Abstract
A striking feature of Chagas' disease is the diversity of clinical presentations. Such variability may be due to the heterogeneity among Trypanosoma cruzi isolates or to the host immune response. Employing two strains which differ in their virulence, we investigated the effect of in vivo infection on professional antigen-presenting cells (APC). Acute infection with the virulent RA strain downregulated the expression of major histocompatibility complex (MHC) class II on splenic dendritic cells (DC) and inhibited its induction on peritoneal macrophages and splenic B cells. It also impaired the ability of DC to prime allogeneic T cells and to form homotypic clusters, suggesting a low maturation state of these cells. In contrast, the low-virulence K98 strain maintained the expression of MHC class II on DC or stimulated it on peritoneal macrophages and B cells and preserved DC's T-cell priming capacity and homotypic clustering. DC from RA-infected mice elicited a lower activation of T. cruzi-specific T-cell proliferation than those from K98-infected mice. APC from RA-infected mice that reached the chronic phase of infection restored MHC class II levels to those found in K98-infected mice and upregulated costimulatory molecules expression, suggesting that the immunosuppression caused by this strain is only transient. Taken together, the results indicate that in vivo infection with T. cruzi modulates APC functionality and that this is accomplished in a strain-dependent manner.
Chagas' disease, caused by the protozoan Trypanosoma cruzi, affects over 18 million people in Latin America (24). The host-parasite relationship determines the onset of the human pathology, which is extremely diverse, varying from a relatively benign indeterminate form to digestive compromise or a fatal cardiac course (35). Clinical forms might depend on host genetic background and/or intraspecific differences of parasite stocks among different areas: in Argentina, myocardiopathy prevails over megacolon, which is the most frequent form in the northeast of Brazil (2, 27).
In order to study the relevance of T. cruzi populations on the host-parasite relationship, we selected two strains with extreme biological differences, RA and CA-I (or its clone K98, with a similar behavior). RA is a highly virulent pantropic/reticulotropic strain, while CA-I/K98 is a low-virulence myotropic strain. RA-infected mice develop an early peak of parasitemia during the acute infection, which drops to undetectable values during the chronic phase, whereas CA-I/K98-infected mice slowly increase the parasitemia to reach its highest levels rather late without a sharp drop, remaining measurable for a longer time (13, 14, 26). During the acute stage of the infection, the RA strain is able to induce a transient impairment of both macrophage microbicidal activity and in vitro mitogen-induced lymphoproliferative response. However, CA-I (or the K98 clone) does not affect macrophage microbicidal activity or lymphoproliferative response (5, 6; our unpublished results). At this stage, mice infected with K98 displayed higher prostaglandin E2 serum levels than RA-infected and normal mice. Evidences were provided that this prostaglandin played a role in resistance to infection with the first strain in mice (7). At the chronic phase in RA-infected mice, the nervous system is a privileged target for damage mediated either by CD4 and CD8 T cells or immunoglobulin G antibodies, while for CA-I, only CD4 T cells are able to induce injury to the muscle, which is the target tissue (23, 36).
The activation of T-cell immune responses is mediated by antigen-presenting cells (APC), which deliver antigen-specific and costimulatory signals. Pathogens or their constituents are able to induce APC to deliver these signals to the resting T cells. Some parasites can modulate the antigen presentation and costimulatory activities of APC. Leishmania major induces upregulation of CD40 and CD86 costimulatory molecules (4), but Leishmania amazonensis and Leishmania donovani subvert the APC function of infected macrophages (12, 18). Toxoplasma gondii and Plasmodium falciparum are able to interfere with the processing and presentation of parasite-derived antigen by macrophages and dendritic cells (DC), respectively (22, 37). The persistence of intracellular parasites in immunocompetent hosts suggests that they have evolved multiple strategies to escape or modulate the antigen presentation- and T-cell-mediated parasiticidal activities of the host.
The effect of T. cruzi in vitro infection on macrophages as APC has been documented: Frosch et al. registered a selective upregulation of B7-2 (CD86) costimulatory molecules, although La Flamme et al. proved that a defective T-cell-macrophage adhesion impaired antigen presentation (11, 20). The parasite population employed in each assay could be responsible for these discordant findings. Van Overtvelt et al. demonstrated that in vitro T. cruzi infection prevented monocyte-derived human DC from optimal maturation and expression of HLA-DR molecules (38). However, no information is available about the in vivo interaction of APC with T. cruzi.
In the present study, we investigated the influence of in vivo infection with two T. cruzi strains which differ in virulence on professional APC expression of major histocompatibility complex (MHC) class II and costimulatory molecules and on the T-cell stimulatory capacity of splenic DC.
MATERIALS AND METHODS
Mice.
Male C3H/HeNk and C57BL/6b mice were supplied by the animal facilities of the Department of Microbiology, School of Medicine, University of Buenos Aires.
Parasites, infection, and T. cruzi lysate.
Bloodstream forms (trypomastigotes) of the RA strain or the K98 clone (derived from the CA-I strain) of T. cruzi were maintained by serial passages in mice. At 8 weeks of age, C3H/HeNk mice were infected with 100 trypomastigotes of either T. cruzi population. Except when indicated, trypomastigotes were inoculated into the hind footpad. By this route, at least 40% of animals infected with the virulent RA strain reached the chronic phase of infection. Uninfected mice of the same age, body weight, and sex were used as controls. For studies at the acute phase of infection, mice were killed when circulating trypanosomes were detected by the microhematocrit technique (10) (15 days for RA-infected and 20 days for K98-infected mice) and 4 months postinfection for those at the chronic phase.
Trypanosomes of the RA and K98 T. cruzi strains were purified from whole blood by density gradient centrifugation as described before (25). Trypomastigotes were subjected to five freeze-thawing cycles, resuspended in phosphate-buffered saline and sonicated (10 cycles of 30 s each at 40 Hz on ice). Suspensions were centrifuged, and the supernatant was filtered and stored at −20°C until use. Protein concentration was determined by the method of Lowry et al. with bovine serum albumin (Sigma, St. Louis, Mo.) as the standard.
Cells.
To prepare dendritic cell (DC)-enriched suspensions, spleens were homogenized at 4°C in a tissue grinder with RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 2-mercaptoethanol, and antibiotics (penicillin at 100 U/ml and streptomycin at 100 μg/ml) (Sigma). Homogenates were subjected to digestion with collagenase (1 mg/ml), DNase I (1 mg/ml), and hyaluronidase (0.5 mg/ml) (Sigma) for 30 min at 37°C. Undigested stromal fragments were removed, and the cells, resuspended in 1.080-g/cm3 density bovine serum albumin solution, were centrifuged at 4,500 × g for 25 min. The low-density fraction was recovered and incubated at 37°C for 2 h in RPMI 1640-10% FBS. Nonadherent cells were removed, and the remaining ones were reincubated in RPMI 1640-10% FBS for 20 h, the time required for DC to detach from plastic.
To purify splenic DC, CD11c+ cells were isolated by magnetic sorting with biotinylated anti-CD11c (HL3) monoclonal antibodies bound to streptavidin-coated magnetic beads and MiniMACS columns (Miltenyi Biotech GmbH, Bergish Gladbach, Germany) following the manufacturer's protocol. Cell populations obtained in this way consisted of approximately 90% CD11c+ cells as determined by flow cytometry analysis.
Peritoneal cells were recovered by intraperitoneal injection of 5 ml of cold RPMI 1640 supplemented with heparin (5 U/ml). After gentle abdomen massage, the fluid was aspirated, and cells were washed twice with RPMI-10% FBS and kept at 4°C until use.
To isolate CD4 and CD8 T cells from lymph nodes, single-cell suspensions were treated for erythrocyte lysis by brief incubation in Tris-buffered 0.83% ammonium chloride solution. CD4+ or CD8+ cells were purified with biotinylated anti-CD4 (L3T4) or anti-CD8 (Ly-2) monoclonal antibodies, streptavidin-coated magnetic beads, and MiniMACS columns (Miltenyi Biotech) following the manufacturer's instructions. The purity of these T-cell populations was routinely 90%, as determined by flow cytometry analysis. In all cases, cell concentration and viability were assessed with a hemacytometer and by the trypan blue dye exclusion test, respectively.
Flow cytometry.
Cells were washed twice in ice-cold phosphate-buffered saline supplemented with 1% bovine serum albumin and 0.1% NaN3 and resuspended at a final cell concentration of 2 × 107/ml. Two percent normal mouse serum was added to avoid unspecific binding through Fc receptors. Then, cells were incubated for 30 min at 4°C with previously optimized amounts of one or more of the following conjugated murine monoclonal antibodies: anti-CD11c-phycoerythrin (PE), anti-CD19-PE, anti-Mac-3-PE, anti-Ie-K (MHC II)-fluorescein isothiocyanate (FITC), anti-CD4-FITC, anti-CD8-FITC, and the biotinylated monoclonal antibodies anti-CD24, anti-CD40, anti-CD80, and anti-CD86. All monoclonal antibodies were purchased from PharMingen, San Diego, Calif. Streptavidin-Cy-chrome (PharMingen) was used as a second-step reagent. As controls, cells were stained with the corresponding isotype-matched monoclonal antibody.
Cells were fixed with 1% paraformaldehyde before being acquired with a flow cytometer (Ortho Cythoron Absolute; Johnson & Johnson). Data were analyzed with the WINMDI software (Joseph Trotter, Scripps Research Institute). Flow cytometry results were expressed as the median fluorescence intensity (median fluorescence intensity of cells with specific monoclonal antibody − median fluorescence intensity of cells with irrelevant isotype-matched monoclonal antibody) or as the percentage of cells which were positive for the surface marker evaluated.
Proliferation assays.
For the allogeneic mixed-lymphocyte reaction, irradiated (20 Gy) splenic DC from infected and uninfected mice were cocultured with untreated CD4 or CD8 T cells from the lymph nodes of C57BL/6 uninfected mice at 37°C in RPMI 1640-10% FBS medium in 96-well microplates in triplicate at a final volume of 200 μl. For evaluation of T. cruzi-specific T-cell responses, irradiated splenic DC from infected and uninfected mice were cocultured with untreated CD4 T cells from the lymph nodes of chronically infected mice and controls in the presence of T. cruzi lysate (50 μg/ml) at 37°C in RPMI 1640-10% FBS medium in 96-well microplates in triplicate at a final volume of 200 μl. Cultures were pulsed with 0.5 μCi of [3H]thymidine (ICN, Costa Mesa, Calif.) per well during the last 24 h of culture. Cells were harvested and analyzed in a Rack Beta liquid scintillation counter (Pharmacia). Results are expressed as the mean counts per minute (cpm) of the culture ± standard deviation.
Statistical analysis.
One-way analysis of variance and Bonferroni's multiple comparison test were used for the analysis of statistical significance.
RESULTS
Effect of infection with T. cruzi strains RA and K98 on surface expression of MHC class II and costimulatory molecules of professional APC.
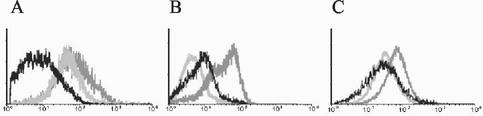
DC located in lymphoid organs constitutively express large amounts of MHC class II molecules at their surface; macrophages and B cells upregulate MHC class II expression in response to infection (1, 28). Flow cytometry analysis showed that constitutive expression of MHC class II in DC was downregulated during the acute phase in mice infected with strain RA while in peritoneal macrophages and B cells, an inhibition of the infection-enhanced MHC class II expression was registered in the same group of animals (Fig. 1). This phenomenon was observed not only as a decrease in the percentage of positive cells but also as a reduction in the number of surface molecules per cell (Table 1). In contrast, MHC class II expression was not altered on DC by infection with strain K98 (Fig. 1A) and was adequately upregulated on peritoneal macrophages (Fig. 1B) and B cells (Fig. 1C). This strain-dependent effect of T. cruzi infection on professional APC was independent of the route of inoculation, since similar results were obtained when the intraperitoneal or the footpad route was used to infect animals (Table 1). It is noteworthy that APC from RA-infected mice who reached the chronic stage of infection attained or even surpassed the levels of MHC class II expression reached by APC from K98-infected mice (Table 1).
FIG. 1.
Acute infection with the high-virulence (RA) T. cruzi strain downregulates or inhibits MHC class II expression on professional APC. Flow cytometry profiles of I-eK (MHC class II) molecules expressed on splenic DC (A), peritoneal macrophages (B), and B cells (C) from mice acutely infected with the RA strain (black line) or K98 strain (dark gray line) or controls (light gray). Enriched splenic DC suspensions were simultaneously stained with anti-CD11c-PE (for DC) and anti-I-eK-FITC. Peritoneal cells were simultaneously stained with anti-Mac-3-PE (for peritoneal macrophages) and anti-I-eK-FITC. Splenic cell suspensions were simultaneously stained with anti-CD19-PE (for B cells) and anti-I-eK-FITC. CD11c+, Mac-3+, and CD19+ events were analyzed for I-eK expression. The mean fluorescence obtained after staining with the corresponding isotype-matched monoclonal antibody was <10. Results of one representative experiment out of five are shown.
TABLE 1.
Surface expression of I-eK (MHC class II) molecules on professional APC from T. cruzi-infected micea
| Strain, inoculation route, and phase | CD11c+ cells
|
Mac-3+ cells
|
CD19+ cells
|
|||
|---|---|---|---|---|---|---|
| Mean MFI ± SD | Mean % I-eK+ ± SD | Mean MFI ± SD | Mean % I-eK+ ± SD | Mean MFI ± SD | Mean % I-eK+ ± SD | |
| Control | 1,242 ± 73 | 78.5 ± 3.9 | 325 ± 62 | 13.5 ± 2.9 | 780 ± 205 | 37.0 ± 6.0 |
| K98, FPI, acute | 1,026 ± 160 | 83.3 ± 3.5 | 1,344 ± 140∗∗ | 83.3 ± 5.2∗∗∗ | 1,215 ± 153 | 87.3 ± 1.8∗∗ |
| RA, FPI, acute | 489 ± 29∗∗ | 11.3 ± 7.3∗∗∗ | 349 ± 176 | 23.0 ± 5.0 | 869 ± 156 | 58.0 ± 3.7 |
| K98, PI, acute | 1,111 ± 161 | 73.0 ± 6.0 | 1,022 ± 249∗ | 67.7 ± 7.4∗∗∗ | 1,189 ± 169 | 78.0 ± 1.0∗∗ |
| RA, PI, acute | 532 ± 238∗ | 31.3 ± 9.3∗∗∗ | 441 ± 38 | 13.0 ± 4.3 | 681 ± 20 | 49.0 ± 10.0 |
| K98, FPI, chronic | 1,309 ± 86 | 95.5 ± 1.5 | 1,134 ± 240∗∗ | 79.5 ± 9.5∗∗∗ | 1,262 ± 85 | 77.5 ± 4.5∗ |
| RA, FPI, chronic | 1,900 ± 136∗∗ | 83.0 ± 3.0 | 1,796 ± 95∗∗ | 86.0 ± 5.0∗∗∗ | 1,537 ± 180∗ | 90.0 ± 9.0∗∗ |
Mice were infected with RA or K98 strain by the foot pad (FPI) or peritoneal (PI) route of inoculation. Cells were obtained at the acute or the chronic phase of infection. CD11c+ (for DC), Mac-3+ (for peritoneal macrophages) and CD19+ (for B cells) events were analyzed for I-eK-FITC expression. Results are expressed as the mean fluorescence intensity (MFI) of I-eK expression or mean percentage of I-eK+ cells. ∗, P < 0.05; ∗∗, P < 0.01; and ∗∗∗, P < 0.001 compared with the control.
As shown in Table 2, surface expression of CD86 on splenic DC was downregulated by acute infection independently of the T. cruzi population employed. Interestingly, at the chronic phase, peritoneal macrophages and B cells of mice surviving the acute phase of infection with RA showed significantly higher levels of CD86 expression than those from K98-infected mice. This was also the case for DC from chronically RA-infected mice, whose CD80 expression was higher than in K98-infected animals. T. cruzi infection had no effect on the basal expression of CD40 ad CD24 molecules (data not shown).
TABLE 2.
Surface expression of costimulatory molecules on professional APC from T. cruzi-infected micea
| Strain and infection phase | Mean fluorescence intensity ± SD
|
|||||
|---|---|---|---|---|---|---|
| CD11c+ cells
|
Mac-3+ cells
|
CD19+ cells
|
||||
| CD80 | CD86 | CD80 | CD86 | CD80 | CD86 | |
| Control | 1,504 ± 174 | 1,453 ± 124 | 1,640 ± 76 | 1,345 ± 20 | 1,123 ± 98 | 1,103 ± 63 |
| K98, acute | 1,196 ± 99 | 928 ± 68∗ | 1,677 ± 48 | 1,216 ± 41 | 1,044 ± 60 | 1,249 ± 75 |
| RA, acute | 1,457 ± 172 | 1,063 ± 24∗ | 1,458 ± 146 | 1,303 ± 88 | 1,012 ± 239 | 1,334 ± 14 |
| K98, chronic | 1,298 ± 141 | 1,179 ± 19 | 1,795 ± 304 | 1,262 ± 310 | 880 ± 293 | 858 ± 74 |
| RA, chronic | 1,864 ± 449∗∗ | 1,299 ± 225 | 1,930 ± 149 | 2,049 ± 165∗∗ | 1,392 ± 323 | 1,340 ± 321∗∗ |
Mice were infected with RA or K98 T. cruzi strains by the foot pad (FPI) route of inoculation. CD11c+, Mac-3+, and CD19+ events were analyzed for CD80 and CD86 expression. ∗, P < 0.05 compared with controls; ∗∗, P < 0.05 comparing K98 chronic and RA chronic.
Effect of acute infection with T. cruzi RA and K98 strains on distribution of APC in spleens.
APC settle in secondary lymphoid organs, where they encounter T cells for antigen presentation (1). As shown in Table 3, flow cytometry analysis of the relative number of professional APC located in the spleen revealed no significant differences in the percentages of CD11c+ (DC) cells among the three groups studied. However, an increase in the percentage of Mac-3+ cells in the spleens of RA-infected mice and a reduction in the relative number of CD19+ cells were found in the same group of animals. This last finding in spleens from RA-infected mice is consistent with the high rate of spontaneous apoptosis of splenic B cells by infection with the virulent Tulahuén strain of T. cruzi reported by Zuñiga et al. (40).
TABLE 3.
Relative number of APC in spleens from mice during the acute phase of infection with T. cruzia
| Infection | Mean % of cells positive (± SD) for surface marker:
|
||
|---|---|---|---|
| CD 11c+ | Mac-3+ | CD19+ | |
| Control | 3.4 ± 0.4 | 4.0 ± 0.5 | 46.3 ± 4.3 |
| RA | 6.4 ± 1.0 | 14.2 ± 3.3∗∗ | 28.3 ± 3.3∗ |
| K98 | 4.1 ± 1.2 | 6.5 ± 0.7 | 43.2 ± 6.7 |
Mice were infected with the RA or K98 T. cruzi strain by footpad inoculation. Splenic single-cell suspensions were stained for PE-conjugated monoclonal antibody against the cell surface markers. Results are expressed as mean percent positive cells. ∗, P < 0.05; ∗∗, P < 0.01 compared with the control.
Acute infection with the RA T. cruzi strain drastically diminishes DC stimulation of CD4 T and CD8 T-cell alloresponse.
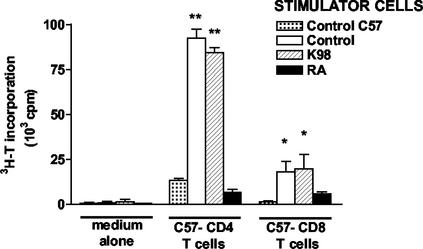
The ability of APC to stimulate an alloresponse strongly depends on the first signal provided by MHC class II molecules. Here we employed DC, the most potent inducers of T-cell immune responses in vitro, to test this capacity on professional APC from T. cruzi-infected mice (3). We performed a mixed-lymphocyte reaction with irradiated DC from acutely RA- or K98-infected and control C3H/HeNk mice as stimulators and purified lymph node CD4 T cells from C57BL/6b mice as responders. Allogeneic CD4 T cells cocultured with DC from control and K98-infected mice proliferated readily, whereas those cocultured with DC from RA-infected mice were unresponsive (Fig. 2). DC are equally important in priming naïve CD8 T cells. They can stimulate the proliferation of allogeneic CD8 T cells in the absence of CD4 T-cell cooperation (16). In another set of experiments with CD8 T cells instead of CD4 T cells, a mixed-lymphocyte reaction was performed to test this trait. DC from RA-infected mice were less efficient at priming allogeneic CD8 T cells for the proliferation response (Fig. 2).
FIG. 2.
Infection with the high-virulence RA T. cruzi strain impairs DC stimulation of CD4 and CD8 T-cell alloresponse. Irradiated splenic DC (2 × 105/well) from acutely RA- or K98-infected and control C3H/HeNk mice were cocultured with CD4 and CD8 T cells (2 × 105/well) from the lymph nodes of uninfected C57BL/6b (C57) mice. As a control for background proliferation, responder CD4 or CD8 T cells (C57BL/6b) were cocultured in the presence of syngeneic splenic DC (control C57). Cell proliferation was measured on day 4 after a 24-h pulse with [3H]-thymidine (3H-T). Values are the mean of triplicate cultures ± standard deviation. *, P < 0.05; **, P < 0.001 compared to control C57BL/6 stimulator cells.
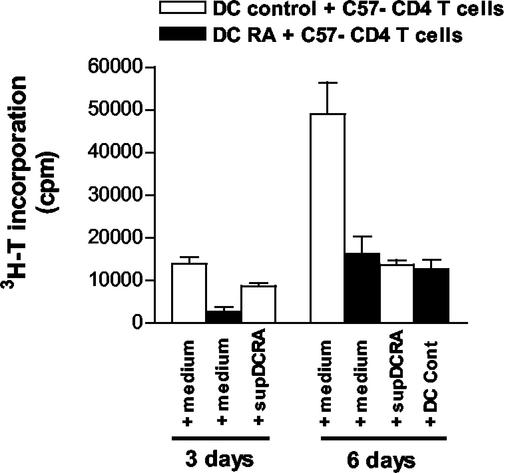
In addition to modulating the allostimulatory capacity of DC, pathogens and pathogen-derived factors themselves can drive DC to produce or induce the release of cytokines promoting different types of immune responses (30). To establish whether DC from RA-infected mice were producing or inducing the production of factors which limited T-cell proliferation (e.g., transforming growth factor beta or interleukin-10), we tested whether addition of DC from uninfected mice (DC control) to the mixed-lymphocyte reaction of DC from RA-infected mice plus CD4 T cells from C57BL/6b mice for the last 3 days of culture would reconstitute the T-cell alloresponse. However, as shown in Fig. 3, culture alloresponse was not restored on the sixth day of culture to the levels found with control DC. Furthermore, addition of supernatant from a mixed-lymphocyte reaction of DC from RA-infected mice plus CD4 T cells from C57BL/6b had an inhibitory effect on T-cell allostimulation induced by splenic DC from control mice.
FIG. 3.
DC from RA-infected mice produce or induce the production of inhibitory factors that limit T-cell proliferation. Irradiated splenic DC (5 × 104/well) from RA-infected and control C3H/HeNk mice were cocultured with CD4 T cells (2 × 104/well) from the lymph nodes of uninfected C57BL/6 mice in the presence of DC from uninfected mice (DC control) during the last 3 days of culture or in the presence of supernatant from the mixed-lymphocyte reaction of DC from RA-infected mice with C57BL/6 (C57) CD4 T cells (supDCRA). Background proliferation of DC alone was ≤400 cpm and of CD4 T cells alone was ≤500 cpm. Cell proliferation was measured after a 24-h pulse with [3H]thymidine. Values are the means of triplicate cultures ± standard deviation. Results for one of three independent experiments are shown.
Effect of in vivo infection with T. cruzi on ability of DC to activate T. cruzi-specific T-cell proliferative responses.
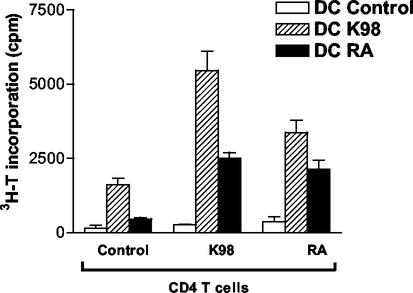
We investigated ex vivo the antigen-presenting activity of DC isolated from the spleens of infected and control mice and monitored their capacity to stimulate CD4 T cells isolated from infected and control mice. As shown in Fig. 4, DC recovered from the spleens of K98-infected mice mediated the highest proliferation of CD4 T cells from infected and control animals. However, stimulation was more efficient with CD4 T cells obtained from mice chronically infected with the homologous T. cruzi strain. In contrast, DC recovered from RA-infected mice induced a weaker proliferation of CD4 T cells than DC from K98-infected mice.
FIG. 4.
Effect of in vivo T. cruzi infection on ability of DC to activate in vitro T. cruzi-specific T-cell proliferative responses. Irradiated splenic DC (4 × 104/well) from acutely RA- or K98-infected and control C3H/HeNk mice were cocultured with CD4 T cells (5 × 105/well) from the lymph nodes of chronically RA- or K98-infected or control mice at 37°C in the presence of T. cruzi lysate (50 μg/ml) in RPMI 1640-10% FBS medium in 96-well microplates in triplicate in a final volume of 200 μl. Background proliferation of DC alone was ≤400 cpm and of T cells alone was ≤500 cpm. Cell proliferation was measured on day 7 after a 24-h pulse with [3H]thymidine. Values are the means of triplicate cultures ± standard deviation. Results for one of three independent experiments are shown.
Splenic DC from acutely RA-infected mice show features of low state of maturation.
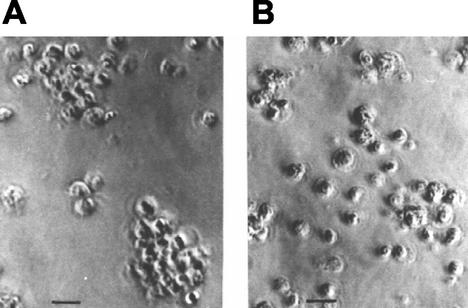
Following antigen capture, immature DC migrate to lymphoid organs, where, after maturation, they display peptide-MHC complexes (3, 15). Homotypic clustering of DC is a close correlate of their state of maturation, as it reflects morphological and physiological changes, including rearrangements of adhesion molecules at the cell surface (8, 34). Here, isolated splenic DC obtained from acutely T. cruzi-infected mice were cultured for 12 h to test this trait. DC isolated from RA-infected mice formed fewer and smaller DC-DC clusters (Fig. 5B) than DC from K98-infected mice (Fig. 5A) or controls (not shown).
FIG. 5.
Cultured splenic DC obtained from mice at the acute phase of infection with RA T. cruzi strain are unable to form homotypic cell clusters. Isolated splenic DC from mice infected with the K98 strain (A) or RA strain (B) at the acute phase of infection are shown. Cells were cultured in RPMI-10% FBS for 12 h at 37°C. Bar, 20 μm.
DISCUSSION
The mechanisms of immune response against T. cruzi infection are still being investigated. Nevertheless, it is clear that the type and state of APC activating T cells are of great importance. Alterations of APC infected in vitro with T. cruzi have been communicated (11, 20, 29, 38), but there are no reports on the effect on professional APC of infection in vivo.
In the present study, we show that murine infection with T. cruzi modulates the expression of MHC class II molecules on professional APC at their plasma membrane in a strain-dependent manner. Indeed, infection with the low-virulence K98 strain preserved the expression of this molecule on DC or stimulated it on peritoneal macrophages and B cells, while the virulent RA strain reduced or inhibited its expression at the surface of professional APC. In the in vivo infection, we found occasional DC and macrophages harboring amastigotes by immunofluorescence staining (less than 5%) without significant differences between strains in the parasite load of APC (data not shown). These results show that factors other than infection of APC themselves are involved in downregulating MHC class II expression during the acute phase of infection with the virulent RA strain. Our results obtained in vivo seem to corroborate what Van Overtvelt et al. demonstrated in vitro: close contact between DC and T. cruzi is not a prerequisite for alteration of the optimal maturation of human DC and expression of HLA-DR molecules (38).
The RA strain almost abrogates the ability of DC to prime allogeneic CD4 T cells, suggesting that the low MHC II expression is responsible for this alteration. Furthermore, DC from RA-infected animals lacked the ability to stimulate alloreactive CD8 T cells, indicating a poor functional capability of these cells. Interestingly, in the mixed-lymphocyte reaction, alloresponse was not restored by addition of DC from uninfected mice to cultures containing DC from RA-infected animals, and supernatants of DC from RA-infected mice inhibited T-cell allostimulation. This finding suggests that infection with the virulent RA strain could be favoring the induction or production of suppressive/regulatory cytokines by DC, thus driving T cells to anergy or to a regulatory phenotype. Even though MHC class II-dependent presentation by DC was not completely lost after infection, DC from K98-infected mice induced a stronger T. cruzi-specific T-cell response than those from RA-infected mice. The fact that DC from RA-infected mice can still elicit a noticeable T-cell response even with low MHC class II expression is not unexpected because it has been proved that only small amounts of MHC class II are necessary to mount a functional cellular response in vivo (39).
DC homotypic interactions stimulate the accessory function of DC by mutual delivery of maturation signals and transfer of antigen between cells (8). The reduced DC-DC clustering of DC from RA-infected mice, together with the low MHC class II expression, might point to an interference with maturation process induced by acute infection with this virulent strain. It has been reported that antigen presentation by immature DC might induce the differentiation of naïve T cells towards a suppressor regulatory phenotype capable of interfering with DC-mediated Th1 differentiation (33). Our observations add the virulent RA strain of T. cruzi to a growing list of stimuli, including measles virus and herpesvirus and Plasmodium falciparum-infected erythrocytes, which appear to inhibit terminal DC differentiation at various stages (17, 34). In contrast, infection with the low-virulence K98 strain preserved DC maturation and allogeneic or T. cruzi-specific T-cell response, allowing the host to control the parasitemia and to survive the acute infection.
The CD80/CD86-CD28 costimulatory pathway participates in the pathogenesis of several infectious diseases (31). As CD86 is the most critical for amplification of T-cell responses (9, 21), its downmodulation on splenic DC from infected mice might contribute to the depression of immune responses observed in acute infection with T. cruzi, a phenomenon of great importance in the pathogenesis of Chagas' disease (19).
It is of interest that APC from mice infected with the virulent RA strain who survived the acute phase of infection recovered or even surpassed MHC class II and costimulatory molecule expression levels to those of K98-infected mice. This finding might be correlated with the kinetics of T- and B-cell proliferation because unresponsiveness to polyclonal activators is seen only at the acute phase of infection with the virulent RA strain. Our data suggest that alteration of APC function by infection with the virulent RA strain is a transient phenomenon during acute infection.
Recent studies reveal that induction of an immune response to pathogens is determined by several variables, including DC function itself, pathogen-derived signals, the local microenvironment, and cytokines released by neighboring T cells and other cells (30, 32). Our results could reflect strain-dependent differences in these microenvironments. Functional modulation of APC by the virulent RA strain may establish an environment that contributes to the great susceptibility shown during acute infection with this strain. An analysis of the immune response focused on the cytokine profiles is currently in progress in our laboratory.
In vitro models are useful to analyze the interactions between APC and T. cruzi. However, the actual relevance of these interactions needs to be demonstrated in vivo, something that has not been done so far. Our results put in evidence for the first time that T. cruzi infection can modulate APC function in vivo. Furthermore, we found that the degree of this alteration correlates with the virulence of the T. cruzi population infecting the host.
Acknowledgments
We thank L. Bukata and D. Fernandez for valuable technical assistance and M. Giordano and V. Tekiel for critical reading of the manuscript.
This work was supported by grants from FONCYT, CONICET, and UBACYT. C.A.S. was supported by a fellowships granted by FONCYT and the Ministry of Health of Argentina. S.M.G.C. is a member of the Research Career from the National Research Council (CONICET) from Argentina.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Amigorena, S., and C. Théry. 2001. The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 13:45-51. [DOI] [PubMed] [Google Scholar]
- 2.Andrade, V., M. Barral-Neto, and S. G. Andrade. 1985. Patterns of resistance of inbred mice to Trypanosoma cruzi are determined by parasite strain. Braz. J. Med. Biol. Res. 18:499-506. [PubMed] [Google Scholar]
- 3.Banchereau, J., F. Briere, C. Caux, J. Davaust, S. Lebecque, L. Yong-Jun, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 4.Brodskyn, C. I., G. K. DeKrey, and R. G. Titus. 2001. Influence of costimulatory molecules on immune response to Leishmania major by human cells in vitro. Infect. Immun. 69:665-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celentano, A. M., and S. M. González Cappa. 1992. Induction of macrophage activation and opsonizing antibodies by Trypanosoma cruzi subpopulations. Parasite Immunol. 14:155-167. [DOI] [PubMed] [Google Scholar]
- 6.Celentano, A. M., and S. M. González Cappa. 1993. In vivo macrophage function in experimental infection with Trypanosoma cruzi subpopulations. Acta Trop. 55:171-180. [DOI] [PubMed] [Google Scholar]
- 7.Celentano, A. M., G. Gorelik, M. E. Solana, L. Sterin-Borda, E. Borda, and S. M. González Cappa. 1995. PGE2 involvement in experimental infection with Trypanosoma cruzi subpopulations. Prostaglandins 49:141-153. [DOI] [PubMed] [Google Scholar]
- 8.Delemarre, F. G., P. G. Hoogeveen, M. De Haan-Meulman, P. J. Simons, and H. A. Drexhage. 2001. Homotypic cluster formation of dendritic cells, a close correlate of their state of maturation. Defects in the biobreeding diabetes-prone rat. J. Leukoc. Biol. 69:373-380. [PubMed] [Google Scholar]
- 9.Freeman, G. J., F. Borriello, R. J. Hodes, H. Resier, J. G. Gribben, J. W. Ng, J. Kim, J. M. Goldberg, K. Hathcock, G. Laszlo, L. A. Lombard, S. Wang, G. S. Gray, L. M. Nadler, and A. H. Sharpe. 1993. Murine B7-2, an alternative CTLA-4 counter-receptor that costimulates T-cell proliferation and interleukin 2 production. J. Exp. Med. 178:2185-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freilij, H., L. Muller, and S. M. González Cappa. 1983. Direct micromethod for diagnosis of acute and congenital Chagas' disease. J. Clin. Microbiol. 18:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frosch, S., D. Kuntzlin, and B. Fleisher. 1997. Infection with Trypanosoma cruzi selectively upregulates B7-2 molecules on macrophages and enhances their costimulatory activity. Infect. Immun. 65:971-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruth, U., N. Solioz, and J. A. Louis. 1993. Leishmania major interferes with antigen presentation by infected macrophage. J. Immunol. 150:1857-1864. [PubMed] [Google Scholar]
- 13.González Cappa, S. M., P. Chiale, G. E. del Prado, A. M. Katzin, G. W. de Martini, E. L. D. de Isola, L. Abramo Orrego, and E. L. Segura. 1980. Isolation of a strain of Trypanosoma cruzi from a patient with chronic Chagas cardiomyopathy and its biological characterization. Medicina (Buenos Aires) 40:63-68. [PubMed] [Google Scholar]
- 14.González Cappa, S. M., A. T. Bijovsky, H. Freilij, L. A. Muller, and A. M. Katzin. 1981. Isolation of a Trypanosoma cruzi strain of predominantly slender form in Argentina. Medicina (Buenos Aires) 41:119-120. [PubMed] [Google Scholar]
- 15.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Théry, and S. Amigorena. 2002. Antigen presentation and T-cell stimulation by dendritic cells. Annu. Rev. Immunol. 20:621-667. [DOI] [PubMed] [Google Scholar]
- 16.Inaba, K., J. W. Young, and R. M. Steinman. 1990. Dendritic cells stimulate primary human cytolytic lymphocyte response in the absence of CD4+ helper T cells. J. Exp. Med. 171:1315-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonuleit, H., E. Schmitt, K. Steimbrink, and A. H. Enk. 2001. Dendritic cells as tools to induce anergic and regulatory cells. Trends Immunol. 22:394-400. [DOI] [PubMed] [Google Scholar]
- 18.Kaye, P. M., N. J. Rogers, A. J. Curry, and J. C. Scott. 1994. Deficient expression of costimulatory molecules on Leishmania-infected macrophages. Eur. J. Immunol. 24:2850-2854. [DOI] [PubMed] [Google Scholar]
- 19.Kierszenbaum, F. 1981. On evasion of Trypanosma cruzi from the host immune response. Lymphoproliferative response to trypanosomal antigens during acute and chronic experimental Chagas' disease. Immunology 44:641-648. [PMC free article] [PubMed] [Google Scholar]
- 20.La Flamme, A. C., S. J. Kahn, A. Y. Rudensky, and W. C. Van Voorhis. 1997. Trypanosoma cruzi-infected macrophages are defective in major histocompatibility complex class II antigen presentation. Eur. J. Immunol. 12:3085-3094. [DOI] [PubMed] [Google Scholar]
- 21.Linsley, P. S., W. Brady, L. Grosmaire, A. Aruffo, N. K. Damle, and J. A. Ledbetter. 1991. Binding of B cell activation antigen B7 to CD28 costimulates T-cell proliferation and interleukin 2 mRNA accumulation. J. Exp. Med. 173:721-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luder, C. G., W. Walter, B. Beurle, M. J. Maeurer, and U. Groos. 2001. Toxoplasma gondii downregulates MHC class II gene expression and antigen presentation by murine macrophages via interference with nuclear translocation of STA1alpha. Eur. J. Immunol. 31:1475-1484. [DOI] [PubMed] [Google Scholar]
- 23.Mirkin, G. A., A. M. Celentano, E. Malchiodi, M. Jones, S. M. González Cappa. 1997. Different Trypanosoma cruzi strains promote neuromyopathic damage mediated by distinct T lymphocyte subsets. Clin. Exp. Immunol. 107:328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moncayo, A. 1997. Progress towards the elimination of transmission of Chagas' disease in Latin America. Wld. Statist. Q. 50:195-198. [PubMed] [Google Scholar]
- 25.Mortatti, R. C., and M. E. Munk. 1985. Separation of bloodstream trypomastigotes of T. cruzi by density gradient centrifugation. J. Parasitol. 71:520-521. [PubMed] [Google Scholar]
- 26.Muller, L., N. Añasco, and S. M. González Cappa. 1986. Trypanosoma cruzi: isolate dependence in the induction of lytic antibodies in the mouse and rabbit. Exp. Parasitol. 61:284-293. [DOI] [PubMed] [Google Scholar]
- 27.Nogueira, S. P., J. Ellis, S. Chaplan, and Z. Cohn. 1981. Trypanosoma cruzi: in vivo and in vitro correlation between T-cell activation and susceptibility in inbred strains of mice. Exp. Parasitol. 51:325-334. [DOI] [PubMed] [Google Scholar]
- 28.Overath, P., and T. Aebischer. 1999. Antigen presentation by macrophages harboring intravesicular parasites. Parasitol. Today 15:325-332. [DOI] [PubMed] [Google Scholar]
- 29.Plasman, N., J. G. Guillet, and B. Vray. 1995. Impaired protein catabolism in Trypanosoma cruzi-infected macrophage: possible involvement in antigen presentation. Immunology 86:636-645. [PMC free article] [PubMed] [Google Scholar]
- 30.Pulendran, B., E. Maraskovsky, J. Banchereau, and C. Maliszewski. 2001. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 22:41-47. [DOI] [PubMed] [Google Scholar]
- 31.Reiser, H., and M. J. Stadecker. 1996. Costimulatory B7 molecules in the pathogenesis of infectious and autoimmune diseases. N. Engl. J. Med. 335:1369-1376. [DOI] [PubMed] [Google Scholar]
- 32.Rescigno, M. 2002. Dendritic cells and the complexity of microbial infection. Trends Microbiol. 10:425-431. [DOI] [PubMed] [Google Scholar]
- 33.Roncarolo, M. G., M. K. Levings, and C. Traversari. 2001. Differentiation of T regulatory cells by immature dendritic cells. J. Exp. Med. 193:F5-F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saemann, M. D., O. Parolini, G. A. Bohming, P. Kelemen, P. M. Krieger, J. Neumuller, K. Knarr, W. Kammlander, W. H. Horl, C. Diakos, K. Stuhlmeier, and G. J. Zlabinger. 2002. Bacterial metabolite interference with maturation of human monocyte-derived dendritic cells. J. Leukoc. Biol. 71:238-246. [PubMed] [Google Scholar]
- 35.Storino, R. 1994. Chagas crónico, p. 247-266. In R. Storino and J. Milei (ed.), Enfermedad de Chagas, 1st ed. Doyma, Buenos Aires, Argentina.
- 36.Tekiel, V., A. Losavio, M. Jones, S. Muchnik, and S. M. González Cappa. 2001. Changes in the mouse sciatic nerve action potential after epineural injection of sera from Trypanosoma cruzi infected mice. Parasite Immunol. 23:533-539. [DOI] [PubMed] [Google Scholar]
- 37.Urban, B. C., D. J. Ferguson, A. Pain, N. Willcox, M. Plebanski, J. M. Austyn, and D. J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73-77. [DOI] [PubMed] [Google Scholar]
- 38.Van Overtvelt, L., N. Vanderheyde, V. Verhasselt, J. Ismail, L. de Vos, M. Goldman, F. Willems, and B. Vray. 1999. Trypanosoma cruzi infects human dendritic cells and prevents their maturation: Inhibition of cytokines, HLA-DR, and costimulatory molecules. Infect. Immun. 67:4033-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf, H. M., V. Thon, H. Gulle, S. Lechleitner, M. M. Eibl, and P. Petzelbauer. 2001. Residual expression of functional MHC class II molecules in twin brothers with MHC class II deficiency is cell type specific. Br. J. Haematol. 115:460-471. [DOI] [PubMed] [Google Scholar]
- 40.Zuñiga, E., C. Motran, C. L. Montes, F. L. Diaz, J. L. Bocco, and A. Gruppi. 2000. Trypanosoma cruzi-induced immunosuppression: B cells undergo spontaneous apoptosis and lipopolysaccharide (LPS) arrests their proliferation during infection. Clin. Exp. Immunol. 119:507-515. [DOI] [PMC free article] [PubMed] [Google Scholar]