Abstract
We previously reported that the aerolysin-like hemolysin of Aeromonas sobria stimulates T84 cells to produce cyclic AMP, which then emerges in the culture medium. In order to clarify the mechanism of action of the hemolysin, we examined the involvement of adenosine nucleotide. The results show that the hemolysin stimulates T84 cells to release ATP, which is then converted to adenosine by ectonucleotidase. The adenosine generated might stimulate the P1 adenosine receptors of T84 cells to produce cyclic AMP.
We purified the enterotoxin from the culture supernatant of Aeromonas sobria and found that it possessed hemolytic activity in addition to enterotoxic activity (5). The analysis of the DNA sequence (GenBank accession number AY157998) showed that the hemolysin is homologous with aerolysin produced by Aeromonas hydrophila. Homology between the two toxins is 68.5% at the amino acid level (5). The aerolysin-like hemolysin and aerolysin bound to cells act to form small pores in the membrane and induce cell damage (5, 11). These results suggest that the mode of action of the hemolysin is identical to that of aerolysin. Since cultured intestinal cells are destroyed by incubation with the hemolysin, we had predicted that the diarrhea caused by the hemolysin was a result of such cytotoxic action of the hemolysin. However, micropathological examination showed that the diarrheic syndrome appeared before the cell damage occurred (5). So, we speculated that the diarrhea was not due to the cytotoxic action of the hemolysin but rather that the hemolysin stimulated the intestinal cells to send some signal(s) to induce diarrhea. Thus, we examined the levels of cyclic nucleotides in T84 cells, i.e., human colonic epithelial cells, after incubation with the hemolysin to obtain a clue as to the signal and found that the level of cyclic AMP in the culture medium increased after the incubation (6). However, it has remained unsolved how the hemolysin induces the elevation of the level in the extracellular cyclic AMP.
In the meantime, the action of adenosine existing outside of the cell has been studied extensively and its receptor, P1, has been found (4). At present, P1 receptors are divided into three groups, A1 to A3. A2 is divided into two subtypes: A2A and A2B. These P1 receptors couple to adenylate cyclase, whose activity is regulated by G proteins. A1 and A3 function to inhibit the cyclase and A2, both A2A and A2B, functions to activate the cyclase (3, 4). It was also shown that T84 cells, which we used to examine the activity of the hemolysin, possessed the A2B receptor (14). Thus, we suspected that the adenosine was involved in the action of the hemolysin.
Addition of papaverine to the medium.
We reported that the level of cyclic AMP in the medium increased on incubation with the hemolysin (6). The excess cyclic AMP produced by the cells is decomposed by phosphodiesterase. Therefore, the amount of cyclic AMP reported in a previous study should be the residual amount after the attack by phosphodiesterase. To examine accurately the effect of hemolysin on the production of cyclic AMP by cells, it is best to eliminate the interference from phosphodiesterase. For that, we added papaverine, a nonxanthine phosphodiesterase inhibitor, at a concentration of 20 μM into the medium 20 min before starting the incubation with hemolysin. The cells were then incubated with the hemolysin for 30 min, and the extracellular cyclic AMP level was measured. The concentration of hemolysin added to the medium ranged from 10 to 320 ng/ml.
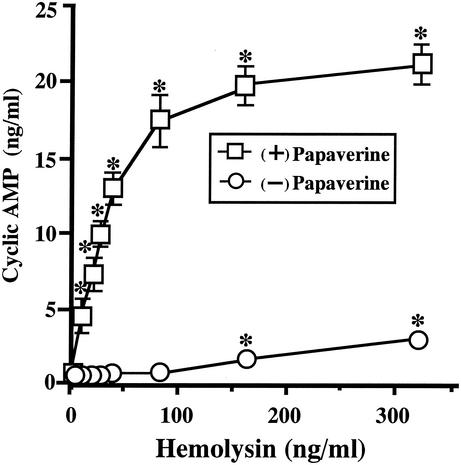
As shown in Fig. 1, the preincubation with papaverine had a remarkable effect on the extracellular level of cyclic AMP. The amount increased linearly with the concentration up to 80 ng/ml in the presence of papaverine. The increase reached almost a plateau at 80 ng of hemolysin/ml. The level of cyclic AMP reached ca. 20 ng/ml with the incubation. In contrast, the increase of cyclic AMP was up to 3 ng/ml without papaverine under the conditions examined (Fig. 1). We added 20 μM papaverine into the culture medium in the subsequent experiments.
FIG. 1.
Effect of papaverine on extracellular cyclic AMP concentration after incubation with the hemolysin. T84 cells were seeded in petri dishes (30 mm in diameter) and grown to 70 to 80% confluence on culture dishes in Dulbecco modified Eagle medium-Ham F-12 (DMEM-F12; 1:1 [vol/vol]) containing 5% fetal bovine serum. The medium of T84 cells was exchanged with 2 ml per culture dish of DMEM-F12 containing 1% fetal bovine serum 60 min before the incubation with hemolysin. At 20 min before the addition of the hemolysin, papaverine was added to half of the dishes (□) at a final concentration of 20 μM. The hemolysin was then added to the medium at the concentration indicated in the figure. After incubation for 30 min at 37°C, the culture medium was recovered. The medium was centrifuged, and the culture supernatant was separated from insoluble materials. To determine the concentratrion of cyclic AMP in the culture supernatant, the culture supernatant was treated with trichloroacetic acid. The insoluble materials yielded were removed by centrifugation, and the concentration of cyclic AMP in the supernatant was determined by using a cyclic AMP enzyme assay kit (Amersham Pharmacia Biotech, Uppsala, Sweden). Data are the means ± the standard error of the mean (SEM) from four independent determinations. ✽, P < 0.05 compared to the control value obtained by incubation without the hemolysin.
Effects of ADA and XAC on the hemolysin-induced increase in cyclic AMP.
Stimulation of the P1 adenosine receptor of the cell membrane enhances the production of cyclic AMP (3, 4). To examine whether this receptor is involved in the action of the hemolysin, the effect of two reagents was examined. One was adenosine deaminase (ADA), which catalyzes the deamination of adenosine into inosine, and the other was 8-(4-[[[[(2-aminoethyl)amino]carbonyl]methyl]oxy]phenyl]-1,3-dipropylxanthine (XAC), which is an antagonist of the P1 adenosine receptor. Since ADA promotes the conversion of adenosine, the amount of adenosine in solution decreases in the presence of ADA. The presence of XAC attenuates the stimulation of P1 adenosine receptors by the stimulants. These agents were added to the medium at concentrations of 1 IU/ml of ADA and 1 μM XAC 10 min before the hemolysin.
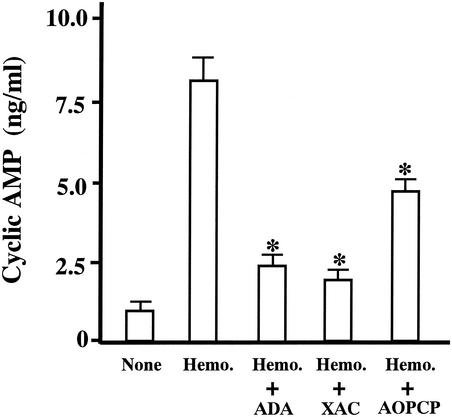
As shown in Fig. 2, these agents reduced the activity of hemolysin to raise the level of cyclic AMP in the culture supernatant. This indicates that adenosine and the P1 receptor are involved in the actions of the hemolysin.
FIG. 2.
Effects of ADA, XAC, and AOPCP on hemolysin-induced cyclic AMP production. T84 cells were preincubated with ADA (1 IU/ml), XAC (1 μM), or AOPCP (100 μM) for 10 min at 37°C. After the preincubation, the hemolysin was added to the medium at 30 ng/ml, and the incubation was continued. Thirty minutes later, the incubation was stopped, and the concentration of cyclic AMP in the medium was determined as described in the legend of Fig. 1. Neither hemolysin nor reagent was added to the medium of cells in the columns labeled as none. Data are the means ± the SEM from four independent determinations. ✽, P < 0.05 compared to experiments with hemolysin alone.
ATP efflux on incubation with the hemolysin.
The results described above suggested that the level of adenosine in the medium increased after exposure to the hemolysin. However, how the increase occurred is still unknown.
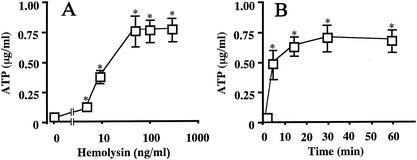
It has been reported that the level of ATP in the medium of some epithelial cells increases when the cells are damaged (1, 2, 8, 15) and that the extracellular ATP is hydrolyzed to adenosine by a cascade of ectonucleotidases involving ecto-ATPase, ecto-ATP diphosphohydrolase, ectonucleotide pyrophosphatase, and ecto-5′-nucleotidase (16). We suspected that T84 cells responded to the release of ATP into the medium after incubation with the hemolysin. To confirm the hypothesis, T84 cells were incubated with hemolysin for 30 min, and the extracellular ATP level was measured (Fig. 3A). The concentration of hemolysin used in this experiment ranged from 5 to 300 ng/ml. The increase in extracellular ATP was progressive from 0.15 to 0.75 μg/ml, where a plateau was formed (Fig. 3A).
FIG. 3.
Dose-dependent (A) and time-dependent (B) ATP production by T84 cells exposed to hemolysin. (A) T84 cells were incubated with different amounts of hemolysin for 30 min at 37°C. The concentration of hemolysin in the medium is indicated in the figure. After the incubation, the extracellular fraction was prepared as described in the legend of Fig. 1, and the concentration of ATP in the medium was determined with a bioluminescence ATP kit (Toyo Ink., Mfg. Co., Tokyo, Japan). Data are the means ± the SEM from four independent determinations. (B) T84 cells were incubated at 37°C with hemolysin (100 ng/ml) for the period indicated in the figure, and the concentration of ATP in the medium was determined. ✽, P < 0.05 compared to the value for the samples prepared without the hemolysin (A) or at 0 min in the presence of hemolysin (B).
The time course of the elevation of ATP was also examined (Fig. 3B). The amount of hemolysin used was 100 ng/ml. The extracellular ATP level increased in the initial 5 min of incubation and continued to increase for up to 15 min of incubation. The level had almost reached a plateau by 15 min of incubation and was maintained until the end of the incubation period (i.e., 60 min).
Participation of ATP in the action of the hemolysin.
We examined the effect of α,β-methyleneadenosine-5′-diphosphate (AOPCP), which is an inhibitor of ectonucleotidases, on the hemolysin-induced increase in cyclic AMP. AOPCP was added to the medium at 100 μM 10 min before the hemolysin. The result is shown in Fig. 2. The agent reduced the activity of hemolysin, suggesting that the ATPs released into the medium by the action of hemolysin were degraded into adenosine by ectonucleotidases and that the adenosines generated stimulated the cells to produce cyclic AMP.
Elevation of cyclic AMP levels by exogenous ATP and effect of reagents.
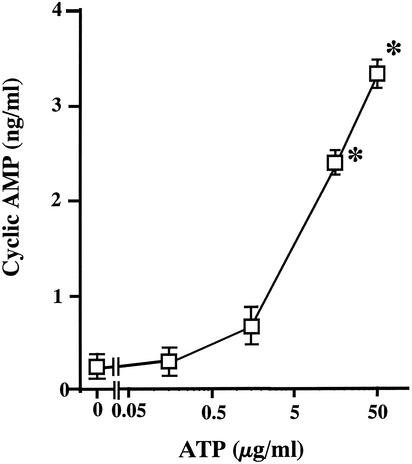
To confirm the participation of ATP in the hemolysin-induced cyclic AMP production, we examined whether the ATP added to the medium of T84 cells enhanced the production of cyclic AMP. The amount of ATP used ranged from 0.25 to 50 μg/ml. The incubation was performed at 37°C for 30 min. The ATP evoked a significant increase in the level of cyclic AMP in a concentration-dependent manner (Fig. 4).
FIG. 4.
Production of cyclic AMP by T84 cells in response to exogenous ATP. T84 cells were incubated for 30 min at 37°C in the presence of ATP. The concentration of ATP in each sample is indicated in the figure. After incubation, the concentration of cyclic AMP in the medium was determined as described in the legend to Fig. 1. Data are the means ± the SEM from four independent determinations. ✽, P < 0.05 compared to the control value obtained for incubation without ATP.
To elucidate how the increase is induced by exogenous ATP, we examined the effects of ADA, XAC, and AOPCP on the production of cyclic AMP. These agents were added to the medium 10 min before the ATP. The concentrations of ATP, ADA, XAC, and AOPCP used were 30 μg/ml, 1 IU/ml, 1 μM, and 100 μM, respectively. After incubation for 30 min with ATP, the amount of cyclic AMP was measured. These three agents reduced the action of ATP to stimulate production of cyclic AMP (data not shown), indicating that the exogenous ATPs are converted into adenosines which stimulate the P1 adenosine receptor to produce cyclic AMP.
The results obtained in the present study indicate that hemolysin acts on T84 cells to elevate the level of ATP in the culture medium and that the ATPs in the medium are converted into adenosines by ectonucleotidases. Finally, the adenosines generated stimulate the P1 adenosine receptor to produce cyclic AMP. Since it has been reported that T84 cells possess a functional adenosine A2B receptor (14), the receptor that functioned in our experiment might be the A2B receptor. Thus, prepared cyclic AMPs were released outside of the cell. However, it is still not known how the cyclic AMP produced in the cell emerges outside of the cell.
As described previously, a large amount of hemolysin damages mammalian cells. We were concerned that the emergence of cyclic AMP in the medium was due to damage of the cell. To make clear how severely the cell was damaged by the incubation with the hemolysin, we examined the release of lactate dehydrogenase (LDH) from the cells into the medium after exposure to the hemolysin. On incubation with the hemolysin at a concentration of <1.45 μg/ml, LDH was not detected (data not shown). The amount of hemolysin we usually used in this experiment was 30 ng/ml. The concentration is about one-fiftieth the minimal amount necessary to induce the release of LDH. We think that the damage to the cells exposed to the hemolysin in the present study was slight. It was unlikely that the cyclic AMP was released because of the cell damage. We believe that the cyclic AMP is specifically released outside of the cell.
The reports from Buckley's group support our hypothesis about the specific release of cyclic AMP (10, 11, 12, 13). These authors showed that channels that are relatively nonselective are formed in the cell membrane after the incubation with aerolysin. The channels are large enough to allow the movement of molecules in the low-kilodalton range. Our hemolysin probably can make such channels in the membrane of T84 cells that are like aerolysin. The cyclic AMP might be released outside of the cell through the channels.
Hemolysin possesses not only cytotoxic but also enterotoxic activity (5). However, the mechanism by which it induces diarrhea has remained unknown. In the meantime, it has been shown that cyclic AMP functions as a intracellular messenger in the diarrhea induced by cholera toxin. The cyclic AMP can activate the cystic fibrosis transmembrane conductance regulator, which is a Cl− channel, and the activation causes an excess efflux of Cl− from the cell. The fluid secretion accompanies the efflux of Cl− (7, 9). The action of the hemolysin to induce the elevation in the content of cyclic AMP in T84 cells may be closely related to the enterotoxic activity of the hemolysin.
Acknowledgments
This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Science, and Culture of Japan.
Editor: B. B. Finlay
REFERENCES
- 1.Born, G. V. R., and M. A. A. Kratzer. 1984. Source and concentration of extracellular adenosine triphosphate during haemostasis in rats, rabbits, and man. J. Physiol. 354:419-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crane, J. K., R. A. Olson, H. M. Jones, and M. E. Duffey. 2002. Release of ATP during host cell killing by enteropathogenic Escherichia coli and its role as a secretory mediator. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G74-G86. [DOI] [PubMed] [Google Scholar]
- 3.Daizeil, H. H., and D. P. Westfall. 1994. Receptors for adenine nucleotides and nucleosides: subclassification, distribution, and molecular characterization. Pharmacol. Rev. 46:449-466. [PubMed] [Google Scholar]
- 4.Fredholm, B. B., A. P. Ijzerman, K. A. Jacobson, K.-N. Klotz, and J. Linden. 2001. International union of pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 53:527-552. [PMC free article] [PubMed] [Google Scholar]
- 5.Fujii, Y., T. Nomura, H. Kanzawa, M. Kameyama, H. Yamanaka, M. Akita, K. Setsu, and K. Okamoto. 1998. Purification and characterization of enterotoxin produced by Aeromonas sobria. Microbiol. Immunol. 42:703-714. [DOI] [PubMed] [Google Scholar]
- 6.Fujii, Y., T. Nomura, and K. Okamoto. 1999. Hemolysin of Aeromonas sobria stimulates production of cyclic AMP by cultured cells. FEMS Microbiol. Lett. 176:67-72. [DOI] [PubMed] [Google Scholar]
- 7.Gabriel, S. E., K. N. Brigman, B. H. Koller, R. C. Boucher, and M. J. Stutts. 1994. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science 266:107-109. [DOI] [PubMed] [Google Scholar]
- 8.Gordon, J. L. 1986. Extracellular ATP: effects, sources and fate. Biochem. J. 233:309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirst, T. R. 1999. Cholera toxin and Escherichia coli heat-labile enterotoxin, p. 104-129. In J. E. Alouf and J. H. Freer (ed.), Bacterial protein toxins. Academic Press, Inc., San Diego, Calif.
- 10.Howard, S. P., and J. T. Buckley. 1982. Membrane glycoprotein receptor and hole-forming properties of a cytolytic protein toxin. Biochemistry 21:1662-1667. [DOI] [PubMed] [Google Scholar]
- 11.Letellier, L., S. P. Howard, and J. T. Buckley. 1997. Studies on the energetics of proaerlysin secretion across the outer membrane of Aeromonas species. Evidence for a requirement for both the protonmotive force and ATP. J. Biol. Chem. 272:11109-11113. [DOI] [PubMed] [Google Scholar]
- 12.Nelson, K. L., and J. T. Buckley. 2000. Channel formation by the glycosylphosphatidylinositol-anchored protein binding toxin aerolysin is not promoted by lipid rafts. J. Biol. Chem. 275:19839-19843. [DOI] [PubMed] [Google Scholar]
- 13.Parker, M. W., J. T. Buckley, J. P. Postma, A. D. Tucker, K. Leonard, F. Pattus, and D. Tsernoglou. 1994. Structure of the Aeromonas toxin proaerolysin in its water-soluble and membrane-channel states. Nature 367:292-295. [DOI] [PubMed] [Google Scholar]
- 14.Strohmeier, G. R., S. M. Reppert, W. I. Lencer, and J. L. Madara. 1995. The A2B adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J. Biol. Chem. 270:2387-2394. [DOI] [PubMed] [Google Scholar]
- 15.Thelestam, M., and R. Mollby. 1979. Classification of microbial, plant and animal cytolysins based on their membrane-damaging effects on human fibroblasts. Biochim. Biophys. Acta 557:156-169. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann, H. 1996. Extracellular purine metabolism. Drug Dev. Res. 39:337-352. [Google Scholar]