Abstract
Nerve damage is a clinical hallmark of leprosy and a major source of patient morbidity. We investigated the possibility that human Schwann cells are susceptible to cell death through the activation of Toll-like receptor 2 (TLR2), a pattern recognition receptor of the innate immune system. TLR2 was detected on the surface of human Schwann cell line ST88-14 and on cultured primary human Schwann cells. Activation of the human Schwann cell line and primary human Schwann cell cultures with a TLR2 agonist, a synthetic lipopeptide comprising the N-terminal portion of the putative Mycobacterium leprae 19-kDa lipoprotein, triggered an increase in the number of apoptotic cells. The lipopeptide-induced apoptosis of Schwann cells could be blocked by an anti-TLR2 monoclonal antibody. Schwann cells in skin lesions from leprosy patients were found to express TLR2. It was possible to identify in the lesions Schwann cells that had undergone apoptosis in vivo. The ability of M. leprae ligands to induce the apoptosis of Schwann cells through TLR2 provides a mechanism by which activation of the innate immune response contributes to nerve injury in leprosy.
For patients with leprosy, nerve damage is a major cause of morbidity. Although antibiotic therapy can eliminate the pathogen, Mycobacterium leprae, therapy is often initiated after nerve damage has occurred. Furthermore, nerve damage can occur during the administration of therapy, in particular, during the reactive states of erythema nodosum leprosum and the reversal reaction.
In all forms of leprosy, M. leprae can be detected in nerves in active lesions (24). The M. leprae-Schwann cell interaction is a complex process, involving multiple bacterial ligands and cellular receptors (21). One initial target for the M. leprae interaction with peripheral nerves is laminin 2, located in the basal lamina of the Schwann cell axon unit (22). A specific glycolipid of M. leprae has been shown to mediate this interaction and hence determine the predilection of M. leprae for nerves (17). Other mycobacteria, including M. tuberculosis, M. chelonae, and M. smegmatis, exhibit laminin-binding capacity for adherence to Schwann cells (14). The colonization of Schwann cells by M. leprae also stimulates granuloma formation and cell-mediated nerve injury (28). However, damage to cutaneous nerves can also occur in the absence of immune cells (23). Therefore, study of the M. leprae-Schwann cell interaction is essential for understanding the mechanisms of nerve injury in leprosy.
The ability of the host to rapidly detect invading pathogens is an important feature of the innate immune system and is mediated in part by pattern recognition receptors that recognize various classes of microbial ligands. In particular, Toll-like receptor 2 (TLR2) has been shown to be involved in the recognition of mycobacterial lipoproteins (2, 5). Evidence implicates the Toll family of receptors as having an important role in host defense. The Toll family of receptors has been conserved throughout hundreds of millions of years of evolution, to include both insects and mammals. Mutation of toll in Drosophila dramatically increases susceptibility to fungal infections (13). Mice with spontaneous or targeted mutations in TLRs are more susceptible to bacterial infections, further implicating TLRs as critical receptors in mammalian host defense (4, 8, 15, 19, 29, 32). TLRs are required for the optimal induction of innate immunity in mouse models of microbial infection (31, 33). Although the activation of TLRs can contribute to host defense through the direct induction of antimicrobial responses (30) or activation of the adaptive immune response (16), the activation of TLRs can also lead to tissue injury, including the manifestations of septic shock (20) and the induction of apoptosis (9).
Previously, whole M. leprae was found to favor Schwann cell survival over apoptosis (23). However, the breakdown of M. leprae either before or during treatment results in the release of bacterial macromolecules that could activate TLRs, including lipoproteins that activate TLR2. In the present study, we sought to determine whether microbial lipopeptides can trigger Schwann cell apoptosis via TLR2 and hence contribute to nerve damage in leprosy.
MATERIALS AND METHODS
Cell line and culture.
The ST88-14 tumor cell line was established from malignant schwannomas (neurofibrosarcomas) from patients with neurofibromatosis type 1 (26) and was generously donated by J. A. Flechter (Dana Farber Cancer Institute, Boston, Mass.). The cells were grown in RPMI 1640 medium supplemented with 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM l-glutamine, and 15% fetal calf serum (HyClone, Logan, Utah) (culture medium) in a humidified CO2 incubator. The purity of Schwann cells was assessed by microscopic examination after immunostaining with antibodies to myelin-associated glycoprotein (MAG) and Ca2+ binding protein (S-100) as described below. For in vitro assays, ST88-14 cells were suspended in culture medium and cultured (7 × 104 cells/well) on 24-well plates (Falcon, Franklin Lakes, N.J.) at 37°C.
Primary human Schwann cell cultures.
Human Schwann cells were prepared from nerve explants from adult human donors as described previously (6). Briefly, nerve fragments were dissected free of connective tissue, cut into small (2- to 4-mm) fragments, and placed in culture medium with enzymes to dissociate the cells. Schwann cells were further purified to homogeneity by using fluorescence cell sorting with the aid of a Schwann cell-specific p75 (low-affinity nerve growth factor receptor) monoclonal antibody (MAb) (N. Tapinos and A. Rambukkana, submitted for publication). The purity of Schwann cells was evaluated by labeling with anti-p75 antibody, which revealed >95% p75-positive cells (see Fig. 3A). These highly purified Schwann cells were seeded on mouse laminin 1 (4 μg/ml) in phosphate-buffered saline (PBS) in 200-μl chamber slide wells and grown for 2 days prior to the experiments.
FIG. 3.
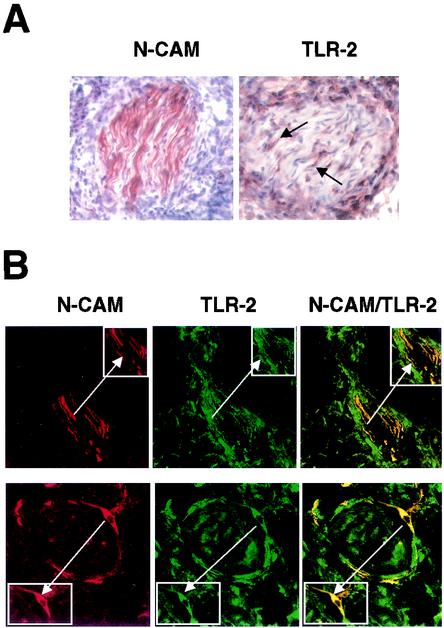
TLR2 is expressed in vivo on Schwann cells in leprosy lesions. (A) Representative sections of skin biopsy specimens from leprosy patients showing the expression of NCAM and TLR2. Arrows indicate cells with wavy nuclei, characteristic of nerve cells. (B) Two-color immunofluorescence staining of skin lesions from leprosy patients. Cryostat sections of skin biopsy specimens were stained with anti-NCAM (red, left panels) and anti-TLR2 (green, center panels) antibodies. The merge of the two images (right panels) showed colocalization of NCAM and TLR2. The presence of nerves in small biopsy specimens was variable. We analyzed almost 20 patients representing the spectrum of leprosy; the lesions shown were from a patient with erythema nodosum leprosum, a reaction in lepromatous patients. The insets duplicate and highlight the doubly positive cells typical of Schwann cells (arrows). Original magnification, ×630.
Patients and clinical specimens.
Patients with leprosy were classified according to the criteria of Ridley and Jopling (25). Scalpel or punch skin biopsy specimens (6-mm diameter) were obtained at the time of diagnosis from patients after they gave informed consent. The specimens were embedded in OCT medium (Ames, Elkhart, Ind.), snap-frozen in liquid nitrogen, and stored at −70°C until sectioned.
Antigens and antibodies.
The M. leprae 19-kDa lipopeptide was synthesized at the University of Texas Southwestern Medical Center (gift of Michael Norgard). The following antibodies were used for immunohistochemical studies: rabbit anti-S-100 (Zymed Laboratories, South San Francisco, Calif.), mouse anti-human MAG (Chemicon, Temecula, Calif.), mouse anti-human neural cell adhesion molecule (anti-human NCAM) (DAKO, Carpinteria, Calif.), mouse anti-human TLR2 (2392; Genentech, San Francisco, Calif.), immunoglobulin G (IgG) controls (Sigma, St. Louis, Mo.), goat anti-rabbit IgG (Caltag, Burlingame, Calif.), and horse anti-mouse IgG (Vector Laboratories, Burlingame, Calif.).
Measurement of cytokines.
Schwann cells were resuspended in culture medium and cultured (7 × 104 cells/well) on 24-well plates (Costar Corporation, Cambridge, Mass.) at 37°C. Human Schwann cell lines were stimulated with the 19-kDa lipopeptide (100 ng/ml) for 96 h. Cytokine levels (interleukin 1β [IL-1β] [BioSource, Camarillo, Calif.] and IL-6, IL-8, IL-12, and tumor necrosis factor alpha [TNF-α] [BD-Pharmingen, San Diego, Calif.]) in the culture supernatants were measured by using enzyme-linked immunosorbent assay (ELISA) kits according to manufacturer instructions.
Immunoperoxidase labeling.
NCAM and TLR2 expression in leprosy lesions was determined by immunoperoxidase labeling of cryostat sections of skin biopsy specimens by using anti-human NCAM, anti-human TLR2, or an isotype control antibody. Cryostat sections (3 to 4 μm) were acetone fixed for 10 min and blocked with normal serum before incubation with the antibodies for 60 min, followed by biotinylated horse anti-mouse IgG for 30 min. Slides were washed with PBS between incubations. Primary antibodies were visualized with the ABC Elite system (Vector Laboratories, Burlingame, Calif.),which uses avidin and a biotin-peroxidase conjugate (ABC) for signal amplification. ABC was incubated for 30 min, followed by incubation with a substrate (3-amino-9-ethylcarbazole; Vector Laboratories) for 10 min. Slides were counterstained with hematoxylin and mounted in aqueous/dry mounting medium (Crystal Mount; Biomeda, Foster City, Calif.).
Immunofluoresence labeling.
Schwann cells were cultured on 24-well plates containing glass coverslips covered with 4% silane (Sigma). The samples were washed (three times for 10 min each time) with PBS and fixed in 4% paraformaldehyde. After the samples were washed (three times for 10 min each time) with PBS, they were incubated with primary antibodies (anti-S-100 and anti-human MAG). Secondary antibodies (fluorescein isothiocyanate [FITC]- goat anti-rabbit IgG and FITC-goat anti-mouse IgG) were added, and the mixture was incubated for 30 min at room temperature. Coverslips were mounted with Vectashield mounting medium (Vector Laboratories).
Double immunofluorescence of leprosy lesions.
Two-color immunofluorescence staining was performed by serial incubation of sections with mouse anti-human NCAM (IgG2a) MAb followed by incubation with an isotype-specific fluorochrome conjugated MAb (goat anti-mouse IgG2a-specific tetramethyl rhodamine isothiocyanate [TRITC]; Southern Biotechnology Associates, Birmingham, Ala.). Sections were washed and incubated with mouse anti-human TLR2 (IgG1) followed by goat anti-mouse IgG1-specific FITC (CalTag).
Confocal microscopy.
Immunofluorescence sections were examined with a Leica-TCS-SP inverted confocal laser scanning microscope fitted with krypton and argon lasers. Sections and cells were illuminated with 488 and 568 nm of light after filtering through an acoustic optical device. Images decorated with FITC and TRITC were recorded simultaneously through separate optical detectors with a 530-nm band-pass filter and a 590-nm long-pass filter, respectively. Pairs of images were superimposed for colocalization analysis.
Flow cytometry.
To assess the cell surface expression of TLR2, standard flow cytometric analysis was performed. Cells were harvested and blocked with human serum for 1 min at 25°C to reduce nonspecific FcR binding. Schwann cells were stained with MAbs to TLR2, major histocompatibility complex class I (MHC-I), and MHC-II for 20 min at 4°C. Cells were washed twice and incubated with phycoerythrin-labeled goat anti-mouse IgG for 20 min at 4°C. After staining, cells were washed and fixed in 2% paraformaldehyde before analysis on a Becton Dickinson (Mountain View, Calif.) FacsScan or FacsCalibur flow cytometer. Gating was set on large granular cells, and 10,000 gated events were collected from each sample. Data were analyzed by using WinMDI 2.8 (Joseph Trotter, Scripps Research Institute, San Diego, Calif.).
Identification of apoptotic cells by Hoechst staining.
Nuclear fragmentation of Schwann cells was assessed as a hallmark of apoptosis. ST88-14 and primary Schwann cells were seeded on 24-well plates containing glass coverslips and incubated in the presence or absence of anti-human TLR2 blocking MAb (10 μg/ml) or isotype control antibody for 30 min. Subsequently, the 19-kDa lipopeptide (100 ng/ml, 20 μg/ml) or actinomycin D (ActD) (10 μg/ml) was added in a total volume of 0.5 ml/well, and the mixture was incubated for 24 h. Control cells were cultured in medium alone. Cells were washed with PBS, fixed in 4% paraformaldehyde, and stained with Hoechst dye 33258 (Sigma) for 15 min. Slides were allowed to air dry, and the nuclear morphology of cells was viewed under UV light exposure. For quantitative analysis, the percentage of nuclear fragmentation was calculated on the basis of the examination of at least 500 cells in multiple vision fields.
TUNEL assay.
Apoptotic cells in leprosy lesions were identified by using the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) method in accordance with supplier instructions (Boehringer Mannheim). Briefly, paraffin skin sections were dewaxed by heating at 60°C for 1 h, washed in xylene, and rehydrated through a graded series of ethanol. Slides were incubated in permeabilization solution (proteinase K [20 μg/ml] in 10 mM Tris-HCl) for 20 min at 37°C, followed by incubation with TUNEL reaction mixture for 60 min at 37°C. After labeling, samples were incubated with Converter-POD for 30 min at 37°C, followed by the addition of the substrate 3-amino-9-ethylcarbazole and incubation for 10 min. Slides were counterstained with hematoxylin and mounted in aqueous dry mounting medium (Crystal Mount). Negative controls included incubation with label solution instead of TUNEL reaction mixture.
Statistical analysis.
Student's t test was used to compare the numbers of apoptotic cells in the primary human Schwann cells and human Schwann cell lines. Differences between means were considered significant when P values were less than 0.05.
RESULTS
Schwann cell line ST88-14 expresses functional TLR2 and undergoes apoptosis in response to TLR2 ligation.
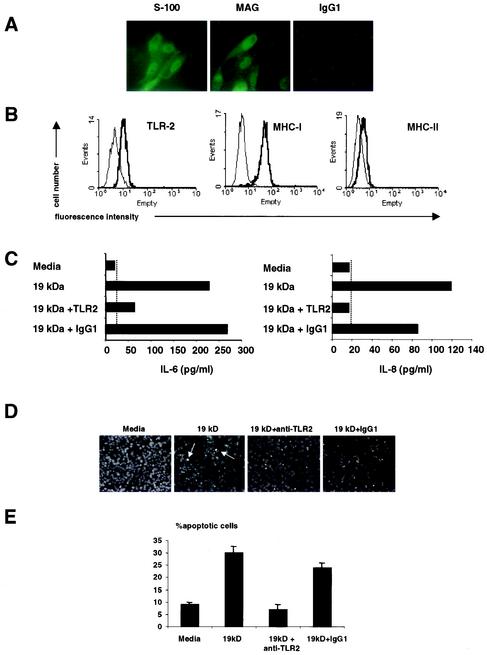
In order to develop an in vitro model to study nerve damage in leprosy caused by M. leprae, we used Schwann cell line ST88-14 (26). Immunohistochemical analysis confirmed that the cell line expressed S-100 and (MAG (Fig. 1A), markers compatible with Schwann cells. We measured TLR2 expression by flow cytometry and found that TLR2 was expressed at moderate levels in comparison to MHC-I but at higher levels in comparison to MHC-II (Fig. 1B). We detected no change in TLR2 expression after activation with a TLR2 ligand, the active N-terminal peptide of the M. leprae 19-kDa lipoprotein (S. R. Krutzik and R. L. Modlin, submitted for publication). These data indicate that Schwann cell line ST88-4 expresses TLR2 constitutively and thus that Schwann cells may be responsive to microbial pattern molecules.
FIG.1.
Expression of TLR2 and TLR2-induced apoptosis in a human Schwann cell line. (A) The human Schwann cell line expressed S-100 and MAG, markers compatible with Schwann cells. (B) Schwann cells were labeled with anti-TLR2, anti-MHC-I, and anti-MHC-II MAbs (thick lines) or isotype control antibodies (thin line), followed by phycoerythrin- or FITC-conjugated goat anti-mouse antibodies, and examined by flow cytometry. The results shown are representative of three independent experiments that gave similar results. (C) Schwann cells produced IL-6 and IL-8 in response to the 19-kDa lipopeptide. The ability of Schwann cells to release IL-6 and IL-8 in response to the 19-kDa lipopeptide was dependent on TLR2. Broken lines indicate the minimum level of detection of the cytokine ELISA. (D) The 19-kDa lipopeptide induced apoptosis in Schwann cells in a TLR2-dependent manner. Arrows indicate apoptotic cells. (E) Quantification of apoptotic cells. The nuclear morphology of apoptotic cells was assessed after Hoechst staining and exposure to UV light. The results from one representative experiment of three are shown. For quantitative analysis, percent nuclear fragmentation was calculated based on an examination of at least 500 target cells in multiple vision fields. The data are shown as mean and standard deviation.
To investigate the functional significance of TLR2 expression on the Schwann cell line in the context of inflammation, we first evaluated cytokine production in response to the 19-kDa lipopeptide of M. leprae. We detected very low levels of inflammatory cytokines (IL-1β, IL-12, and TNF-α were not detectable; IL-6 and IL-8 are shown in Fig. 1C) in response to the 19-kDa lipopeptide. Lipopeptide activation of IL-6 and IL-8 from the Schwann cell line was inhibited by antibodies to TLR2, demonstrating a functional TLR2 response in Schwann cells.
While evaluating their response to the 19-kDa lipopeptide, we were surprised to find that the normally adherent Schwann cells detached from cell culture plates. TLR2 ligation promotes apoptosis (3) of Schwann cells, thus, we evaluated apoptotic cell death by monitoring changes in nuclear morphology with Hoechst dye. Cells with condensed nuclei, characteristic of apoptosis, comprised 9% ± 0.8% (mean and standard error of the mean) of the cultures in the absence of the 19-kDa lipopeptide (Fig. 1D and E), but the frequency of apoptotic cells increased to 29% ± 2.6% in the presence of the 19-kDa lipopeptide. When cultures were incubated with an anti-TLR2 MAb prior to exposure to the lipopeptide, nuclear damage was diminished to 7% ± 2.1% (P < 0.05), whereas the frequency of apoptotic cells in the presence of an isotype control antibody was 24% ± 2.1% (P < 0.001). These data indicate that TLR2 ligation induces apoptosis in a human Schwann cell line.
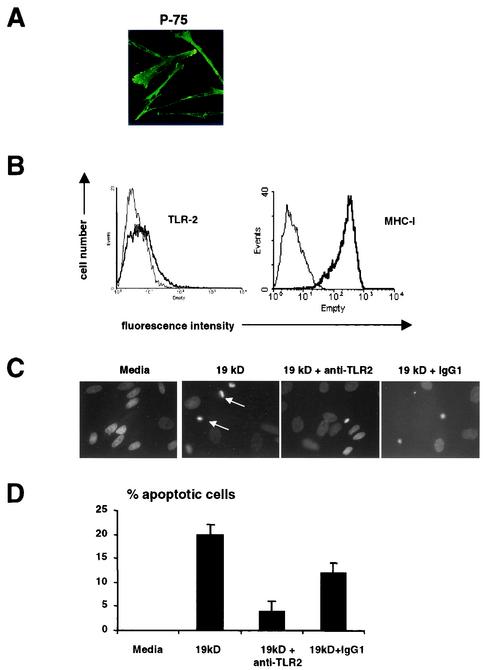
TLR2 activation on primary human Schwann cells induces apoptosis.
We next wanted to determine whether primary human Schwann cells expressed functional TLR2. Primary human Schwann cells isolated from nerves showed typical Schwann cell morphology when labeled with a MAb to Schwann cell-specific p75 (Fig. 2A). Primary Schwann cells also expressed TLR2 and high levels of MHC-I (Fig. 2B). To determine whether TLR2 might trigger apoptosis in primary Schwann cell cultures, we cultured the cells in the presence or absence of the 19-kDa lipopeptide and assessed morphologic changes characteristic of apoptosis by using Hoechst dye. Cells with nuclear fragmentation, indicative of apoptosis (Fig. 2C), were not detectable in cultures containing medium but increased to 20% ± 2.1% in the presence of the 19-kDa lipopeptide. Apoptosis was inhibited in the presence of an anti-TLR2 MAb, with 4% ± 2.0% (P < 0.001) of cells showing evidence of nuclear damage, whereas the frequency of apoptotic cells in the presence of an isotype control antibody was 12% ± 2.1% (P < 0.05) (Fig. 2D). These data indicate that the activation of TLR2 induces apoptosis in primary human Schwann cells.
FIG. 2.
Expression of TLR2 and TLR2-induced apoptosis in a primary human Schwann cell line. (A) Primary human Schwann cells isolated from nerve explants showed typical Schwann cell morphology when labeled with a MAb to Schwann cell-specific p75. (B) Primary Schwann cells expressed TLR2 and the antigen-presenting molecule MHC class I (MHC-I). Specific antibodies are indicated by thick lines; isotype control antibodies are indicated by thin lines. (C) The 19-kDa lipopeptide induced apoptosis in Schwann cells in a TLR2-dependent manner. (D) Quantification of apoptotic cells. The nuclear morphology of apoptotic cells was assessed after Hoechst staining and exposure to UV light. For quantitative analysis, percent nuclear fragmentation was calculated based on an examination of at least 100 target cells in multiple vision fields. The data are shown as mean and standard error of the mean. The data are from a single donor, since this experiment was performed to confirm the data from the cell lines.
Schwann cells in leprosy lesions express TLR2.
To determine whether Schwann cells in vivo express TLR2, skin biopsy specimens from leprosy patients were labeled with MAbs to NCAM, a Schwann cell marker, and TLR2. Although we analyzed almost 20 patients representing the clinical spectrum of leprosy, it was difficult to localize intact nerve cells in the skin. Therefore, we evaluated TLR2 expression in lesions where nerve cells were evident, regardless of the form of leprosy. The NCAM antibody identified cells containing wavy nuclei typical of nerve cells in all lesions (Fig. 3A, left panel). Cells with characteristic nerve morphology were also found to express TLR2 (Fig. 3A, right panel). Immunofluorescence staining for both TLR2 and NCAM demonstrated that NCAM-positive Schwann cells expressed TLR2 (Fig. 3B). The results obtained with isotype control antibodies were negative (data not shown). These data indicate that TLR2 is expressed on Schwann cells in vivo and therefore may be susceptible to apoptosis through TLR2 ligation.
Apoptosis of Schwann cells in leprosy tissues.
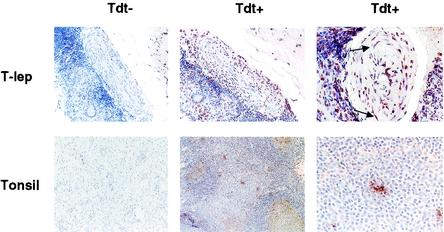
To determine whether TLR2 expression on Schwann cells in leprosy lesions correlated with apoptosis, DNA strand breaks were evaluated as indicative of cellular apoptosis. Nerve cells within the skin lesions of leprosy patients were stained positive by the TUNEL technique (Fig. 4), which detects DNA strand breaks. Lymphoid tissue (tonsil) was used as a control to demonstrate background levels of apoptosis. These data indicate that in leprosy, human Schwann cells undergo apoptosis in vivo, and this apoptosis correlates with the expression of TLR2 on Schwann cells in lesions.
FIG. 4.
Apoptotic Schwann cells in leprosy tissues. DNA strand breaks were detected on the basis of terminal deoxynucleotidyltransferase (Tdt) activity and were found in cells with a morphology typical of Schwann cells in leprosy tissues. Arrows indicate apoptosis-positive cells with wavy nuclei, characteristic of Schwann cells. Original magnifications, ×100 (left and middle panels) and ×400 (right panels). Tdt−, no enzyme added (negative control); Tdt+, Tdt enzyme added; T-lep, tuberculoid leprosy.
DISCUSSION
We investigated the contribution of TLR2, a pattern recognition receptor of the innate immune system, to nerve damage in leprosy. We found that TLR2 was expressed by a human Schwann cell line, by primary human Schwann cell cultures, and on Schwann cells in skin biopsy specimens from leprosy patients. The activation of Schwann cells with a lipopeptide comprising the functional N-terminal portion of the putative M. leprae 19-kDa lipoprotein triggered nuclear apoptosis. Schwann cells in leprosy lesions were also found to have undergone nuclear apoptosis. These data suggest that the activation of TLR2 on Schwann cells contributes to nerve damage in leprosy.
Several mechanisms of Schwann cell injury have been thought to contribute to nerve damage in leprosy. Previously, Spierings et al. provided striking evidence that human Schwann cells process and present M. leprae antigen to CD4+ T cells in an MHC-II-restricted manner (28). The ability of these T cells to kill antigen-pulsed Schwann cells provides a mechanism by which cell-mediated immunity to M. leprae can contribute to nerve injury in leprosy. The release of TNF-α locally in leprosy lesions and the binding of TNF-α to its receptors on Schwann cells may also contribute to peripheral nerve injury in leprosy (12, 27, 34). Other cytokines, including IL-1β and gamma interferon, can also induce apoptosis in cultured Schwann cells (7). Nerve damage can also occur in the absence of apoptosis or lysis because of demyelination upon exposure to M. leprae in the absence of immune cells (23).
Here we found that human Schwann cells express TLR2 and that activation of TLR2 can lead to apoptosis. Recent studies showed that bacterial lipoproteins induce the apoptosis of monocytes through a TLR2-dependent pathway (2). Although we measured TLR2-dependent IL-6 and IL-8 production from Schwann cells, it is not clear at present whether inflammatory cytokines are involved in TLR2-mediated apoptosis. We also found that TLR2-positive Schwann cells in leprosy lesions undergo apoptosis, potentially contributing to nerve damage in leprosy. These findings do not exclude the contribution of other mechanisms, including T cells or TNF-α, to apoptosis in leprosy; rather, they allow for an additional pathway of nerve damage.
Although the induction of apoptosis following the activation of TLR2 may lead to tissue injury, apoptosis can also be a beneficial component of the host response to the pathogen. First, apoptosis, like lysis, can lead to the release of intracellular bacteria, allowing their uptake by freshly activated macrophages, which can dispose of them (11). Second, apoptosis can lead to the release of antigen, which can be taken up and presented, via MHC-I and MHC-II molecules, by professional antigen-presenting cells (1, 10). Although Schwann cells can express MHC-II molecules and present antigen to T cells (28), the activation of TLR2 in monocytes has been shown to downregulate MHC-II expression and presentation (18). Further studies are required to determine whether TLR activation on Schwann cells directly affects antigen presentation by Schwann cells or, through apoptosis, enhances presentation by professional antigen-presenting cells. The fact that whole M. leprae does not induce apoptosis (23) suggests that the bacteria, when intact, can evade detection by this aspect of the innate immune system.
TLR2-induced apoptosis has been shown to involve a novel pathway requiring myeloid differentiation factor 88 and nuclear factor κB and involving Fas-associated death domain protein and caspase 8 (3). However, the mechanism of TLR-induced apoptosis of Schwann cells remains to be investigated. The use of TLR agonists as therapeutic agents must be carefully evaluated to achieve a balance in generating proinflammatory responses without tissue injury. On the other hand, TLR antagonists could be useful in preventing immunopathologic manifestations of the innate immune response to microbial infection.
Acknowledgments
We thank Michael Norgard for lipopeptide; Patrick Wood, Linda White, Nikos Tapinos, and Clare Eastby for help in generating primary human Schwann cells; and the UCLA Human Tissue Research Center for preparing paraffin-embedded slides.
This work was supported in part by NIH grants (AI07126, AI22553, and AI47866), a grant from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) (to R.L.M.), and NIH grant AI45816 (to A.R.).
Editor: J. M. Mansfield
REFERENCES
- 1.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R.-B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 3.Aliprantis, A. O., R. B. Yang, D. S. Weiss, P. Godowski, and A. Zychlinsky. 2000. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 19:3325-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, G. W. J., and J. V. Osterman. 1980. Host defenses in experimental rickettsial pox: genetics of natural resistance to infection. Infect. Immun. 28:132-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 6.Casella, G. T., R. P. Bunge, and P. M. Wood. 1996. Improved method for harvesting human Schwann cells from mature peripheral nerve and expansion in vitro. Glia 17:327-338. [DOI] [PubMed] [Google Scholar]
- 7.Conti, G., A. De Pol, E. Scarpini, F. Vaccina, M. De Riz, P. Baron, M. Tiriticco, and G. Scarlato. 2002. Interleukin-1 beta and interferon-gamma induce proliferation and apoptosis in cultured Schwann cells. J. Neuroimmunol. 124:29-35. [DOI] [PubMed] [Google Scholar]
- 8.Cross, A., L. Asher, M. Seguin, L. Yuan, N. Kelly, C. Hammack, J. Sadoff, and P. J. Gemski. 1995. The importance of a lipopolysaccharide-initiated, cytokine-mediated host defense mechanism in mice against extraintestinally invasive Escherichia coli. J. Clin. Investig. 96:676-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 10.Inaba, K., S. Turley, F. Yamaide, T. Iyoda, K. Mahnke, M. Inaba, M. Pack, M. Subklewe, B. Sauter, D. Sheff, M. Albert, N. Bhardwaj, I. Mellman, and R. M. Steinman. 1998. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 188:2163-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann, S. H. E. 1988. CD8+ T lymphocytes in intracellular microbial infections. Immunol. Today 9:168-174. [DOI] [PubMed] [Google Scholar]
- 12.Khanolkar-Young, S., N. Rayment, P. M. Brickell, D. R. Katz, S. Vinayakumar, M. J. Colston, and D. N. Lockwood. 1995. Tumour necrosis factor-alpha (TNF-alpha) synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions. Clin. Exp. Immunol. 99:196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 14.Marques, M. A., n. Ant, V., E. N. Sarno, P. J. Brennan, and M. C. Pessolani. 2001. Binding of alpha2-laminins by pathogenic and non-pathogenic mycobacteria and adherence to Schwann cells. J. Med. Microbiol. 50:23-28. [DOI] [PubMed] [Google Scholar]
- 15.Medina, E., and R. J. North. 1998. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology 93:270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medzhitov, R., P. Preston-Hurlburt, and C. A. J. Janeway. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 17.Ng, V., G. Zanazzi, R. Timpl, J. F. Talts, J. L. Salzer, P. J. Brennan, and A. Rambukkana. 2000. Role of the cell wall phenolic glycolipid-1 in the peripheral nerve predilection of Mycobacterium leprae. Cell 103:511-524. [DOI] [PubMed] [Google Scholar]
- 18.Noss, E. H., R. K. Pai, T. J. Sellati, J. D. Radolf, J. Belisle, D. T. Golenbock, W. H. Boom, and C. V. Harding. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167:910-918. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien, A. D., D. L. Rosenstreich, I. Scher, G. H. Campbell, R. P. MacDermott, and S. B. Formal. 1980. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J. Immunol. 124:20-24. [PubMed] [Google Scholar]
- 20.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 21.Rambukkana, A. 2001. Molecular basis for the peripheral nerve predilection of Mycobacterium leprae. Curr. Opin. Microbiol. 4:21-27. [DOI] [PubMed] [Google Scholar]
- 22.Rambukkana, A., J. L. Salzer, P. D. Yurchenco, and E. I. Tuomanen. 1997. Neural targeting of Mycobacterium leprae mediated by the G domain of the laminin-alpha2 chain. Cell 88:811-821. [DOI] [PubMed] [Google Scholar]
- 23.Rambukkana, A., G. Zanazzi, N. Tapinos, and J. L. Salzer. 2002. Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science 296:927-931. [DOI] [PubMed] [Google Scholar]
- 24.Ridley, D. S. 1972. The pathogenesis of the early lesions in leprosy. J. Pathol. 111:191-206. [DOI] [PubMed] [Google Scholar]
- 25.Ridley, D. S., and W. H. Jopling. 1966. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. 34:255-273. [PubMed] [Google Scholar]
- 26.Ryan, J. J., K. A. Klein, T. J. Neuberger, J. A. Leftwich, E. H. Westin, S. Kauma, J. A. Fletcher, G. H. DeVries, and T. F. Huff. 1994. Role for the stem cell factor/KIT complex in Schwann cell neoplasia and mast cell proliferation associated with neurofibromatosis. J. Neurosci. Res. 37:415-432. [DOI] [PubMed] [Google Scholar]
- 27.Sarno, E. N., and M. C. Pessolani. 2001. Leprosy. Oldest and most feared disease. Lancet 358(Suppl.):S39. [DOI] [PubMed] [Google Scholar]
- 28.Spierings, E., T. de Boer, B. Wieles, L. B. Adams, E. Marani, and T. H. Ottenhoff. 2001. Mycobacterium leprae-specific, HLA class II-restricted killing of human Schwann cells by CD4+ Th1 cells: a novel immunopathogenic mechanism of nerve damage in leprosy. J. Immunol. 166:5883-5888. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 30.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 31.Wang, X., C. Moser, J. P. Louboutin, E. S. Lysenko, D. J. Weiner, J. N. Weiser, and J. M. Wilson. 2002. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J. Immunol. 168:810-815. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein, D. L., C. R. Lissner, R. N. Swanson, and A. D. O'Brien. 1986. Macrophage defect and inflammatory cell recruitment dysfunction in Salmonella susceptible C3H/HeJ mice. Cell. Immunol. 102:68-77. [DOI] [PubMed] [Google Scholar]
- 33.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]
- 34.Yamamura, M., X.-H. Wang, J. D. Ohmen, K. Uyemura, T. H. Rea, B. R. Bloom, and R. L. Modlin. 1992. Cytokine patterns of immunologically mediated tissue damage. J. Immunol. 149:1470-1475. [PubMed] [Google Scholar]