungs were homogenized mechanically with a Tissue-tearor (Biospec Products, Bartlesville, Okla.). A 100-μl aliquot was removed for CFU assay. The homogenate was then pelleted, and the supernatant was passed through a 0.45-μm-pore-size syringe filter (Nalgene, Rochester, N.Y.). Homogenate supernatants were stored at −20°C.
Harvesting of tissues.
Extrapulmonary organs were harvested subsequent to removal of lungs. Lung-associated lymph nodes were collected by dissecting the nodes from the junction of the azygos vein and the superior vena cava. Brains were collected by first removing the top of the cranium and excising the brain from the brain stem. Organs were placed in tubes containing 2 ml of sterile water and homogenized mechanically with a Tissue-tearor (Biospec Products).
CFU assay.
Aliquots of the lung digests, whole-lung homogenates, whole-brain homogenates, and lung-associated lymph node homogenates were plated out on Sabouraud dextrose agar (Difco) in 10-fold dilutions and incubated at room temperature. Colonies were counted 2 to 3 days later, and the numbers of CFU per organ were calculated.
Cell staining.
Leukocyte differentials (neutrophils, eosinophils, macrophages, and monocytes or lymphocytes) were visually counted after Wright-Giemsa staining of lung leukocyte samples cytospun onto glass slides (Shandon Cytospin, Pittsburgh, Pa.). The percentage of a leukocyte subset was multiplied by the total number of leukocytes to yield the absolute number of that leukocyte subset.
Histological analysis.
The lungs were excised from the experimental animals at various times after infection. The lungs were perfused with phosphate-buffered saline, inflated, and fixed with 10% buffered formalin. The fixed lungs were then sectioned and stained with mucicarmine, which stains the polysaccharide capsule of C. neoformans. The sections were examined for the presence of leukocytic infiltrate and cryptococcal cells.
Quantitation of cytokine levels in whole-lung homogenates by ELISA.
Supernatants from whole-lung homogenates were assayed in duplicate for murine interleukin 12 (IL-12), monocyte chemoattractant protein 1 (MCP-1), IL-10, gamma interferon, IL-4, and tumor necrosis factor alpha (TNF-α) by using monoclonal enzyme-linked immunosorbent assay (ELISA) kits (PharMingen, San Diego, Calif.) as previously described (16). The sensitivity limit for detection was approximately 15 to 40 pg/ml.
Determination of eicosanoid concentration from whole-lung homogenates by ELISA.
Supernatants from whole-lung homogenates were filtered with 0.45-μm-pore-size syringe filters (Nalgene). Lipids were purified from filtered lung homogenate supernatants with Sep-Pac C18 cartridges according to the instructions of the manufacturer (Waters Corp., Milford, Mass.). Eluted samples were dried under a continuous flow of N2 and stored at −80°C. Samples were resuspended in enzyme immunoassay (EIA) buffer (Cayman Chemicals, Ann Arbor, Mich.) and were analyzed for prostaglandin E2 (PGE2), PGF2α, and cysteinyl LT (cysLT) with monoclonal EIA kits (Cayman Chemicals). Background signal from uninfected lung homogenates was subtracted from the results.
Determination of PG concentration by ELISA.
C. neoformans H99 and the plb1 and plb1rec mutants were grown in SDB at 25°C while being shaken. PG production was measured with a PG screening EIA kit (Cayman Chemicals). This ELISA detects PGE2, PGD2, and thromboxane B2 along with PGE1, PGE3, PGF1α, PGF2α, and PGF3α. It does not detect PGA, PGB1, 15-keto PGE2, 13,14-dihydro-15-keto PGF2α, or misopristol.
Determination of eicosanoid concentration from phospholipids. C. neoformans H99 and the plb1 and plb1rec mutants were grown in SDB at 25°C for 3 days. Cultures were centrifuged and resuspended in RPMI 1640 containing 1 mM AA (Cayman Chemicals) or 1 mM arachidonoyl-phosphatidylcholine, which is a symmetric phospholipid containing AA at both the sn-1 and sn-2 positions (Avanti Polar Lipids, Alabaster, Ala.). Cultures were incubated for an additional 2 h at 37°C. Culture supernatants from 3-day SDB-, AA-, and arachidonoyl-phosphatidylcholine-fed yeast were analyzed for PGE2, PGF2α, and cysLTs (LTC4, LTD4, and LTE4) with monoclonal EIA kits (Cayman Chemicals).
Macrophage antifungal assay.
The MH-S cell line (ATCC CRL-2019), a murine alveolar macrophage (AM) cell line, was plated out into 24-well tissue culture dishes. The MH-S cell line displays many of the properties of primary AM, including functional and phenotypic heterogeneity (25). MH-S macrophages are adherent, phagocytic, esterase positive, and peroxidase negative and suppress leukocyte activation (19, 30). Similar to primary AM, MH-S macrophages express Mac-1 antigen, major histocompatibility complex class II, the CR3 receptor, and the Fc receptor (19). Cells were allowed to rest for 24 h at 37°C prior to infection. C. neoformans strain H99 and the plb1 and plb1rec mutants were grown in SDB for 24 h at 37°C. Yeast was opsonized in 100% fresh mouse serum for 1 h at 37°C, and macrophages were infected at a multiplicity of infection of 0.1. Control wells contained equivalent amounts of either yeast cells or macrophages alone in culture medium. At 24 h postinfection, culture supernatants were removed and saved, and macrophages were lysed with sterile H2O for 15 min. Macrophage lysates and culture supernatants were harvested and analyzed for numbers of CFU by plating serial dilutions on Sabouraud dextrose agar.
Statistical analysis.
Student's t test (two-tailed, unequal levels of variance) was used to analyze the significance of differences between the experimental groups. Data with a P value of 0.05 or less were considered to be significant.
RESULTS AND DISCUSSION
Role of cryptococcal PLB1 in virulence during pulmonary infection.
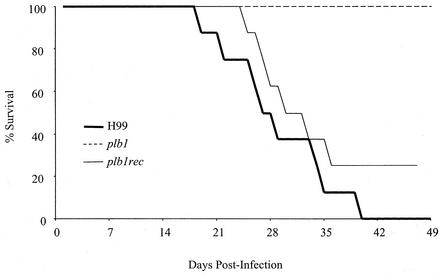
CBA/J mice were inoculated intratracheally with 104 CFU of C. neoformans strain H99 or the plb1 or plb1rec mutant and were monitored for survival on a daily basis (Fig. 1). By day 18 postinfection, mice infected with parental strain H99 began to die, with a 100% mortality rate reached by day 40. In contrast, no mortality was observed in mice infected with the plb1 mutant strain by day 49 postinfection. Mice infected with the plb1rec mutant began to die by day 24 postinfection, with a 75% mortality rate reached by day 36 postinfection. The mean survival time of mice infected with the plb1 mutant was >72 days, which was significantly longer than that of either mice infected with H99 (29.0 days) or mice infected with the plb1rec mutant (40.4 days) (P < 0.005). These results indicate that PLB1 is required by C. neoformans for virulence during a pulmonary infection.
FIG. 1.
Effect of PLB1 on survival of mice following infection. CBA/J mice were infected intratracheally with 104 CFU of C. neoformans strain H99 or the plb1 or plb1rec mutant. Mice were monitored daily for survival. Survivors were euthanized at 10 weeks postinfection.
Role of PLB1 in the growth of C. neoformans in the lungs.
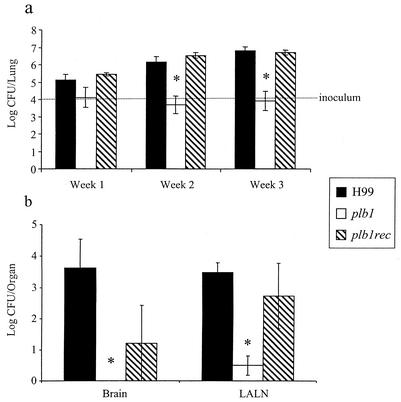
Our first objective was to determine whether PLB1 was required for the growth and/or survival of C. neoformans in the lungs. CBA/J mice were infected intratracheally with 104 CFU of C. neoformans parent strain H99, the plb1 mutant, or the plb1rec mutant, and numbers of CFU were analyzed at weeks 1, 2, and 3. Time points after week 3 were not examined because significant numbers of mice infected with either H99 or the plb1rec mutant died between weeks 3 and 4 (Fig. 1). From 0 to 3 weeks postinfection, the number of pulmonary CFU increased 1,000-fold in the H99- and plb1rec mutant-infected mice (Fig. 2a). In contrast, the numbers of pulmonary CFU in the plb1 mutant-infected mice remained at or below the level inoculated at day 0 (Fig. 2a). At earlier time points (weeks 1 and 2), a greater percentage of animals infected with H99 and the plb1rec mutant contained detectable organisms in extrapulmonary sites (data not shown). At week 3, numbers of pulmonary lymph node CFU and brain CFU for plb1 were also significantly lower than those for the PLB1-expressing strains of C. neoformans (Fig. 2b). This finding suggests that the plb1 mutant exhibits both a defect in dissemination and an inability to grow in the lymph nodes or brain following dissemination. Through day 70, the plb1 mutant was still readily detectable in the lungs (mean, 5.5 ± 1.5 log CFU) but dissemination was minimal (2.08 ± 0.9 log brain CFU). These results indicate that PLB1 is not required for the survival of C. neoformans in the lungs. However, PLB1 is required for the virulence of C. neoformans by promoting both its growth in the lungs and its extrapulmonary dissemination and growth.
FIG. 2.
Effect of PLB1 on pulmonary burden (a) and organ burden (b). CBA/J mice were infected intratracheally with 104 CFU of C. neoformans strain H99 or the plb1 or plb1rec mutant. Lungs were excised at weeks 1, 2, and 3 postinfection, and the cryptococcal burden was determined. Results are expressed as the mean CFU per organ ± standard errors of the means (SEM). The number of mice per time point pooled from two separate experiments was 7 to 8. *, P < 0.05 (values for H99 compared with values for the plb1 mutant). LALN, lung-associated lymph nodes.
Role of PLB1 in the pulmonary inflammatory response to C. neoformans.
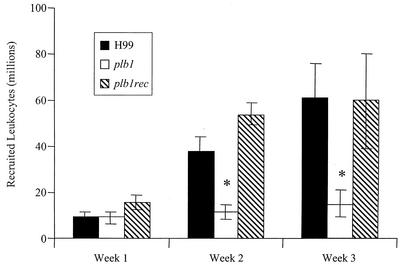
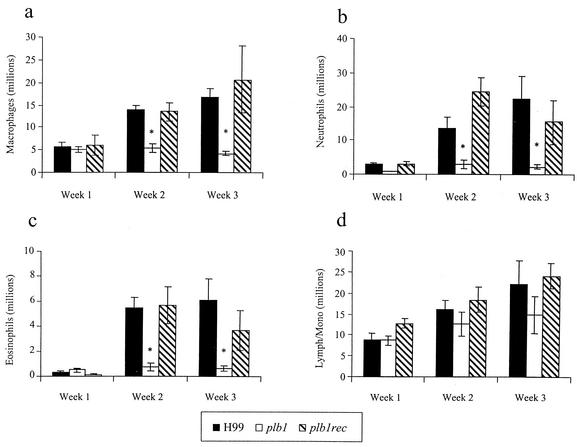
To investigate the nature of the inflammatory response in the lungs, the leukocytic infiltrate was examined quantitatively and qualitatively. At weeks 2 and 3, total numbers of leukocytes in the lungs of mice infected with the plb1 mutant were significantly less than those in the lungs of mice infected with H99 and the plb1rec mutant (Fig. 3). Of the leukocyte subsets, there were significantly fewer macrophages, neutrophils, and eosinophils in the lungs of mice infected with the plb1 strain (Fig. 4). One interpretation of these data is that the smaller organism loads in the lungs of plb1 mutant-infected mice produced less inflammation. However, the pulmonary burdens of the three cryptococcal strains were within 10-fold of each other at the onset of the inflammatory response (week 1), and this relative difference in numbers of CFU did not affect the magnitude of the pulmonary inflammatory response in other studies (G. B. Huffnagle et al., unpublished data). Further insight is provided by histological analysis of the lungs (Fig. 5). At week 2 postinfection, there were fewer cryptococci and much less inflammation in the lungs of mice infected with the plb1 strain than in those infected with H99 or the plb1rec strain. Further, few if any extracellular plb1 organisms were found in areas of inflammation, while large numbers of both H99 and plb1rec organisms were growing in areas of inflammation (evidence of budding). In addition, areas of the lung devoid of inflammatory cell infiltration contained more extracellular plb1 organisms than areas populated with inflammatory cells (data not shown). Thus, at this point, we favored the alternative explanation that only a limited inflammatory response is necessary to control the growth of PLB1-deficient C. neoformans.
FIG. 3.
Effect of PLB1 on pulmonary leukocyte recruitment. CBA/J mice were infected intratracheally with 104 CFU of C. neoformans strain H99 or the plb1 or plb1rec mutant. Lungs were excised at weeks 1, 2, and 3 postinfection. Leukocytes were isolated from whole lungs by enzymatic digestion and mechanical dispersion. The number of recruited leukocytes in an infected mouse was equal to the total number of leukocytes in the infected mouse minus the mean number of leukocytes in uninfected mice. Results are expressed as the mean number of leukocytes per lung ± the SEM. The number of mice per time point pooled from two separate experiments was 7 to 8. *, P < 0.01 (values for H99 compared with values for the plb1 mutant).
FIG. 4.
Effect of PLB1 on the recruitment of leukocyte subsets into the lungs of mice. CBA/J mice were infected intratracheally with 104 CFU of C. neoformans strain H99 or the plb1 or plb1rec mutant. Lungs were excised at weeks 1, 2, and 3 postinfection. Leukocytes were isolated from whole lungs by mechanical and enzymatic dispersion and then phenotyped by Wright-Giemsa staining of samples cytospun onto slides. Subsets included macrophages (a), neutrophils (b), eosinophils (c), and grouped lymphocytes and monocytes (Lymph/Mono) (d). The percentage of a leukocyte subset was multiplied by the total number of leukocytes to yield the absolute number of that leukocyte subset. Results are expressed as the mean numbers of leukocytes per mouse ± the SEM.The number of mice per time point pooled from two separate experiments was 7 to 8. *, P < 0.01 (values for H99 compared with values for the plb1 mutant).
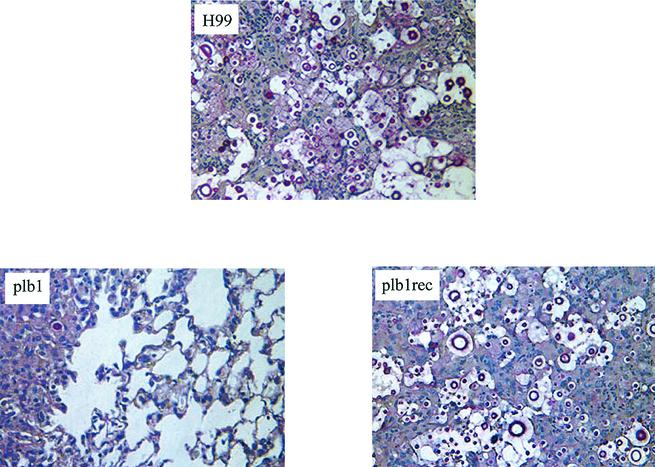
FIG. 5.
Photomicrographs of the lungs of mice infected with C. neoformans strain H99 or the plb1 or plb1rec mutant at week 2 postinfection. CBA/J mice were infected intratracheally with 104 CFU of C. neoformans strain H99 or the plb1 or plb1rec mutant. Lungs were excised at week 2 postinfection and fixed in 10% buffered formalin. Lung sections were stained with mucicarmine, which stains the polysaccharide capsule of C. neoformans. The increased leukocytic infiltrate of lungs of H99- and plb1rec mutant-infected mice compared with that of plb1 mutant-infected mice is consistent with total leukocyte recruitment reported in Fig. 3. A pervasive cryptococcal burden and inflammation are evident within the lungs of mice infected with H99 and the plb1rec mutant but not in plb1 mutant-infected lungs. Magnification, ×400.
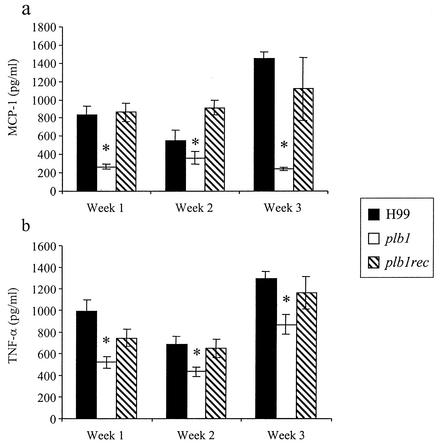
Our next objective was to determine whether the expression of PLB1 altered the pulmonary inflammatory response to C. neoformans. To determine if PLB1 expression altered inflammatory cytokine production, lung homogenates from all three groups of mice were analyzed (Fig. 6). Statistically equivalent amounts of IL-12, IL-10, IL-4, and gamma interferon at weeks 1 to 3 (P > 0.05; data not shown) were detected in lung homogenates from mice infected with H99, the plb1 mutant, or the plb1rec mutant. However, at week 1 the TNF-α and MCP-1 production in lung homogenates from mice infected with the plb1 strain was statistically significantly less than that in mice infected with H99 (Fig. 6). TNF-α production is required for neutrophil recruitment during a pulmonary cryptococcal infection (A. Herring, submitted for publication), while MCP-1 is a well-known macrophage chemotactic factor (24). These results help to explain the lack of neutrophil and macrophage recruitment observed with the plb1 strain.
FIG. 6.
Comparison of MCP-1 (a) and TNF-α (b) levels in lung homogenates prepared from mice infected with H99 or the plb1 or plb1rec mutant. The cytokines were measured by ELISA. Results are expressed as means ± SEM. Four mice per time point were assayed in duplicate experiments. *, P < 0.01 (relative to values for H99-infected mice).
Role of PLB1 in cryptococcus-macrophage interactions.
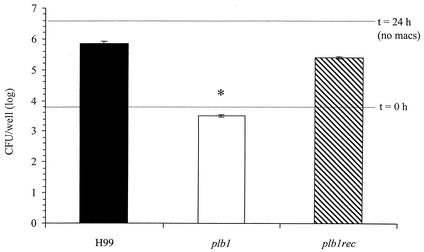
The next objective was to determine whether PLB1 was required for the evasion of alveolar macrophage fungicidal activity. It was previously reported that there was no difference in levels of phagocytosis by the macrophage cell line J774 among H99, the plb1 mutant, and the plb1rec mutant but that there was a defect in intracellular budding following phagocytosis of the plb1 mutant (6). We cocultured the H99, plb1, and plb1rec strains with the murine AM-derived cell line MH-S in the presence of fresh serum (source of C3). AMs can readily phagocytize C3b-opsonized C. neoformans but cannot exert fungicidal activity unless they are activated (2). Levels of phagocytosis of all three C. neoformans strains by the MH-S cell line were statistically equivalent (P > 0.05) as measured by the percentages of uptake at 1 h postinfection (H99, 3.2%; plb1 strain, 3.5%; and plb1rec strain, 3.6%). Similar to primary AM, the AM cell line could phagocytize the two PLB1-expressing C. neoformans strains (H99 and the plb1rec mutant) but did not seem to inhibit their growth (Fig. 7). There were 2.5 logs more H99 and plb1rec organisms at 24 h than there were of the input inoculum, suggesting that these strains grow well in the presence of macrophages. However, the plb1 mutant strain was phagocytized and its growth was inhibited by the AM cell line (Fig. 7 and data not shown). The doubling times of all three strains in RPMI 1640 alone were similar (H99, 2.84 h; plb1 strain, 2.79 h; plb1rec strain, 2.82 h), ruling out the possibility that a defect in the growth of the PLB1 mutant strain accounted for the reduced numbers observed in the presence of macrophages at 24 h. Thus, PLB1 appears to be required for the evasion of AM-mediated growth inhibition.
FIG. 7.
Effect of PLB1 on cryptococcal survival during coculture with AM. Murine AM cell line MH-S was plated out into tissue culture dishes. Cells were allowed to rest for 24 h at 37°C prior to infection. C. neoformans strain H99 and the plb1 and plb1rec mutants were grown in SDB for 24 h at 37°C. Yeast cells were opsonized in 100% fresh mouse serum for 1 h at 37°C, and macrophages were infected at a multiplicity of infection of 0.1. Phagocytosis data for all three C. neoformans strains by the MH-S cell line were statistically equivalent (P > 0.5) as measured by percentages of uptake at 1 h postinfection (H99, 3.2%; plb1 mutant, 3.5%; and plb1rec mutant, 3.6%). Bars represent combined numbers of CFU in macrophage lysates and supernatants, which were harvested at 24 postinfection. In separate wells, C. neoformans was cultured in the absence of macrophages (no macs) and analyzed for numbers of CFU at 24 h (upper dotted line). All strains exhibited similar levels of growth in complete media. Results are expressed as the mean numbers of CFU per well ± the SEM for triplicate cultures. The experiments were repeated two times with similar results. *, P < 0.01 (relative to values for H99-infected mice).
What is the mechanism of action of PLB1?
C. neoformans secretes one phospholipase with multiple functions. PLB1 can release fatty acids from phospholipids at both the sn-1 and sn-2 positions (PLB activity), release fatty acids attached to a lysophospholipid (lysophospholipase activity), and attach free fatty acids to lysophospholipids (transacylase activity) (5). It is likely that the phospholipid degradation activity of PLB will damage host cell membranes and lead to cell lysis. It has been suggested that phospholipase activity in C. neoformans culture supernatants is responsible for their toxicity toward neutrophils (26). In our studies, PLB1 production by C. neoformans did cause a slight but statistically insignificant decrease in macrophage viability (78% for H99 and 82% for the plb1rec strain versus 99% for the plb1 strain). That difference alone seemed unlikely to account for the 100-fold increase in fungistatic activity of the AM toward the PLB1-deficient cryptococci compared to the fungistatic activities of other strains. Thus, we hypothesized that other activities of PLB1 might account for changes in macrophage activity.
PLB1 may provide an alternative nutrient source, especially during intracellular growth. For instance, fungi are able to metabolize fatty acids as a sole carbon source and mutants of C. albicans that cannot utilize fatty acids as an energy source (isocitrate lyase mutants) are less virulent (17). However, despite the observation that isocitrate lyase is up-regulated in C. neoformans during infection, icl1 mutants are not attenuated for virulence in either mice or rabbits (J. Perfect, unpublished data). On the other hand, it has previously been reported that the time before the onset of budding following phagocytosis is longer for the plb1 strain than for H99 and the plb1rec strain (6). One possibility is that PLB1 may liberate fatty acids more efficiently for the energy utilization that is needed for optimal intracellular survival. However, the H99, plb1rec, and plb1 strains are all able to grow intracellularly (6) and a block in the glyoxylate pathway had no apparent impact on in vivo growth (J. Perfect, unpublished data). Thus, another possibility is that PLB1 plays a role in the production of a virulence factor that affects macrophage activation, which is crucial to anticryptococcal activity.
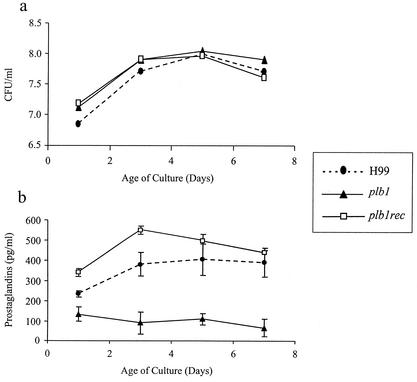
We recently reported that C. neoformans can produce a variety of PGs and LTs. Since PLB1 may play a role in the elaboration of the fatty acid precursors (e.g., AA) required for PG and LT production, we assayed whether PLB1 was required for PG production. Production of PGs by the H99, plb1, and plb1rec strains was assessed over time in nutrient-rich SDB. A polyclonal PG screening ELISA was used to analyze total PG levels. This ELISA detects PGE2, PGD2, and thromboxane B2 along with PGE1, PGE3, PGF1α, PGF2α, and PGF3α. It does not detect PGA, PGB1, 15-keto PGE2, 13,14-dihydro-15-keto PGF2α, or misopristol. Levels of growth of all three strains in SDB were indistinguishable, but there was decreased production of PGs in the PLB1-deficient C. neoformans strain (Fig. 8) (P < 0.01). Reconstitution of plb1 with a wild-type copy of the PLB1 gene restored PG production to the levels seen in the wild-type strain. These data suggest that PLB1 is involved in PG production by C. neoformans in SDB.
FIG. 8.
Effect of PLB1 on cryptococcal PG production and growth rate. C. neoformans strain H99 and the plb1 and plb1rec mutants were grown in SDB at 25°C while being shaken. Culture supernatants were analyzed at various time points for PG production (a) and culture CFU concentration (b). PG production was measured with a PG screening EIA kit (Cayman Chemicals). This ELISA detects PGE2, PGD2, and thromboxane B2 along with PGE1, PGE3, PGF1α, PGF2α, and PGF3α. It does not detect PGA, PGB1, 15-keto PGE2, 13,14-dihydro-15-keto PGF2α, or misopristol. Results are expressed as mean numbers of CFU per well ± SEM for triplicate cultures. The experiments were repeated two times with similar results. *, P < 0.01 (relative to values for H99-infected mice).
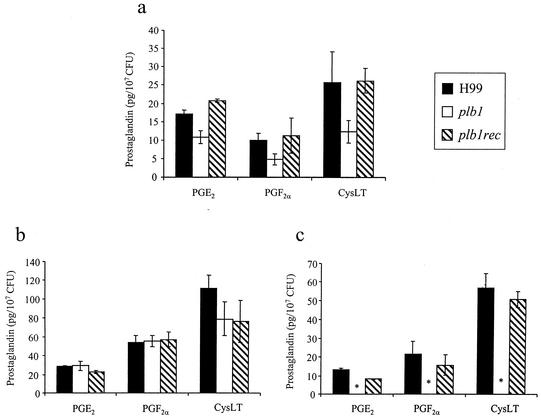
Phospholipase A2 (PLA2) is the enzyme used by mammalian leukocytes to preferentially cleave AA from the sn-2 position of phospholipids to be used in the PG-LT synthetic pathways. PLB1 exhibits PLA activity (PLA1 and PLA2), as well as lysophospholipase and lysophospholipase transacylase activities. To determine whether it was the PLA activity of PLB1 that was required for PG production, the three strains of C. neoformans were assayed for PGE2 and PGF2α production after incubation in defined medium supplemented with either AA or phosphatidylcholine (PC)-AA (Fig. 9). The PLB1-expressing C. neoformans strains readily produced PGE2 and PGF2α from both PC-AA and AA. In contrast, the PLB1-deficient strain could produce PGE2 and PGF2α only from AA and not from PC-AA, indicating that PLB1 has PLA activity. Furthermore, the mutation of PLB1 did not affect eicosanoid enzymatic pathways downstream of phospholipase action (such as the cyclooxygenase or PG-synthase activity). cysLT production was also assayed. cysLTs are also produced from AA, but this is done via a lipoxygenase activity. Figure 9 demonstrates that the plb1 mutant could produce cysLT from AA but not from PC-AA and that the plb1rec and H99 strains could produce cysLT from both AA and PC-AA. Thus, PLB1 liberates eicosanoid precursors (AA) from phospholipids in C. neoformans, and deletion of PLB1 does not appear to affect downstream enzymes in the biosynthesis pathways for PGE2, PGF2α, and cysLT.
FIG. 9.
Role of PLB1 in cryptococcal PG and LT production from phospholipids. C. neoformans strains H99 and the plb1 and plb1rec mutants were grown in SDB at 25°C for 3 days. Cultures were centrifuged and resuspended in RPMI 1640 containing 1 mM AA or 1 mM arachidonoyl-phosphatidylcholine (Avanti Polar Lipids). Cultures were incubated for an additional 2 h at 37°C. Culture supernatants from yeast cells fed 3 days with SDB (a), AA (b), and (c) arachidonoyl-phosphatidylcholine were analyzed for PGE2, PGF2α, and cysLT with EIA kits (Cayman Chemicals). Results are expressed as the mean PG concentration divided by the mean CFU concentration ± SEM for duplicate cultures. The experiments were repeated two times with similar results. *, P < 0.01 (relative to values for H99-infected mice).
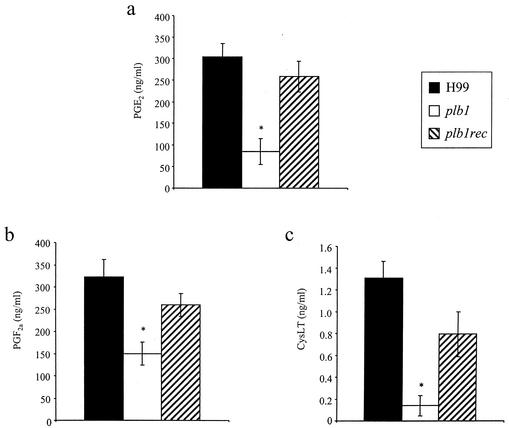
Does cryptococcal phospholipase play a role in eicosanoid generation in vivo? To examine this possibility, lung eicosanoid levels were measured in mice infected with the H99, plb1, or plb1rec strain (Fig. 10). At week 1 postinfection, mice infected with H99 and the plb1rec mutant had significantly higher levels of PGs (PGE2 and PGF2α) and cysLTs in their lungs than did mice infected with the PLB1-deficient strain (the plb1 mutant). At this time point, there were approximately equivalent numbers of recruited leukocytes in the lungs of mice infected with either the H99, plb1, or plb1rec strain (Fig. 3). The source of eicosanoids in the lungs during infection may be the yeast, the host, or both. However, these data are consistent with the observed inability of the PLB1 mutant to produce eicosanoids from phospholipids and demonstrate that PLB1 plays a role in the elaboration of eicosanoids during C. neoformans infection.
FIG. 10.
Eicosanoid production in the lungs of mice following infection with H99 or the plb1 or plb1rec mutant. CBA/J mice were infected intratracheally with 104 CFU of C. neoformans strain H99 or the plb1 or plb1rec mutant. Lungs were excised at week 1 postinfection and homogenized. Lung homogenate supernatants were filtered, and lipids were extracted with C18 Sep-Pac cartridges. PGE2 (a), PGF2α (b), and cysLT (c) levels in purified samples were measured using EIA kits (Cayman Chemicals). Results are expressed as the means ± SEM. Seven to eight mice per time point were used. *, P < 0.05 (relative to values for H99-infected mice).
PGE2 and other eicosanoids (AA metabolites) are well known to down-regulate macrophage function (13, 23, 29, 31). Eicosanoids are membrane diffusible, and many are potent ligands of the intracellular receptors peroxisome proliferator-activated receptor α (PPARα) and PPARγ in macrophages (33). Binding to PPARα and PPARγ causes macrophage deactivation. Thus, one potential mechanism of the intracellular survival of cryptococci in macrophages is the deactivation of macrophages by PGs and LTs produced by C. neoformans (either in phagosomes or extracellularly). Unfortunately, we cannot conclude at this time whether the activity of PLB1 in down-regulating the fungistatic activity of AM is due solely to the production of eicosanoids rather than to the other activities of PLB1 on phagocyte membranes, because the cyclooxygenase or lipoxygenase enzymes in C. neoformans have not been identified (to create mutant strains). However, our studies clearly identify previously unreported activities for a fungal PLB that may enhance virulence: provision of AA from phospholipids for fungal PG and LT production and subsequent down-regulation of macrophage activation.
Summary.
Phospholipases are present in several pathogenic fungi, including members of the genera Aspergillus and Candida (11). A PLB gene from Candida albicans (caPLB1) has been cloned and disrupted. The resulting null mutant was found to be less virulent in a murine intravenous model of disseminated candidiasis than were the parent and reconstituted mutant strains (15, 20). Eicosanoid production has been reported for C. albicans; however, a role for caPLB1 in this process has yet to be examined (21). Because C. albicans possesses multiple phospholipase genes (caPLB1, caPLB2, and caPLD), the single-knockout mutant may not exhibit measurable differences in its levels of eicosanoid production from that of the wild type (11), and its effect on pathogenesis may be due to direct tissue invasion. However, we have recently reported that numerous species of pathogenic fungi produce eicosanoids (22), and it is likely that phospholipases are also produced by all pathogenic fungi. Based on the studies of Candida and Cryptococcus, these enzymes may be required for the virulence composite of fungal pathogens.
Along with host cell membrane phospholipids such as AA-PC, lung surfactant can also serve as a substrate for fungal PLB (4, 26). With surfactant as a phospholipid source, the production of PGs and LTs by fungi in the lungs may also play a role in modulating the T1-T2 balance of the immune response and may promote eosinophil recruitment or survival in the lungs (32). Eosinophil infiltrates are a common feature of many chronic fungal infections, and fungi are a common cause of atopic diseases (14). We report here that C. neoformans strain H99 failed to induce significant pulmonary eosinophilia if PLB1 production was deficient, which had a major impact on cellular immunity. Furthermore, production of PGs and LTs by fungi represents a potential virulence mechanism that can cause macrophage deactivation and immune deviation leading to chronic infections and atopic (T2) diseases. Cryptococcal PLB1 (and, we predict, other fungal PLBs) is one of the enzymes involved in the biosynthesis of these bioactive lipids from exogenous sources of phospholipids and may have a major impact on the growth of fungi in vivo.
Acknowledgments
We thank Deirdra Williams, Cara Chrisman, and Rod McDonald for their work on the murine infections and harvests. We also thank Galen Toews for scholarly contributions and support.
This work was supported by New Investigator Awards in Molecular Pathogenic Mycology from the Burroughs-Wellcome Fund (G.B.H. and G.M.C.). M.C.N. was supported by NIH-NIAID training grant T32AI07528. Additional support was provided by the following grants from the National Institutes of Health: RO1-HL65912 (G.B.H.), RO1-HL63670 (G.B.H.), and RO1-AI28388 (J.R.P.).
Editor: T. R. Kozel
REFERENCES
- 1.Betz, M., and B. S. Fox. 1991. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J. Immunol. 146:108-113. [PubMed] [Google Scholar]
- 2.Chen, G. H., J. L. Curtis, C. H. Mody, P. J. Christensen, L. R. Armstrong, and G. B. Toews. 1994. Effect of granulocyte-macrophage colony-stimulating factor on rat alveolar macrophage anticryptococcal activity in vitro. J. Immunol. 152:724-734. [PubMed] [Google Scholar]
- 3.Chen, S. C., M. Muller, J. Z. Zhou, L. C. Wright, and T. C. Sorrell. 1997. Phospholipase activity in Cryptococcus neoformans: a new virulence factor? J. Infect. Dis. 175: 414-420. [DOI] [PubMed] [Google Scholar]
- 4.Chen, S. C., L. C. Wright, J. C. Golding, and T. C. Sorrell. 2000. Purification and characterization of secretory phospholipase B, lysophospholipase and lysophospholipase/transacylase from a virulent strain of the pathogenic fungus Cryptococcus neoformans. Biochem. J. 347:431-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, S. C. A., L. C. Wright, R. T. Santangelo, M. Muller, V. R. Moran, P. W. Kuchel, and T. C. Sorrell. 1997. Identification of extracellular phospholipase B, lysophospholipase, and acyltransferase produced by Cryptococcus neoformans. Infect. Immun. 65:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox, G. M., H. C. McDade, S. C. Chen, S. C. Tucker, M. Gottfredsson, L. C. Wright, T. C. Sorrell, S. D. Leidich, A. Casadevall, M. A. Ghannoum, and J. R. Perfect. 2001. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 39:166-175. [DOI] [PubMed] [Google Scholar]
- 7.Cox, G. M., D. L. Toffaletti, and J. R. Perfect. 1996. Dominant selection system for use in Cryptococcus neoformans. J. Med. Vet. Mycol. 34:385-391. [PubMed] [Google Scholar]
- 8.Demeure, C. E., L.-P. Yang, C. Desjardins, P. Raynauld, and G. Delespesse. 1997. Prostaglandin E2 primes naive T cells for the production of anti-inflammatory cytokines. Eur. J. Immunol. 27:3526-3531. [DOI] [PubMed] [Google Scholar]
- 9.Falkow, S. 1988. Molecular Koch's postulates applied to microbial pathogenicity. Rev. Infect. Dis. 10:5274-5276. [DOI] [PubMed] [Google Scholar]
- 10.Ford-Hutchinson, A. W. 1990. Leukotriene B4 in inflammation. Crit. Rev. Immunol. 10:1-12. [PubMed] [Google Scholar]
- 11.Ghannoum, M. A. 2000. Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol. Rev. 13:122-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonsson, E. W., and S. Dahlen. 1999. The role of eicosanoids in inflammation and allergy. In F. Marks and G. Furstenberger (ed.), Prostaglandins, leukotrienes, and other eicosanoids. Wiley-VCH, Weinheim, Germany.
- 13.Kunkel, S. L., M. Spengler, M. A. May, R. Spengler, J. Larrick, and D. Remick. 1988. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J. Biol. Chem. 263:5380-5384. [PubMed] [Google Scholar]
- 14.Kwon-Chung, K. J. 1992. Medical mycology. Lea & Febiger, Philadelphia, Pa.
- 15.Leidich, S. D., A. S. Ibrahim, Y. Fu, A. Koul, C. Jessup, J. Vitullo, W. Fonzi, F. Mirbod, S. Nakashima, Y. Nozawa, and M. A. Ghannoum. 1998. Cloning and disruption of caPLB1, a phospholipase B gene involved in the pathogenicity of Candida albicans. J. Biol. Chem. 273:26078-26086. [DOI] [PubMed] [Google Scholar]
- 16.Lindell, D. M., T. J. Standiford, P. Mancuso, Z. J. Leshen, and G. B. Huffnagle. 2001. Macrophage inflammatory protein 1α/CCL3 is required for clearance of an acute Klebsiella pneumoniae pulmonary infection. Infect. Immun. 69:6364-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenz, M. C., and G. R. Fink. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412:83-86. [DOI] [PubMed] [Google Scholar]
- 18.Matsuoka, T., M. Hirata, H. Tanaka, Y. Takahashi, T. Murata, K. Kabashima, Y. Sugimoto, T. Kobayashi, F. Ushikubi, Y. Aze, N. Eguchi, Y. Urade, N. Yoshida, K. Kimura, A. Mizoguchi, Y. Honda, H. Nagai, and S. Narumiya. 2000. Prostaglandin D2 as a mediator of allergic asthma. Science 287:2013-2017. [DOI] [PubMed] [Google Scholar]
- 19.Mbawuike, I. N., and H. B. Herscowitz. 1989. MH-S, a murine alveolar macrophage cell line: morphological, cytochemical, and functional characteristics. J. Leukoc. Biol. 46:119-127. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee, P. K., K. R. Seshan, S. D. Leidich, S. D. Chandra, G. T. Cole, and M. A. Ghannoum. 2001. Reintroduction of the PLB1 gene into Candida albicans restores virulence in vivo. Microbiology 147:2585-2597. [DOI] [PubMed] [Google Scholar]
- 21.Noverr, M. C., S. M. Phare, G. B. Toews, M. J. Coffey, and G. B. Huffnagle. 2001. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 69:2957-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noverr, M. C., G. B. Toews, and G. B. Huffnagle. 2002. Production of prostaglandins and leukotrienes by pathogenic fungi. Infect. Immun. 70:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters-Golden, M. 1997. Lipid mediator synthesis by lung macrophages, p. 151-182. In M. F. Lipscomb and S. W. Russell (ed.), Lung macrophages and dendritic cells in health and disease, vol. 102. Marcel Dekker, Inc., New York, N.Y.
- 24.Rollins, B. J. 1996. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol. Med. Today 2:198-204. [DOI] [PubMed] [Google Scholar]
- 25.Sankaran, K., and H. B. Herscowitz. 1995. Phenotypic and functional heterogeneity of the murine alveolar macrophage-derived cell line MH-S. J. Leukoc. Biol. 57:562-568. [DOI] [PubMed] [Google Scholar]
- 26.Santangelo, R. T., M. H. Nouri-Sorkhabi, T. C. Sorrell, M. Cagney, S. C. Chen, P. W. Kuchel, and L. C. Wright. 1999. Biochemical and functional characterization of secreted phospholipase activities from Cryptococcus neoformans in their naturally occurring state. J. Med. Microbiol. 48:731-740. [DOI] [PubMed] [Google Scholar]
- 27.Sergeeva, M. G., M. V. Gonchar, A. T. Mevkh, and S. D. Varfolomeyev. 1997. Prostaglandin E2 biphasic control of lymphocyte proliferation inhibition by picomolar concentrations. FEBS Lett. 418:235-238. [DOI] [PubMed] [Google Scholar]
- 28.Snijdewint, F. G., P. Kalinski, E. A. Wierenga, J. D. Bos, and M. L. Kapsenberg. 1993. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J. Immunol. 150:5321-5329. [PubMed] [Google Scholar]
- 29.Standiford, T. J., S. L. Kunkel, M. W. Rolfe, H. L. Evanoff, R. M. Allen, and R. M. Strieter. 1992. Regulation of human alveolar macrophage- and blood monocyte-derived interleukin-8 by prostaglandin E2 and dexamethasone. Am. J. Respir. Cell Mol. Biol. 6:75-81. [DOI] [PubMed] [Google Scholar]
- 30.Steele, M. G., and H. B. Herscowitz. 1993. Suppression of murine IgM, IgG, IgA and IgE antibody responses by alveolar macrophages. Immunology 80:62-67. [PMC free article] [PubMed] [Google Scholar]
- 31.Strassmann, G., V. Patil-Koota, F. Finkelman, M. Fong, and T. Kambayashi. 1994. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J. Exp. Med. 180:2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szczeklik, A., R. Gryglewski, and J. R. Vane (ed.). 1998. Eicosanoids, aspirin, and asthma, vol. 114. Marcel Dekker, Inc., New York, N.Y.
- 33.Vamecq, J., and N. Latruffe. 1999. Medical significance of peroxisome proliferator-activated receptors. Lancet 354:141-148. [DOI] [PubMed] [Google Scholar]