Abstract
The periodontopathogen Porphyromonas gingivalis is an obligate anaerobe that is devoid of catalase but exhibits a relatively high degree of resistance to peroxide stress. In the present study, we demonstrate that P. gingivalis contains a Dps homologue that plays an important role in the protection of cells from peroxide stress. The Dps protein isolated from P. gingivalis displayed a ferritin-like spherical polymer consisting of 19-kDa subunits. Molecular cloning and sequencing of the gene encoding this protein revealed that it had a high similarity in nucleotide and amino acid sequences to Dps proteins from other species. The expression of Dps was significantly increased by exposure of P. gingivalis to atmospheric oxygen in an OxyR-dependent manner, indicating that it is regulated by the reactive oxygen species-regulating gene oxyR. The Dps-deficient mutants, including the dps single mutant and the ftn dps double mutant, showed no viability loss upon exposure to atmospheric oxygen for 6 h. In contrast to the wild type, however, these mutants exhibited the high susceptibility to hydrogen peroxide, thereby disrupting the viability. On the other hand, no significant difference in sensitivity to mitomycin C and metronidazole was observed between the wild type and the mutants. Furthermore, the dps single mutant, compared with the wild type, showed a lower viability in infected human umbilical vein endothelial cells.
Atmospheric oxygen is metabolically converted to reactive oxygen species (ROS), including superoxide anion radical, hydrogen peroxide, hydroxy radical, and singlet oxygen, in bacterial cells. ROS are also generated by phagocytic host cells such as polymorphonuclear leukocytes and macrophages and attack invading bacterial cells. It is widely recognized that two cellular systems function to protect organisms from oxidative stresses (15, 33). One is regulated by antioxidant enzymes in which molecular oxygen and ROS are diminished or eliminated (42). Superoxide dismutase (SOD), catalase, peroxidase, and oxidase are involved in this reaction. The other is catalyzed by endonucleases by which oxidatively damaged nucleic acids are repaired. This includes Escherichia coli exonuclease III and endonuclease IV (51). These two systems cooperatively function to minimize the detrimental effects of ROS upon cells, as evidenced by the presence of common regulatory genes such as oxyR (43).
Porphyromonas gingivalis is a gram-negative obligate anaerobe belonging to the division Cytophagales (23). This bacterium is one of the organisms that is most strongly associated with chronic adult periodontitis and expresses numerous potential virulence factors, such as fimbriae, hemagglutinins, lipopolysaccharides, and various proteases that are capable of hydrolyzing collagen, immunoglobulins, iron-binding proteins, and complement factors (21, 27). P. gingivalis, by definition, cannot grow in aerobic conditions but exhibits a high degree of aerotolerance. This aerotolerance enables the organism to survive in periodontal pockets that are occasionally exposed to aerobic conditions.
P. gingivalis posseses SOD that is essential for tolerance to atmospheric oxygen, as revealed by the finding that P. gingivalis sod mutant shows a rapid viability loss by exposure to atmospheric oxygen (34), although it exhibits a marked resistance to peroxide stress. It has been demonstrated that the Dps (DNA-binding protein from starved cells) protein in E. coli plays an important role in the protection of cells from peroxide stress (1, 2). This protein is produced primarily in the stationary-phase cells, and its expression is regulated by σ38, σ70, and OxyR. Structurally, the Dps protein forms a ferritin-like spherical oligomeric structure. In addition, the Dps monomer displays essentially the same protein fold (four-helix bundle) as the ferritin monomer (17). It is of special importance that E. coli Dps exhibits DNA- and iron-binding activities by which the cells probably gain the resistance to oxidative stresses. Recent studies have demonstrated that a diverse group of Dps homologues are found in various prokaryotes, including Synechococcus sp., Bacillus subtilis, Listeria innocua, Streptococcus mutans, and Bacteroides fragilis (5, 6, 36, 38, 49), and are related to the ferritin-bacterioferritin-rubrerythrin superfamily (3, 17).
In the present study, we provide the first evidence indicating the presence of a Dps homologue in P. gingivalis. We have also constructed the Dps-deficient mutants to analyze the function of this protein in the organism. The results clearly indicate that the Dps protein is implicated in the protection of the organism from peroxide stress, thereby contributing to its survival in periodontal pockets and host cells.
MATERIALS AND METHODS
Bacterial strains and plasmids.
All bacterial strains and plasmids used in the present study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Source or reference |
|---|---|---|
| Strains | ||
| P. gingivalis | ||
| ATCC 33277 | Wild type | ATCC |
| KDP139 | ftn::Emr Emr | 37 |
| KDP141 | dps::Tcr Tcr | This study |
| KDP142 | ftn::Emrdps::Tcr Emr Tcr | This study |
| KDP143 | oxyR::Tcr Tcr | This study |
| KDP146 | dps+dps′-′lacZ Emr | This study |
| KDP148 | dps+dps′-′lacZ oxyR::Tcr Emr Tcr | This study |
| E. coli | ||
| BL21 (DE3) | ompT hsdSB with a λ prophage carrying the T7 RNA polymerase gene | 44 |
| DH5α | supE44 ΔlacU169(φ89lacZΔM15) hsdR17 relA1 endA1 gyrA96 thi-1 | 18 |
| TA4112 | araD139 Δ(argF-lac)205 flbB5301 non-9 gyrA219 relA1 rpsL150 metE70 Δ(oxyR btuB)3 | 8 |
| Plasmids | ||
| pET11a | Apr, overexpression plasmid | Novagen |
| pUC19 | Apr, cloning vector plasmid | 50 |
| pCR2.1 | Apr, PCR TA cloning vector plasmid | Invitrogen |
| pKD355 | Apr Emr, contains the ermF ermAM DNA block of pVA2198 (16) between EcoRI and BamHI of pUC18 | This study |
| pKD375 | Apr Tcr, contains the tetQ DNA block | 40 |
| pKD386 | Aprdps+, contains 2.6-kb Sau3AI chromosomal fragment (dps region) of P. gingivalis in pUC19 | This study |
| pKD387 | Aprdps (deletion), contains 4 bp deletion at BstXI within dps of pKD386 | This study |
| pKD388 | Aprdps::Tcr, contains the tetQ DNA block at BstXI within dps of pKD386 | This study |
| pKD390 | Apr, pET11a containing P. gingivalis dps | This study |
| pKD391 | Apr, contains 3.1-kb lacZ fragment PCR-amplified from pRS414 (40) in pCR2.1 | This study |
| pKD392 | Apr Emr, contains 3.1-kb EcoRI lacZ fragment of pKD39 in EcoRI site of pKD355 | This study |
| pKD393 | Apr Emr, lacZ reporter suicide/integration plasmid, contains unique EcoRI and BamHI at lacZ fusion sites | This study |
| pKD394 | Apr Emrdps′-′lacZ, contains promoter and 5′-terminal region of dps in pKD393 | This study |
| pKD396 | AproxyR+, contains P. gingivalis oxyR+ in pUC19 | This study |
| pKD397 | AproxyR::Tcr, contains the tetQ DNA block at BspEI within oxyR of pKD396 | This study |
Media and growth conditions.
Unless otherwise specified, P. gingivalis was grown in an anaerobic atmosphere (10% CO2, 10% H2, 80% N2) at 37°C. Enriched brain heart infusion (BHI) medium (containing 37 g of BHI [Difco Laboratories, Detroit, Mich.], 5 g of yeast extract (Difco), 1 g of cysteine, 5 mg of hemin, and 1 mg of vitamin K1/liter), enriched tryptic soy (TS) agar (containing 40 g of Trypto-Soya agar [Nissui, Tokyo, Japan], 5 g of BHI, 1 g of cysteine, 5 mg of hemin, and 1 mg of vitamin K1/liter), and blood agar prepared by adding hemolyzed defibrinated sheep blood to enriched TS agar at 5% were used for P. gingivalis. E. coli was grown at 37°C in L broth or on L agar (L broth solidified with 1.5% agar). Erythromycin (10 μg/ml), tetracycline (0.5 μg/ml), and ampicillin (50 μg/ml) were added as required for selection and maintenance of the strains.
Purification of P. gingivalis ferritin-like protein and determination of its N-terminal amino acid sequence.
P. gingivalis ATCC 33277 was grown in enriched BHI broth for 48 h. The cells were harvested by centrifugation at 15,000 × g for 20 min at 4°C. The pellet was suspended in 30 ml of buffer A (10 mM Tris-HCl buffer [pH 7.5]), and N-α-p-tosyl-l-lysine chloromethyl ketone (Sigma Chemical Co., St. Louis, Mo.) and leupeptin (Peptide Institute, Osaka, Japan) were added to final concentrations of 0.1 and 1 mM, respectively. The cells were broken by sonication (25 W; 30 pulses/min; 1-s pulse length) in a Branson sonicator with 1-min intervals for 10 min on ice. The sonicate was shaken for 15 min at 37°C and centrifuged to remove the unbroken cells. The supernatant was saved, and CsCl was added to a final concentration of 40% (wt/vol), followed by centrifugation at 80,000 × g for 24 h at room temperature. The solution was carefully separated into fractions, and each fraction was dialyzed overnight against buffer A by changing the buffer at 6-h intervals. The fractions were then examined by electron microscopy. The fraction containing ferritin-like particles was concentrated by ultrafiltration with a Microcon YM-10 (Millipore Corp., Bedford, Mass.). For further purification, the concentrated ferritin-like particles were subjected to gel filtration on a Sephadex FPLC column (FPLC column TM200; Pharmacia, Uppsala, Sweden). Proteins were eluted with buffer A at a flow rate of 0.5 ml/min. Protein elution was monitored by measuring the absorbance at 280 nm. Each fraction was examined by electron microscopy and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for the presence of ferritin-like particles.
To determine the amino-terminal amino acid sequence of this ferritin-like protein, the protein on the polyacrylamide gel was electrophretically transferred onto polyvinylidene difluoride membrane and stained with Coomassie brilliant blue R-250. The stained protein band was cut out and analyzed in an automatic protein sequencer (Applied Biosystems model 476A; Perkin-Elmer Cetus, Norwalk, Conn.).
Molecular cloning of P. gingivalis dps and construction of recombinant plasmids and bacterial strains.
Sau3AI plasmid library (pUC19) of P. gingivalis ATCC 33277 chromosomal DNA was introduced to E. coli TA4112 (oxyR) after amplification of the library in DH5α and a recombinant plasmid clone (pKD386) that conferred resistance to t-butyl hydroperoxide on the oxyR strain was obtained. The BstXI site within dps of pKD386 was eliminated with T4 DNA polymerase to yield pKD387. A tetQ DNA block of pKD375 (40) was inserted into the BstXI site of pKD386 to yield pKD388. The dps gene DNA was PCR amplified from pKD386 with primers (an upper primer [CCATATGAAAAAGATTCTTGAAGTAACGGG] and a lower primer [GGGATCCTTACTTGGCAGCGTAGGCAGACA]) and introduced to pET11a, resulting in pKD390.
For construction of the lacZ reporter suicide-integration plasmid, a 3.1-kb lacZ region DNA was PCR amplified from pRS414 DNA (41) with PCR primers (an upper primer [CGGAATTCCCGGGGATCCCGTCGTT] and a lower primer [AAGATCTTATTTTTGACACCAGACCAACTGG]) and ligated to the linear pCR2.1 DNA by using the TA cloning method, resulting in pKD391. E. coli DH5α harboring pKD391 showed β-galactosidase activity, indicating that the subcloned lacZ gene was functional. A 3.1-kb EcoRI fragment of pKD391 containing the lacZ gene was then inserted to the EcoRI site of pKD355, resulting in pKD392. EcoRI and BamHI sites other than the EcoRI-SmaI-BamHI multiple cloning sites were eliminated in pKD392 by partial restriction digestion and Klenow filling, giving rise to pKD393.
For construction of a dps′-′lacZ fusion plasmid, a DNA fragment comprising the 5′-terminal region of dps and its upstream region was PCR amplified from the chromosomal DNA of P. gingivalis ATCC 33277 with primers (an upper primer [CGAATTCCTCTAGAGGATCTTCTTC] and a lower primer [GGGATCCAAACCCGTTACTTCAAGA]). The upper primer can hybridize to the chromosomal DNA 1.6-kb upstream of the start codon of dps and generate an EcoRI site at one end of the PCR product, whereas the lower primer can hybridize to the chromosomal DNA within the dps gene and generate a BamHI site at the other end of the PCR product. The amplified DNA fragment was cloned into pCR2.1, sequenced, excised by double digestion with EcoRI and BamHI, and ligated to the EcoRI-BamHI region of pKD393. The resulting plasmid pKD394 produced the dps′-′lacZ fusion protein with the N-terminal 10 amino acids of Dps.
P. gingivalis oxyR preliminary sequence data was obtained from The Institute for Genomic Research website (http://www.tigr.org). The P. gingivalis oxyR gene region (927 bp) was PCR amplified from the chromosomal DNA of ATCC 33277 with PCR primers (an upper primer [CCATATGAATATACAGCAGCTCGAA] and a lower primer [CGGATCCTCAAGCCAAATGCTGCCCTGT]), cloned into pCR2.1, sequenced, excised with EcoRI, and ligated to the EcoRI site of pUC19, resulting in pKD396. The BspEI site within the oxyR gene of pKD396 was converted to BglII by using a BglII linker DNA and the tetQ DNA block was inserted into the BglII site, resulting in pKD397. ATCC 33277 and KDP146 (dps+ dps′-′lacZ) were transformed to tetracycline resistance by electrotransformation with the PstI-linearized pKD397 DNA to yield KDP143 (oxyR::Tcr) and KDP148 (oxyR::Tcr dps+ dps′-′lacZ), respectively.
For construction of P. gingivalis dps mutants, ATCC 33277 and KDP139 were transformed to tetracycline resistance with linearized pKD388 (dps::Tcr) DNA to yield KDP141 (dps::Tcr) and KDP142 (ftn::Emr dps::Tcr), respectively.
Purification of Dps from a dps-overexpressing E. coli.
E. coli BL21(DE3) harboring pKD390 was grown to an optical density at 540 nm (OD540) of 0.5 in 300 ml of L broth containing 50 μg of ampicillin/ml. IPTG (isopropyl-β-d-thiogalactopyranoside) was added to the culture at 1 mM, followed by incubation for 2 h to overproduce the recombinant Dps protein. The cells were collected by centrifugation at 9,000 × g for 20 min, resuspended in 10 ml of 20 mM phosphate buffer (pH 7.0), and disrupted by sonic oscillation (1 min each, 10 times). After centrifugation at 10,000 × g for 15 min, the supernatant was saved, to which ammonium sulfate was added at 85% saturation, and the precipitate was dissolved in 3 ml of phosphate buffer. After centrifugation to remove insoluble materials, the supernatant was dialyzed against the same phosphate buffer with a seamless cellulose tube (UC36-32-100; Sankohjunyaku Co., Tokyo, Japan) for 24 h and applied to a column (2.0 by 25 cm) of DEAE-Sepharose (Pharmacia) equilibrated with the same buffer. Proteins were eluted with 180 ml of a 0 to 1,000 mM linear NaCl gradient in the same buffer. The recombinant Dps protein was usually eluted at 400 mM NaCl. After concentration of the protein with a Microcon YM-10, the protein sample was applied to Mono-Q column (Pharmacia) and eluted with 180 ml of a 0 to 1,000 mM linear NaCl gradient in the same buffer. The Dps protein fraction that was eluted at 400 mM NaCl was then subjected to gel filtration on Superdex 200 column (Pharmacia). The system was equilibrated with the same buffer, proteins were eluted at a flow rate of 0.3 ml/min and 2-ml fractions were collected. The fractions constituting the prominent protein peak were pooled and concentrated with a Microcon YM-10. The iron-loaded Dps was obtained by incubating 50 μg of the recombinant Dps with 1 mM ferrous ammonium sulfate in 20 mM morpholinepropanesulfonic acid-NaOH (pH 7.0) at 4°C for 1 h (45). To remove excess iron, the mixture was extensively dialyzed against the same morpholinepropanesulfonic acid buffer by using the seamless cellulose tube (UC36-32-100). The iron-loaded Dps was concentrated by using the Microcon YM-10.
DNA-binding activity.
Recombinant P. gingivalis Dps (10 μg) treated with or without ferrous ammonium sulfate was mixed with 500 ng of linear DNA (1-kb DNA Ladder; Promega, Madison, Wis.) and kept at 4°C for 1 h. The mixture was subjected to agarose gel electrophoresis (1%; Tris-acetate buffer).
Iron starvation.
To determine the ability to grow under iron starvation, cells of test strains were first grown in the presence of hemin and then deprived of the iron source. The initial inoculum that had been prepared by growing the strains in hemin-containing enriched BHI medium overnight was diluted 10-fold with hemin-free enriched BHI medium and incubated. Every 24 h, the OD540 of the cultures was measured, and a 10-fold dilution of the cultures with hemin-free enriched BHI medium was repeated.
Agar diffusion assay.
P. gingivalis cells were anaerobically grown in enriched BHI medium and spread on enriched TS plates, and a sterile disk containing 4 μl of 6% hydrogen peroxide, 15% hydrogen peroxide, 1 mg of mitomycin C/ml, or 0.5 mg of metronidazole/ml was placed at the center of each plate and incubated anaerobically at 37°C for 4 days.
Sensitivity of P. gingivalis to hydrogen peroxide in aerobic conditions.
P. gingivalis cells grown in enriched BHI medium for 48 h were diluted twofold with fresh enriched BHI medium with or without 1.0 mM hydrogen peroxide and incubated aerobically at 37°C with shaking (125 cpm). To determine the number of survivals, cultures were withdrawn at intervals and plated on enriched TS plates after adequate dilution. The plates were anaerobically incubated for 7 days at 37°C
Cell culture and infection of HUVEC with P. gingivalis.
Human ambilical vein endothelial cells (HUVEC) obtained from Cell Applications, Inc. (San Diego, Calif.) was maintained in MCDB151 medium (Sigma) containing 15% fetal calf serum, acidic fibroblast growth factor (Sigma), heparin (Sigma), and kanamycin sulfate. For infection, cells were seeded at 5 × 105 cells per well in six-well tissue culture dishes with 2 ml of the same medium. P. gingivalis cells were grown in enriched BHI medium to a mid-logarithmic phase, washed with phosphate-buffered saline (PBS), and suspended into kanamycin sulfate- and fetal bovine serum-free MCDB151 medium. The bacterial suspension was then added to the HUVEC monolayer at a multiplicity of infection of 10,000. After incubation at 37°C for the indicated period, the infected cells were collected, washed with the same medium containing 300 μg of gentamicin and 400 μg of metronidazole/ml, suspended in the antibiotic-containing medium, and incubated at 37°C for 1 h to kill extracellular bacterial cells. The infected HUVEC were collected, washed with PBS, and suspended in MCDB151 medium. After freezing and thawing of the HUVEC suspension, enriched BHI medium was added to the suspension. The mixture was vortexed for 15 s and serially diluted, and each dilution was plated on enriched TS agar and then incubated anaerobically at 37°C for 5 days to determine the survival of the intracellular bacterial cells. All assays were conducted independently at least five times. The results are expressed as the means ± the standard deviations (SD) of multiple experiments.
Confocal laser scanning microscopy.
P. gingivalis cells of a fresh overnight culture were precipitated by centrifugation at 6,000 rpm for 10 min, washed, and suspended in PBS. The cell suspension was supplemented with 2,7-bis-(2-carboxyethyl)-5-(and-6-)-carboxyfluorescein (BCECF; 10 mM stock solution; Molecular Probes, Inc., Eugene, Oreg.) at a final concentration of 5 μM and incubated anaerobically at 37°C for 30 min. After centrifugation at 6,000 rpm for 5 min, the precipitated bacterial cells were suspended in MCDB151 medium without antibiotics. HUVEC that had been grown on a glass coverslip in a six-well tissue culture plate were washed twice with PBS and infected with the BCECF-labeled P. gingivalis cells at a multiplicity of infection of 10,000. After a 10-min incubation at 37°C, the infected HUVEC were washed twice with PBS and fixed in 10% formaldehyde in PBS at room temperature for 15 min. The fixed cells were washed twice with PBS and treated with PBS containing 50 mM NH4Cl and 0.3% Tween 20 for 10 min at room temperature. After being washed with PBS twice, the HUVEC were subjected to confocal laser scanning microscopy (DMIRB/E; Leica Microsystems, Wetzlar, Germany). From confocal laser scanning images, incorporated bacterial cells were counted in a depth of 1.0 μm at the central section of a single HUVEC. Data were expressed as the means of more than 50 determinations ± the SD.
Other methods.
Iron staining with Ferene S and heme staining with 3,3′,5,5′-tetramethylbenzidine (TMBZ) were performed as described previously (37). Electrotransformation of P. gingivalis cells was done as previously described (35). SDS-PAGE was performed under reducing conditions on 15% gels essentially as described previously (25). The gels were stained with 0.1% Coomassie blue R-250. For immunoblot analysis, proteins on SDS-PAGE gels were electrophretically transferred to nitrocellulose membranes as previously described (46). The membranes were immunostained with a 5,000-fold dilution of an anti-P. gingivalis Dps antiserum. The antiserum was prepared from a rabbit immunized with P. gingivalis Dps purified from the E. coli strain overproducing P. gingivalis Dps. The reacting proteins were detected by using the ECL Western blotting system (Amasham).
DNA sequencing was carried out by using a dideoxy sequencing kit (Auto Read Sequencing kit; Pharmacia) with plasmid templates and an automated DNA sequencer (ALF DNA Sequencer; Pharmacia). The sequence data were analyzed with the GeneWorks software program (IntelliGenetics, Mountain View, Calif.).
Colony hybridization and Southern blot hybridization were performed by standard methods (39, 47).
Statistical analysis.
The Student t test was used to compare differences in CFU numbers among bacterial strains by using StatView J4.5 software (Abacus Concepts, Inc., Berkeley, Calif.).
Nucleotide sequence accession number.
The nucleotide sequence reported in the present study was deposited in the DDBJ/EMBL/GenBank database under accession no. AB025779.
RESULTS
Isolation and characterization of a Dps homologue from P. gingivalis.
In the process of purification of ferritin from P. gingivalis (37), we found that the organism contained other ferritin-like particles in the cell extracts. The fraction containing ferritin-like particles was separated from the ferritin fraction by CsCl density gradient centrifugation, followed by gel filtration on Superdex 200. The final preparation of ferritin-like particles gave a single protein band with an apparent molecular mass of 19 kDa when examined by SDS-PAGE under reducing conditions (data not shown). Since the apparent molecular mass of the protein was estimated to be more than 200 kDa, the protein appeared to be a dodecamer consisting of the 19-kDa protein. The N-terminal amino acid sequence of the protein was found to start with MKKILEVTGLKEQQV, which was highly homologous to those of Dps from other bacterial species.
Molecular cloning of a P. gingivalis gene conferring peroxide resistance on the E. coli oxyR mutant.
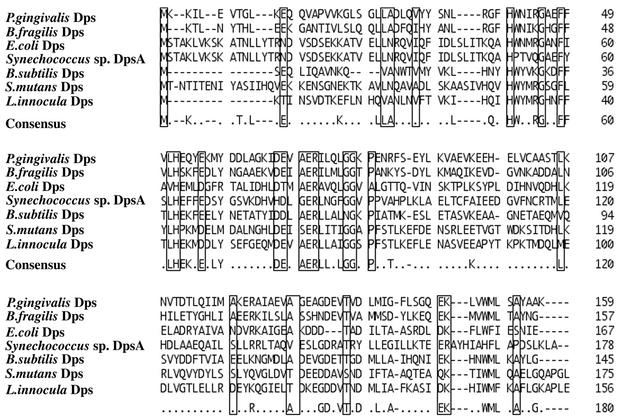
Apart from the biochemical characterization of the ferritin-like protein, we tried to isolate P. gingivalis genes responsible for peroxide resistance. P. gingivalis chromosomal DNA library was introduced into the E. coli oxyR mutant and t-butyl hydroperoxide-resistant transformants were isolated. All of the transformants harbored recombinant plasmids containing the same chromosomal DNA region, revealed by restriction enzyme analysis (data not shown). One of the recombinant plasmids (pKD386) contained a 2.6-kb chromosomal DNA fragment. Subcloning analysis located the peroxide-resistant gene on a 1.1-kb DNA region within the 2.6-kb fragment. Unexpectedly, one open reading frame in the 1.1-kb region encoded a protein with 159 amino acid residues, the N-terminal sequence of which was the same as that of the ferritin-like particle protein. As shown in Fig. 1, the protein had a marked resemblance of the amino acid sequence to Dps proteins from other bacterial species. Therefore, this protein was referred to as P. gingivalis Dps.
FIG. 1.
Alignment of the amino acid sequence of P. gingivalis Dps with those of the Dps previously isolated and determined in other prokaryotes.
Verification of P. gingivalis dps gene.
To determine whether the dps gene is responsible for protection against peroxide stress, we constructed a derivative (pKD387) of pKD386 in which 4 bp were deleted at the BstXI site in the dps gene and examined the oxyR mutant harboring pKD387 for sensitivity to t-butyl hydroperoxide by spreading these cells on L plates containing t-butyl hydroperoxide (60 ng/ml). The plating efficiencies of the oxyR mutants harboring pUC19 (vector), pKD386 (dps+), and pKD387 (dps with deletion) were 1.8 × 10−4, 4.5 × 10−1, and 5.7 × 10−4, respectively, indicating that P. gingivalis dps gene has the ability to confer peroxide resistance on the E. coli oxyR mutant.
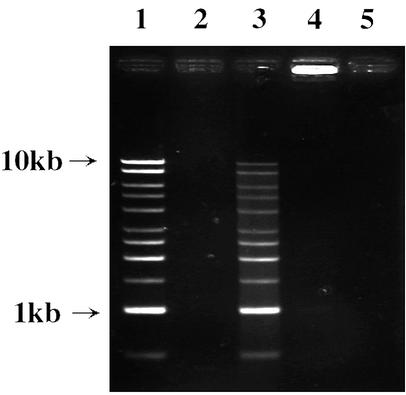
P. gingivalis Dps purified from the recombinant E. coli cells was mixed with ferrous ammonium sulfate, electrophoresed through nondenaturing gels, and stained with Ferene S and TMBZ. Ferene S but not TMBZ stained P. gingivalis Dps, suggesting that P. gingivalis Dps is able to bind nonheme iron (data not shown). The DNA-binding activity of Dps was assayed by using linear plasmid DNA as described previously (1). The linear DNA mixed with the iron-loaded Dps did not migrate into an agarose gel, indicating the DNA-binding activity of Dps (Fig. 2).
FIG. 2.
DNA-binding activity of P. gingivalis Dps. Recombinant P. gingivalis Dps purified from the E. coli overexpressing P. gingivalis dps or the recombinant P. gingivalis Dps treated with ferrous ammonium sulfate was incubated with linear DNA (1-kb DNA ladder) at 4°C for 1 h. The mixture was then subjected to agarose gel electrophoresis. DNA on the gel was stained with ethidium bromide. Lanes: 1, DNA (500 ng) alone; 2, recombinant Dps (10 μg) alone; 3, recombinant Dps (10 μg) and DNA (500 ng); 4, iron-loaded recombinant Dps (10 μg) and DNA (500 ng); 5, iron-loaded recombinant Dps (10 μg) alone.
Expression of the dps gene.
To investigate the dps expression in P. gingivalis, we first constructed the lacZ reporter suicide-integration plasmid (pKD393) for P. gingivalis and introduced the promoter region of dps to pKD393. The resulting plasmid (pKD394) containing the dps′-′lacZ protein fusion gene was then introduced to P. gingivalis ATCC 33277 (wild type) to yield KDP146 (dps+ dps′-′lacZ). An oxyR::Tcr mutation was then introduced to ATCC 33277 and KDP146, resulting in KDP143 (oxyR::Tcr) and KDP148 (oxyR::Tcr dps+ dps′-′lacZ), respectively. Aerobic incubation caused a slight increase of the dps expression in the wild-type background, and such an increase was not observed in the oxyR background, indicating that the dps expression was partially controlled by oxyR (Fig. 3).
FIG. 3.

Time course of induction of β-galactosidase activity in the dps′-′lacZ fusion strains. P. gingivalis KDP146 (dps+ dps′-′lacZ) (circles) and KDP148 (oxyR dps+ dps′-′lacZ) (triangles) were grown anaerobically in enriched BHI medium at 37°C. At an A600 of 0.3, the cultures were shifted under aerobic conditions (solid symbols) or kept under anaerobic conditions (open symbols). Samples were withdrawn after the indicated time, and the activity of β-galactosidase was determined by the method of Miller (32). The β-galactosidase activity of the wild-type parent strain ATCC 33277 was <4 U under both aerobic and anaerobic conditions.
Construction of a dps mutant of P. gingivalis.
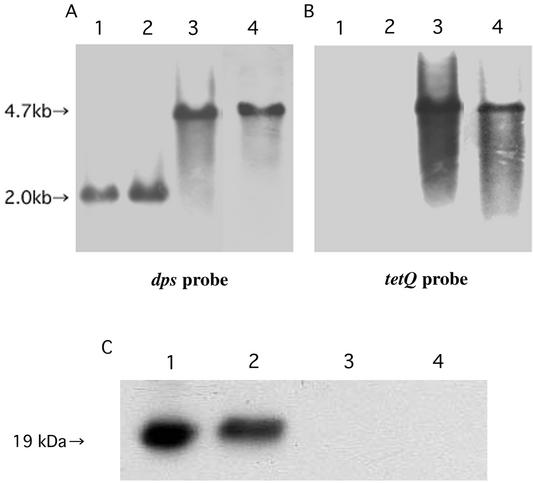
To gain insight into the biological significance of Dps in P. gingivalis cells, a Dps-deficient mutant was constructed. The dps gene DNA disrupted by insertion of the tetQ cartridge DNA was introduced into cells of P. gingivalis wild-type strain (ATCC 33277) and the ftn mutant (KDP139) by electroporation. A number of tetracycline-resistant colonies were obtained in both strains, and KDP141 (dps::Tcr) and KDP142 (ftn::Emr dps::Tcr) were chosen for further characterization. Southern blot hybridization and immunoblot analyses revealed the proper construction of KDP141 and KDP142 (Fig. 4). A gene encoding a putative transporter was located downstream of the dps gene. However, expression of the putative transporter gene would not be affected by insertion of the drug resistance cartridge into the dps gene since the direction of transcription of the gene was opposite to that of dps.
FIG. 4.
Proof of authenticity of the P. gingivalis dps mutant KDP141 and the ftn dps double mutant KDP142. (A and B) Southern blot analyses of the chromosomal DNA. The chromosomal DNAs of the wild-type ATCC 33277 (lane 1) and the ftn mutants KDP139 (lane 2), KDP141 (lane 3), and KDP142 (lane 4) were digested with NcoI. The resulting DNA fragments were subjected to agarose gel electrophresis, followed by blotting. Hybridization was performed by using the 0.6-kb NdeI-BglII fragment of pKD390 as a dps probe (A) and the 2.7-kb BamHI-BglII fragment of pKD375 as a tetQprobe (B). (C) Immunoblot analysis. After purified P. gingivalis Dps (lane 1) and the cell extracts of ATCC 33277 (lane 2), KDP141 (lane 3), and KDP142 (lane 4) were electrophoresed through an SDS-polyacrylamide gel, the proteins were transferred to a nitrocellulose membrane and immunoreacted with antiserum against P. gingivalis Dps.
Characterization of the Dps-deficient mutants of P. gingivalis. (i) Growth under iron depletion.
To determine the contribution of Dps to intracellular iron storage, the wild-type (ATCC 33277), ftn (KD139), dps (KD141), and ftn dps (KD142) strains were iron starved after growth in enriched BHI broth containing hemin as the iron source. The dps mutant showed the same growth depression as the wild type, whereas the ftn mutant showed earlier depression than the wild type, as previously reported (37). There was no difference in growth depression between the ftn and ftn dps mutants. These results suggested that Dps made no contribution to intracellular iron storage (data not shown).
(ii) Sensitivity to hydrogen peroxide, mitomycin C, and metronidazole.
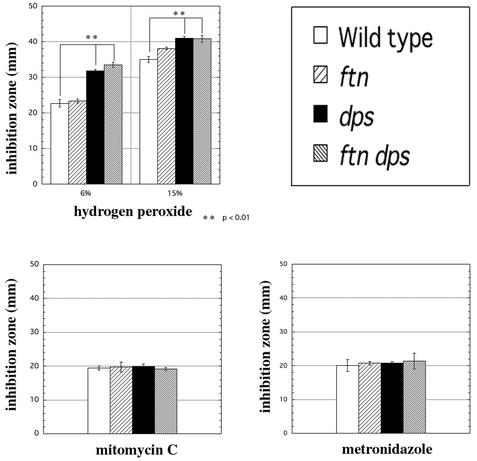
To determine the sensitivity of the dps mutants to hydrogen peroxide, mitomycin C, and metronidazole, we used agar diffusion assays under anaerobic conditions (Fig. 5). The dps and ftn dps mutants were more sensitive to hydrogen peroxide than were the wild-type and ftn strains (Fig. 5A). There was no difference in sensitivity to mitomycin C and metronidazole among these strains (Fig. 5B and C). These results indicated that Dps contributed to protection against peroxide in P. gingivalis cells.
FIG. 5.
Sensitivity of P. gingivalis cells to hydrogen peroxide, mitomycin C, and metronidazole. P. gingivalis ATCC 33277 (wild type), KDP139 (ftn), KDP141 (dps), and KDP142 (ftn dps) were anaerobically grown in enriched BHI medium for 48 h. The cells were spread on enriched TS plates, and a paper disk containing hydrogen peroxide (A), mitomycin C (B), or metronidazole (C) was placed at the centers of the plates, followed by incubation at 37°C anaerobically for 7 days. The diameters of the clear zones next to the disks were measured (in millimeters). The data shown are the means and SD of triplicate experiments.
(iii) Sensitivity to atmospheric oxygen with or without hydrogen peroxide.
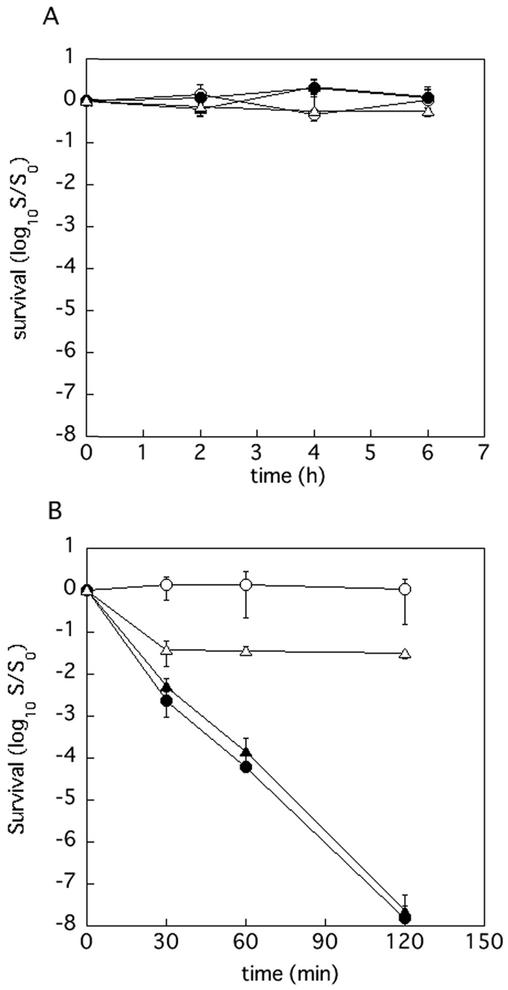
Although P. gingivalis is an obligate anaerobe, it exhibits a relatively high degree of aerotolerance. In order to determine whether Dps contributes to the aerotolerance of this organism and protection against hydrogen peroxide in aerobic condition, bacterial cells that had been grown anaerobically in enriched BHI medium overnight were diluted twice with fresh enriched BHI with or without hydrogen peroxide (final concentration, 0.5 mM) and aerobically incubated. It was shown that the dps, ftn, and ftn dps mutants were as tolerant to atomospheric oxygen as was the wild-type parent (Fig. 6A). Compared to the wild-type and ftn strains, the dps and ftn dps mutants were very sensitive to hydrogen peroxide under aerobic conditions (Fig. 6B). These results clearly indicated that the dps gene had an important role in protecting the cells from hydrogen peroxide.
FIG. 6.
Sensitivity of P. gingivalis mutants to air (A) and air with hydrogen peroxide (B). P. gingivalis cells that had been anaerobically cultured in enriched BHI medium overnight were diluted twice with fresh enriched BHI medium (A) or enriched BHI medium containing hydrogen peroxide (final concentration, 0.5 mM) (B) and then incubated aerobically with vigorous shaking. Samples were withdrawn at intervals and plated, after dilution in enriched BHI medium, on enriched TS plates. The plates were incubated anaerobically at 37°C for 7 days. Symbols: ○, ATCC 33277 (wild type); ▵, KDP139 (ftn); •, KDP141 (dps); ▴, KDP142 (ftn dps).
(iv) Survival in HUVEC.
P. gingivalis can invade endothelial cells, including HUVEC (11-13). To investigate the role of Dps in survival of P. gingivalis cells in HUVEC, we determined intracellular viability of the P. gingivalis dps mutant and the wild-type parent strain in HUVEC upon treatment with metronidazole and gentamicin after infection (Fig. 7). Although the wild-type parent strain and the dps mutant showed decrease of viability in HUVEC in a time-dependent manner, the dps mutant showed fewer surviving cells than did the wild-type parent strain. Since this difference in the number of intracellular survivals might result from a difference in invasion, we counted the intracellular bacterial cells in a block within a single HUVEC after infection by confocal laser scanning microscopy. The numbers of bacterial cells of the wild-type parent and the dps mutant incorporated into a depth of 1.0 μm at the central section of a single HUVEC were 48.4 ± 12.2 and 46.6 ± 15.2, indicating that there was no significant difference in invasion between these strains. These results suggested that Dps contributed to survival of P. gingivalis cells in HUVEC.
FIG. 7.
Survival of P. gingivalis cells in HUVEC. Monolayers of HUVEC were infected by P. gingivalis ATCC 33277 (wild type) and KDP141 (dps). Experiments were done at least five times, and the data are presented as the mean and the SD of the CFU per HUVEC.
DISCUSSION
We purified ferritin-like particles from P. gingivalis cell extracts and determined the N-terminal amino acid sequence of the ferritin-like particle protein. On the other hand, we cloned a P. gingivalis gene conferring peroxide resistance on the E. coli oxyR mutant and found that the gene product had the same molecular mass and N-terminal sequence as the ferritin-like particle protein, indicating that the peroxide resistance gene encodes the ferritin-like particle protein of P. gingivalis. Database analysis of the deduced amino acid sequence of the protein indicated that the P. gingivalis ferritin-like protein belonged to the Dps protein family, resulting in the gene designation dps. An E. coli catalase-null (katG katE) mutant was also used as a host strain for cloning of P. gingivalis peroxide resistance genes. All of the chromosomal DNA fragments obtained contained dps (unpublished data), suggesting that in P. gingivalis genes dps might be the only gene which could suppress increased peroxide sensitivity of E. coli oxyR and katG katE mutants.
Expression of Dps homologue genes of various microorganisms is upregulated by exposure to oxidative stress (1, 2, 6, 22, 38). In E. coli and B. fragilis, the stress response of their dps genes is regulated by OxyR (2, 38). In the present study, we constructed the lacZ reporter suicide-integration plasmid for analysis of gene expression of P. gingivalis. Using this lacZ reporter system, we found that the P. gingivalis dps gene was constitutively expressed but was upregulated by exposure to atmospheric oxygen and that this induction was totally dependent on OxyR. There was an approximately 10-fold increase in B. fragilis dps expression upon exposure to atmospheric oxygen (38), whereas P. gingivalis dps expression increased by only 25%, indicating that the degree of constitutive expression of P. gingivalis dps was much greater than that of B. fragilis dps. B. fragilis possesses catalase, whereas P. gingivalis does not possess catalase (31, 38). The high degree of constitutive expression of Dps in P. gingivalis might compensate for the absence of catalase in this organism.
Analysis of the P. gingivalis dps mutant revealed that P. gingivalis Dps was responsible for protection against peroxides, especially against hydrogen peroxide. Previous studies suggest that the ability of Dps to protect cells from oxidative damage may be derived from DNA condensation and masking with the Dps protein and from sequestration of iron ions that might otherwise generate detrimental free radicals (17, 24, 29, 48, 49). If DNA condensation and mechanical masking with Dps contributes to the protection of chromosomal DNA from peroxides, Dps-deficient mutants might show sensitivity to other DNA-damaging agents as well. However, the P. gingivalis dps mutant had no sensitivity to mitomycin C or metronidazole that can damage DNA. Nondenaturing PAGE profiles showed that P. gingivalis Dps contained nonheme iron. However, P. gingivalis Dps may not contribute to the iron storage of this organism, as revealed by the iron deprivation experiment. In addition, the ftn dps double mutant showed the same sensitivity to hydrogen peroxide as did the dps single mutant. These results suggest that, in P. gingivalis Dps, the sequestration of iron ions might not be plausible to explain the ability to protect cells from peroxides. We found in a previous study (34) that P. gingivalis sod mutant shows a rapid viability loss upon exposure to atmospheric oxygen. In contrast, we found in the present study that the dps mutant showed no viability loss upon exposure to atmospheric oxygen for 6 h. On the other hand, the dps mutant showed a viability loss by the treatment of hydrogen peroxide in aerobic conditions and that this viability loss was not enhanced by the addition of the ftn mutation. These results strongly suggest that P. gingivalis Dps is responsible for protection against specific ROS such as hydrogen peroxide. In this context, Dunkan and Touati (14) have shown that the E. coli dps mutant as well as a catalase-null mutant shows increased sensitivity to both hypochlorous acid and a catalase-null mutant. Hassett et al. (20) reported hat overproduction of Pseudomonas aeruginosa Dps provides protection against hydrogen peroxide but increases sensitivity to cumen hydroperoxide in P. aeruginosa oxyR mutant. Synechococcus sp. DpsA has catalase activity (36). In addition, rubrerythrin belonging to the ferritin-bacterioferritin-rubrerythrin superfamily, which is in turn related to the Dps family, has been found to have NADH peroxidase activity (9). P. gingivalis Dps might have peroxide-reducing activity, although we have not yet found this activity. To elucidate the mechanism of the peroxide resistance conferred by P. gingivalis Dps, the molecular and catalytic properties of the Dps should be explored.
Recently, a number of epidemiological studies have revealed a positive correlation between periodontal disease and coronary heart disease (4, 30). Patients with periodontal disease are more likely to experience transient bacteremias produced by flossing, mastication, and toothbrushing, which can occasionally let periodontal bacteria localize to endothelial cells (10). In fact, P. gingivalis has been immunolocalized in the shoulders of atherosclerotic plaques (7), and P. gingivalis DNA has been found in endarterectomy samples of patients with carotid stenosis by PCR analysis with primers specific to P. gingivalis DNA (19). The microorganism can invade various host cells, including epithelial cells and endothelial cells (11, 13, 26, 28). Survival of P. gingivalis cells in these host cells is important for the development of infection. The present study has provided the finding that Dps contributed to the intracellular survival of P. gingivalis cells. As far as we know, the present study is the first description of the significance of Dps in the survival of microorganisms in host cells.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture (Japan).
Editor: V. J. DiRita
REFERENCES
- 1.Almiron, M., A. J. Link, D. Furkong, and R. Kolter. 1992. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 6:2646-2654. [DOI] [PubMed] [Google Scholar]
- 2.Altuvia, S., M. Almiron, G. Huisman, R. Kolter, and G. Storz. 1994. The dps promoter is activated by OxyR during growth and IHF and σs in stationary phase. Mol. Microbiol. 13:265-272. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, S. C. 1998. Iron storage in bacteria. Adv. Microb. Physiol. 40:281-351. [DOI] [PubMed] [Google Scholar]
- 4.Beck, J. D., J. Pankow, H. A. Tyroler, and S. Offenbacher. 1999. Dental infections and atherosclerosis. Am. Heart J. 138:528-533. [DOI] [PubMed] [Google Scholar]
- 5.Bozzi, M., G. Mignogna, S. Stefanini, D. Barra, C. Longsh, P. Valenti, and E. Chiancone. 1997. A novel non-heme iron-binding ferritin relared to the DNA-binding proteins of the Dps family in Listeria innocua. J. Biol. Chem. 272:3259-3265. [DOI] [PubMed] [Google Scholar]
- 6.Chen, L., and J. D. Helman. 1995. Bacillus subtilis MrgA is a Dps (PexB) homologue: evidence for metalloregulation of an oxidative-stress gene. Mol. Microbiol. 18:295-300. [DOI] [PubMed] [Google Scholar]
- 7.Chiu, B. 2000. Multiple infections in carotid atherosclerotic plaques. Am. Heart J. 138:S534-S536. [DOI] [PubMed] [Google Scholar]
- 8.Christman, M. F., R. W. Morgan, F. S. Jacobson, and B. N. Ames. 1985. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell 41:753-762. [DOI] [PubMed] [Google Scholar]
- 9.Coulter, E. D., N. V. Shenvi, and D. M. Kurtz, Jr. 1999. NADH peroxidase activity of rubrerythrin. Biochem. Biophys. Res. Commun. 255:317-323. [DOI] [PubMed] [Google Scholar]
- 10.Daly, C., D. Mitchell, D. Grossberg, J. Highfield, and D. Stewart. 1997. Bacteremia caused by periodontal probing. Aust. Dent. J. 42:77-80. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande, R. G., M. B. Khan, and C. A. Genco. 1998. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect. Immun. 66:5337-5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorn, B. R., W. A. Dunn, Jr., and A. Progulske-Fox. 1999. Invasion of human coronary artery cells by Porphyromonas gingivalis. Infect. Immun. 67:5792-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorn, B. R., W. A. Dunn, Jr., and A. Progulske-Fox. 2001. Porphyromonas gingivalis traffics to autophagosomes in human coronary artery endothelial cells. Infect. Immun. 69:5698-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunkan, S., and D. Touati. 1996. Hypochlorous acid stress in Escherichia coli: resistance, DNA damage, and comparison with hydrogen peroxide stress. J. Bacteriol. 178:6145-6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farr, S. B., and T. Kogoma. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55:561-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher, H. M., H. A. Schenkein, R. M. Morgan, K. A. Bailey, C. R. Berry, and F. L. Macrina. 1995. Virulence of Porphyromonas gingivalis W83 mutant defective in prtH gene. Infect. Immun. 63:1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant, R. A., D. J. Filman, S. E. Finkel, R. Kolter, and J. M. Hogle. 1998. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat. Struct. Biol. 5:294-303. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan, D. 1985. Techniques for transformation of E. coli, p. 109-136. In D. M. Glover (ed.), The practical approach, vol. 1. DNA cloning. IRL Press, Ltd., Oxford, England.
- 19.Haraszthy, V. I., J. J. Zambon, M. Trevian, M. Zeid, and R. J. Genco. 2000. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 71:1554-1560. [DOI] [PubMed] [Google Scholar]
- 20.Hassett, D. J., E. Alsabbagh, K. Parvatiyar, M. L. Howell, R. W. Wilmontt, and U. A. Ochsner. 2000. A protease-resistant catalase, KatA, released upon cell lysis during stationary phase is essential for aerobic survival of a Pseudomonas aeruginosa oxyR mutant at low cell densities. J. Bacteriol. 182:4557-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holt, S. C., L. Kesavalu, S. Walker, and C. A. Genco. 1999. Virulence factors of Porphromonas gingivalis. Periodontol. 2000 20:168-238. [DOI] [PubMed] [Google Scholar]
- 22.Horsburgh, M. J., M. O. Clements, H. Crossley, E. Ingham, and S. J. Foster. 2001. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect. Immun. 69:3744-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hugenholtz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krispin, D. F., S. L. Zaidman, E. Shimoni, S. G. Wolf, E. J. Wachtel, T. Arad, S. E. Finkel, R. Kolter, and A. Minsky. 2001. Regulated phase transitions of bacterial chromatin: a non-enzymatic pathway for generic DNA protection. EMBO J. 20:1184-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 26.Lamont, R. J., A. Chan, C. M. Belton, K. T. Izutsu, D. Vasel, and A. Weinberg. 1995. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect. Immun. 63:3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamont, R. J., and H. F. Jenkinson. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madianos, P. N., P. N. Papapanou, U. Nannmark, G. Dahlen, and J. Sandros. 1996. Porphyromonas gingivalis FDC381 multiplies and persists within human oral epithelial cells in vitro. Infect. Immun. 64:660-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez, A., and R. Kolter. 1997. Protection DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J. Bacteriol. 179:5188-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattila, K. J., V. V. Valtonen, M. Nieminen, and J. K. Huttunen. 1995. Dental infection and the risk of new coronary events: prospective study of patients with documented coronary artery disease. Clin. Infect. Dis. 20:588-592. [DOI] [PubMed] [Google Scholar]
- 31.Mayrand, D., and S. C. Holt. 1988. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol. Rev. 52:134-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 33.Miller, R. A., and B. E. Britigan. 1997. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 10:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakayama, K. 1994. Rapid viability loss on exposure to air in a superoxide dismutase-deficient mutant of Porphyromonas gingivalis. J. Bacteriol. 176:1939-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama, K., T. Kadowaki, K. Okamoto, and K. Yamamoto. 1995. Construction and characterization of arginine-specific cysteine proteinase (Arg-gingipain)-deficient mutants of Porphyromonas gingivalis: evidence for significant contribution of Arg-gingipain to virulence. J. Biol. Chem. 270:23619-23626. [DOI] [PubMed] [Google Scholar]
- 36.Pena, M. M. O., and G. S. Bullerjahn. 1995. The DpsA protein of Synechococcus sp. strain PCC7942 is a DNA-binding hemoprotein. J. Biol. Chem. 270:22478-22482. [DOI] [PubMed] [Google Scholar]
- 37.Ratnayake, D. B., S. N. Wai, Y. Shi, K. Amako, H. Nakayama, and K. Nakayama. 2000. Ferritin from the obligate anaerobe Porphyromonas gingivalis: purification, gene cloning, and mutant studies. Microbiology 146:1119-1127. [DOI] [PubMed] [Google Scholar]
- 38.Rocha, E. R., G. Owens, Jr., and C. J. Smith. 2000. The redox-sensitive transcriptional activator OxyR regulates the peroxide response regulon in the obligate anaerobe Bacteroides fragilis. J. Bacteriol. 182:5059-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 40.Shi, Y., D. B. Ratnayake, K. Okamoto, N. Abe, K. Yamamoto, and K. Nakayama. 1999. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis: construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J. Biol. Chem. 274:17955-17960. [DOI] [PubMed] [Google Scholar]
- 41.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 42.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 43.Storz, G., L. A. Tartaglia, S. B. Farr, and B. N. Ames. 1990. Bacterial defenses against oxidative stress. Trends Genet. 6:363-368. [DOI] [PubMed] [Google Scholar]
- 44.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 45.Tonello, F., W. G. Dundon, B. Satin, M. Molinari, G. Tognon, G. Grandi, G. D. Giudice, R. Rappuoli, and C. Montecucco. 1999. The Helicobacter pulori neutrophil-activating protein is an iron-binding protein with dodecameric structure. Mol. Microbiol. 34:238-246. [DOI] [PubMed] [Google Scholar]
- 46.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace, R. B., and C. G. Miyada. 1987. Oligonucleotide probes for the screening of recombinant DNA libraries. Methods Enzymol. 152:432-442. [DOI] [PubMed] [Google Scholar]
- 48.Wolf, S. G., D. Frenkiel, T. Arad, S. E. Finkel, R. Kolter, and A. Minsky. 1999. DNA protection by stress-induced biocrystallization. Nature 400:83-85. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto, Y., M. Higuchi, L. B. Poole, and Y. Kamio. 2000. Role of the dpr product in oxygen tolerance in Streptococcus mutans. J. Bacteriol. 182:3740-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, Q. M., T. Takemoto, S. Mito, and S. Yonei. 1996. Induction of repair capacity for oxidatively damaged DNA as a component of peroxide stress response in Escherichia coli. J. Radiat. Res. 37:171-176. [DOI] [PubMed] [Google Scholar]