Abstract
Cytotoxic necrotizing factor 1 (CNF1), a protein produced by pathogenic strains of Escherichia coli, activates the p21 Rho-GTP-binding protein, inducing a profound reorganization of the actin cytoskeleton. CNF1 binds to its cell surface receptor on HEp-2 cells with high affinity (Kd = 20 pM). In HEp-2 cells the action of CNF1 is not blocked in the presence of filipin, a drug described to reduce cholera toxin internalization by the caveolae-like mechanism. Moreover, HEp-2 cells, which express a dominant negative form of proteins that impair the formation of clathrin coated-vesicles and internalization of transferrin (Eps15, dynamin or intersectin-Src homology 3), are still sensitive to CNF1. In this respect, the endocytosis of CNF1 is similar to the plant toxin ricin. However, unlike ricin toxin, CNF1 does not cross the Golgi apparatus and requires an acidic cell compartment to transfer its enzymatic activity into the cytosol in a manner similar to that required by diphtheria toxin. As shown for diphtheria toxin, the pH-dependent membrane translocation step of CNF1 could be mimicked at the level of the plasma membrane by a brief exposure to a pH of ≤5.2. CNF1 is the first bacterial toxin described that uses both a clathrin-independent endocytic mechanism and an acidic-dependent membrane translocation step in its delivery of the catalytic domain to the cell cytosol.
INTRODUCTION
Bacterial protein toxins are among the most powerful virulence factors produced by pathogenic microorganisms. In general, these toxins are divided into three groups according to their mechanism of action (Boquet and Gill, 1991). Group I toxins such as the heat-stable Escherichia coli enterotoxin (reviewed by Sears and Kaper, 1996) act on cell surface where they induce transmembrane signaling. Group II toxins or “pore-forming toxins” (e.g., perfringolysin O, Staphylococcus aureus alpha toxin, and aerolysin [reviewed by Bhakdi et al., 1996]) act by disrupting the integrity of the plasma membrane. Finally, group III toxins, such as diphtheria, cholera, or tetanus toxins, transfer an enzymatically active domain into the cytosol and modify a eukaryotic target, which gives rise to toxicity (reviewed by Montecucco et al., 1994). The latter group is clearly the most potent, because it is believed that one or a few copies of the toxin enzymatic domain, for instance of the diphtheria toxin (DT) fragment A, introduced into the cytosol can kill a cell within 2 d (Yamaizumi et al., 1978). Group III toxins are also known as A–B toxins on the basis of their structure–function relationships, which involve a catalytic domain (the A subunit) and cell binding and membrane translocation domains (the B subunit).
Translocation of the catalytic domain of the type III toxins into the eukaryotic cytosol appears to be accomplished by either one or two mechanisms (reviewed by Johannes and Goud, 1998; Lord and Roberts, 1998; Montecucco, 1998). In the first instance, for which the paradigm is DT (Sandvig and Olsnes, 1991), the toxin first binds to its cell surface and is endocytosed and trafficked into early endosomes. After acidification of this compartment, a small portion of toxin molecules undergo a conformational change exposing hydrophobic segments within the B fragment. These domains (hydrophobic helixes) then spontaneously insert into the lipid bilayer of the endosomal lining membrane and facilitate the translocation of the toxin catalytic domain into the cytosol as shown in model membranes (Senzel et al., 1998). The second mechanism used by some toxins (e.g., cholera toxin [Orlandi et al., 1993], Shiga toxin [Sandvig et al., 1992; Johannes et al., 1997], Pseudomonas aeruginosa exotoxin A [Seetharam et al., 1991], and the plant toxin ricin [van Deurs et al., 1988]) appears to involve an injection of their catalytic subunit into the cytosol by a different mechanism. After binding to their respective surface receptors, these toxins are internalized by endocytosis and reach the trans-Golgi network (TGN) (reviewed by Johannes and Goud, 1998; Lord and Roberts, 1998; Montecucco, 1998). From the TGN, these toxins are transported, in a retrograde manner, through the Golgi apparatus toward the endoplasmic reticulum (ER), where translocation of the catalytic fragment into the cytosol is probably achieved (reviewed by Johannes and Goud, 1998; Lord and Roberts, 1998). In this instance an acidic pH is not generally required for the translocation the catalytic domain into the cytosol. However, in the case of P. aeruginosa exotoxin A, it has been observed that drugs that block the acidification of intracellular compartments impair the activity of this toxin (Fitzgerald et al., 1983). The exact mechanism by which these toxins transfer their catalytic fragments into the cytosol from either the Golgi or ER compartments is still unknown.
In general, bacterial toxins of group III raise three questions: 1) what is the nature and the number of their cell receptors; 2) by which endocytic pathway (clathrin dependent or independent) is a given toxin internalized into the cell; and 3) does a given type III toxin either transfer its catalytic domain into the cytosol, upon reaching an endosomal compartment, or undergo a longer endocytic journey to the endoplasmic reticulum before its catalytic domain is translocated to the cytosol?
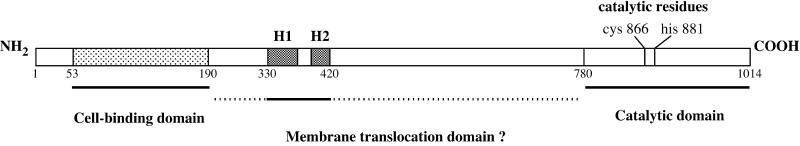
Cytotoxic necrotizing factor 1 (CNF1) is a recently described type III toxin belonging to the “dermonecrotizing” toxins group (Boquet and Fiorentini, 2000). This toxin is most often produced by uropathogenic E. coli strains (Blanco et al., 1996). CNF1 (relative molecular mass, 113 kDa) is a single-chain toxin molecule (Falbo et al., 1993), which has been shown to induce ruffling of eukaryotic cell membranes, actin stress fiber formation, and spreading and multinucleation through the permanent activation of the small GTPase Rho (Fiorentini et al., 1995; Flatau et al., 1997; Schmidt et al., 1997). CNF1 is a deamidase that is specifically directed toward glutamine 63 of the Rho protein both in vivo and in vitro (Flatau et al., 1997; Schmidt et al., 1997). The deamidation of the Rho glutamine 63 into glutamic acid leads to permanent activation of the GTP-binding protein by impairing the Rho intrinsic or Rho GTPase-activating protein-mediated hydrolysis of GTP (Flatau et al., 1997; Schmidt et al., 1997). The enzymatic domain of CNF1 lies within a 300-amino-acid domain located at the carboxyl terminus of the toxin (Lemichez et al., 1997b; Schmidt et al., 1998), and the cell–receptor binding domain is located within amino acids 53–190 (Fabbri et al., 1999). Figure 1 depicts the molecular organization of CNF1.
Figure 1.
Schematic structure of the E. coli CNF1. Numbers located underneath the schematic representation of CNF1 indicate amino acid residues delimiting the different toxin domains. Black boxes are the two hydophobic helices (H1 and H2). The gray box located at the amino terminus of the protein is the actual delimitation of residues implicated in the binding of the toxin to its cell receptor (Fabbri et al., 1999).
It is well established that the cellular effects of CNF1 can be antagonized by agents that increase endosomal pH such as ammonium chloride (Falzano et al., 1993) and methylamine (Lacerda et al., 1997). By analogy with DT (Sandvig and Olsnes, 1991), it has been assumed that CNF1 must reach an acidic cell compartment to translocate its deamidase activity into the cytosol. However, the number of CNF1 cell receptors, their affinity for the toxin, the mode by which CNF1 is taken up by cells, the nature of the endocytic compartment from which this molecule enters the cytosol, and the mechanism by which the CNF1 catalytic activity crosses the lipid membrane remain unknown.
In the present work, we show that CNF1 binds tightly to its cell surface receptor. After binding, the toxin is internalized by both clathrin- and caveolae-like–independent pathways and is subsequently transferred to an endosomal compartment by a microtubule-dependent mechanism. From this compartment the catalytic activity of the toxin appears to translocate the eukaryotic membrane by an acidic pH-driven mechanism comparable with that of DT fragment A.
MATERIALS AND METHODS
Bacterial Strains, Plasmids, and Cloning and Mutagenesis Procedures
The human uropathogenic E. coli strain J96 (O4:K6) (Low et al., 1984; Blum et al., 1995) was used as the source of chromosomal DNA for cloning the cnf1 gene together with the promoter region of the toxin into E. coli TG1 (Sambrook et al., 1989). Plasmid pCR2.1 was used for cloning cnf1 into E. coli INVaF′ (TA cloning kit; Amersham, Les Ulis, France). The cnf1-C866S mutant gene was obtained by site-directed mutagenesis using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Cysteine 866 of CNF1 was mutated to a serine to abolish the catalytic activity of the toxin (Schmidt et al., 1998). cnf1-C866S cloned in pCR2.1 plasmid was transformed into the E. Coli XL1-Blue. CNF1-C866S and the wild-type CNF1 were purified as previously reported (Falzano et al., 1993). DT was produced and purified as previously described (Boquet and Pappenheimer, 1976).
Cell Culture
HEp-2, Madin–Darby canine kidney (MDCK), and HeLa cells were grown in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Grand Island, NY) supplemented with 7% calf serum (Life Technologies) and 1% glutamine (Life Technologies) at 37°C in a 5% CO2 atmosphere in 6- or 24-well cell culture plates (Nunc; Life Technology, Cergy-Pontoise, France).
Schild Plot Analysis
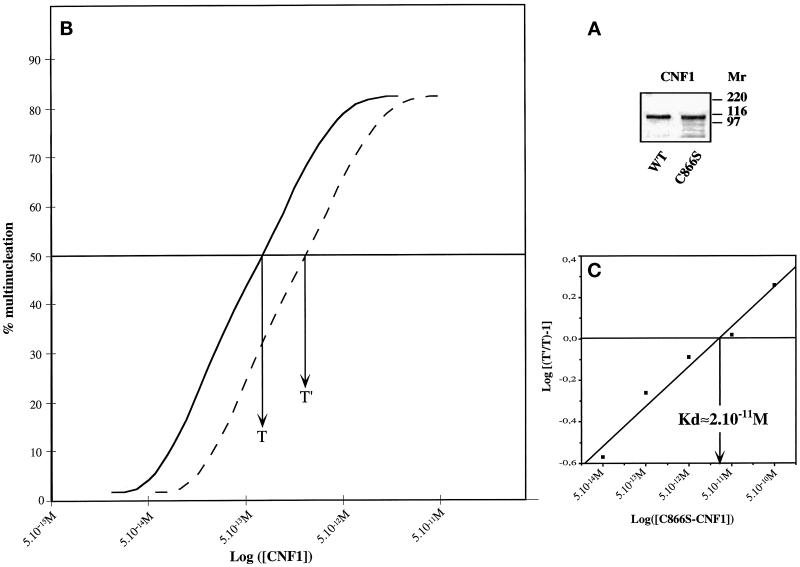
To determine the apparent affinity of CNF1 for its cell surface receptor, a Schild plot analysis (Schild, 1957) was performed as described by Ittelson and Gill (1973) for DT. This method is based on competition experiments between a wild-type toxin and its nonenzymatically active counterpart for cell toxicity. CNF1-C866S was produced and purified (Figure 2A). This nontoxic mutant form of CNF1 was then shown to block the action of wild-type CNF1 as demonstrated by its ability to inhibit the CNF1 multinucleating activity on HEp-2 cells in vitro.
Figure 2.
Schild plot analysis for the determination of the CNF1 affinity to its cell receptor. (A) Purified wild-type CNF1 and CNF1-C866S used in this study. Approximately 2 μg of each protein were loaded on 10% SDS-PAGE, and the gel was fixed and stained with Coomassie blue. Molecular masses are given in kilodaltons. (B) Typical curves showing the displacement of CNF1 effects (multinucleation of HEp-2 cells) after addition of a fixed concentration of CNF1-C866S; T, concentration of CNF1 to obtain 50% of multinucleated cells in 48 h; T′, that of CNF1 after addition of a given amount of CNF1-C866S, which gives the same percentage of multinucleated cells. (C) Data plotted according to the method of Schild (1957). The arrow indicates the affinity of CNF1-C866S determined by the Schild method.
DNA Cell Transfection and Analysis by Immunofluorescence Confocal Microscopy
HEp-2 cells were transfected with a 2 μg/ml concentration of each plasmid construct into 2 ml of DMEM (for cells grown in a 35-mm-diameter culture dish) using the DOTAP transfection kit (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's instructions. The following plasmids were used: pEGFP-C2 for the green fluorescent protein (GFP)-Eps15 mutant (ED95/295) (Benmerah et al., 1999) and the GFP-Src homology 3 (SH3) intersectin (Simpson et al., 1999) (a gift from Dr. P.S McPherson, McGill University, Montreal, Quebec, Canada), and pCMV5d for the hemagglutinin (HA)-tagged dynamin-2 mutant (dyn-K44A) (Damke et al., 1995) (a gift from Dr Schmid, Scripps Research Institute, La Jolla, CA). Transfected cells were incubated overnight for expression and then incubated for 90 min with 10−9 M CNF1 or 90 min with 2 μg/ml Texas Red-transferrin (Molecular Probes, Eugene, OR) to monitor the activity of the coated pit pathway. CNF1-intoxicated cells were incubated in medium supplemented with 100 nM bafilomycin A1, which blocks further entry of CNF1. Cells were further incubated for 24 h with bafilomycin A1. Cells were then fixed in PBS containing 3.7% paraformaldehyde (Sigma, St. Louis, MO), permeabilized in PBS containing 0.1% Triton X-100 (Sigma) and 0.2% BSA, stained with Texas Red-phalloidin (Molecular Probes), and mounted in 150 mM Tris, pH 8.5 (Sigma) containing 12% Mowiol (Calbiochem, La Jolla, CA) and 30% glycerol (Sigma). For the HA-tagged dyn-K44A mutant, the 12CA5 mouse mAb and the anti-mouse FITC-labeled mAb (1:70; Dako, Copenhagen, Denmark) were used. Preparations were observed and analyzed with a laser confocal microscope (Leica, Heidelberg, Germany).
Assays for Inhibitors of Endocytosis, Endosome Acidification, Microtubule Polymerization, and Golgi Vesicle Trafficking
Cells were intoxicated with 10−10 M CNF1 in the presence or absence of the following drugs: brefeldin A, 5 μg/ml; nocodazole, 30 μM; filipin III, 1.5 μM; chlorpromazine, 10 μg/ml; amiloride, 5 or 10 μM; bafilomycin A1, 100 nM; monensin, 2 μM; and NH4Cl, 2–10 mM. All products were from Sigma except for brefeldin A (Molecular Probes).
For tests with brefeldin A, filipin, chlorpromazine, and amiloride, cells were preincubated for 1 h with the drug at the given concentration. For nocodazole experiments, preincubation was performed on ice to increase microtubule depolymerization. Cells were then incubated for 90 min with 10−10 M CNF1 in the presence of the drug. Cells were washed, bafilomycin A1 was then added to the medium to block further entry of CNF1, cell multinucleation was recorded 24 h later, and results were expressed as percent of control without drugs.
Effects of nocodazole on microtubule depolymerization were controlled by immunofluorescence using an anti-tubulin mAb (Amersham). The effects of brefeldin A on the Golgi apparatus were controlled by immunofluorescence with the CTR433 anti-Golgi mAb (Bornens et al., 1987) (a gift from Dr. Bornens, Institut Curie, Paris, France). Filipin effects were controlled by their ability to inhibit cholera toxin activity on intracellular cAMP accumulation as described by Orlandi and Fishman (1998). Extraction and detection of cAMP were performed with the enzyme immunoassay system kit Biotrak (Amersham).
To decrease or inhibit endocytic compartment acidification, the v-ATPase inhibitor bafilomycin A1, the proton ionophore monensin, and the weak base NH4Cl were used. As previously described, cells were preincubated for 1 h with these drugs before addition of CNF1, then washed, and bafilomycin A1 was added to the medium until 24 h of incubation.
Assay for the Transfer of CNF1 Bound to the Cell Surface into the Cytosol by a Short Acidic pH Exposure
HEp-2 cells were seeded 24 h before the experiment into six-well cell culture plates at a subconfluent density. Cell monolayers were washed with PBS and incubated at 37°C for 10 min in DMEM and 25 mM HEPES (Life Technologies) containing 0.5% BSA (DMEM-HEPES-BSA). CNF1 (10−8 M) was bound to cells at 4°C in cold DMEM-HEPES-1% BSA for 4 h. Cells were washed twice with PBS at 4°C and incubated for 10 min at 37°C at different pH values (from 7.2 to 4.0) according to the procedure described by Sandvig and Olsnes (1980), in the presence of bafilomycin A1 to block the normal entry of CNF1 into cells. Control cultures were incubated in DMEM-HEPES-BSA in the presence or not of bafilomycin A1. Cells were further incubated for 3 h in DMEM containing 7% calf serum and bafilomycin A1. CNF1 molecules translocated into the cytosol were detected by their ability to upshift the molecular weight of the Rho GTP-binding protein (Flatau et al., 1997; Schmidt et al., 1997). Cell monolayers were incubated on ice for 45 min, washed twice in 3 mM imidazole buffer, pH 7.5, containing 250 mM sucrose, detached from the Petri dishes with a rubber policeman, and suspended in 100 μl of the same solution supplemented with proteases inhibitors (Complete EDTA free; Boehringer Mannheim). Cell suspensions were transferred to microfuge tubes and incubated for 10 min at 4°C and then lysed by three cycles of freeze–thaw in liquid nitrogen and 37°C incubation. Lysates were centrifuged (5000 × g, 20 min, 4°C), and supernatants were collected. Total protein content was measured by means of a Bio-Rad (Ivry-sur Seine, France) assay kit. Each supernatant (30 μg of protein) was incubated for 90 min at 37°C with 15 ng of purified exoenzyme C3 as described previously (Chardin et al., 1989) and 106 cpm of [32P]NAD (New England Nuclear, Boston, MA; 53 Ci/mmol) in 20 mM Tris buffer, pH 7.5, containing 2 mM MgCl2 and 100 mM NaCl. The in vitro Rho ADP ribosylation reactions were performed as described previously (Chardin et al., 1989). Samples were then subjected to SDS-PAGE (12%), and the Rho protein was detected by autoradiography.
RESULTS
CNF1 Exhibits a Very High Affinity for Specific Binding Sites on HEp-2 Cells
Within 24 h after exposure to 10−12 M CNF1, membrane ruffling, stress fiber formation, or the measurable multinucleating effect of CNF1 can be clearly observed on HEp-2 cells (Falzano et al., 1993). Our first aim was to estimate the number of cell surface receptors and to measure the affinity of CNF1 to its receptor using 125I-labeled CNF1. As yet, we have not been able to label CNF1 with 125-INa (using various techniques) without a concomitant loss of toxin activity. As a result, we measured the apparent affinity of CNF1 toward its cell receptor using the Schild plot technique (Schild, 1957). This method, based on competition experiments between a wild-type toxin and its nonenzymatically active counterpart for cell toxicity, has been successfully used to measure the affinity of DT to its membrane receptor on HeLa cells (Ittelson and Gill, 1973). The percent multinucleation resulting from several competition experiments, using different concentrations of the catalytically inactive CNF1-C866S and wild-type CNF1, was plotted as shown in Figure 2B. Schild analysis of these data suggests that the apparent affinity between CNF1 and its cell receptor is Kd 20 pM (Figure 2C). Thus CNF1 appears to exhibit a high affinity for its cell membrane receptor.
CNF1 Does Not Require the Clathrin- or Caveolin-like–dependent Pathways of Endocytosis to Be Taken up by HEp-2 Cells
It is well known that the endocytosis of proteins into cells can be achieved by several mechanisms. The first and most extensively described is the clathrin-dependent pathway (Gruenberg and Maxfield, 1995; reviewed by Mellman, 1996). The second, a clathrin-independent mode of entry (reviewed by Sandvig and van Deurs, 1994; Lamaze and Schmid, 1995), was first described for bacterial toxins (Montesano et al., 1982) and the plant ricin toxin (RT) endocytosis (Moya et al., 1985; Sandvig et al. 1987). The third, more recently studied, relies on the presence of cholesterol- and glycolipid-rich domains, defined as caveolae (Simons and Ikonen, 1997), in the cell membrane. And finally, macropinocytosis, best described in phagocytic cells, depends on membrane ruffling (Steinman and Swanson, 1995). To investigate which of these mechanisms was involved in the uptake of CNF1, we used either specific inhibitors or cell transfections with DNA-encoding proteins known to impair the formation of coated vesicles.
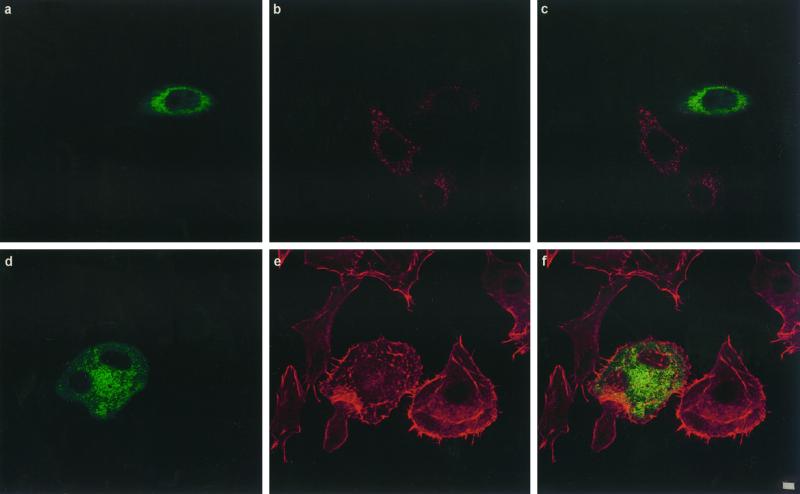
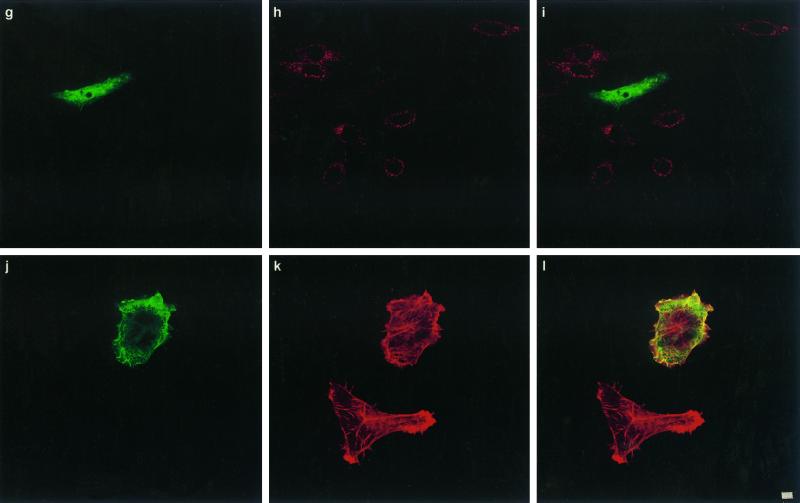
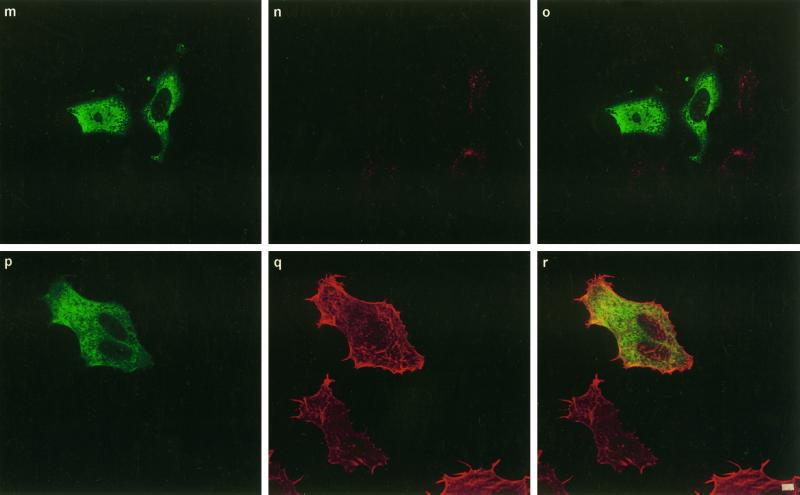
To test whether CNF1 was taken up by a clathrin-dependent mechanism, we transfected HEp-2 cells with different plamid DNAs encoding proteins well known to inhibit the clathrin-coated pathway of endocytosis. These included the dominant negative mutant of the Eps15 molecule (ED95/295) (Benmerah et al., 1999), dynamin GTPase (dyn-K44A) (Damke et al., 1994), and the SH3 domain of intersectin (Simpson et al., 1999). Eps15 is involved in the early steps of clathrin-coated vesicle formation (Carbone et al., 1997; Benmerah et al., 1998). Indeed, the ED95/295 dominant negative mutant impairs clathrin-coated pit assembly at the plasma membrane and therefore strongly inhibits clathrin-dependent endocytosis of transferrin (Benmerah et al., 1999). As shown in Figure 3, a–c, compared with nontransfected cells, HEp-2 cells expressing the GFP-tagged ED95/295 protein did not accumulate transferrin. Expression of ED95/295, however, did not block the formation of cell spreading, expression of membrane ruffling, and multinucleation typically induced by CNF1, and these results were comparable with nontransfected cells (Figure 3, d–f). We then transfected HEp-2 cells with the DNA encoding the dominant negative form of dynamin dyn-K44A (Damke et al., 1994). Dynamin is a GTP-binding protein involved in the constriction of coated pit collars, separating vesicles when they invaginate from the membrane (van der Bliek et al., 1993). Whether dynamin is a GTPase endowed with mechanical activity, a signaling activity, or both is not clear (reviewed by van der Bliek, 1999). The dynamin-2 isoform was used in this work, because it is the ubiquitous form of this protein and seems to be more potent in inhibiting receptor-mediated endocytosis than dynamin-1 (Altschuler et al., 1998). When mutated in the dynamin GTP-binding domain, the molecule becomes a dominant negative protein and blocks the formation of coated vesicles (Damke et al., 1994). As shown in Figure 3, g–i, expression of dyn-K44A impaired the uptake of the transferrin molecule but did not block CNF1 activity (Figure 3, j–l). Finally, to confirm that the clathrin-coated pathway of endocytosis was not strictly necessary for CNF1 uptake, HEp-2 cells were transfected with plasmid DNA encoding the SH3A domain of intersectin (Simpson et al., 1999). Intersectin has been shown to interact through SH3 domains with dynamin (Roos and Kelly, 1998; Yamabhai et al., 1998; Sengar et al., 1999). Expression of the SH3A intersectin domain disrupts this interaction and blocks clathrin-coated vesicles constriction, resulting in a strong inhibition of endocytosis (Simpson et al., 1999). As observed with ED95/295 or dyn-K44A, SH3A also efficiently blocked the entry of transferrin into HEp-2 cells (Figure 3, m–o) but did not inhibit effects of CNF1 (Figure 3, p–r).
Figure 3.
Confocal immunofluorescence microscopic analysis of coated vesicle inhibition in HEp-2 cells by transfection of DNAs encoding ED95/295, dyn-K44A, and int-SH3: effects on transferrin internalization and CNF1 activity. Transfected cells are identified either by GFP tags, ED95/295 (a and d) and int-SH3 domain (m and p), or by the HA tag using mAb 12CA5 anti-HA followed by binding of an anti-mouse FITC-labeled antibody (g and j). Cells were controlled for transferrin endocytosis by incubation with 2 mg/ml Texas Red-labeled transferrin for 90 min at 37°C (b, ED95/295; h, dyn-K44A; n, int-SH3 domain), (c, i, and o) Merged images of a and b, g and h, and m and n, respectively. Cells transfected with ED95/295 (e), dyn-K44A (k), or int-SH3 (q) were incubated with 10−9 M CNF1 for 90 min. Bafilomycin A1 was then added to block further entry of the toxin (see MATERIALS AND METHODS), and cells were incubated for an additional period of 24 h with bafilomycin A1 to develop the CNF1 phenotype consisting of cell spreading, membrane ruffling, stress fiber formation, and multinucleation (clearly shown in e, k, and q). Cells were then stained for F-actin with Texas Red-phalloidin (e, k, and q). (i, f, and r) Merged images of d and e, j and k, and p and q, respectively. Bars, 2 mm.
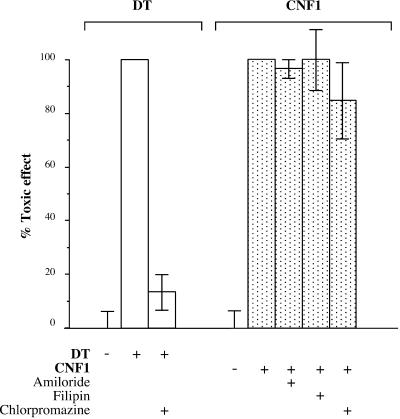
Cationic amphiphilic drugs such as chlorpromazine inhibit receptor-mediated endocytosis by disrupting the assembly of AP2 and clathrin, thereby causing the accumulation of these proteins in endosomes and reducing the number of coated pits at the cell surface (Hunt and Marshall-Carlson, 1986; Wang et al., 1993). Although almost completely inhibiting the toxicity of DT on HEp-2 cells, chlorpromazine did not significantly alter CNF1-induced HEp-2 cell multinucleation (Figure 4).
Figure 4.
Effects of amiloride, filipin, and chlorpromazine on CNF1-induced multinucleation of HEp-2 cells. Cells grown in 24-well plates were intoxicated with 10−10 M CNF1 in the presence or absence of the following drugs: filipin, 1.5 μM; chlorpromazine, 10 μg/ml, and amiloride, 1 μM. Cells were washed briefly in HEPES-buffered medium and incubated for 1 h with or without each drug before incubation with CNF1. Then cells were incubated with 10−10 M CNF1 still in the presence of the drug. After 90 min cells were washed, and bafilomycin A1 was added to the medium to block further entry of CNF1. Cell multinucleation, induced by the toxin, was recorded 24 h later and expressed as percentage of control without drugs. Effects of chlorpromazine on DT are given as a positive control.
As shown in Figure 4, amiloride, which inhibits macropinocytosis (West et al., 1989), was not able to antagonize the CNF1-induced multinucleation of HEp-2, although it efficiently blocked the toxin-induced membrane ruffling (our unpublished data).
Filipin is used to sequester cholesterol in membranes and thereby to modulate the endocytosis of certain ligands (e.g., cholera toxin) by cholesterol- and sphingolipid-rich membrane domains by the caveolae-like pathway of endocytosis (Orlandi and Fishman, 1998). Filipin has been shown to reduce to ∼40% the internalization of cholera toxin (CT) in Caco 2 cells and to block the cAMP accumulation induced by CT (Orlandi and Fishman, 1998). Filipin did not block CNF1 effects on HEp-2 cells (Figure 4), although it inhibited the cholera toxin-induced accumulation of cAMP (our unpublished results).
From these studies we concluded that the HEp-2 cell internalization of CNF1 does not strictly require either the clathrin coated pit coated vesicle or the caveolae-like pathways of endocytosis. Thus the uptake and internalization of CNF1 are similar to the plant toxin ricin.
CNF1 Must Reach an Endosomal Compartment, in a Microtubule-dependent Way, to Express Its Activity
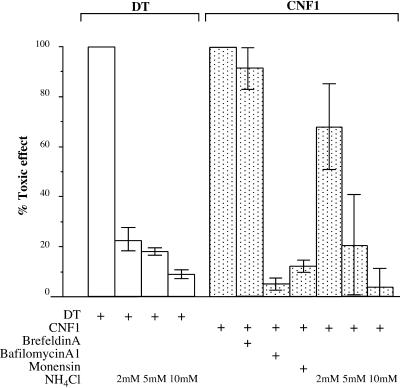
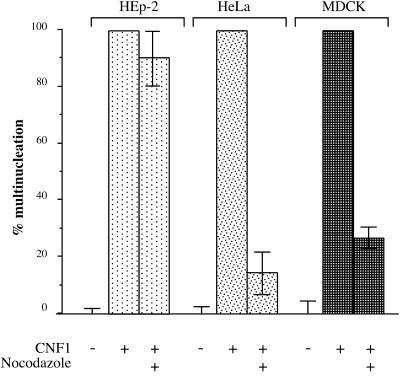
An important goal of the present work was to localize the endocytic compartment involved in the entry of the CNF1 enzymatic activity into the cytosol. To date, two main compartments have been found to be gateways for toxins: early endosomes and the ER (reviewed by Johannes and Goud, 1998; Lord and Roberts, 1998; Montecucco, 1998). The fungal drug brefeldin A inhibits ARF 1 (reviewed by Chardin and McCormick, 1999). This provokes an inhibition of vesicle formation at the level of the Golgi apparatus, resulting in the disruption of the traffic between the TGN and the ER (Klausner et al., 1992), without impairing endosomal and lysosomal function (Wood and Brown, 1992; Strous et al., 1993). Brefeldin A has been shown to block the action of those toxins that transit toward the ER such as Shiga toxin, cholera toxin, and Pseudomonas exotoxin A, but not that of DT, which enters the cytosol at the level of early endosomes (reviewed by Johannes and Goud, 1998; Lord and Roberts, 1998; Montecucco, 1998). Treatment of HEp-2 cells with a concentration of brefeldin A, which completely disrupted the Golgi apparatus, as monitored by immunofluorescence staining using an antibody directed against a Golgi protein (our unpublished data), had no effect on CNF1 multinucleating activity (Figure 5). This result strongly suggests that CNF1 was entering the cytosol through a compartment located upstream of the Golgi apparatus and indicates that the toxin, unlike ricin, does not translocate into the cytosol via the TGN–Golgi route. The CNF1 translocating compartment could thus be either early or late endosomes. To either mature into or communicate with late endosomes early endosomes must use microtubules (Gruenberg and Maxfield, 1995). Inhibition of microtubule polymerization with nocodazole blocks the transport of endocytosed ligands from early to late endosomes (Gruenberg and Maxfield, 1995). However, because the maturation of endosomal compartments in HEp-2 cells appears to be poorly microtubule dependent (van Deurs et al., 1993), we used MDCK and HeLa cells to test whether CNF1 activity was sensitive to nocodazole. As shown in Figure 6, CNF1 activity was totally blocked by nocodazole in MDCK and HeLa cells but not in HEp-2 cells. These results suggest that CNF1, unlike DT (Papini et al., 1993; Lemichez et al., 1997a), requires transport from the early to the late endocytic compartment to translocate its enzymatic activity to the cytosol.
Figure 5.
Effects of brefeldin A, bafilomycin A1, and monensin on CNF1-induced multinucleation in HEp-2 cells. Cells cultivated in 24-well plates were washed briefly with a HEPES-buffered medium and incubated with brefeldin A (5 μg/ml) for 1 h. This treatment resulted in the total disruption of the Golgi apparatus, as indicated by immunofluorescence labeling with the anti-Golgi mAb CTR433 (see MATERIALS AND METHODS). Cells were then incubated with CNF1 (10−10 M) for 90 min at 37°C still in the presence of brefeldin A. Then the medium was removed, and cells were washed and incubated with fresh medium containing bafilomycin A1 to block further entry of CNF1. Cells were incubated for an additional 24 h, and multinucleation was recorded. Identical experiments were performed with bafilomycin A1 and monensin. Effects of increased concentrations of NH4Cl on DT activity are given as controls. Results are the average of duplicate determinations.
Figure 6.
Effects of nocodazole on CNF1-induced cell multinucleation. HEp-2, MDCK, or HeLa cells grown in 24-well plates were washed briefly with HEPES-buffered medium and incubated with nocodazole for 1 h at 4°C. CNF1 (10−10 M) was added for 90 min at 37°C still in the presence of nocodazole. Then the medium was removed, and cells were incubated with fresh medium containing bafilomycin A1 to block further entry of CNF1. Cells were incubated for an additional 24 h in the presence of bafilomycin A1, and multinucleation was recorded and expressed as in Figure 5.
CNF1 Requires an Acidic pH-dependent Mechanism to Translocate across the Membrane
We and others have shown that the cellular effects of CNF1 can be antagonized by weak bases such as ammonium chloride (Falzano et al., 1993) and methylamine (Lacerda et al., 1997), suggesting that a low pH step is necessary for the toxin to penetrate into the cytosol. As shown in Figure 6, bafilomycin A1 and monensin, which block acidification of endosomal and lysosomal compartments, are strong inhibitors of CNF1 cellular activity. Although these results strongly suggest that CNF1 requires an acidic compartment to translocate its enzymatic domain into the cytosol, the possibility that inhibition of vesicular acidification might also affect the recycling of CNF1 receptors still remains.
An important difference between the entry of DT and CNF1 is their relative sensitivity to the concentration of NH4Cl required to their respective action. In the case of DT, 2 mM is required to completely block DT, whereas >5 mM is necessary to decrease the CNF1 multinucleating activity (Figure 6). One interpretation of these results is that CNF1, as indicated by data obtained with nocodazole, must reach a late endosomal compartment in which the pH is lower than that of early endosomes to deliver its catalytically active domain to the cytosol. Thus a higher concentration of NH4Cl would be necessary to inhibit the toxin activity.
To better understand the mechanism of CNF1–membrane translocation, we performed an experiment mimicking the possible transfer of CNF1 across the endosomal membrane of HEp-2 cells by an acidic pH. This type of experiment has clearly shown that DT could be translocated across a lipid membrane when enviromental pH is acidic (Draper and Simon, 1980; Sandvig and Olsnes, 1980; Moskaug et al., 1988; Sandvig and Olsnes, 1991). Taking advantage of the tight binding of CNF1 to its receptor, HEp-2 cells were incubated at 4°C for 4 h in the presence of the toxin and then thoroughly washed to remove all unbound molecules. Bafilomycin A1 was then added to monolayers to block CNF1 entry by its normal route, and cells were incubated for 10 min at different pH values ranging from 7 to 4. Monolayers were then incubated in medium containing bafilomycin A1 for an additional period of 3 h at 37°C. Cells were then detached from culture dishes and lysed. Lysates were ADP ribosylated by the Clostridium botulinum exoenzyme C3 with [32P]NAD to specifically label the Rho GTPase (Chardin et al., 1989). By catalyzing the deamidation of glutamine 63, CNF1 activity results in a mobility shift of Rho to a slightly higher molecular weight by SDS-PAGE (Flatau et al., 1997; Schmidt et al., 1997). As shown in Figure 7, when HEp-2 cells were exposed to CNF1 at 4°C, in the presence of bafilomycin A1, and then briefly exposed to pH 5.2 (and below this value), SDS-PAGE analysis of Rho revealed an upshift in electrophoretic mobility characteristic of deamidation. No shift of the GTPase could be observed at neutral pH, indicating that the entry of the toxin was efficiently blocked by bafilomycin A1. Accordingly, HEp-2 cells, into which CNF1 was introduced from the plasma membrane by a 10-min pulse at pH 5.2 (and below this value), demonstrated membrane ruffling and stress fiber formation typically induced by this toxin (our unpublished results). This result clearly indicates that CNF1 catalytic activity was transferred across the plasma membrane by a low-pH-driven mechanism, probably comparable with that of the DT catalytic domain to the cytosol.
Figure 7.
Direct transfer of CNF1 catalytic activity from the plasma membrane by a short exposure to an acidic pH. HEp-2 cells cultivated to confluence in six-well plates were briefly washed with cold 0.5% BSA-HEPES-buffered medium and incubated at 4°C for 4 h with 10−8 M CNF1 in the same medium. Monolayers were washed thoroughly to remove all unbound toxin molecules with cold PBS on ice. Then the cells were incubated for 10 min in the indicated pH buffers containing bafilomycin A1 to block CNF1 entry by its normal route. Monolayers were then briefly washed with cold medium and incubated in DMEM and 7% calf serum containing bafilomycin A1 for an additional 3 h at 37°C. Cells were then washed and lysed. Lysates were ADP ribosylated by the C. botulinum exoenzyme C3 with [32P]NAD as described in MATERIALS AND METHODS. Arrows indicate the Rho upshift (i.e., deamidation by CNF1). It was found that a 10-min period of endocytosis at pH 7.2 (in the absence of bafilomycin A1) of CNF1 did not allow the transfer of enough toxin molecules into the cytosol to upshift Rho (lane *). To obtain a positive control of CNF1 activity on Rho when CNF1 enters cells by its own system of endocytosis, HEp-2 cells were treated with CNF1 for 30 min at pH 7.2 before addition of bafilomycin A1 to allow a sufficient number of CNF1 molecules to act on Rho (lane **).
DISCUSSION
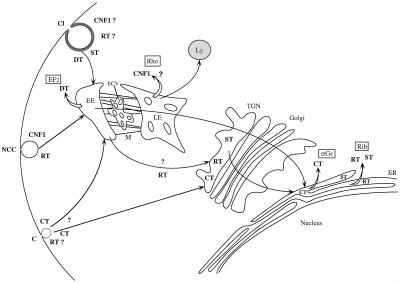
The molecular mechanisms by which enzymatic activities of bacterial toxins are delivered to the cell cytosol constitute an important topic in cell biology. Unraveling these processes has led to important clues on endocytic mechanisms such as the existence of a clathrin-independent pathway system (Montesano et al., 1982; Moya et al., 1985; Sandvig et al., 1987) or the existence of a retrograde transport of proteins across the Golgi apparatus (Sandvig et al., 1992; reviewed by Johannes and Goud, 1998). Furthermore, the mechanism of toxin translocation through the membrane is an important issue concerning the physical chemistry of macromolecules interacting with lipid membranes (London, 1992). In the present work we show that CNF1, a single-chain toxin, is endocytosed by a mechanism that does not depend strictly on both clathrin and caveolae. Once the toxin is transferred to an appropriate acidic compartment, most likely late endosomes, the catalytic activity is translocated across the lipid bilayer and into the cytosol by a low-pH-induced mechanism. CNF1 thereby appears to follow the RT model for endocytosis and the DT model for membrane translocation. We have summarized in Figure 8 the endocytic and intracellular routing and site of entry of CNF1 into the cytosol, with the entry of other well-studied toxins.
Figure 8.
Endocytosis and site of entry of CNF1. Comparison with others toxin. RT, ricin toxin; CT, cholera toxin; ST, Shiga toxin; DT, diphtheria toxin; CNF1, cytotoxic necrotizing factor 1; ER, endoplasmic reticulum; M, microtubules; ECV, endocytic carrier vesicles; EE, early endosome; LE, late endosome; Ly, lysosome; Cl, clathrin-coated invagination; C, caveolae invagination; NCC, non-clathrin noncaveolae endocytic invagination; EF2, elongation factor 2; aGs, heterotrimeric GTP-binding protein a component; Rib, 28S ribosome; Rho, Rho GTP-binding protein; TGN, trans-Golgi network. Open arrows indicate sites where toxins are supposed to be released into the cytosol. Boxed abbreviations indicate the intracellular targets of toxins.
We have shown by Schild analysis that CNF1 binds to HEp-2 to cell surface receptors with high affinity. Because of technical difficulties in radiolabeling CNF1 without an appreciable loss of toxic activity, we are, at present, unable to quantify the number of toxin receptors on the HEp-2 cell surface. The high affinity of CNF1 for its receptor is uncommon for a single-chain toxin. For example, the affinity between DT and its HB-epidermal growth factor receptor is approximately nanomolar (Brown et al., 1993). In contrast, enterotoxins such as cholera toxin and the heat-labile E. coli toxin, because of the oligomeric structure of their binding components, exhibit a very high affinity toward their cell surface receptors (MacKenzie et al., 1997). Such a high affinity might be required to firmly bind toxins to the epithelial surface, because in a liquid milieu the toxin would be effectively washed from the cell surface. Because CNF1 is almost exclusively produced by uropathogenic strains of E. coli (Blanco et al. 1996), this toxin might also be washed from the epithelial surface, and in this case a high receptor affinity might be necessary to compensate for the urine flow. The high affinity of CNF1 for its receptor may also be reflected in its action at very low concentration. Because there is no specific secretion mechanism that has been found for CNF1 in uropathogenic E. coli strains, the current hypothesis for its release into the medium is that a small proportion of the bacterial population could release the toxin upon lysis (Falzano et al., 1993).
The entry of CNF1 into eukaryotic cells, like RT, appears not to strictly require the clathrin-dependent pathway of endocytosis. However, we cannot exclude that the entry of a fraction of CNF1 molecules may use this pathway of endocytosis, as shown for RT (Moya et al., 1985; Sandvig et al., 1987). Indeed, as shown in the present work, CNF1 cellular effects could not be antagonized or clearly decreased in HEp-2 cells that expressed the dominant negative forms of proteins inhibiting the formation of coated vesicles (Eps15, dynamin, and intersectin); although the transferrin uptake was impaired in these cells, it could be argued that these dominant negative proteins do not completely block the entry of some CNF1 molecules by the clathrin-dependent pathway, thereby leading to intoxication. This is unlikely, because the CNF1 phenotype of transfected cells was identical to that of the nontransfected cells (Figure 3). Furthermore, in these experiments cells were exposed to CNF1 for an identical period, during which transferrin uptake was negligible (Figure 3). In addition, CNF1 activity was not affected by chlorpromazine, which is known to inhibit clathrin-dependent endocytosis (Wang et al., 1993) and to block DT toxicity (Orlandi and Fishman, 1998 and this work). These results suggest that the clathrin-dependent pathway of endocytosis may participate but is not the only route of internalization used for the uptake of CNF1 into HEp-2 cells.
The cholesterol-sequestering drug filipin has been shown to reduce the internalization of CT by ∼40% and to inhibit the activity of this toxin, probably by disruption of caveolae-like structures (Orlandi and Fishman, 1998). Filipin was not able to inhibit the CNF1-mediated multinucleation in HEp-2 cells. However, we must point out that filipin, in addition to decreasing the internalization of CT, was also shown to exhibit an inhibitory effect by acting on the reduction of the disulfide bridge linking the A1 catalytic subunit to the B cell-binding subunits of the toxin and thereby reducing its intracellular enzymatic activity (Orlandi and Fishman, 1998).
Experiments with brefeldin A demonstrated that CNF1, unlike RT, does not reach the Golgi apparatus and must be transferred to the cytosol upstream of this compartment.
CNF1 activity in MDCK and HeLa cells is sensitive to the microtubule-depolymerizing drug nocodazole. However, in HEp-2 cells CNF1 activity was not sensitive to nocodazole. In HEp-2 cells, ligands are transported by progressive maturation of endosomal compartments (van Deurs et al., 1993) rather than using endocytic carrier vesicles moving on microtubules between late and early endosomes (Gruenberg and Maxfield, 1995). We have shown with both MDCK and HeLa cells that CNF1 toxicity is sensitive to nocodazole, and thus the toxin appears to be transferred from an early to a late endocytic compartment to gain entry into the cytosol. This hypothesis is further substantiated by the requirement of relatively high concentrations of the weak base NH4Cl, compared with those that inhibit DT entry from early endosomes (Papini et al., 1993; Lemichez et al., 1997a), to neutralize CNF1 activities. However, we cannot rule out the possibility that nocodazole might reduce the number of CNF1 receptors at the surface of HeLa or MDCK cells.
Transfer of RT from early endosomes to the TGN and subsequent toxicity are blocked by expression of dyn-K44A (Llorente et al., 1998). Dynamin is most likely part of the machinery involved in vesicles formation that is located on endosomes and required for ricin to be transported to the TGN (Llorente et al., 1998). However, it is not yet clear whether RT is transferred directly from early endosomes to the TGN or if it transits by late endosomes (Simpson et al., 1995). CNF1 activity is not blocked by expression of dyn-K44A. Thus, if RT joins the TGN via late endosomes, CNF1 might use the same endocytic pathway as ricin to reach late endosomes but might escape into the cytosol from this compartment. This would explain why CNF1 is unsensitive to dyn-K44A. An alternative explanation is that a minor amount of ricin molecules is taken up by the dynamin-dependent mechanism from early endosomes and transferred to late endosomes, whereas CNF1 will not follow this pathway but rather the degradative pathway to proceed to late endosomes. It has been shown that ligands going to late endosomes for degradation into lysosomes are not sensitive to dyn-K44A effects (Damke et al., 1994; Llorente et al., 1998).
The transfer of the CNF1 deamidase activity to the cytosol can be induced directly across the plasma membrane of HEp-2 cells by a brief exposure to pH 5.2. This value is comparable with that required for the entry of DT fragment A to the cytosol from cell surface-bound toxin (5.5) (Moskaug et al., 1988). Although it is tempting to speculate that entry of CNF1 into the cytosol is directly analogous to that of DT, it is worth noting that a cleavage site that would release the catalytic domain of CNF1 into the cytosol has not been found. The fact that CNF1 might gain entry into the cytosol from the late endocytic compartment might suggest that a proteolytic cleavage could be accomplished by one or several protease(s) present in this prelysosomal cell compartment. Interestingly, Rho B, a target for CNF1, has been localized on the late endosomal compartment (Adamson et al., 1992). Although Rho B is a growth factor early induced protein (Jähner and Hunter, 1991), it might be the first intracellular target of CNF1 when the toxin translocates the membrane.
CNF1, like DT, seems to be divided into three functional domains involved in different steps of the intoxication process: the cell-binding domain is localized in the first 190 amino-terminal residues (Fabbri et al., 1999); two hydrophobic polypeptides (H1 and H2) with transmembrane properties are individualized immediately downstream of the binding domain (Falbo et al., 1993; Oswald et al., 1994); and the enzymatic deamidase activity is located within the last 300 residues of the toxin (Lemichez et al., 1997b; Schmidt et al., 1998). We would therefore hypothesize that the CNF1 hydrophobic helices H1 and H2 might play a role in CNF1 membrane translocation similar to that of the TH8 and TH9 hydrophobic helices of DT (Silverman et al., 1994).
In conclusion, CNF1 is the first microbial toxin described that is internalized by a clathrin- and caveolae-independent endocytic pathway and as DT is translocated across an endosomal membrane by a low-pH-dependent mechanism. CNF1 might be a useful probe for cell biological studies aimed at further understanding the link between the clathrin- and caveolae-independent pathways of endocytosis with endosomal compartments. This toxin might also be used to address the question of how a large hydrophilic protein can be transferred, via a low-pH-driven mechanism, through a lipid membrane.
ACKNOWLEDGMENTS
We thank Michel Gauthier (Institut National de la Santé et de la Recherche Médicale Unité 452), Ingo Just (Institut für Pharmakologie und Toxikologie, Universität Freiburg, Freiburg, Germany), John R. Murphy (Boston University Medical School, Boston, MA), Ellen Van Obberghen-Schillig (Centre National de la Recherche Scientifique, Center Antoine Lacassagne, Nice, France), and Cesare Montecucco (University of Padova, Padova, Italy) for scientific discussions and Aurore Grima (Institut Fédératif 50, Nice, France) for artwork. S.C. is recipient of a fellowship from the Ministère de l'Education Nationale de la Recherche et de la Technologie. This work was performed as partial fullfilment of a Ph.D. degree (to S.C.).
Abbreviations used:
- CNF1
cytotoxic necrotizing factor 1
- CT
cholera toxin
- DMEM
Dulbecco's modified Eagle's medium
- DT
diphtheria toxin
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- HA
influenza virus hemagglutinin epitope
- MDCK
Madin–Darby canine kidney
- RT
ricin toxin
- SH3
Src homology 3
- TGN
trans-Golgi network
REFERENCES
- Adamson P, Paterson HF, Hall A. Intracellular localization of the p21Rho proteins. J Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler Y, Barbas SM, Terlecky LJ, Tang K, Hardy S, Mostov KE, Schmid SL. Redundant and distinct function for dynamin-1 and dynamin-2 isoforms. J Cell Biol. 1998;143:1871–1881. doi: 10.1083/jcb.143.7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J Cell Sci. 1999;112:1303–1311. doi: 10.1242/jcs.112.9.1303. [DOI] [PubMed] [Google Scholar]
- Benmerah A, Lamaze C, Begue B, Schmid SL, Dautry-Varsat A, Cerf-Bensussan N. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J Cell Biol. 1998;140:1055–1062. doi: 10.1083/jcb.140.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S, Bayley H, Valeva A, Walev I, Walker B, Weller U, Kehoe M, Palmer M. Staphylococcal alpha-toxin, streptolysin-O and Escherichia coli hemolysin: prototypes of pore-forming bacterial cytolysins. Arch Microbiol. 1996;165:73–79. doi: 10.1007/s002030050300. [DOI] [PubMed] [Google Scholar]
- Blanco M, Blanco JE, Alonso MP, Blanco. J. Virulence factors and O groups of Escherichia coli isolates from patients with acute pyelonephritis, cystitis and asymptomatic bacteriuria. Eur J Epidemiol. 1996;12:191–198. doi: 10.1007/BF00145506. [DOI] [PubMed] [Google Scholar]
- Blum G, Falbo V, Caprioli A, Hacker J. Gene clusters encoding the cytotoxic necrotizing factor type 1, Prs-fimbriae and α-hemolysin from the pathogenicity island II of the uropathogenic Escherichia coli strain J96. FEMS Microbiol Lett. 1995;126:189–195. doi: 10.1111/j.1574-6968.1995.tb07415.x. [DOI] [PubMed] [Google Scholar]
- Boquet P, Fiorentini C. The cytotoxic necrotizing factor 1 (CNF1) from Escherichia coli. In: Aktories K, Just I, editors. Handbook of Experimental Pharmacology: Bacterial Protein Toxins. Berlin: Springer-Verlag; 2000. (in press). [Google Scholar]
- Boquet P, Gill DM. Modulation of cell function by ADP-ribosylating bacterial toxins. In: Alouf JE, Freer JH, editors. Sourcebook of Bacterial Protein Toxins. London: Academic Press; 1991. pp. 23–44. [Google Scholar]
- Boquet P, Pappenheimer AM., Jr Interaction of diphtheria toxin with mammalian cell membranes. J Biol Chem. 1976;251:5770–5778. [PubMed] [Google Scholar]
- Bornens M, Paintrand M, Berges J, Marty MC, Karsenti E. Structural and chemical characterization of isolated centrosomes. Cell Motil Cytoskeleton. 1987;8:238–249. doi: 10.1002/cm.970080305. [DOI] [PubMed] [Google Scholar]
- Brown JG, Almond BD, Naglich JG, Eidels L. Hypersensitivity to diphtheria toxin by mouse cells expressing both diphtheria receptor and CD9 antigen. Proc Natl Acad Sci USA. 1993;90:8184–8188. doi: 10.1073/pnas.90.17.8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone R, Fre S, Iannolo G, Belleudi F, Mancini P, Pellici PG, Torrisi MR, DI Fiore PP. eps15 and eps15R are essential components of the endocytic pathway. Cancer Res. 1997;57:5498–5504. [PubMed] [Google Scholar]
- Chardin P, Boquet P, Madaule P, Popoff MR, Rubin EJ, Gill DM. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P, McCormick F. Brefeldin A: the advantage of being uncompetitive. Cell. 1999;97:153–155. doi: 10.1016/s0092-8674(00)80724-2. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper RK, Simon MI. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980;87:849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri A, Gauthier M, Boquet P. The 5′ region of cnf1 harbours a translational regulatory mechanism for CNF1 synthesis and encodes the cell-binding domain of the toxin. Mol Microbiol. 1999;33:108–118. doi: 10.1046/j.1365-2958.1999.01453.x. [DOI] [PubMed] [Google Scholar]
- Falbo V, Pace T, Picci L, Pizzi E, Caprioli A. Isolation and nucleotide sequence of the gene encoding cytotoxic necrotizing factor 1 of Escherichia coli. Infect Immun. 1993;61:4909–4914. doi: 10.1128/iai.61.11.4909-4914.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzano L, Fiorentini C, Donelli G, Michel E, Kocks C, Cossart P, Cabanie L, Oswald E, Boquet P. Induction of phagocytic behavior in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol Microbiol. 1993;9:1247–1254. doi: 10.1111/j.1365-2958.1993.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Donelli G, Matarrese P, Fabbri A, Paradisi S, Boquet P. Escherichia coli cytotoxic necrotizing factor 1: evidence for induction of actin assembly by constitutive activation of the p21 Rho GTPase. Infect Immun. 1995;63:3936–3944. doi: 10.1128/iai.63.10.3936-3944.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald D, Morris RE, Salinger CB. Inhibition of the activity of Pseudomonas toxin by methylamine. Rev Infect Dis. 1983;5(suppl 5):S985–S991. doi: 10.1093/clinids/5.supplement_5.s985. [DOI] [PubMed] [Google Scholar]
- Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Hunt RC, Marshall-Carlson L. Internalization and recycling of transferrin and its receptor. Effect of trifluoroperazine on recycling in human erythroleukemic cells. J Biol Chem. 1986;261:3681–3686. [PubMed] [Google Scholar]
- Ittelson TR, Gill DM. Diphtheria toxin: specific competition for cell receptors. Nature. 1973;242:330–332. doi: 10.1038/242330b0. [DOI] [PubMed] [Google Scholar]
- Jähner D, Hunter T. The ras-related gene rhoB is an immediate-early gene inducible by v-Fps, epidermal growth factor, and platelet-derived growth factor in rat fibroblasts. Mol Cell Biol. 1991;11:3682–3690. doi: 10.1128/mcb.11.7.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes L, Goud B. Surfing on a retrograde wave: how does Shiga toxin reach the endoplasmic reticulum? Trends Cell Biol. 1998;8:158–162. doi: 10.1016/s0962-8924(97)01209-9. [DOI] [PubMed] [Google Scholar]
- Johannes L, Tenza D, Antony C, Goud B. Retrograde transport of KDEL-bearing B-fragment of Shiga toxin. J Biol Chem. 1997;272:19554–19561. doi: 10.1074/jbc.272.31.19554. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda HM, Pullinger GD, Lax AJ, Rozengurt E. Cytotoxic necrotizing factor 1 from Escherichia coli and dermonecrotic toxin from Bordetella bronchiseptica induce p21 (rho)-dependent tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 cells. J Biol Chem. 1997;272:9587–9596. doi: 10.1074/jbc.272.14.9587. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Schmid SL. The emergence of clathrin-independent pinocytic pathway. Curr Opin Cell Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- Lemichez E, Bomsel M, Devilliers G, vanderSpek J, Murphy JR, Lukianov EV, Olsnes S, Boquet P. Membrane translocation of diphtheria toxin fragment A exploits early to late endosome trafficking machinery. Mol Microbiol. 1997a;23:445–457. doi: 10.1111/j.1365-2958.1997.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Lemichez E, Flatau G, Bruzzone M, Boquet P, Gauthier M. Molecular localization of the Escherichia coli cytotoxic necrotizing factor 1 cell-binding and catalytic domains. Mol Microbiol. 1997b;24:1061–1070. doi: 10.1046/j.1365-2958.1997.4151781.x. [DOI] [PubMed] [Google Scholar]
- Llorente A, Rapak A, Schmid SL, van Deurs B, Sandvig K. Expression of mutant dynamin inhibits toxicity and transport of endocytosed ricin to the Golgi apparatus. J Cell Biol. 1998;140:553–563. doi: 10.1083/jcb.140.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E. How bacterial protein toxins enter cells: the role of partial unfolding in membrane translocation. Mol Microbiol. 1992;6:3277–3282. doi: 10.1111/j.1365-2958.1992.tb02195.x. [DOI] [PubMed] [Google Scholar]
- Lord JM, Roberts LM. Toxin entry: retrograde transport through the secretory pathway. J Cell Biol. 1998;140:733–736. doi: 10.1083/jcb.140.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D, David V, Lark D, Schoolnick G, Falkow S. Gene clusters governing the production of hemolysin and mannose-resistant hemagglutination are closely linked in Escherichia coli serotype O4 and O6 isolates from urinary tract infections. Infect Immun. 1984;43:353–358. doi: 10.1128/iai.43.1.353-358.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie CR, Hirama T, Lee KK, Altman E, Young NM. Quantitative analysis of bacterial toxin affinity and specificity for glycolipid receptors by surface plasmon resonance. J Biol Chem. 1997;272:5533–5538. doi: 10.1074/jbc.272.9.5533. [DOI] [PubMed] [Google Scholar]
- Mellman I. Membranes and sorting. Curr Opin Cell Biol. 1996;8:497–498. doi: 10.1016/s0955-0674(96)80026-3. [DOI] [PubMed] [Google Scholar]
- Montecucco C. Protein toxins and membrane transport. Curr Opin Cell Biol. 1998;100:530–536. doi: 10.1016/s0955-0674(98)80069-0. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Papini E, Schiavo G. Bacterial protein toxins penetrate cells via a four-step mechanism. FEBS Lett. 1994;346:92–98. doi: 10.1016/0014-5793(94)00449-8. [DOI] [PubMed] [Google Scholar]
- Montesano R, Roth J, Robert A, Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982;296:651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- Moskaug JO, Sandvig K, Olsnes S. Low pH-induced release of diphtheria toxin A-fragment in Vero cells. Biochemical evidence for transfer to the cytosol. J Biol Chem. 1988;263:2518–2525. [PubMed] [Google Scholar]
- Moya M, Dautry-Varsat A, Goud B, Louvard D, Boquet P. Inhibition of coated pit formation in HEp-2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin toxin. J Cell Biol. 1985;101:548–559. doi: 10.1083/jcb.101.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi PA, Curran PK, Fishman PH. Brefeldin A blocks the response of cultured cells to cholera toxin. Implications for intracellular trafficking in toxin action. J Biol Chem. 1993;268:12010–12016. [PubMed] [Google Scholar]
- Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald E, Sugai M, Labigne A, Wu HC, Fiorentini C, Boquet P, O'Brien AD. Cytotoxic necrotizing factor 2 produced by virulent Escherichia coli modifies the small GTP-binding protein Rho involved in assembly of actin stress fibers. Proc Natl Acad Sci USA. 1994;91:3814–3818. doi: 10.1073/pnas.91.9.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini E, Rappuoli R, Murgia M, Montecucco C. Cell penetration of diphtheria toxin. Reduction of the interchain disulfide bridge is the rate-limiting step of translocation in the cytosol. J Biol Chem. 1993;268:1567–1574. [PubMed] [Google Scholar]
- Roos J, Kelly RB. Dap160, a neural-specific Eps15 homology and multiple SH3 domain-containing protein that interacts with Drosophila dynamin. J Biol Chem. 1998;273:19108–19119. doi: 10.1074/jbc.273.30.19108. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sandvig K, Garred O, Prydz K, Kozlov JV, Hansen SH, van Deurs B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature. 1992;358:510–512. doi: 10.1038/358510a0. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980;87:828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K, Olsnes S. Membrane translocation of diphtheria toxin. In: Alouf JE, Freer JH, editors. Sourcebook of Bacterial Protein Toxins. London: Academic Press; 1991. pp. 57–73. [Google Scholar]
- Sandvig K, Olsnes S, Petersen OW, van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987;105:679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K, van Deurs B. Endocytosis without clathrin. Trends Cell Biol. 1994;4:275–277. doi: 10.1016/0962-8924(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Schild HO. Drug antagonism and pAx. Pharmacol Rev. 1957;9:242–246. [PubMed] [Google Scholar]
- Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Selzer J, Lerm M, Aktories K. The Rho-deamidating cytotoxic necrotizing factor 1 from Escherichia coli possesses transglutaminase activity. Cysteine 866 and histidine 881 are essential for enzyme activity. J Biol Chem. 1998;273:13669–13674. doi: 10.1074/jbc.273.22.13669. [DOI] [PubMed] [Google Scholar]
- Sears CL, Kaper JB. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharam S, Chaudhary VK, Fitzgerald D, Pastan I. Increased cytotoxic activity of Pseudomonas exotoxin and two chimeric toxins ending in KDEL. J Biol Chem. 1991;266:17376–17381. [PubMed] [Google Scholar]
- Sengar AS, Wang W, Bishay J, Cohen S, Egan SE. The EH and SH3 domain Ese proteins regulate endocytosis by linking to dynamin and Eps15. EMBO J. 1999;18:1159–1171. doi: 10.1093/emboj/18.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzel L, Huynh PD, Jakes KS, Collier RJ, Finkelstein A. The Diphtheria toxin channel-forming T domain translocates its own NH2-terminal region across planar bilayers. J Gen Physiol. 1998;112:317–324. doi: 10.1085/jgp.112.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JA, Mindell JA, Zhan H, Finkelstein A, Shen WH, Collier RJ. Structure-function relationships in diphtheria toxin channels: determining a minimal channel-forming domain. J Membr Biol. 1994;137:17–28. doi: 10.1007/BF00234995. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simpson F, Hussain NK, Qualmann B, Kelly RB, Kay BK, McPherson PS, Schmid SL. SH3-domain-containing proteins function at distinct steps in clathrin-coated vesicle formation. Nat Cell Biol. 1999;1:119–124. doi: 10.1038/10091. [DOI] [PubMed] [Google Scholar]
- Simpson JC, Dascher C, Roberts LM, Lord JM, Balch WE. Ricin cytotoxicity is sensitive to recycling between the endoplasmic reticulum and the Golgi complex. J Biol Chem. 1995;270:20078–20083. doi: 10.1074/jbc.270.34.20078. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Swanson J. The endocytic activity of dendritic cells. J Exp Med. 1995;182:283–288. doi: 10.1084/jem.182.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous GJ, van Kerkhof P, van Meer G, Rijnboutt S, Stoorvogel W. Differential effects of brefeldin A on transport of secretory and lysosomal proteins. J Biol Chem. 1993;268:2341–2347. [PubMed] [Google Scholar]
- van der Bliek AM. Is dynamin a regulator motor or a master regulator? Trends Cell Biol. 1999;9:253–254. doi: 10.1016/s0962-8924(99)01591-3. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K, Hansen SH. Multivesicular bodies in HEp-2 cells are maturing endosomes. Eur J Cell Biol. 1993;61:208–224. [PubMed] [Google Scholar]
- van Deurs B, Sandvig K, Petersen OW, Olsnes S, Simons K, Griffiths G. Estimation of the amount of internalized ricin that reaches the trans-Golgi network. J Cell Biol. 1988;106:253–267. doi: 10.1083/jcb.106.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989;109:2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SA, Brown WJ. The morphology but not the function of lysosomes is altered by brefeldin A. J Cell Biol. 1992;119:273–285. doi: 10.1083/jcb.119.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamabhai M, Hoffman NG, Hardison NL, McPherson PS, Castagnoli L, Cesarini G, Kay BK. Intersectin, a novel adaptor protein with Eps 15 homology and five Src homology 3 domains. J Biol Chem. 1998;273:31401–31407. doi: 10.1074/jbc.273.47.31401. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M, Mekada E, Uchida T, Okada Y. One molecule of diphteria toxin fragment A introduced into a cell can kill the cell. Cell. 1978;15:245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]