Abstract
DsbA is a periplasmic thiol:disulfide oxidoreductase which contributes to the process of protein folding by catalyzing the formation of disulfide bonds. In this study, we demonstrate that the dsbA gene is required for the expression of the type III secretion system under low-calcium inducing conditions, intracellular survival of P. aeruginosa upon infection of HeLa cells, and twitching motility. The diverse phenotypes of the dsbA mutant are likely due to its defect in the folding of proteins that are involved in various biological processes, such as signal sensing, protein secretion, and defense against host clearing. In light of its effect on various virulence factors, DsbA could be an important target for the control of P. aeruginosa infections.
As an opportunistic human pathogen, Pseudomonas aeruginosa causes infections ranging from minor skin diseases to life-threatening complications in severe-burn patients and patients with leukemia, AIDS, cystic fibrosis, and cancer (2, 4, 29, 36). P. aeruginosa is able to grow in diverse environments by utilizing a wide variety of carbon and nitrogen sources (26). This adaptability and its intrinsic resistance to many common antibiotics as well as the ability to form biofilms make P. aeruginosa difficult to eradicate from the hospital environment (1, 7, 28). Moreover, P. aeruginosa has many virulence factors, such as proteases, cytotoxins, phospholipases, neuraminidase, capsular polysaccharides, and lipopolysaccharides, contributing to its ability to colonize, penetrate, and survive the host immune defense (9, 26, 36). The P. aeruginosa clinical isolate PA103 has been categorized as a noninvasive (cytolytic) strain based on its interaction with nonphagocytic corneal epithelial cells (12). This noninvasive strain carries exoT and exoU, whose products are translocated into the host cells via type III secretion machinery (11). Expression of these exoenzymes is coordinately regulated by a transcriptional activator, ExsA, in response to various environmental signals, including low calcium and direct contact with tissue culture cells (11, 19, 35).
DsbA is a periplasmic protein in gram-negative bacteria and functions as a soluble thiol:disulfide oxidoreductase. It contains a conserved motif, Cys-X-X-Cys, which is commonly found in other disulfide oxidoreductases (10). In a catalytic cascade pathway, the activity of DsbA is maintained by the function of DsbB (14). DsbA is required for catalyzing the oxidative folding and assembly of many secreted proteins, such as cholera toxin, Escherichia coli heat-labile toxin, pertussis toxin, elastase, alkaline phosphatase, and lipase (27, 31, 34, 38). Besides having a role in facilitating protein folding, DsbA is essential in balancing periplasmic redox potential, and a dsbA mutant is sensitive to reducing reagents such as dithiothreitol (25).
In this study, we demonstrate that dsbA is required for the expression of the type III secretion system as well as the intracellular survival of P. aeruginosa during infection of HeLa cells. Furthermore, we show that DsbA affects the expression of pilA, which could partially explain the twitching motility defect in the dsbA mutant. Overall, DsbA affects multiple virulence factors and thus may be important in the pathogenesis of P. aeruginosa.
Secretion of type III effector molecules is defective in a dsbA mutant.
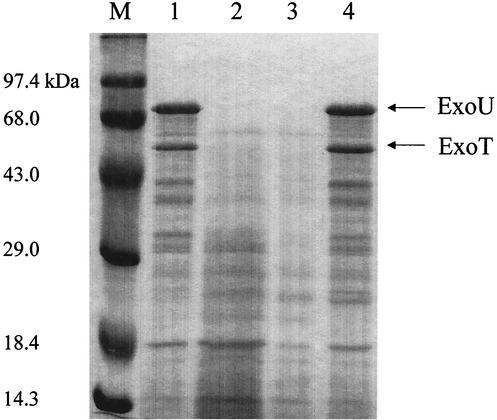
In a screening of a transposon insertion library for P. aeruginosa mutants that are noncytotoxic to HeLa cells, two types of mutants were identified, a type III-defective mutant and a dsbA mutant, suggesting that the type III secretion system could be defective in the dsbA mutant background. To test the effect of the dsbA gene on the secretion of type III effector molecules in P. aeruginosa, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to examine the secreted proteins under a type III inducing condition. Because the type III secretion system of P. aeruginosa is known to respond to a low-calcium signal (37), treatment with EGTA, a strong chelator for divalent cations, was used to activate the type III secretion system. To generate a defined dsbA mutant (Table 1), the dsbA gene of PA103 was amplified by PCR by using the oligonucleotides 5-dsbA (5′-CGC CTA CTT CGC CAG CCA GAA GAT GAG CGT-3′) and dsbA-3 (5′-GCA GGG GCG AGT TTT CCA GAA GAT CGA CGG-3′). An amplified 1.8-kb DNA fragment was cloned into a PCR cloning vector, pCR2.1-TOPO (Invitrogen), resulting in pHW0206. A 1.6-kb blunt-ended gentamicin resistance cassette was then inserted into the unique MluI site, located 5′ of dsbA, in pHW0206, and the resulting construct was named pHW0212. A 3.4-kb HindIII-XbaI fragment from pHW0212 containing the dsbA gene disrupted by the insertion of a gentamicin resistance cassette was cloned into HindIII-XbaI-digested pEX18Tc, yielding pHW0216. pHW0216 was transformed into the wild-type PA103 background to generate a PA103 dsbA mutant through homologous recombination into its chromosome by double crossover (18). The genotype of the resulting mutant was confirmed by Southern hybridization (data not shown). Then, pHW0210 containing the intact dsbA gene was further transformed into PA103 dsbA for a complementation test. Bacterial strains were cultured in L broth containing appropriate antibiotics at 37°C overnight. The cultured bacteria were reinoculated into L broth containing 5 mM EGTA to an optical density at 600 nm of 0.1 and then vigorously shaken at 37°C for 12 h. Supernatant of bacterial culture was collected and precipitated with 15% trichloroacetic acid at 4°C for 12 h. The precipitated pellet of the protein sample was completely suspended by sonication in 1× protein sample buffer and subjected to SDS-12% PAGE. As shown in Fig. 1, PA103 dsbA did not secrete any detectable amount of ExoU and ExoT proteins, unlike wild-type PA103. However, complementation with dsbA fully restored the ability of PA103 dsbA to secrete the two effector molecules, to the level of wild-type PA103. These results indicate that the DsbA of P. aeruginosa is required for the secretion of type III effector molecules.
TABLE 1.
Strains and plasmids used in this study
| Strain, phage, or plasmid | Descriptiona | Reference or source |
|---|---|---|
| P. aeruginosa | ||
| PA103 | Clinical isolate of wild-type cytotoxic strain | 22 |
| PA103 exsA | PA103 with chromosomal disruption of the exsA locus; Spr Smr | This study |
| PA103 ΔexoU exoT | PA103 with exoU deletion and exoT disruption; Tcr | 35 |
| PA103 dsbA | PA103 with chromosomal disruption of the dsbA locus; Spr Smr | This study |
| PA103 exsA dsbA | PA103 with chromosomal disruption of both exsA and dsbA loci; Spr Smr Gmr | This study |
| PO4 phage | Pillus-specific lytic phage | David Bradley |
| Plasmids | ||
| pCR2.1-TOPO | PCR cloning vector, Apr Kmr | Invitrogen |
| pDN19lacΩ | Broad-host-range plasmid containing a promoterless lacZ gene; Spr Smr Tcr | 33 |
| pEX18Tc | Suicide vector for recombination by site-specific excision; Sucs Tcr | 18 |
| pHW0018 | exoT promoter of PA103 fused to promoterless lacZ in pDN19lacΩ; Spr Smr Tcr | 15 |
| pHW0203 | exsA promoter of PA103 fused to promoterless lacZ in pDN19lacΩ; Spr Smr Tcr | This study |
| pHW0206 | PCR-amplified dsbA gene from PA103 in pCR2.1-TOPO; Apr Kmr | This study |
| pHW0210 | dsbA gene of PA103 cloned in pUCP19; Apr | This study |
| pHW0212 | Disruption of dsbA gene by Gmr cassette in pHW0206; Apr Kmr Gmr | This study |
| pHW0216 | dsbA gene with Gmr cassette insertion cloned in pEX18Tc; Tcr | This study |
| pMSZ5 | PAK pilA promoter fused to a promoterless lacZ in pDN19lacΩ; Spr Smr Tcr | 20 |
| pUCP19 | Broad-host-range shuttle vector, Apr | 30 |
Resistance markers: Apr, ampicillin; Kmr, kanamycin; Gmr, gentamicin; Spr, spectinomycin; Smr, streptomycin; Tcr, tetracycline. Sucs, sucrose-sensitive marker encoded by sacB.
FIG. 1.
The dsbA mutant is defective in the secretion of type III effector molecules. Bacterial strains were cultured in L broth containing appropriate antibiotics at 37°C overnight. The cultured bacterial cells were reinoculated into fresh L broth containing 5 mM EGTA and vigorously shaken at 37°C for 12 h. The bacterial culture supernatant was subjected to trichloroacetic acid precipitation. Standard SDS-PAGE was used to observe the secreted protein profiles. M, high-molecular-weight protein marker; lane 1, wild-type PA103; lane 2, PA103 ΔexoU exoT; lane 3, PA103 dsbA; lane 4, PA103 dsbA complemented by pHW0210 harboring an intact dsbA gene.
DsbA is required for the expression of the type III secretion system.
The defect in the secretion of type III effector molecules by the dsbA mutant could be due to either low expression of the type III effector molecules or defects in the type III secretion apparatus. To determine whether DsbA is essential for the expression of the type III secretion system, the promoter activity of exoT, one of the major type III effector molecules expressed by PA103, was monitored. Both the pDN19lacΩ vector and pHW0018, an exoT::lacZ fusion construct, were first introduced into wild-type PA103 and PA103 dsbA. Then, pUCP19 vector or pHW0210, containing the intact dsbA gene, was further transformed into the resulting strains for the purpose of complementation. The resulting transformants were cultured in L broth containing appropriate antibiotics with or without 5 mM EGTA at 37°C overnight. As shown in Fig. 2A, the expression of exoT in PA103 dsbA with pUCP19 vector did not respond to EGTA treatment. However, complementation of PA103 dsbA with a dsbA gene fully restored the expression level of exoT in response to EGTA treatment, comparable to that of wild-type PA103. Since the expression of exoT is regulated by the transcriptional activator ExsA, the expression of exsA promoter was also monitored. Similar to earlier experiments, both pDN19lacΩ vector and pHW0203 containing an exsA::lacZ fusion were transformed into the wild-type PA103 and PA103 dsbA. pUCP19 vector or pHW0210 was further transformed into the resulting transformants. As shown in Fig. 2B, there was no expression of exsA in the dsbA mutant background under a type III inducing condition. However, complementation of PA103 dsbA with the dsbA gene fully restored the expression of exsA in response to EGTA treatment. These results indicate that the function of DsbA is required for type III gene expression in response to the type III inducing signal; thus, the signal sensor molecule might need a disulfide bond to be functional.
FIG. 2.
The dsbA mutant is defective in the expression of both the type III effector molecule ExoT (A) and the type III transcriptional activator ExsA (B). Bacterial strains were cultured in L broth with (L/EGTA) or without (L) 5 mM EGTA at 37°C overnight. The cultured bacterial cells were subjected to a standard β-galactosidase assay. 103, wild-type strain PA103; 103d, dsbA mutant of PA103; Vlac, pDN19lacΩ vector; V, pUCP19 vector; 0210, pHW0210 harboring an intact dsbA gene in pUCP19 vector; 0018, pHW0018 harboring lacZ fusion to the exoT promoter in pDN19lacΩ; 0203, pHW0203 harboring lacZ fusion to the exsA promoter in pDN19lacΩ. Average values from four separate tests are shown.
DsbA is required for intracellular survival during infection of HeLa cells.
In several bacteria, including P. aeruginosa PAO1, mutations in the dsbA gene conferred sensitivity to strong reducing reagents, such as dithiothreitol (23, 25). Since the host intracellular compartment is known to be a reduced environment due to the high ratio of reduced to oxidized glutathione, estimated to be between 30:1 and 100:1 (13), the dsbA mutant is likely less able to survive within the host cells. To test this, a dsbA mutant was generated in a PA103 exsA background; PA103 exsA is a type III secretion null mutant which has no cytotoxic activity and is capable of maintaining viability within HeLa cells. The viability of PA103 exsA dsbA was compared to that of its parental strain, PA103 exsA, during HeLa cell infection. Bacterial strains were cultured in L broth containing appropriate antibiotics at 37°C overnight. HeLa S3 epithelial cells (3.0 × 105) in 3 ml of Dulbecco's modified Eagle medium (DMEM) containing 5% fetal bovine serum (FBS) were seeded into six-well plates and incubated at 37°C in 5% CO2 for 24 h. After two washes with warmed phosphate-buffered saline (PBS), 1 ml of DMEM containing 5% FBS was added to the HeLa cells, followed by the addition of 0.1 ml of bacterial suspension in DMEM containing 5% FBS, giving a multiplicity of infection of 100. The infected HeLa cells were incubated at 37°C in 5% CO2 for 2 h, followed by three washes with warmed PBS and the addition of 1.5 ml of DMEM containing 5% FBS and 400 μg of amikacin per ml to kill extracellular bacteria. At each incubation time, the HeLa cells on the plate were lysed with 0.25% Triton X-100 and plated by serial dilution on L-agar plates containing appropriate antibiotics to count the number of internalized bacteria. As shown in Fig. 3, the viability of PA103 exsA dsbA complemented with vector control (103EdsbA/V) was dramatically reduced, over 100-fold, after 24 h of infection, and no bacteria were recovered after 60 h postinfection. In contrast, complementation of PA103 exsA dsbA with the dsbA gene (103EdsbA/0210) fully restored its viability compared to the parental strain, PA103 exsA (103E) (Fig. 3). The dsbA mutant, however, was not defective in growth in either rich or minimal medium, compared to its parental strain PA103 exsA (data not shown); thus, the defect of the dsbA mutant in intracellular survival is not due to a defect in its growth rate.
FIG. 3.
Effect of DsbA on the intracellular survival of P. aeruginosa during infection of HeLa cells. Stationary-phase bacteria were used to infect HeLa S3 cells and incubated at 37°C in 5% CO2 for 2 h. After three washes with warmed PBS, the HeLa cells were incubated at 37°C in 5% CO2 for 2, 24, 48, and 60 h in the presence of 400 μg of amikacin per ml to kill extracellular bacteria. The number of intracellular bacteria was calculated by colony counting. Average values for three repeated tests are shown. 103E, PA103 exsA; 103EdsbA, PA103 exsA dsbA; V, pUCP19 vector; 0210, pHW0210 harboring an intact dsbA gene in the pUCP19 vector.
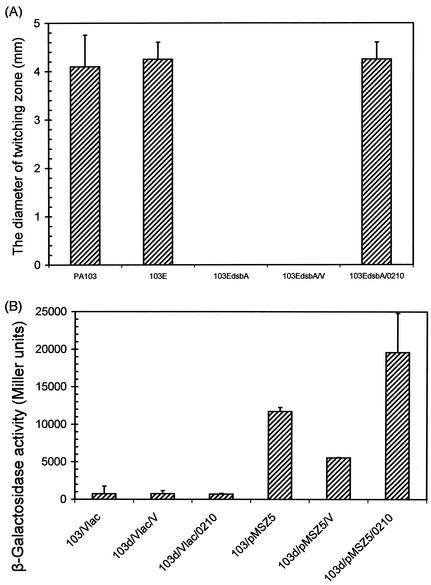
A dsbA mutant strain of PA103 is completely defective in twitching motility.
During the intracellular survival assay described above, it was observed that binding of the dsbA mutant to HeLa cells decreased about 100-fold compared to that of the parental strain (data not shown), indicating that the dsbA mutant is also defective in host cell attachment. Since pili are known to be the major adhesin of P. aeruginosa, the effect of dsbA on pili was further examined. To test the pilus function, bacterial twitching motility was tested with PA103 exsA dsbA, which was used in the intracellular survival assay. As shown in Fig. 4A, PA103 exsA dsbA completely lost the ability to form a twitching zone, compared to wild-type PA103 and the parental strain, PA103 exsA (103E), which formed twitching zones with average diameters of 4.1 and 4.25 mm, respectively. Complementation of PA103 exsA dsbA with a dsbA gene fully restored the twitching motility, with an average diameter of 4.25 mm, whereas the mutant complemented with vector did not form any twitching zone (Fig. 4A). To understand the defect in twitching motility, we examined the expression level of pilA in the dsbA mutant background. As shown in Fig. 4B, pilA expression in the dsbA mutant background was half the level seen in wild-type PA103. pilA expression was restored by the introduction of dsbA. Indeed, despite its nontwitching phenotype, the mutant was sensitive to the pilus-specific lytic phage PO4 (data not shown), indicating that the dsbA mutant still has pili that act as phage receptors. Therefore, it may be that the dsbA mutant has a reduced number of pili on the cell surface but is unable to retract them, thus conferring nontwitching motility.
FIG. 4.
The dsbA mutant is completely defective in twitching motility and has decreased expression of pilA. (A) Freshly grown bacterial strains on L-agar plates containing appropriate antibiotics were transferred with a sharp needle to the bottoms of thin-layer L-agar plates without antibiotics. The plates were incubated at 37°C for 12 h and then at 25°C for 48 h. (B) Bacterial strains were cultured in L broth containing appropriate antibiotics at 37°C overnight. The cultured bacterial cells were subjected to a standard β-galactosidase assay. 103, wild-type strain PA103; 103d, PA103 dsbA; 103E, PA103 exsA; 103EdsbA, PA103 exsA dsbA; Vlac, pDN19lacΩ vector; V, pUCP19 vector; 0210, pHW0210 harboring an intact dsbA gene in the pUCP19 vector; pMSZ5, lacZ fusion to the pilA promoter in pDN19lacΩ.
Conclusions.
Overall, this study describes the essential role of DsbA for several important virulence factors that affect P. aeruginosa pathogenesis. The molecular mechanisms by which DsbA affects type III secretion systems, intracellular survival, and twitching motility are not clear yet. It is reasonable to assume that the direct requirement of DsbA could be due to its catalytic activity of forming a disulfide bond for the functional folding of various proteins required for its virulence. However, it was previously reported that a dsbA mutant of Yersinia pestis also secretes a dramatically reduced amount of Yop proteins by the type III secretion system due to reduced amounts of full-sized YscC protein, which is required for the formation of a ring-shaped structure in a type III secretion apparatus (21). Like Yersinia, the DsbA protein of P. aeruginosa might also be required for the formation of the type III secretion apparatus, in addition to its effect on the expression of type III genes. The defect of the type III secretion system in the dsbA mutant was also supported by a recent study in which several genetic loci, including a dsbA gene of P. aeruginosa, were identified by screening of a transposon insertion library for type III secretion system-deficient noncytotoxic mutants (8). Recently, three proteins in the plant pathogen Ralstonia solanacearum were reported to be required for transmitting a type III inducing signal to initiate the transcription of type III secretion system across the bacterial membrane (6). P. aeruginosa could also encode similar signal sensing and transducing factors, and the DsbA protein might be required for the formation of functional signal sensor molecules, presumably through catalyzing disulfide bond formation. In Shigella flexneri, the dsbA gene was also reported to be required for intracellular survival (39), although the exact mechanism is not known. Besides possibly having sensitivity to the reduced environments found in host intracellular compartments (13), the dsbA mutant was reported to be defective in the synthesis of c-type cytochromes during anaerobic growth (24). This implies that DsbA could be critically involved in the assembly of electron transfer chains induced under anaerobic conditions. Therefore, DsbA could have a vital role in bacterial survival by enabling proper folding of membrane or secreted proteins involved in adapting to stressful conditions or physiological changes. The decrease in the expression of pilA could partially explain the defect of dsbA mutants in twitching motility. It is also possible that the loss of the disulfide loop at the C terminus of the type IV pilin subunit, important for adhesion to epithelial cells (16), contributes to the reduced twitching motility, since twitching motility requires adhesion and traction on solid surfaces (23). Although the dsbA mutation in the PA103 background caused a complete loss of twitching motility, mutation of dsbA in a PAO1 background was reported to result in about 60% reduction in the twitching zone compared to that of wild-type PAO1 (23), indicating that the dsbA effect on the twitching motility in P. aeruginosa seems to be a strain-dependent phenomenon. The defect in twitching motility was also supported by previous reports presenting the requirement of DsbA in the biogenesis of type 4 bundle-forming pili in enteropathogenic E. coli and type 4 pili in Burkholderia cepacia (3, 17). DsbA of P. aeruginosa has been shown to be essential for the production of important virulence factors such as elastase, alkaline phosphatase, and lipase (5, 34), and infection of Caenorhabditis elegans with the dsbA mutant causes slower killing (32). These previously observed effects of DsbA combined with the roles of DsbA in intracellular survival, the expression of a type III secretion system, and twitching motility shown in this study suggest that DsbA could be an important target for the control of P. aeruginosa infections.
Acknowledgments
We thank members of S. Jin's laboratory for helpful discussion and suggestions.
Work in our laboratory is supported by grants from the NIH, American Cancer Society, and Cystic Fibrosis Foundation.
Editor: V. J. DiRita
REFERENCES
- 1.Anwar, H., J. L. Strap, and J. W. Costerton. 1992. Establishment of aging biofilms: possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrob. Agents Chemother. 36:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asboe, D., V. Gant, H. M. Aucken, D. A. Moore, S. Umasankar, J. S. Bingham, M. E. Kaufmann, and T. L. Pitt. 1998. Persistence of Pseudomonas aeruginosa strains in respiratory infection in AIDS patients. AIDS 12:1771-1775. [DOI] [PubMed] [Google Scholar]
- 3.Bieber, D., S. W. Ramer, C. Y. Wu, W. J. Murray, T. Tobe, R. Fernandez, and G. K. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 4.Bodey, G. P., R. Bolivar, V. Fainstein, and L. Jadeja. 1983. Infections caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 5:279-313. [DOI] [PubMed] [Google Scholar]
- 5.Braun, P., C. Ockhuijsen, E. Eppens, M. Koster, W. Bitter, and J. Tommassen. 2001. Maturation of Pseudomonas aeruginosa elastase. Formation of the disulfide bonds. J. Biol. Chem. 276:26030-26035. [DOI] [PubMed] [Google Scholar]
- 6.Brito, B., D. Aldon, P. Barberis, C. Boucher, and S. Genin. 2002. A signal transfer system through three compartments transduces the plant cell contact-dependent signal controlling Ralstonia solanacearum hrp genes. Mol. Plant-Microbe Interact. 15:109-119. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 8.Dacheux, D., O. Epaulard, A. De Groot, B. Guery, R. Leberre, I. Attree, B. Polack, and B. Toussaint. 2002. Activation of the Pseudomonas aeruginosa type III secretion system requires an intact pyruvate dehydrogenase aceAB operon. Infect. Immun. 70:3973-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doring, G., M. Maier, E. Muller, Z. Bibi, B. Tummler, and A. Kharazmi. 1987. Virulence factors of Pseudomonas aeruginosa. Antibiot. Chemother. 39:136-148. [DOI] [PubMed] [Google Scholar]
- 10.Fabianek, R. A., H. Hennecke, and L. Thony-Meyer. 2000. Periplasmic protein thiol:disulfide oxidoreductases of Escherichia coli. FEMS Microbiol. Rev. 24:303-316. [DOI] [PubMed] [Google Scholar]
- 11.Finck-Barbancon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. Fleiszig, C. Wu, L. Mende-Mueller, and D. W. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 12.Fleiszig, S. M., J. P. Wiener-Kronish, H. Miyazaki, V. Vallas, K. E. Mostov, D. Kanada, T. Sawa, T. S. Yen, and D. W. Frank. 1997. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect. Immun. 65:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman, R. B. 1989. Protein disulfide isomerase: multiple roles in the modification of nascent secretory proteins. Cell 57:1069-1072. [DOI] [PubMed] [Google Scholar]
- 14.Guilhot, C., G. Jander, N. L. Martin, and J. Beckwith. 1995. Evidence that the pathway of disulfide bond formation in Escherichia coli involves interactions between the cysteines of DsbB and DsbA. Proc. Natl. Acad. Sci. USA 92:9895-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha, U., and S. Jin. 2001. Growth phase-dependent invasion of Pseudomonas aeruginosa and its survival within HeLa cells. Infect. Immun. 69:4398-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn, H. P. 1997. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa—a review. Gene 192:99-108. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi, S., M. Abe, M. Kimoto, S. Furukawa, and T. Nakazawa. 2000. The dsbA-dsbB disulfide bond formation system of Burkholderia cepacia is involved in the production of protease and alkaline phosphatase, motility, metal resistance, and multi-drug resistance. Microbiol. Immunol. 44:41-50. [DOI] [PubMed] [Google Scholar]
- 18.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 19.Hovey, A. K., and D. W. Frank. 1995. Analyses of the DNA-binding and transcriptional activation properties of ExsA, the transcriptional activator of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 177:4427-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishimoto, K. S., and S. Lory. 1992. Identification of pilR, which encodes a transcriptional activator of the Pseudomonas aeruginosa pilin gene. J. Bacteriol. 174:3514-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson, M. W., and G. V. Plano. 1999. DsbA is required for stable expression of outer membrane protein YscC and for efficient Yop secretion in Yersinia pestis. J. Bacteriol. 181:5126-5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, P. V. 1966. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J. Infect. Dis. 116:481-489. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra, S., L. A. Silo-Suh, K. Mathee, and D. E. Ohman. 2000. Proteome analysis of the effect of mucoid conversion on global protein expression in Pseudomonas aeruginosa strain PAO1 shows induction of the disulfide bond isomerase, DsbA. J. Bacteriol. 182:6999-7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metheringham, R., L. Griffiths, H. Crooke, S. Forsythe, and J. Cole. 1995. An essential role for DsbA in cytochrome c synthesis and formate-dependent nitrite reduction by Escherichia coli K-12. Arch. Microbiol. 164:301-307. [DOI] [PubMed] [Google Scholar]
- 25.Missiakas, D., and S. Raina. 1997. Protein folding in the bacterial periplasm. J. Bacteriol. 179:2465-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicas, T. I., and B. H. Iglewski. 1985. The contribution of exoproducts to virulence of Pseudomonas aeruginosa. Can. J. Microbiol. 31:387-392. [DOI] [PubMed] [Google Scholar]
- 27.Peek, J. A., and R. K. Taylor. 1992. Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 89:6210-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole, K., and R. Srikumar. 2001. Multidrug efflux in Pseudomonas aeruginosa: components, mechanisms and clinical significance. Curr. Top. Med. Chem. 1:59-71. [DOI] [PubMed] [Google Scholar]
- 29.Roilides, E., K. M. Butler, R. N. Husson, B. U. Mueller, L. L. Lewis, and P. A. Pizzo. 1992. Pseudomonas infections in children with human immunodeficiency virus infection. Pediatr. Infect. Dis. J. 11:547-553. [DOI] [PubMed] [Google Scholar]
- 30.Schweizer, H. P. 1991. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene 97:109-121. [DOI] [PubMed] [Google Scholar]
- 31.Stenson, T. H., and A. A. Weiss. 2002. DsbA and DsbC are required for secretion of pertussis toxin by Bordetella pertussis. Infect. Immun. 70:2297-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan, M. W., L. G. Rahme, J. A. Sternberg, R. G. Tompkins, and F. M. Ausubel. 1999. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA 96:2408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Totten, P. A., and S. Lory. 1990. Characterization of the type a flagellin gene from Pseudomonas aeruginosa PAK. J. Bacteriol. 172:7188-7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urban, A., M. Leipelt, T. Eggert, and K. E. Jaeger. 2001. DsbA and DsbC affect extracellular enzyme formation in Pseudomonas aeruginosa. J. Bacteriol. 183:587-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallis, A. J., V. Finck-Barbancon, T. L. Yahr, and D. W. Frank. 1999. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect. Immun. 67:2040-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Delden, C., and B. H. Iglewski. 1998. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yahr, T. L., L. M. Mende-Mueller, M. B. Friese, and D. W. Frank. 1997. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 179:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamanaka, H., M. Kameyama, T. Baba, Y. Fujii, and K. Okamoto. 1994. Maturation pathway of Escherichia coli heat-stable enterotoxin I: requirement of DsbA for disulfide bond formation. J. Bacteriol. 176:2906-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu, J. 1998. Inactivation of DsbA, but not DsbC and DsbD, affects the intracellular survival and virulence of Shigella flexneri. Infect. Immun. 66:3909-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]