Abstract
Chlamydia trachomatis is a human pathogen causing trachoma, urogenital disease, and lymphogranuloma venereum (LGV). A family of nine polymorphic membrane protein genes (pmpA to pmpI), resembling autotransporter proteins, has recently been discovered in C. trachomatis. pmp genes are large and predicted to be outer membrane proteins. We hypothesized that they would contain useful nucleotide sequence variability for epidemiologic studies. Since sequence information is available only for serovars D and L2, we sought to determine the amount of diversity within an individual pmp gene among serovars. We used restriction fragment length polymorphism (RFLP) analysis as a primary screen to assess the amount of sequence divergence among the pmp genes for serovars A to L3 of C. trachomatis. RFLP analysis showed little variation for some of the genes, such as pmpA, but substantial variation in others, such as pmpI. pmpH and pmpE yielded RFLP patterns that clustered the 15 serovars into ocular, urogenital, and LGV groups, and both proteins have been localized to the outer membrane. Therefore, we chose to sequence pmpE, pmpH, and pmpI from each of the 15 serovars. Evolutionary analysis showed three distinct divergence patterns. PmpI was least variable, resulting in an ambiguous evolutionary pattern. PmpE showed a high degree of diversity in the ocular strains compared to the other strains. Finally, the evolution of PmpH shows three groups that reflect disease groups, suggesting this protein may play a role in pathogenesis.
Chlamydia trachomatis is an obligate intracellular human pathogen that causes trachoma, urogenital disease, and lymphogranuloma venereum (LGV). The strains of C. trachomatis are divided into at least 18 different serovars based on immunological cross-reactivity of the major outer membrane protein (MOMP) (30, 31, 32). Additionally, the serovars of C. trachomatis are classified into two groups, the trachoma biovar and the LGV biovar, based upon differences in disease presentation and in vitro cell growth characteristics. The trachoma biovar includes serovars that cause ocular trachoma (A, B, Ba, and C) and serovars that cause urogenital disease (D through K, Da, and Ia), while the LGV biovar includes serovars L1, L2, L2a, and L3.
A superfamily of genes encoding predicted polymorphic membrane proteins (pmp) was discovered as part of the Chlamydia genome project (16, 23, 26). Several proteins encoded by these genes had previously been investigated in C. psittaci (6, 11, 12, 20, 25). Nine genes are found in C. trachomatis (pmpA to pmpI) (26). The Pmp proteins are large proteins, 90 to 187 kDa in mass, and they have no obvious homologs in nonchlamydial species (29). Transcription of all nine genes has been shown for both biovars in serovars D and L2 (19). Of the nine genes, all but pmpA have an apparent signal peptide leader sequence and are predicted outer membrane proteins (13). While expression has not been verified for all nine proteins in all serovars, expression of PmpE, G, and H, and localization of these three proteins to the outer membrane of C. trachomatis serovar L2 has been documented (21, 28).
The pmp genes are highly diverse in nucleotide sequence. None of the pmp genes are more than 52% similar to each other (13). However, the amount of sequence diversity within an individual pmp gene among the different serovars has not been investigated since sequence information is available only for serovars D and L2. Considering that the Pmp proteins are large, and thought to be expressed on the outer membrane (28, 29), we hypothesized that pmp genes might contain useful variability for epidemiologic studies. Currently, the MOMP gene serves as a molecular marker in epidemiologic studies because of its high degree of sequence variability. However, the MOMP gene for some serovars, like E and F, exhibits a high degree of conservation at the nucleotide level, decreasing its usefulness in epidemiologic studies (9, 27). Other markers, like the pmp, could improve discrimination between strains. Therefore, we examined the pmp genes as possible molecular epidemiologic markers by determining the amount of sequence diversity within the pmp genes among serovars.
We used restriction fragment length polymorphism (RFLP) analysis as an initial screen to assess the amount of sequence variability among the pmp genes of 15 serovars of C. trachomatis. RFLP analysis showed limited to no variation among serovars for some of the pmp genes, such as pmpA, but substantial variation was seen for other genes, such as pmpI. Interestingly, pmpH yielded RFLP patterns that clustered the 15 serovars into ocular, urogenital, and LGV disease groups. The RFLP patterns for pmpE and pmpF also showed potential segregation of serovars by disease group. However, unlike PmpE, expression of PmpF has not been demonstrated. Therefore, we chose to sequence pmpE, pmpH, and pmpI from 15 serovars of C. trachomatis. We chose pmpE and pmpH because of their provocative RFLP patterns and demonstration of their surface location and pmpI because of its apparent nucleotide diversity.
MATERIALS AND METHODS
Strains and isolates.
Elementary bodies (EBs) were partially purified (22) by differential centrifugation followed by density gradient centrifugation in Percoll from the following laboratory strains: A/571-B/OT, B/TW-5/OT, Ba/Ap-2/OT, C/TW-3/OT, D/UW-3/Cx, E/UW-5/Cx, F/UW-6/Ur, G/UW-57/Cx, H/UW-4/Cx, I/UW-12/Ur, J/UW-36/Cx, K/UW-53/Cx, L1/440, L2/434, and L3/404.
DNA extraction.
DNA was extracted from purified EBs by using previously described methods (27). The only modification was the addition of dithiothreitol (4 mM final concentration) before boiling to assist in disruption of EB outer membrane proteins. DNA from purified EBs was resuspended into 50 μl of Tris buffer (10 mM Tris-Cl [pH 8.5]).
pmp PCR amplification.
The pmp genes were amplified from C. trachomatis serovars A to L3 by using PCR primers (Table 1) based on the published serovar D sequences. We were unable to amplify the pmpB gene from any serovar after trying three separate sets of primers. All PCR products were of the expected sizes based on the published serovar D genes (26).
TABLE 1.
pmp PCR primers
| Primer | Sequence | Nucelotide locationa and direction |
|---|---|---|
| pmpA1-FOR | 5′-ATG AAT CGA GTT ATA GAA ATC CAT GC | 145, forward |
| pmpA1-REV | 5′-TTA GAA GCC AAC ATA GCC TCC G | 3061, reverse |
| pmpC1-FOR | 5′-GAC TGC AAT GTT AGC AAA TTA GGA TA | 94, forward |
| pmpC1-REV | 5′-CAT ACG AGC ACC GCA GTT CA | 5288, reverse |
| pmpD1-FOR | 5′-CCT CAA GCG GTT TTA TTG TTA GAC C | 181, forward |
| pmpD1-REV | 5′-CAA TCC AGT ATT CGC CTC ATA TCC T | 4590, reverse |
| pmpE1-FOR | 5′-CTT ATG CCC AAC TCA GTT CCA GA | 151, forward |
| pmpE1-REV | 5′-TCG CAG AGC AAT TTC CCC AT | 2942, reverse |
| pmpF1-FOR | 5′-ATG AAA CGG ATA CGC TAC AGT TC | 83, forward |
| pmpF1-REV | 5′-GAC CAG AGC TCC TCC TGC AT | 3086, reverse |
| pmpG1-FOR | 5′-CAA GGA ATT TAC GAT GGG GAG A | 127, forward |
| pmpG1-REV | 5′-TCC TGC ACT CAA ACC ATA ACC | 3031, reverse |
| pmpH N1 | 5′-TCT CCT CAA GTG TTA ACA CCT AAT G | 76, forward |
| pmpH1-REV | 5′-AAA GAT TCT ATT CAA GCC CAT GG | 3035, reverse |
| pmpI1-FOR | 5′-CAG GAT CCC TTA GGT GAA ACC G | 73, forward |
| pmpI1-REV | 5′-CAG GGA GTA ACT ATG GCT TTG CC | 2610, reverse |
Primer locations indicate base position of each primer in serovar D for each pmp gene.
DNA amplification was done with the Expand high-fidelity kit (Roche Molecular Biochemicals, Indianapolis, Ind.). Reaction mixtures were 50 μl and consisted of 5 μl of template DNA, 15 pmol of each primer, 1.5 mM MgCl2, 200 μM concentrations of each deoxynucleoside triphosphate, and 0.75 μl of Expand polymerase in a 1× reaction buffer. Cycling conditions began with an initial 5-min denaturation step at 95°C followed by 35 cycles of denaturation (95°C, 30 s), annealing (58°C, 30 s), and extension (68°C, 4 min). An additional 10-min extension at 68°C was performed at the end of the 35 cycles.
RFLP analysis.
PCR products were purified with the QIAquick PCR purification kit (QIAGEN). Products were eluted from QIAquick columns into 50 μl of elution buffer (10 mM Tris [pH 8.5]). Restriction endonuclease reactions were carried out according to the manufacturer's instructions. Reaction mixtures were 10 μl in volume and consisted of 5 μl of pmp PCR product and 1 to 5 U of enzyme in a 1× reaction buffer. Computer-generated restriction digests of the pmp genes from serovar D (Clone Manager, version 4.01; Scientific and Educational Software) were used to determine which enzymes would be appropriate for RFLP analysis and included CfoI, BamHI, HinfI, TaqI, and Tru9I (all from Roche Molecular Biochemicals). PCR products were digested with at least two different restriction endonucleases that cleaved from 3 to 19 times within each gene. The restriction products were then electrophoresed through 2 to 4% agarose gels, stained with ethidium bromide, and digitally photographed with a UV transilluminator.
DNA sequencing.
PCR products for pmpE, pmpH, and pmpI were each amplified in duplicate and pooled for sequence analysis to insure sequence fidelity. All sequencing reactions were done with Big Dye terminator chemistry and an ABI Prism 310 automated DNA sequencer (Applied Biosystems, La Jolla, Calif.). To obtain full-length sequences from pmpE, pmpH, and pmpI, several internal primers were used in addition to the PCR amplification primers (Table 2). Sequences were collected from both strands in order to resolve ambiguities and ensure consistency. In addition, electropherograms were manually checked for computer miscalls.
TABLE 2.
pmpE, pmpH, and pmpI internal sequencing primers
| Primer | Sequence (5′-3′) | Nucleotide locationc and direction |
|---|---|---|
| pmpE-560 | TAC CTT TGT TGT GAG CGA GAA TC | 560, forward |
| pmpE-570R | AAC AAG ACT GAT TCT CGC TCA C | 570, reverse |
| pmpE-1259 | CAA TAA GGA AGC TGA TCA AAC AG | 1259, forward |
| pmpE-1510R | CCW ATA TGA TTM AGT GTT AYA GAG G | 1510, reverse |
| pmpE-2359R | TCT CCT TGR GTA TAG AAA TGG TGA C | 2359, reverse |
| pmpE-2499 | GCT CTT GGT ATT TAT TCT AGC CTG | 2499, forward |
| pmpH-443REV | GGC ACA ATA GAC AAA GGA GAA TAC C | 443, reverse |
| pmpH-1026 | TCT TGT AGC AGA TGA TTC GGT TGT C | 1026, forward |
| pmpH-1097REVa | CCA TTC GTG CTC ATA TTG ATA GTA G | 1097, reverse |
| pmpH-1174REVa | ACC AGA GCT TGC TTC ACT CAC | 1174, reverse |
| pmpH-1914a | CAC TAT TAA TGA TGC AGA TGC TGC | 1914, forward |
| pmpH-2011a | GTA CCT CCT AAT ACC AAT AAC ACT CTG | 2011, forward |
| pmpH-2188REV | CAT GCA GAG ATG CTA CAA ATC CAA C | 2188, reverse |
| pmpH-2619 | TCG TCA GTT CGC TAG AGA TTA TGA G | 2619, forward |
| pmpI-435R | GCA GAG ATG GCT CCT CCA GAG | 435, reverse |
| pmpl-627 | CGG CGC TAT TTG CTG TAG TAA TC | 627, forward |
| pmpI N2b | TCC CCC GGG CGT GAC TTC TCC CAC CCC | 1118, forward |
| pmpI C2b | AGA AAG CTT AGG GGC GTG GAT AGT TAC | 1438, reverse |
| pmpI-1659 | TTC TTG GAA AGA TTC TGA TGA AGG G | 1659, forward |
| pmpI-1729REV | ACG AGT GTA GAT TGA CGT TCT GG | 1729, reverse |
| pmpI-2301 | TGA GGA GAG TTC GGG AGA GAT TC | 2301, forward |
pmpH-1174REV and pmpH-2011 did not work on all strains due to mismatches in those regions with LGV and ocular strains. Therefore, pmpH-1097REV and pmph-1914 were made as alternate primers.
Underlined bases in pmpI N2 and pmpI C2 indicate restriction sites added to the primer and are not part of the pmpI gene.
Primer locations indicate base position of each primer in serovar D for each pmp gene.
Nucleotide and amino acid sequence alignment.
Sequences were aligned with the assistance of the program ESEE (5) based on both nucleotide and amino acid residues. In regions of high variability, the alignment was based primarily on the most-conservative amino acid substitutions and secondarily on nucleotide substitutions.
The primers used to amplify pmpE extended from nucleotide 80 of the coding region to nucleotide 2881 based on the serovar D pmpE gene. Therefore, the first 79 bases and the last 15 bases are not included in the alignment. The sequence alignment for pmpE contained 2,811 nucleotide sites (nucleotides plus alignment gaps) and 936 amino acid sites (amino acids plus alignment gaps).
The primers used to amplify pmpH extended from nucleotide 76 of the coding region to nucleotide 3051 (terminal stop codon) based on the serovar D pmpH gene. Therefore, the first 75 bases are not included in the alignment. The sequence alignment for pmpH contained 2,991 nucleotide sites (nucleotides plus alignment gaps) and 996 amino acid sites (amino acids plus alignment gaps).
The primers used to amplify pmpI extended from nucleotide 73 of the coding region to nucleotide 2610 based on the serovar D pmpI gene. Therefore, the first 72 bases and the last 27 bases are not included in the alignment. The sequence alignment for the pmpI gene contained 2,538 nucleotides and 846 amino acids. There were no in/del events in pmpI.
Nucleotide and amino acid comparisons.
After sequences were aligned, nucleotide and amino acid sequences were compared manually in a pairwise fashion for differences. Contiguous in/dels at the nucleotide and amino acid levels were counted as individual differences, i.e., a deletion of six nucleotides between two pairs was counted as six differences rather than one deletion event, and the resulting two-amino-acid deletion was counted as two deletions rather than as one deletion event.
Phylogenetic analysis. (i) Nucleotide sequences.
Because the 5′ and 3′ terminal sequences of the pmp genes are primer defined, those regions were excluded from the phylogenetic analyses. Consequently, 2,783 of 2,811 nucleotides were compared for pmpE, 2,940 of 2,991 nucleotides were compared for pmpH, and 2,493 of 2,538 nucleotides of pmpI were compared.
All phylogenetic and evolutionary analyses were conducted with MEGA (molecular evolutionary genetics analysis), version 2.1 (17). Once aligned, the corrected nucleotide divergence of sequences was calculated in MEGA, incorporating the Kimura two-parameter correction for multiple substitutions at a single site. The branching pattern of taxa was estimated from the distance matrix by the neighbor-joining method (24) as implemented in MEGA. A cladistic reconstruction of the phylogenetic relationships among taxa also was performed by using maximum parsimony as implemented in MEGA with close-neighbor interchange. Confidence levels for the branching pattern were estimated by a bootstrap resampling of the data. Bootstrap values for the trees were obtained from a consensus tree based on 1,000 replications. Although pmp genes have been identified in C. trachomatis, Chlamydiapneumoniae, and Chlamydia psittaci, no homologues exist for C. trachomatis pmp genes. Therefore, an appropriate outgroup could not be identified, and the trees were not rooted.
(ii) Amino acid sequences.
Because 5′ and 3′ terminal sequences of the pmp genes are primer defined, 927 of 936 amino acids of PmpE, 974 of 996 amino acids of PmpH, and 831 of 846 amino acids of PmpI were included in the analyses.
All phylogenetic and evolutionary analyses were conducted using MEGA, version 2.1 (17). Phylogenetic reconstructions based on the inferred amino acid sequences were performed by using both distance and parsimony methods as implemented in MEGA. As with the nucleotide data, bootstrap values for the trees were obtained from a consensus tree based on 1,000 randomly generated datasets and the trees were not rooted.
Nucleotide sequence accession numbers.
Nucleotide sequences determined as part of this study can be found using the following GenBank accession numbers (pmpE, pmpH, and pmpI, respectively): A/571-B/OT, no. AY184140, AY184155, and AY184170; B/TW-5/OT, no. AY184141, AY184156, and AY184171; Ba/Ap-2/OT, no. AY184142, AY184157, and AY184172; C/TW-3/OT, no. AY184143, AY184158, and AY184173; D/UW-3/Cx, no. AY184144, AY184159, and AY184174; E/UW-5/Cx, no. AY184145, AY184160, and AY184175; F/UW-6/Ur, no. AY184146, AY184161, and AY184176; G/UW-57/Cx, no. AY184147, AY184162, and AY184177; H/UW-4/Cx, no. AY184148, AY184163, and AY184178; I/UW-12/Ur, no. AY184149, AY184164, and AY184179; J/UW-36/Cx, no. AY184150, AY184165, and AY184180; K/UW-53/Cx, no. AY184151, AY184166, and AY184181; L1/440, no. AY184152, AY184167, and AY184182; L2/434, no. AY184153, AY184168, and AY184183; and L3/404, no. AY184154, AY184169, and AY184184. Previously published nucleotide sequences for pmpE, pmpH, and pmpI from serovar D/UW3 can be found at the Chlamydia genome website (http://chlamydia-www.berkeley.edu:4231/).
RESULTS
RFLP analysis shows differing degrees of variation within the pmp gene family.
RFLP results are summarized in Table 3. Serovars that are grouped within parentheses in the table had the same RFLP pattern for the particular enzyme listed. For example, digestion of pmpA with TaqI produced two RFLP patterns, dividing the 15 serovars into two RFLP groups. One group consisted of serovars J and K while the remaining serovars had a different RFLP pattern. Interestingly, pmpA, the only pmp gene without a predicted leader peptide sequence, exhibited the least amount of variation, with two of the three enzymes demonstrating no differences among serovars. Variation in RFLP patterns was seen for the other pmp genes, but the amount of variation differed.
TABLE 3.
RFLP analysis of pmp genes from C. trachomatis serovars A to L3
| pmp locus | Enzyme | No. of expected sitesa | RFLP groupings (serovars with the same RFLP pattern) |
|---|---|---|---|
| A | CfoI | 3 | No variation |
| HinfI | 12 | No variation | |
| TaqI | 12 | (ABBaCDEFGHI and LGV) (JK) | |
| C | TaqI | 16 | (ABCEF and LGV) (Ba) (DGHIJK) |
| Tru9I | 19 | (ABBaC and LGV) (EF) (DGH) (IJK) | |
| D | CfoI | 10 | No variation |
| HinfI | 17 | (B) (C) (EF) (ABaDGHIJK and LGV) | |
| E | Tru9I | 9 | (ABBaC) (DEFGH) (IJK and LGV) |
| HinfI | 14 | (ABBaC) (DEFGH) (IJK) (LGV) | |
| F | HinfI | 10 | (ABBaC) (DEFGH) (IJK) (LGV) |
| CfoI | 5 | (ABBaC) (DEFGH) (IJK) (LGV) | |
| TaqI | 6 | (ABa) (BC) (DEFGH) (IJK) (LGV) | |
| G | CfoI | 6 | No variation |
| TaqI | 11 | (BC) (ABaDEFGHIJK) (LGV) | |
| HinfI | 15 | (ABBaCI) (DEFGHJK) (LGV) | |
| H | CfoI | 7 | (ABBaC) (DEFGHIJK) (LGV) |
| Tru9I | 16 | (ABBaC) (DEFGHIJK) (LGV) | |
| TaqI | 14 | (ABBaC) (DEFGH) (IJK) (L1, L2) (L3) | |
| I | CfoI | 10 | (ABBaCDEFGHIJK) (LGV) |
| HinfI | 12 | (ABC) (Ba) (EF) (DGHIJK) (LGV) | |
| TaqI | 6 | (ABaC) (B) (DEFGHI and LGV) (JK) |
The number of expected sites is based upon published serovar D sequences for each pmp gene.
Digestion of pmpF with three different enzymes produced nearly the same patterns, and the patterns indicate a segregation of the ocular, urogenital, and LGV serovars based on this gene. HinfI and CfoI digests were identical, producing the same four RFLP groups: one containing the ocular serovars; one containing D through H; one including I, J, and K; and one with the LGV serovars. The TaqI digest exhibited some variation within the ocular serovars, producing one pattern for serovars A and Ba and another pattern for serovars B and C.
A very similar arrangement of serovars was seen for pmpE digests. Digestion of pmpE with HinfI produced the same serovar groupings as seen for the HinfI and CfoI digests of pmpF. Tru9I produced a similar pattern, the only difference being that LGV serovars also had the same Tru9I pattern as serovars I, J, and K.
The most interesting RFLP pattern was seen for pmpH. Two of the three enzymes used produced an arrangement of serovars for this gene that mirrors the three disease groups of C. trachomatis. Both Tru9I and CfoI produced three RFLP groups, one containing the ocular serovars, one containing the urogenital serovars, and one containing the LGV serovars. The TaqI digest showed some variation within the urogenital and LGV groups, but for all three enzymes serovars in different disease groups never shared the same RFLP pattern.
RFLP analysis of pmpI suggests that it is the most variable pmp gene, but sequence analysis shows pmpI to be highly conserved.
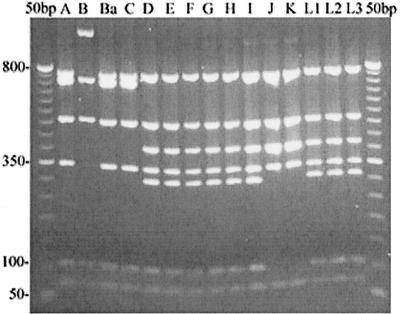
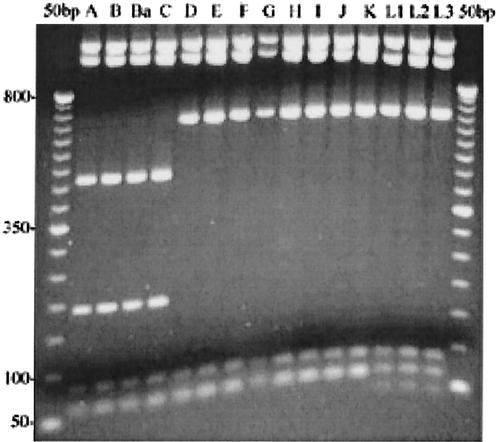
pmpI exhibited the most variation of any of the pmp genes by RFLP analysis (Fig. 1). Three different enzymes were used to digest pmpI, and each enzyme produced a different set of RFLP patterns. Since pmpI exhibited interserovar variation, we chose to search for intraserovar variation in pmpI by using 13 serovar E clinical isolates. We selected serovar E isolates because the MOMP gene is remarkably conserved among serovar E isolates (9, 27). However, no variation was evident in the pmpI gene from the 13 clinical isolates by using either TaqI or CfoI (data not shown).
FIG. 1.
RFLP analysis suggests pmpI is most variable. pmpI PCR products amplified from 15 serovars (indicated above their respective lanes) of C. trachomatis digested with TaqI endonuclease and separated on a 2% agarose gel are shown. Numbers on the left are size standards in base pairs.
Contrary to the RFLP results, sequences from 15 serovars showed that pmpI is highly conserved, with no more than 1% sequence dissimilarity across all serovars, including the LGV strains (Table 4). There also were no insertion or deletion (in/del) events in pmpI among the serovars. The sequence for pmpI from serovar D/UW3 was identical to the sequence deposited at the Chlamydia genome website for pmpI from serovar D (http://chlamydia-www.berkeley.edu:4231/).
TABLE 4.
Sequence diversity in the pmpI gene among serovars of C. trachomatis
| Sero- var | No. of dissimilarities with serovara:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | Ba | C | D | E | F | G | H | I | J | K | L1 | L2 | L3 | |
| A | 1 | 2 | 2 | 5 | 6 | 5 | 6 | 5 | 5 | 9 | 10 | 6 | 6 | 6 | |
| B | 5 | 3 | 1 | 6 | 7 | 6 | 7 | 6 | 6 | 8 | 9 | 5 | 5 | 5 | |
| Ba | 2 | 7 | 4 | 7 | 8 | 7 | 8 | 7 | 7 | 11 | 12 | 8 | 8 | 8 | |
| C | 3 | 4 | 5 | 7 | 8 | 7 | 8 | 7 | 7 | 9 | 10 | 6 | 6 | 6 | |
| D | 8 | 13 | 10 | 11 | 7 | 6 | 1 | 0 | 0 | 4 | 5 | 7 | 7 | 7 | |
| E | 10 | 15 | 12 | 13 | 14 | 1 | 8 | 7 | 7 | 11 | 12 | 8 | 8 | 8 | |
| F | 9 | 14 | 11 | 12 | 13 | 1 | 7 | 6 | 6 | 10 | 11 | 7 | 7 | 7 | |
| G | 9 | 14 | 11 | 12 | 1 | 15 | 14 | 1 | 1 | 5 | 6 | 8 | 8 | 8 | |
| H | 8 | 13 | 10 | 11 | 0 | 14 | 13 | 1 | 0 | 4 | 5 | 7 | 7 | 7 | |
| I | 6 | 11 | 8 | 9 | 2 | 12 | 11 | 3 | 2 | 4 | 5 | 7 | 7 | 7 | |
| J | 15 | 16 | 17 | 14 | 9 | 21 | 20 | 10 | 9 | 9 | 1 | 9 | 9 | 9 | |
| K | 16 | 17 | 18 | 15 | 10 | 22 | 21 | 11 | 10 | 10 | 1 | 10 | 10 | 10 | |
| L1 | 19 | 20 | 21 | 18 | 23 | 23 | 22 | 24 | 23 | 21 | 25 | 27 | 0 | 0 | |
| L2 | 18 | 19 | 20 | 17 | 22 | 22 | 21 | 23 | 22 | 20 | 25 | 26 | 1 | 0 | |
| L3 | 18 | 19 | 20 | 17 | 22 | 22 | 21 | 23 | 22 | 20 | 25 | 26 | 1 | 0 | |
Values below the diagonal are nucleotide dissimilarities out of 2,538 nucleotides matched. Values above the diagonal are amino acid differences out of 846 amino acids matched. Within-disease group comparisons can be made for serovars A to C, D to K, and L1 to L3.
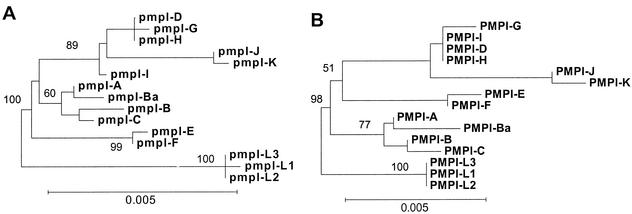
Phylogenetic analyses of the nucleotide and amino acid sequences of pmpI did not produce highly stable trees (Fig. 2), likely due to the lack of diversity among the serovars. Neighbor-joining and parsimony trees based upon the nucleotide sequence of pmpI were nearly identical; Fig. 2A shows the neighbor-joining tree. Branch lengths are proportional to distances between taxa, but because there is so little diversity in pmpI, the scale bar and branch lengths in Fig. 2 are inflated (compared to trees drawn for pmpE and pmpH) in order to clearly illustrate branching patterns. Evolutionary analysis of the inferred amino acid sequences of PmpI produced a slightly different tree (Fig. 2B). In this tree, serovars diverge into three clades consistent with disease groups, whereas in the nucleotide tree, serovars E and F diverge prior to a clade composed of a mixture of ocular and urogenital serovars.
FIG. 2.
Evolution of pmpI shows weak segregation of serovars compatible with disease groups. Neighbor-joining trees based on the nucleotide (A) and inferred amino acid (B) sequences of pmpI are shown. Bootstrap values are shown only at the nodes separating major clades and only when they exceeded 50%. The scale bar equals a corrected sequence divergence of 0.005, which is the same as the scale used in Fig. 4 and 6. However, due to lesser divergence in pmpI, the branches and scale bar here are lengthened in order to plainly illustrate the branching patterns.
Sequence analysis of pmpE indicates substantial genetic evolution in the ocular serovars for this protein.
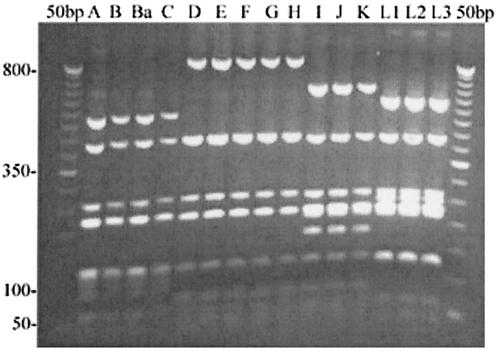
The RFLP pattern for pmpE (Table 3) with HinfI (Fig. 3) was identical to those seen for pmpF, showing a separation of ocular, urogenital, and LGV serovars but with additional differences in serovars I, J, and K that separate them from the rest of the urogenital serovars. Since PmpE has been shown to be surface exposed (28), we obtained sequence data for pmpE from the 15 serovars.
FIG. 3.
PmpE digested with HinfI restriction endonuclease shows segregation of ocular, urogenital, and LGV serovars. An 8-μl aliquot of the pmpE PCR products was digested with HinfI endonuclease, and restriction fragments were separated on a 2% agarose gel. Serovars are indicated above their respective lanes. Numbers on the left are size standards in base pairs.
The nucleotide and inferred amino acid sequences of pmpE were nearly identical among serovars within a disease group, with less than 0.5% dissimilarity, but they were more different when comparing serovars across disease groups (Table 5). Unlike PmpI, five in/del events have occurred in PmpE, all occurring in the N-terminal portion of the protein, between amino acids 147 and 533 (based on serovar D/UW3). The events range from one to four amino acids in length, and in three of the five events, the ocular serovars contain an in/del relative to the other 11 serovars.
TABLE 5.
Sequence diversity in the pmpE gene among serovars of C. trachomatis
| Sero- var | No. of dissimilarities with serovara:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | Ba | C | D | E | F | G | H | I | J | K | L1 | L2 | L3 | |
| A | 4 | 4 | 4 | 64 | 63 | 63 | 64 | 64 | 58 | 57 | 57 | 66 | 66 | 63 | |
| B | 6 | 0 | 2 | 63 | 62 | 62 | 63 | 63 | 57 | 56 | 56 | 63 | 63 | 60 | |
| Ba | 4 | 2 | 2 | 63 | 62 | 62 | 63 | 63 | 57 | 56 | 56 | 63 | 63 | 60 | |
| C | 6 | 4 | 4 | 63 | 62 | 62 | 63 | 63 | 57 | 56 | 56 | 63 | 63 | 60 | |
| D | 179 | 181 | 179 | 180 | 1 | 1 | 0 | 0 | 22 | 21 | 21 | 22 | 22 | 19 | |
| E | 175 | 177 | 175 | 176 | 4 | 0 | 1 | 1 | 21 | 20 | 20 | 21 | 21 | 18 | |
| F | 175 | 177 | 175 | 176 | 4 | 0 | 1 | 1 | 21 | 20 | 20 | 21 | 21 | 18 | |
| G | 179 | 181 | 179 | 180 | 0 | 4 | 4 | 0 | 22 | 21 | 21 | 22 | 22 | 19 | |
| H | 179 | 181 | 179 | 180 | 0 | 4 | 4 | 0 | 22 | 21 | 21 | 22 | 22 | 19 | |
| I | 145 | 147 | 145 | 146 | 69 | 65 | 65 | 69 | 69 | 1 | 1 | 19 | 19 | 16 | |
| J | 144 | 146 | 144 | 145 | 69 | 65 | 65 | 69 | 69 | 2 | 0 | 18 | 18 | 15 | |
| K | 144 | 146 | 144 | 145 | 68 | 64 | 64 | 68 | 68 | 1 | 1 | 18 | 18 | 15 | |
| L1 | 163 | 163 | 161 | 162 | 57 | 53 | 53 | 57 | 57 | 39 | 39 | 38 | 0 | 3 | |
| L2 | 164 | 164 | 162 | 163 | 58 | 54 | 54 | 58 | 58 | 40 | 40 | 39 | 1 | 3 | |
| L3 | 161 | 161 | 159 | 160 | 52 | 48 | 48 | 52 | 52 | 37 | 37 | 36 | 8 | 9 | |
Values below the diagonal are nucleotide dissimilarities out of 2,783 nucleotides matched. Values above the diagonal are amino acid differences out of 927 amino acids matched. Within-disease group comparisons can be made for serovars A to C, D to K, and L1 to L3.
Overall, sexually transmitted disease (STD) serovars differed from LGV serovars by 1.6 to 2.5% at the amino acid level and 1.3 to 2.5% at the nucleotide level (Table 5). There was also an increased amount of divergence seen in serovars I, J, and K compared to the other STD serovars, again consistent with the RFLP patterns. At the sequence level, the most remarkable thing about pmpE was the amount of diversity in the ocular serovars compared to the two other groups. Ocular serovars exhibited three times more variation in pmpE than the other two disease groups: 5.1 to 6.5% at the nucleotide level and 6.1 to 7.0% at the amino acid level (Table 5). The sequence for pmpE from serovar D/UW3 was identical to the sequence deposited at the Chlamydia genome website for pmpE from serovar D (http://chlamydia-www.berkeley.edu:4231/).
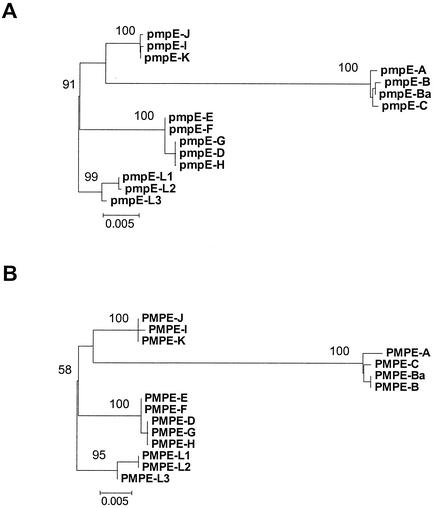
Neighbor-joining and parsimony analyses of the nucleotide sequences of pmpE produced the same tree (Fig. 4A). Branch lengths are proportional to nucleotide divergence, and while the scale bar represents the same amount of divergence as seen in Fig. 2, note that the actual bar is smaller due to the increased amount of divergence in pmpE compared to pmpI. The evolutionary analysis of pmpE nucleotide sequences was consistent with the RFLP patterns seen in Fig. 3 and Table 3, with serovars I, J, and K forming a distinct group separate from the rest of the urogenital serovars. The evolutionary tree obtained using the amino acid sequences of PmpE (Fig. 4B) was the same as the nucleotide tree. Most remarkable, however, is the long branch separating the ocular serovars from the remaining serovars, suggesting either an increased rate of evolution in the ocular serovars or a deceleration in the nonocular strains for this protein.
FIG. 4.
Sequence analysis of pmpE shows an extraordinary amount of evolution in ocular serovars. Phylogenetic trees based on the nucleotide (A) and inferred amino acid (B) sequences of pmpE. Bootstrap values are shown only at the nodes separating major clades and only when they exceeded 50%. The scale bar equals a corrected sequence divergence of 0.005, which is the same as the scale used in Fig. 2 and 6. However, because there is significantly more divergence in pmpE and pmpH than in pmpI, the actual bars here and in Fig. 6 are similar in length compared to the elongated bar used in Fig. 2.
RFLP and sequencing analysis illustrates pmpH evolution parallels the separation ofC. trachomatis serovars into three disease groups.
Digestion of pmpH (Table 3) with CfoI (Fig. 5) and Tru9I divided the serovars into three groups compatible with the three disease groups of C. trachomatis. Because no other gene in C. trachomatis has exhibited such a divergence pattern, we obtained sequences of pmpH for 15 serovars.
FIG. 5.
RFLP analysis of pmpH yields three groups consistent with the three disease groups of C. trachomatis. An 8-μl aliquot of the pmpH PCR products was digested with CfoI endonuclease, and restriction fragments were separated on a 4% Metaphor agarose gel. In the LGV strains, the loss of the 54-bp fragment is difficult to see because it comigrates with the 58-bp fragment in the other serovars, and the new 15-bp band in the LGV strains is not visible. However, the new 39-bp fragment in the LGV strains is faintly visible below the 58-bp fragment. Serovars are indicated above their respective lanes. Numbers on the left are size standards in base pairs.
The nucleotide and inferred amino acid sequences of pmpH were nearly identical among serovars within a disease group but quite different for serovars compared across disease groups. For instance, ocular serovars differed from LGV serovars by approximately 8% and from urogenital serovars by about 6% (Table 6). However, compared to each other, ocular serovars differed by no more than 0.1%. Similarly, urogenital serovars differed from each other by no more than 0.7%, and LGV serovars differed from each other by no more than 0.2% (Table 6). The sequence for pmpH from serovar D/UW3 was identical to the sequence deposited at the Chlamydia genome website for pmpH from serovar D (http://chlamydia-www.berkeley.edu:4231/).
TABLE 6.
Sequence diversity in the pmpH gene among serovars of C. trachomatis
| Sero- var | No. of dissimilarities with serovara:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | Ba | C | D | E | F | G | H | I | J | K | L1 | L2 | L3 | |
| A | 1 | 1 | 1 | 47 | 47 | 48 | 47 | 47 | 46 | 47 | 47 | 79 | 78 | 80 | |
| B | 4 | 0 | 0 | 47 | 46 | 47 | 46 | 46 | 46 | 47 | 47 | 79 | 78 | 80 | |
| Ba | 2 | 2 | 0 | 47 | 46 | 47 | 46 | 46 | 46 | 47 | 47 | 79 | 78 | 80 | |
| C | 2 | 2 | 0 | 47 | 46 | 47 | 46 | 46 | 46 | 47 | 47 | 79 | 78 | 80 | |
| D | 168 | 168 | 166 | 166 | 2 | 3 | 0 | 0 | 3 | 4 | 4 | 58 | 57 | 59 | |
| E | 169 | 169 | 167 | 167 | 11 | 1 | 2 | 2 | 5 | 6 | 6 | 58 | 57 | 59 | |
| F | 170 | 170 | 168 | 168 | 12 | 1 | 3 | 3 | 6 | 7 | 7 | 59 | 58 | 60 | |
| G | 168 | 168 | 166 | 166 | 0 | 11 | 12 | 0 | 3 | 4 | 4 | 58 | 57 | 59 | |
| H | 168 | 168 | 166 | 166 | 0 | 11 | 12 | 0 | 3 | 4 | 4 | 58 | 57 | 59 | |
| I | 169 | 170 | 168 | 168 | 8 | 19 | 20 | 8 | 8 | 1 | 1 | 55 | 54 | 56 | |
| J | 171 | 172 | 170 | 170 | 10 | 21 | 22 | 10 | 10 | 2 | 0 | 56 | 55 | 57 | |
| K | 170 | 171 | 169 | 169 | 9 | 20 | 21 | 9 | 9 | 1 | 1 | 56 | 55 | 57 | |
| L1 | 247 | 248 | 246 | 246 | 215 | 210 | 211 | 215 | 215 | 207 | 209 | 208 | 2 | 1 | |
| L2 | 245 | 246 | 244 | 244 | 211 | 206 | 207 | 211 | 211 | 203 | 205 | 204 | 6 | 3 | |
| L3 | 247 | 248 | 246 | 246 | 215 | 210 | 211 | 215 | 215 | 207 | 209 | 208 | 2 | 6 | |
Values below the diagonal are nucleotide dissimilarities out of 2,991 nucleotide sites matched. Values above the diagonal are amino acid differences out of 996 amino acid sites matched. Within-disease group comparisons can be made for serovars A to C, D to K, and L1 to L3.
There were six in/del events in pmpH, all of which occur in the first ∼1,800 bp of the gene. The most notable was a large 36-bp deletion in the LGV serovars compared to serovars A through H around position 480 in the gene (Table 7). Serovars I, J, and K also have a 6-bp deletion in that same region compared to serovars A through H. The other in/dels found in the gene were small, only 3 or 6 bases (1 to 2 amino acids) in length.
TABLE 7.
Sequence alignment of pmpH in variable region around bp 500
| Serovar | Sequence (from bp 476)a |
|---|---|
| A | CGCCTGCTCTAGATCCATCCCCTACCGCTTCAAGCTCTTCATCTCCCACAGTCAGTGATGCTCGCCAGGGATCTATTTTTTCTATAGAGACCAGTTTAGA |
| B | .........C.......................................................................................... |
| Ba | .........C.......................................................................................... |
| C | .........C.......................................................................................... |
| D | .T..A..A.C..CA...G.T...G.T.............T......A.....T...........GA.A..G............G.......T.....G.. |
| E | .T..A..A.C..CA...G.T...G.T.............T......A.....T...........GA.A..G............G.......T.....G.. |
| F | .T..A..A.C..CA...G.T...G.T.............T......A.....T...........GA.A..G............G.......T.....G.. |
| G | .T..A..A.C..CA...G.T...G.T.............T......A.....T...........GA.A..G............G.......T.....G.. |
| H | .T..A..A.C..CA...G.T...G.T.............T......A.....T...........GA.A..G............G.......T.....G.. |
| I | .T..A..A.C..C------T...G.T.............T......A.....T...........GA.A..G............G.......T.....G.. |
| J | .T..A..A.C..C------T...G.T.............T......A.....T...........GA.A..G............G.......T.....G.. |
| K | .T..A..A.C..C------T...G.T.............T......A.....T...........GA.A..G............G.......T.....G.. |
| L1 | .......------------------------------------...A.....T...........GA.A..G............G.......T.....G.. |
| L2 | .......------------------------------------...A.....T...........GA.A..G............G.......T.....G.. |
| L3 | .......------------------------------------...A.....T...........GA.A..G............G.......T.....G.. |
pmpH sequences for all serovars were compared to that of serovar A on the top line. ., identity with serovar A; -, deletion compared to serovar A.
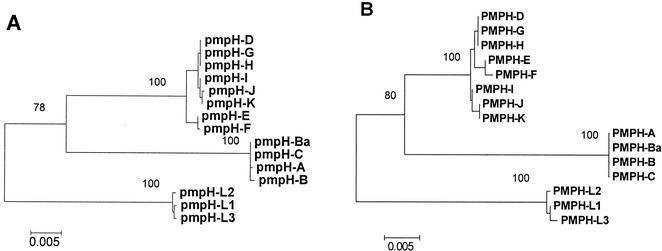
Neighbor-joining and parsimony analyses of the nucleotide sequences of pmpH produced the same tree (Fig. 6A). Branch lengths are proportional to nucleotide divergence, and the scale bar measures the same amount of divergence as the scale bars in Fig. 2 and 4, although the amount of diversity in pmpH is more similar to that seen in pmpE than to that seen in pmpI. The evolutionary analysis of pmpH nucleotide sequences was consistent with the RFLP patterns seen in Fig. 5 and Table 3. The evolutionary tree obtained by using the amino acid sequences of PmpH (Fig. 6B) was slightly different than the nucleotide tree. In the nucleotide tree, serovars E and F diverge prior to the DGHIJK cluster, but in the amino acid tree, E and F are sister taxa to DGH, with IJK diverging first. In addition, there was some rotation within the ocular clade when comparing the neighbor-joining tree to the parsimony tree based on amino acid sequences, but no single pattern was well supported by bootstrap analysis. Most essential, in all four analyses of pmpH, the 15 serovars reliably formed three clades that were congruent with the three disease groups of C. trachomatis: ocular disease, urogenital disease, and LGV.
FIG. 6.
Evolution of pmpH indicates strong divergence of serovars compatible with disease groups. Phylogenetic trees based on the nucleotide (A) and inferred amino acid (B) sequences of pmpH. Bootstrap values are shown only at the nodes separating major clades and only when they exceeded 50%. Based on pmpH, the 15 serovars clearly evolve into three distinct disease groups, with strong bootstrap support (∼100%) for the three clades. The scale bar equals a corrected sequence divergence of 0.005, which is the same as the scale used in Fig. 2 and 4. However, because there is significantly more divergence in pmpE and pmpH than in pmpI, the actual bars here and in Fig. 4 are similar in length compared to the elongated bar used in Fig. 2.
DISCUSSION
The genomes of obligate intracellular organisms have been streamlined via a reductive mode of evolution that is thought to rid the genome of unnecessary coding sequences (1). For example, while free-living bacteria, such as Escherichia coli, have multiple copies of the rRNA gene operon (E. coli has seven [4]), obligate intracellular and parasitic bacteria sometimes have only 1 to 2 copies (1, 2, 8, 10). Therefore, the discovery of a superfamily of large, protein-encoding genes with unknown function in the C. trachomatis genome is of considerable interest.
The pmp genes are large, ranging from 2,600 to 5,300 bp. An exhaustive sequencing survey of nucleotide diversity within these large genes for the 15 serovars of C. trachomatis would result in about 500,000 bp of nucleotide sequence data. Therefore, we chose instead to first screen for diversity by using RFLP analysis and to then further investigate three of the potentially interesting genes with sequence analysis.
The original goal of our study was to investigate the pmp genes for use as molecular epidemiologic markers. We obtained RFLP analysis of eight genes and sequenced three that looked the most interesting by RFLP analysis. Based on our results, however, it is unlikely that there is significant variation among strains of the same serovar. pmpI was highly conserved, and a recent report suggests that pmpG is also highly conserved (W. J. Jackson, R. B. Taylor, J. H. Tian, K. Johnson, X. Ding, N. Chang, and H. H. Yang, Abstr. 102nd Gen. Meet. Am. Soc. Microbiol., abstr. E-53, 2002). pmpE exhibited up to 6.5% nucleotide and amino acid dissimilarity among serovars in different disease groups, but between serovars within a disease group, pmpE differed by less than 0.5%. A similar result was obtained for pmpH, which showed 8% nucleotide dissimilarity among serovars in different disease groups, but nearly identical sequence for serovars within a disease group. Thus, it is unlikely there will be substantial intraserovar variation in any of the pmp genes.
Evolutionary analysis of pmpE, pmpH, and pmpI produced trees that are significantly different from the published MOMP/omp1 tree (9, 27). In the MOMP evolutionary tree, ocular, urogenital, and LGV serovars are intermixed and form three clades that are not congruent with disease groups: the B serogroup (B, Ba, D, L1, and L2), the C serogroup (A, C, H, I, J, K, and L3), and the intermediate group (serovars F and G). Analysis of pmpE, pmpH, and pmpI indicated that these genes have evolved such that the serovars of C. trachomatis diverge into clades that are more consistent with disease groups. However, the evolutionary pattern of each pmp gene is somewhat unique.
We were surprised to find that pmpI is highly conserved. The paucity of genetic variability resulted in an ambiguous phylogenetic pattern (Fig. 2). In evolutionary terms, highly conserved genes either code for important proteins for which primary amino acid sequence as well as tertiary structure must be conserved or are only recently evolved and have not had time to accumulate mutational variation (18). The high degree of sequence conservation suggests three possibilities for PmpI: (i) PmpI may play a general role in pathogenesis, (ii) PmpI may not be expressed, or (iii) PmpI may not be exposed on the cell surface and thus not under selective pressure by host defenses. Consistent with the latter two suggestions, PmpI has not been demonstrated yet to be in the outer membrane of C. trachomatis (28).
PmpE showed a remarkable and quite unexpected evolutionary pattern. The amount of diversity in PmpE in the ocular strains compared to the STD and LGV strains was three times the amount of diversity between STD and LGV strains, and the majority of nucleotide substitutions were nonsynonymous. The amount of genetic change taking place in PmpE within the ocular group suggests that PmpE may be under different selective pressure in the ocular strains versus the STD and LGV strains. Two possible hypotheses regarding the source of this difference are (i) pressure for variability in the ocular strains, for instance, due to selection at the cell surface and (ii) pressure towards conservation in the nonocular strains, for instance, due to constraints on the amino acid structure of the protein. However, until the function of PmpE is known, it is difficult to speculate on the source of the difference. Furthermore, while one study has demonstrated PmpE to be surface exposed in serovar L2 (28), a different study failed to find this protein on the surface of serovar L2 (21). Therefore, further study of PmpE is necessary.
Although we did not sequence pmpF for this study, the RFLP patterns for this gene are very similar to those seen for pmpE, suggesting a possible divergence of serovars consistent with disease group. In all three digests of pmpF, serovars in different disease groups do not share the same RFLP pattern. In addition, pmpE and pmpF are separated by only 3 bp, and they are located in the same cluster of pmp genes as pmpG and pmpH (26). Therefore, sequencing pmpF may demonstrate that evolution of this gene is comparable to patterns seen in pmpE and/or pmpH.
PmpH exhibited up to 8% nucleotide and amino acid dissimilarity and several in/del events among serovars when comparing sequences among disease groups. However, within a disease group, the serovars were nearly identical in sequence (Table 6). Phylogenetic analysis of pmpH nucleotide and inferred amino acid sequences (Fig. 6) indicates that, based on this gene, the serovars of C. trachomatis diverge distinctly into three clades that are compatible with disease groups: an ocular-trachoma clade (serovars A, B, Ba, and C), a urogenital-STD clade (serovars D, E, F, G, H, I, J, and K), and an LGV clade (serovars L1, L2, and L3). No other C. trachomatis gene studied to date shows a pattern of evolution that parallels disease. The bootstrap support for this pattern was very strong for both the nucleotide and amino acid trees, with values hovering around 100%. In addition, two studies have shown PmpH to be one of the proteins located in the outer membrane of C. trachomatis serovar L2 (21, 28). Therefore, based on its location on the cell surface and its evolutionary pattern consistent with disease groups, we hypothesize that PmpH may be essentially involved in the mechanisms of pathogenesis, perhaps as a ligand on the cell surface involved in attachment or invasion.
The three pmp genes investigated here each show a different pattern of evolution. The evolution of PmpH is most provocative, however, especially with recent evidence that the Chlamydia spp. pmp family closely resembles the autotransporter or type V secretion family of proteins (3, 7, 14). Proteins within this family are known toxins, adhesins, and mediators of intracellular motility, including Bordetella pertactin, FHA, and TcfA; E. coli AIDA-I and TibA; Haemophilusinfluenzae Hap, Hia, and Hsf; Rickettsia spp. rOmpA and rOmpB; Moraxella catarrhalis UspA1 and UspA2 h; and Shigella flexneri IcsA (reviewed in reference 15). Therefore, we hypothesize that C. trachomatis PmpH may play an important role in pathogenesis based on its evolutionary pattern, together with its outer membrane location and similarity to type V secretion proteins. It is important to further investigate the function of PmpH and those of the other pmp proteins to determine if they play a role in pathogenesis. It is possible that PmpH may be a ligand responsible for attachment or invasion and would thus be an attractive vaccine candidate.
Editor: J. T. Barbieri
REFERENCES
- 1.Andersson, S. G. E., A. Zomorodipour, J. O. Andersson, T. Sicheritz-Ponten, U. C. Alsmark, R. F. Podowski, A. K. Naslund, A.-S. Eriksson, H. H. Winkler, and C. G. Kurland. 1998. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396:133-140. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, S. G. E., D. R. Stothard, P. A. Fuerst, and C. G. Kurland. 1999. Molecular phylogeny and rearrangement of rRNA genes in Rickettsia species. Mol. Biol. Evol. 16:987-995. [DOI] [PubMed] [Google Scholar]
- 3.Birkelund, S., G. Christiansen, B. Vandahl, and A. S. H. Pedersen. 2002. Are the Pmp proteins parallel beta-helices?, p. 551-554. In J. Schachter, G. Christiansen, I. N. Clarke, M. R. Hammerschlag, B. Kaltenboeck, C.-C. Kuo, R. G. Rank, G. L. Ridgway, P. Saikku, W. E. Stamm, R. S. Stephens, J. T. Summersgill, P. Timms, and P. B. Wyrick (ed.), Chlamydial infections: proceedings of the Tenth International Symposium on Human Chlamydial Infections. International Chlamydia Symposium, San Francisco, Calif.
- 4.Blattner, F. R., G. Plunkett III, C. A. Bloch, et al. 1997. The complete genome sequence of Escherichia coli K12. Science 277:1453-1462. [DOI] [PubMed] [Google Scholar]
- 5.Cabot, E. L., and A. T. Beckenbach. 1989. Simultaneous editing of multiple nucleic acid and protein sequences with ESEE. Comput. Appl. Biosci. 5:233-234. [DOI] [PubMed] [Google Scholar]
- 6.Cevenini, R., M. Donati, E. Brocchi, F. DeSimone, and M. LaPlaca. 1991. Partial characterization of an 89-kDa highly immunoreactive protein from Chlamydia psittaci A/22 causing ovine abortion. FEMS Microbiol. Lett. 81:111-116. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen, G., A.-S. Pedersen, K. Hjerno, B. Vandahl, and S. Birkelund. 2000. Potential relevance of Chlamydia pneumoniae surface proteins to an effective vaccine. J. Infect. Dis. 181:S528-S537. [DOI] [PubMed] [Google Scholar]
- 8.Clark, J. 1990. Ph.D. thesis. The Ohio State University, Columbus.
- 9.Dean, D., and K. Millman. 1997. Molecular and mutation trends analyses of omp1 alleles for serovar E of Chlamydia trachomatis. Implications for the immunopathogenesis of disease. J. Clin. Investig. 99:475-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser, C. M., J. D. Gocayne, O. White, et al. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397-403. [DOI] [PubMed] [Google Scholar]
- 11.Giannikopoulou, P., K. Bini, O. D. Simitsek, V. Pallini, and E. Vretou. 1997. Two-dimensional electrophoretic analysis of the protein family at 90 kDa of abortifacient Chlamydia psittaci. Electrophoresis 18:2104-2108. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths, P. C., H. L. Phillips, M. Dawson, and M. J. Clarkson. 1992. Antigenic and morphological differentiation of placental and intestinal isolates of Chlamydia psittaci of ovine origin. Vet. Microbiol. 30:165-177. [DOI] [PubMed] [Google Scholar]
- 13.Grimwood, J., and R. S. Stephens. 1999. Computational analysis of the polymorphic protein superfamily of Chlamydia trachomatis and Chlamydia pneumoniae. Microb. Comp. Genomics 4:187-201. [DOI] [PubMed] [Google Scholar]
- 14.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370-378. [DOI] [PubMed] [Google Scholar]
- 15.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalman, S., W. Mitchell, R. Marathe, C. Lammel, J. Fan, W. Hyman, L. Olinger, J. Grimwood, R. W. Davis, and R. S. Stephens. 1999. Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat. Genet. 21:385-389. [DOI] [PubMed] [Google Scholar]
- 17.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 18.Li, W.-H., and D. Gauer. 1991. Fundamentals of molecular evolution. Sinauer Associates, Sunderland, Mass.
- 19.Lindquist, E. A., and R. S. Stephens. 1998. Transcriptional activity of a sequence variable protein family in Chlamydia trachomatis, p. 259-262. In R. S. Stephens, G. I. Byrne, G. Christiansen, I. N. Clarke, J. T. Grayston, R. G. Rank, G. L. Ridgway, P. Saikku, J. Schachter, and W. E. Stamm (ed.), Chlamydial infections: proceedings of the Ninth International Symposium on Human Chlamydial Infections. International Chlamydia Symposium, San Francisco, Calif.
- 20.Longbottom, D., M. Russell, G. E. Jones, F. A. Lainson, and A. J. Herring. 1996. Identification of a multigene family coding for the 90kDa proteins of the ovine abortion subtype of Chlamydia psittaci. FEMS Microbiol. Lett. 142:277-281. [DOI] [PubMed] [Google Scholar]
- 21.Mygind, P. H., G. Christiansen, P. Roepstorff, and S. Birkelund. 2000. Membrane proteins PmpG and PmpH are major constituents of Chlamydia trachomatis L2 outer membrane complex. FEMS Microbiol. Lett. 186:163-169. [DOI] [PubMed] [Google Scholar]
- 22.Newhall, W. J., B. Batteiger, and R. B. Jones. 1982. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect. Immun. 38:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockey, D. D., J. Lenart, and R. S. Stephens. 2000. Genome sequencing and our understanding of chlamydiae. Infect. Immun. 68:5473-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 25.Souriau, A., J. Salinas, C. De Sa, K. Layachi, and A. Rodolakis. 1994. Identification of subspecies- and serotype 1-specific epitopes on the 80- to 90-kilodalton protein region of Chlamydia psittaci that may be useful for diagnosis of chlamydial induced abortion. Amer. J. Vet. Res. 55:510-514. [PubMed] [Google Scholar]
- 26.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 27.Stothard, D. R., G. Boguslawski, and R. B. Jones. 1998. Phylogenetic analysis of the Chlamydia trachomatis major outer membrane protein and examination of potential pathogenic determinants. Infect. Immun. 66:3618-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanzer, R. J., and T. P. Hatch. 2001. Characterization of outer membrane proteins in Chlamydia trachomatis LGV serovar L2. J. Bacteriol. 183:2686-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanzer, R. J., D. Longbottom, and T. P. Hatch. 2001. Identification of polymorphic membrane proteins of Chlamydia psittaci 6BC. Infect. Immun. 69:2428-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, S. P., C.-C. Kuo, and J. T. Grayston. 1973. A simplified method for immunological typing of trachoma-inclusion conjunctivitis-lymphogranuloma venereum organisms. Infect. Immun. 8:356-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, S. P., C.-C. Kuo, R. C. Barnes, R. S. Stephens, and J. T. Grayston. 1985. Immunotyping of Chlamydia trachomatis with monoclonal antibodies. J. Infect. Dis. 152:791-800. [DOI] [PubMed] [Google Scholar]
- 32.Wang, S. P., and J. T. Grayston. 1991. Three new serovars of Chlamydia trachomatis: Da, Ia, and L2a. J. Infect. Dis. 163:403-405. [DOI] [PubMed] [Google Scholar]