Abstract
The hemolytic Streptococcus pyogenes can use a variety of heme compounds as an iron source. In this study, we investigate hemoprotein utilization by S. pyogenes. We demonstrate that surface proteins contribute to the binding of hemoproteins to S. pyogenes. We identify an ABC transporter from the iron complex family named sia for streptococcal iron acquisition, which consists of a lipoprotein (siaA), membrane permease (siaB), and ATPase (siaC). The sia transporter is part of a highly conserved, iron regulated, 10-gene operon. SiaA, which was localized to the cell membrane, could specifically bind hemoglobin. The operon's first gene encodes a novel bacterial protein that bound hemoglobin, myoglobin, heme-albumin, and hemoglobin-haptoglobin (but not apo-haptoglobin) and therefore was named Shr, for streptococcal hemoprotein receptor. PhoZ fusion and Western blot analysis showed that Shr has a leader peptide and is found in both membrane-bound and soluble forms. An M1 SF370 strain with a polar mutation in shr was more resistant to streptonigrin and hydrogen peroxide, suggesting decreased iron uptake. The addition of hemoglobin to the culture medium increased cell resistance to hydrogen peroxide in SF370 but not in the mutant, implying the sia operon may be involved in hemoglobin-dependent resistance to oxidative stress. The shr mutant demonstrated reduced hemoglobin binding, though cell growth in iron-depleted medium supplemented with hemoglobin, whole blood, or ferric citrate was not affected, suggesting additional systems are involved in hemoglobin utilization. SiaA and Shr are the first hemoprotein receptors identified in S. pyogenes; their possible role in iron capture is discussed.
Iron deprivation hinders the attempts of pathogenic bacteria to colonize the human body, and bacteria that can use heme and hemoglobin can take advantage of a major iron-reservoir in their host environment. Consequently, mechanisms for iron acquisition from hemoproteins are very common among bacterial pathogens (19, 70) and often are found to be important for full virulence (1, 4, 24, 40, 61, 68).
Hemoprotein utilization is best understood in gram-negative bacteria, since most use TonB-dependent outer membrane receptors that directly bind to hemoproteins, extract, and transport the heme or the iron through the receptor cavity (19, 70). A few bacteria produce hemophores that remove the heme and convey it to surface receptors (8, 30, 31, 50). Surface or secreted proteases that can release heme from hemoglobin are produced by Vibrio vulnificus, pathogenic Escherichia coli, and Porphyromonas gingivalis (32, 38, 41). Heme or iron crosses the cytoplasmic membrane by binding protein-dependent transport systems (PBT), which consist of a periplasmic substrate-binding protein, membrane-spanning protein(s), and hydrophilic ATPase(s) that energize the transport process (19, 70). The proteins of heme-PBT systems share homology and sequence signatures with transporters of siderophores and vitamin B12; the most conserved components are the membrane permeases and the ATPases (28, 52).
In gram-positive bacteria, proteins, and genes involved in heme or hemoglobin utilization have been described only in Corynebacterium species (11, 53) and Streptococcus pneumoniae (4, 64, 65). The first bacterial heme oxygenase (HmuO), the enzyme that carries out heme oxidative cleavage, was discovered and characterized in Corynebacterium (53). Mutants in hmuO cannot utilize heme or hemoglobin to satisfy their iron needs. Complementation of Corynebacterium ulcerans hmuO mutants with the C. diphtheriae genomic library led to the discovery of a heme ABC transporter (HmuTUV) (11, 54). Disruption of hmuT, the binding protein, in C. diphtheriae had no effect on heme utilization, suggesting this bacterium may have additional heme uptake pathways. Direct evidence for the role of HmuTUV proteins came from the related bacterium C. ulcerans, in which a mutation in hmuT is significantly impaired in heme utilization (54).
S. pneumoniae can utilize heme and hemoglobin as an iron source (64). A surface heme-binding protein of about 43 kDa was described, but its physiological role has not been established (65). Recently, two pneumococcal operons piu and pia (formerly pit1 and pit2), encoding homologous ABC transporters, were identified (4, 5). Growth and iron transport assays indicated that the piu and pia transporters are required for the transport of inorganic iron, as well as for iron capture from hemoglobin. Significant defects in hemoglobin utilization were observed only when both loci where inactivated. Mutants in piu and/or pia transporters were also attenuated in a mouse pneumonia model, with the most dramatic effect observed in the double mutant strain (4).
The gram-positive pathogen S. pyogenes is capable of producing a diverse array of skin and mucus membrane infections, as well as aggressive deep tissue diseases and streptococcal toxic shock syndrome. Untreated streptococcal infections can lead to the serious complications of rheumatic fever and acute glomerulonephritis (10). The role of iron in the physiology and virulence of S. pyogenes is poorly understood. We have previously established that iron is necessary for growth of the S. pyogenes class I strain JRS4 (M6 serotype), and iron limitation results in multiple changes in protein production and secretion, including the secretion of the streptococcal plasmin receptor, Plr (13). Reduced iron availability also resulted in decreased transcription of the emm6.1 gene encoding the antiphagocytic M6 protein (36). Recent analysis revealed that in S. pyogenes cells grown at 40°C, iron restriction had a widespread effect on gene expression, including the induction of genes encoding a putative iron transporter, several hemolysins, a putative superoxide dismutase, a bacterioferritin homologue, and iron-dependent repressors (60). Unlike the other hemolysins, slo expression was downregulated under these conditions (60). Together, these results suggest that iron plays a significant role in the physiology of S. pyogenes and is used as an important regulatory cue by this bacterium.
Among the abundant iron complexes in the human body, hemolytic S. pyogenes could use the hemoglobin-haptoglobin complex, hemoglobin, myoglobin, heme-albumin, and catalase but not transferrin or lactoferrin (14, 18). The means by which S. pyogenes obtains iron from hemoproteins have not been defined. In the present study, we show that S. pyogenes can directly bind heme and hemoproteins to its surface. We have identified an ABC transporter (named siaABC for streptococcal iron acquisition) with homology to transporters that mediate the uptake of iron complexes and investigate its role in iron acquisition.
MATERIALS AND METHODS
Strains, media, and growth conditions.
S. pyogenes strains used in the present study are listed in Table 1. S. pyogenes was grown in Todd-Hewitt broth (TH; Difco Laboratories), TH with 0.2% yeast extract (THY), or TH medium with 10 mM Tris, adjusted to pH 6.9 prior to autoclaving (ZTH) (14). ZTH was depleted of iron by the addition of the chelating agent nitrilotriacetic acid (NTA; Sigma-Aldrich). Medium containing 10 mM NTA was supplemented with 0.55 mM MgCl2, MnCl2, CaCl2, and ZnCl2. S. pyogenes was grown statically at 37°C in screw-cap polypropylene tubes or Klett flasks. Defibrinated sheep blood was purchased from Colorado Serum Company (lot 2170) and horse serum from either Sigma (lot 61k8407) or Atlas Biologicals (lot H20919A). All glassware was washed by soaking for 30 min in chromic-sulfuric acid (Sulfuric Acid and Chromerge; Fisher Scientific) and rinsing with double-distilled water. The E. coli strains JM83, DH5α, and Top10 (Invitrogen) were used for cloning and gene expression. E. coli cells were grown in Luria broth (LB). When necessary, the antibiotic spectinomycin was used at 100 μg/ml, ampicillin was used at 100 μg/ml, and chloramphenicol was used at 15 μg/ml for E. coli and at 5 μg/ml for S. pyogenes. Human hemoglobin (Sigma) was prepared as a 10-mg/ml stock solution in phosphate-buffered saline (PBS) and filter sterilized. Heme (0.4 mg/ml; Sigma) was mixed with 4 mg of bovine serum albumin (BSA; Sigma)/ml in PBS and filter sterilized.
TABLE 1.
S. pyogenes strains used in this study
| Strain (M serotype) | Descriptiona (mo/day/yr) | Source or referenceb |
|---|---|---|
| JRS4 (M6) | 57 | |
| NZ131 (M49) | 58 | |
| SF370 (M1) | 16 | |
| ZE11 (M1) | SF370 shr::pCB2 | This study |
| GA02581 (M1) | Clinical isolate (5/5/94) | GaEIP |
| GA03198 (M11) | Clinical isolate (9/24/94) | GaEIP |
| GA04425 (M1) | Clinical isolate (2/6/95) | GaEIP |
| GA05982 (M1) | Clinical isolate (4/20/96) | GaEIP |
| GA06439 (M114) | Clinical isolate (10/14/96) | GaEIP |
| GA08067 (M12) | Clinical isolate (4/11/97) | GaEIP |
| GA07346 (M22) | Clinical isolate (8/20/97) | GaEIP |
| GA10156 (M75) | Clinical isolate (3/10/98) | GaEIP |
| GA10878 (M18) | Clinical isolate (11/16/98) | GaEIP |
Clinical strains were isolated from sterile sites of patients with S. pyogenes infections.
GaEIP, Georgia Emerging Infections Program.
Whole-cell binding assay.
Cells grown overnight on TH (S. pyogenes) or LB (E. coli) were harvested, washed, and resuspended in 20 mM phosphate buffer (pH 7.4) containing 0.14 M NaCl (PBS). Cell suspensions were each adjusted to an optical density at 600 nm (OD600) of 1, diluted in PBS, and applied to a nitrocellulose membrane (100 μl/dot, by using BioDot apparatus; Bio-Rad). Biotinylated hemoglobin-binding assays were done as previously described (62). Briefly, cell blots were blocked (with PBS, 0.0025% Tween 20, and 5% skim milk), rinsed, and incubated with 50 nM biotinylated hemoglobin (prepared according to manufacturer's protocol). Binding to hemoglobin was visualized with streptavidin conjugated to alkaline phosphatase (Roche). Heme-binding assays were performed as described by Mazoy and Lemos (35). In short, membranes were incubated for 5 h at room temperature with 10 μM heme (in double-distilled H2O) and then rinsed; the color was developed in dimethoxybenzidine buffer (7.11 mM o-dianisidine, 50 mM sodium citrate [pH 4.4], 0.067% hydrogen peroxide).
Binding assays with proteinase K.
Cells from overnight cultures were harvested, washed, resuspended in 1 ml of buffer (10 mM Tris [pH 8.0], 10 mM EDTA, 10 mM NaCl) containing 0.04 μg of proteinase K (Sigma), and incubated for 1 h at 37°C with periodic mixing. The protease inhibitor 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (Roche) was subsequently added (to a final concentration of 8.2 mM) to stop the reaction, and the cells were harvested and washed three times with 1.5 ml of saline containing 4.2 mM AEBSF. Cell suspensions were then adjusted to an OD600 of 1, applied to membranes, and tested for hemoglobin as described above.
DNA manipulations.
Cloning, plasmid construction, chromosomal DNA extraction, restriction analysis, DNA transformation, and Southern hybridization were performed by published methods (15, 43, 51). The primers used in the present study are listed in Table 2.
TABLE 2.
Primers used in this study
| Primer | Location | Sequence |
|---|---|---|
| 204Y-S | shr | 5′-TGAAGACGTTACTATTACCG-3′ |
| 204Y-A | shr | 5′-TCTACCTGATAACCACTCCA-3′ |
| 204X-S | spy1796 | 5′-AGCATTCCTTTGATATTGGT-3′ |
| 204X-A | spy1796 | 5′-GGCTTTTGACTGTCTTTTAC-3′ |
| 204A-Fwd | siaA | 5′-GCTATGATGCTGTTAAGCGTGTGG-3′ |
| 204A-Rev | siaA | 5′-TCTGGAATGGCATGAGCTGTTC-3′ |
| 204B-Fwd | siaB | 5′-GTTATTGCCTTATCCCTTGGTGG-3′ |
| 204B-Rev | siaB | 5′-TACTTCCAACCAGCAAGCGG-3′ |
| 204C-Fwd | siaC | 5′-ACTATTATGGGGGCAAATGG-3′ |
| 204C-Rev | siaC | 5′-GATCAAGGAGGTCTCTTGGTTG-3′ |
| 204-ORF1-A | spy1791 | 5′-CCCCAAACTGCAGAATTCCCAGATACATAACTGCA |
| 204-ORF5-S | spy1787 | 5′-ATTGCCAAGCAGGCAGAGACAGTGT-3′ |
| 204-ORF5-A | spy1787 | 5′-TCCAAGCCCGTTTGATCCTG-3′ |
| ORFX-3-Fwd | shr | 5′-GCCAGCATGTTAAAAACTGACTTAGCATCTG-3′ |
| CP-ShuA-S | siaA | 5′-CCCGGATCCCGGTCTAAAAAAGAC-3′ |
| CP-ShuA-A | siaA | 5′-CCCCTGCAGGTAAATCTGATGCTG-3′ |
| ORFX-DEL-S | shr | 5′-CCCGAATTCAAAAGATATCAATGGTAGCT-3′ |
| ORFX-DEL-A | shr | 5′-CCCGAATTCTGATTGAACTTCACATCTAA-3′ |
| Y-His-Fwd | shr | 5′-CCCCGAATTCAAATCACAAGAGCCTTTAGTT-3′ |
| Y-His-Rev | shr | 5′-CCCCGAATTCAAATCACAAGAGCCTTTAGTT-3′ |
| ShuAF | siaA | 5′-CCCCAACTCGAGGTGAATCAGCACCCTAAAAC-3′ |
| ShuAR | siaA | 5′-CCCCTTGAATTCTTACGGATGATCTCCCACG-3′ |
| Spec-F | aad9 | 5′-GTGAGGAGGATATATTTGAATACATACGAA-3′ |
| Spec-R | aad9 | 5′-GTCCATTCAATATTCTCTCCAAGATAACTAC-3′ |
| SRAR | recA | 5′-CTGATGCTACTGCCATAGCAG-3′ |
| SRAL | recA | 5′-GCGTTCAGGAGGTCTAGCTC-3′ |
| X-Pho-Fwd | shr | 5′-GGGGGAAGCTTATTGTGATGAATATGCAG-3′ |
| X-Pho-Rev | shr | 5′-GGGGAAGCTTACTGATTGACTAGCGTAAGGG-3′ |
PCR analysis of the sia locus in S. pyogenes isolates.
PCR analysis was done with S. pyogenes chromosomal DNA extracted from different strains as a template. The fragments shr-spy1796, spy1796-siaC, and siaC-spy1787 were amplified from each strain by using the primer pairs 204Y-S and 204X-A, 204X-S and 204C-Rev, and 204C-Fwd and 204-ORF5-A, respectively.
Construction of plasmids and strains. (i) Construction of SiaA expression vector (pSiaA-His).
siaA was amplified from the chromosome of SF370 without its leader peptide by using the primers ShuAF and ShuAR and cloned into XhoI and EcoRI sites in the plasmid pBAD/His (Invitrogen). The resulting plasmid, pSiaA-His, expresses the rSiaA (SiaA N-terminal fusion to His-Xpress epitope) from the arabinose-regulated promoter, PBAD (23). An enterokinase site exists downstream of the His-Xpress epitope.
(ii) Construction of SiaA under the P23 promoter (pCPSiaA).
A fragment that carries the siaA gene was amplified with CP-ShuA-S and CP-ShuA-A primers and was cloned into the BamHI and PstI sites of pOri23 (46). The resulting plasmid, pCPSiaA, expresses the native SiaA protein from the lactococcal P23 promoter.
(iii) Construction of Shr expression vector (pCB1).
shr was amplified from SF370 chromosome without its putative signal sequence by using the Y-His-Fwd and Y-His-Rev primers. The PCR product was cloned into the EcoRI site of pBAD/His (Invitrogen), resulting in pCB1, where the expression of an amino-terminal fusion of the His-Xpress epitope to Shr (rShr) is placed under control of the PBAD promoter. An enterokinase site exists downstream of the His-Xpress epitope.
(iv) Construction of the Shr-PhoZ fusion (pCW1).
A 550-bp fragment of 5′ shr was amplified with X-Pho-Fwd and X-Pho-Rev primers and cloned into the HindIII site of pDC123 (7), ligating a fragment containing the ribosome-binding site and the first 62 amino acids of Shr to phoZ coding sequence. This construct, pCW1, carries the in-frame fusion of the leader sequence of shr with phoZ, replacing PhoZ's native leader with that of Shr. Restriction enzyme analysis determined the insert orientation in the resulting clones. Plasmid pCW1R carries the shr fragment in the reverse orientation.
(v) Construction of ZE11 mutant strain.
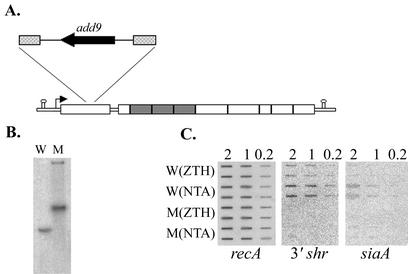
A 1,068-bp internal fragment of shr was amplified from SF370 by using the ORFX-DEL-S and ORFX-DEL-A primers and cloned into the EcoRI site of the vector pUCSpec, which carries the spectinomycin resistance gene, aad9 (26). The resulting construct, pCB2, was introduced into SF370 cells by electroporation (Bio-Rad Gene Pulser). Spectinomycin-resistant clones, resulting from the integration of pCB2 into the chromosome via homologous recombination, were selected by plating on TH agar plates containing spectinomycin. The structure of the chromosomal shr::pCB2 mutation was verified by PCR and Southern blot analysis (Fig. 6). The 204Y-S and Y-His-Rev primer set was used to amplify the shr fragment outside of the insertion from the wild type and from the mutant strain (ZE11), and the 204Y-S and Spec-R primer set was used to verify the formation of the chromosome-pCB2 junction in the mutant strain. The Southern blot analysis was done with both an aad9 specific probe, amplified with the Spec-F and Spec-R primers, and an shr probe, amplified with the ORFX-DEL-S and ORFX-DEL-A primers.
FIG. 6.
Construction and analysis of an insertion duplication mutant in the sia operon. (A) Schematic presentation of the shr::pCB2 mutation. The insertion site and the structure of plasmid pCB2 are indicated. The dashed boxes indicate the internal shr fragment cloned into pCB2. (B) Southern blot analysis. Chromosomal DNA of the parent strain SF370 (W) and the mutant strain ZE11 (M) was digested with BglII and hybridized with a [α-32P]dATP-labeled probe specific to the shr gene. (C) RNA slot blot analysis of sia genes in both the wild-type (W) and the mutant (M) strains. The same as in Fig. 3C, RNA was extracted from mid-log-phase SF370 grown in complete medium (ZTH) or iron-restricted medium (NTA).
RNA methods. (i) RNA preparation.
S. pyogenes cells were harvested at the mid-log phase (40 Klett units) after the addition of 0.06% sodium azide. RNA was extracted by using the Fast RNA Kit-Blue and FastPrep FP120 (Bio 101, Inc.). RNA was treated with DNase I and quantified by measuring the absorbance at 260 nm, and its integrity was verified on agarose gel. PCR analysis done with 2 μg of RNA as a template was used to confirm the absence of DNA contamination.
(ii) RT-PCR analysis.
cDNA was generated with Superscript II reverse transcriptase (RT; Life Technologies) according to the manufacturer's recommendations by using 2 μg of total RNA as a template. The reactions were terminated by heat inactivation of the enzyme. The 204-ORF5-A and 204-ORF1-A primers were used to produce two cDNA molecules, and 1/10 of each RT reaction (2 μl) served as a template in the subsequent PCRs, which was done with the following primer pairs: 204Y-S and 204Y-A, 204X-S and 204X-A, 204A-Fwd and 204A-Rev, 204B-Fwd and 204B-Rev, 204C-Fwd and 204C-Rev, and 204C-Fwd and 204-ORF1-A.
(iii) RNA slot blot.
RNA was transferred to a Zeta probe membrane (Bio-Rad) as previously described (67) by using a BioDot SF apparatus (Bio-Rad) and was cross-linked by baking for 2 h at 80°C. The membrane reacted with probes generated by PCR amplifications and was labeled with [α-32P]dATP by random priming (Roche). Probes were made by using the following primer pairs: 204Y-S and 204Y-A for shr, 204X-S and 204X-A for spy1796, 204A-Fwd and 204A-Rev for siaA, 204B-Fwd and 204B-Rev for siaB, 204C-Fwd and 204C-Rev for siaC, 204-ORF5-S and 204-ORF5-A for spy1787, and SRAR and SRAL for recA. PCR products were gel purified by using the Concert extraction system (Life Technologies).
Purification of rSiaA.
rSiaA was purified from Top10/pSiaA-His cells by using the HisTrap Kit (Amersham), according to the manufacturer's instructions. Expression was induced with 0.02% arabinose for 4 h. Cells were lysed by cycles of sonication, freezing, and thawing in the presence of a protease inhibitor cocktail (Sigma). After elution, rSiaA was concentrated by a Centricon spin column (10,000-molecular-weight cutoff) and quantified by Bradford assay (Bio-Rad). When digested with enterokinase, 20 μg of rSiaA was incubated with 1 U of EnterokinaseMax (Invitrogen) in 50 ml of EkMax buffer for 16 h at 37°C.
Purification of rShr.
rShr was purified from DH5α/pCB1 by using the Probond purification system (Invitrogen) according to the manufacturer's protocol. Protein expression was induced with 0.002% arabinose for 4 h. Cells were lysed in lysis buffer (6 M guanidine-HCl, 20 mM sodium phosphate, 500 mM sodium chloride; final pH 7.8) by sonication. rShr protein was eluted from the column by using the lysis buffer at pH 4.0. After elution, the lysis buffer was subsequently replaced with 10 mM Tris (pH 7.4) by using Centricon-10 concentrators (10,000-molecular-weight cutoff, Amicon). The protein concentration was measured by using the Bradford assay (Bio-Rad). When digested with enterokinase, 1.2 μg of rShr were incubated with 0.01 U of EnterokinaseMax (Invitrogen) in 50 μl of EkMax buffer for 16 h at room temperature.
PhoZ enzyme activity in cell-free culture supernatant.
Samples from SF370 cultures grown in TH to a density of 40 Klett units were obtained, and the cells were removed by centrifugation. Then, 200 μl of cell-free supernatant were incubated for 1.5 h with 800 μl of alkaline phosphatase substrate, 10% p-nitrophenyl phosphate in 1.0 M Tris-HCl (pH 8.03; Sigma). The absorbance was measured at 405 nm.
SDS-PAGE, Western blotting, and in vitro hemoprotein-binding assays.
Proteins were prepared in Laemmli sample buffer, separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE), and stained with Coomassie brilliant blue (29). For immunological analysis, proteins were transferred to a nitrocellulose membrane and reacted with the indicated antibody (6). For hemoprotein-binding assays, 3 μg of SiaA and 0.3 μg of Shr were resuspended in a sample buffer (65 mM Tris-HCl [pH 6.8], 10% glycerol, 0.03% bromophenol blue), fractionated at 4°C by SDS-PAGE without preboiling (17), and transferred to a nitrocellulose membrane. Membranes were blocked for 2 h, rinsed, and reacted with 50 nM biotinylated hemoproteins for 1 h. Binding was visualized with streptavidin-conjugated alkaline phosphatase (Roche).
Antiserum preparation.
The purified protein (rSiaA or rShr) was emulsified with Freund complete adjuvant (Sigma) and injected into New Zealand female rabbits. The rabbits were immunized with two booster injections (in Freund incomplete adjuvant) at 3 to 4 weeks after each injection. Antiserum was collected 1 week after each booster. This protocol was approved by the Georgia State University Institutional Animal Care and Use Committee.
Protein preparation from S. pyogenes cells and culture supernatant.
Protein preparation was done based on the method of Pancholi and Fischetti (42). Cells from overnight cultures were harvested, washed with saline, and resuspended in either PED (20 mM phosphate buffer, 5 mM EDTA, 10 mM dithiothreitol) for the preparation of total cellular proteins or in PERD (PED with 40% raffinose) for cellular fractionation. For total protein extract, cells were treated with the muralytic enzyme lysin for 1 h at 37°C to dissolve the cell wall and then boiled in sample buffer for 10 min. For cell fractionation, 20 mM MgCl2 (final concentration) was added to lysin-treated cells, and protoplasts were separated from the cell surface fraction by centrifugation, resuspended in PE (20 mM phosphate buffer, 5 mM EDTA), and lysed by successive rounds of flash freezing, thawing, and vortexing. Membrane proteins were separated from the soluble intracellular proteins by centrifugation and were dissolved by boiling in sample buffer. Proteins were prepared from culture supernatant by microfiltration by using a Centricon-10 (10-molecular-weight cutoff; Amicon) at 4°C. Proteins extracted from whole cells, different cellular fractions, or supernatant were standardized with respect to the cell number in the corresponding culture.
Streptonigrin sensitivity.
TH medium containing 1.6 μM streptonigrin was inoculated with cells from a glycerol stock (1:1,000 dilution) and incubated at 37°C. Cell growth was determined after overnight incubation.
β-Glucuronidase activity.
Plasmid pNZ276 is a shuttle vector that carries the gusA gene fused to the lacA promoter of L. lactis; this promoter is significantly expressed in S. pyogenes in the absence of lactose (15a). Enzyme activity associated with ZE11 cells harboring plasmid pNZ276 and in the corresponding supernatant was determined as previously described (15a). Briefly, cells from 6-ml portions of overnight cultures grown in THY were harvested, resuspended in 0.4 ml of lysis buffer, and lysed with a FastPrep cell disrupter (model EP120; Bio 101, Inc.). Then, 5 ml of cell-free supernatant was concentrated ca. 100-fold by using Centricon and Microcon (Amicon) YM10 centrifugal filter devices. Next, 0.1 ml of cell lysate and all of the concentrated culture supernatant were assayed for β-glucuronidase activity by placement in 0.9 ml of reaction buffer and adding 0.2 ml of 4 mg of p-nitrophenyl-β-d-glucuronidase/ml and incubation at 28°C until a yellow color developed in the samples from the whole cells. The reactions were stopped by the addition of 0.5 ml of 1 M Na2CO3, and the OD420 was determined. The enzyme activity was corrected for the size of the processed sample (1.5 ml of whole cells and 5 ml for supernatant), and the OD420 obtained with ZE11 was used to determine the background values. β-Glucuronidase activity (in units) is expressed as 1,000 times the increase in the OD420 per min per the OD600 of the original culture.
Hydrogen peroxide susceptibility.
Cells from overnight cultures were used to inoculate THY medium to an OD600 of 0.01 to 0.02 and then incubated at 37°C. Hydrogen peroxide was added (5 mM final concentration) to the culture at the mid-log phase of growth (OD600 0.3 to 0.45). Culture samples, taken immediately before and 30 min after the addition of hydrogen peroxide, were plated on THY plates to determine viable counts. For hemoglobin protection assays, cells were grown in THYB with 15 μM human hemoglobin. Cell growth is expressed as the fraction (percentage) of CFU/ml obtained after 30 min of incubation with hydrogen peroxide versus that obtained before treatment.
Computer analysis of nucleic and amino acid sequence.
Preliminary S. pyogenes sequence data was obtained from the streptococcal genome database (http://dna1.chem.ou.edu/strep.html) and from the NCBI microbial genome website (http://www.ncbi.nlm.nih.gov/PMGifs/Genomes/micr.html) when it became available later. The streptococcal numbers used in the present study represent the gene numbers in the SF370 database. The sequence data was analyzed and manipulated by using MacVector (International Biotechnologies) and Vector NTI (InforMax). BLAST searches of available nucleotide and protein databases and of incomplete microbial genomes were performed by using the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/blast). Cell blots were quantified by using the program Image Gauge version 3.0 (Fuji Film).
RESULTS
Heme compounds support S. pyogenes M1 SF370 growth in iron-depleted medium.
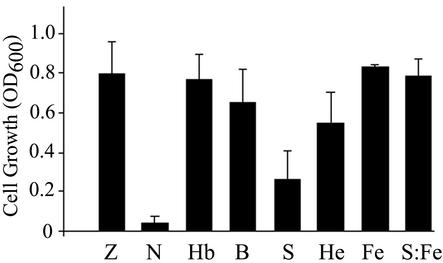
We previously reported that the chelating agent NTA inhibited the growth of S. pyogenes M6 strain JRS4 on complete medium (ZTH) and that growth inhibition was specifically reversed by the addition of iron and heme compounds such as hemoglobin (13, 14). In the present study, we used a similar method to characterize the growth of S. pyogenes strain SF370 (serotype M1) on iron-depleted medium; this strain was chosen since its genome sequence has been determined (16). Growth of SF370 in ZTH containing 10 mM NTA and a mixture of bivalent cations was inhibited (Fig. 1). The concentration that allows optimal bacterial growth under these conditions was found to be 22 μM hemoglobin, 6 μM heme-albumin, and 0.125% whole blood. Hemoglobin iron is the most abundant source of iron provided by whole blood (11 g/100 ml, for adult female sheep blood) (2). Addition of 0.125% whole blood provides ca. 2 μM hemoglobin, and it does not add significantly to the concentration of other metals such as Mg, Ca, Mn, and Zn, which are already present in concentrations of 0.5 mM in the ZTH-NTA medium. Therefore, streptococcal growth in the medium supplemented with blood is likely to result from the presence of hemoglobin and heme. Cell growth in 0.125% serum was only partial. It is likely that the inability of serum to support full streptococcal growth is the result of iron limitation rather than serum sensitivity since 10 mM ferric citrate restores full growth in the presence of NTA and serum.
FIG. 1.
Use of host iron sources by S. pyogenes strain SF370. Cells were used to inoculate (1:1,000 dilution from glycerol stocks) fresh ZTH medium (Z), ZTH medium containing 10 mM NTA (N), ZTH-NTA medium supplemented with 22 μM hemoglobin (Hb), 0.125% whole sheep blood (B), 0.125% horse serum (S), 6 μM heme-BSA (He), 10 mM ferric citrate (Fe), or 0.125% serum and 10 mM ferric citrate (S:Fe). Cell growth is expressed as the culture OD600 after overnight incubation (17 h). The results are from at least four separate experiments; the error bars indicate the standard deviation.
S. pyogenes produces surface proteins that directly bind hemoglobin and other hemoproteins.
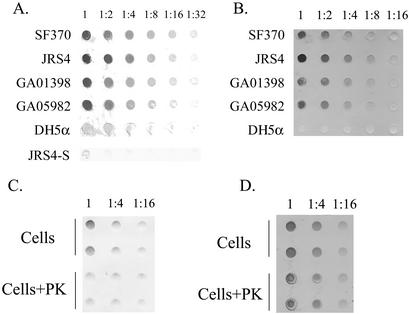
Since surface receptors are the most prevalent mechanism used by bacteria to capture iron from hemoproteins, we first sought to determine whether S. pyogenes could directly bind hemoglobin and free heme. Cells from SF370, JRS4, and nine clinical isolates (that represent seven different M serotypes; Table 1) were filtered onto a nitrocellulose membrane and tested for binding to biotinylated hemoglobin by using a streptavidin alkaline phosphatase reporter system (62). Each of the examined streptococcal strains bound hemoglobin in a cell concentration-dependent manner (Fig. 2A and data not shown). In the control, only very weak binding directly to streptavidin was observed (JRS4-S, in Fig. 2A), indicating that binding is specific to hemoglobin. In contrast to the S. pyogenes strains, the E. coli strain DH5α exhibited significantly lower binding to hemoglobin. Using the same method, we have also determined that Streptococcus binds heme-albumin and myoglobin (data not shown). Conversely, S. pyogenes did not bind transferrin and bound lactoferrin poorly, both of which cannot support its needs for iron (14). To test for heme binding, we used a method that is based on the intrinsic peroxidase activity of heme and was used several times to detect heme binding with whole cells or purified proteins (35). Cell blots were incubated with free heme (10 μm) and subsequently stained with the chromogenic substrate dimethoxybenzidine (DMB). All of the tested streptococcal strains bound heme, although to a different extent (Fig. 2B).
FIG. 2.
Hemoglobin and heme binding to S. pyogenes surface. (A) Binding to human hemoglobin by S. pyogenes isolates. Cell suspensions at an OD600 of 1 were immobilized onto a nitrocellulose membrane from a serial dilution. Binding to biotinylated hemoglobin was detected with streptavidin-conjugated alkaline phosphatase. Binding to S. pyogenes strains SF370, JRS4, GA01398, and GA05982 and to E. coli DH5α is shown. JRS4-S represents JRS4 cells treated only with streptavidin. (B) Heme binding to S. pyogenes surface. The results here are the same as in panel A except that the membranes were stained with the chromogenic substrate DMB. (C) Surface proteins mediate hemoglobin binding to S. pyogenes. SF370 cells were incubated for an hour in buffer (cells) or were treated with proteinase K (cells+PK) prior to membrane immobilization in duplicates. Hemoglobin binding was assayed as in panel A. (D) Nonprotein constituent(s) mediates most of the heme binding to S. pyogenes. The results here are the same as in panel C except that the membrane was stained with DMB.
To determine the nature of the streptococcal receptors for hemoproteins, we tested the binding sensitivity to protease treatment. Treatment of intact cells with proteinase K reduced hemoglobin binding by 60% ± 9% (n = 6, P < 0.01, Fig. 2C), and a similar reduction in heme-albumin binding was observed as well (data not shown). A small but statistically insignificant reduction in binding to heme was observed following the treatment (19 ± 3, n = 6, P < 0.2; Fig. 2D). These observations suggest that S. pyogenes produces surface proteins that bind hemoglobin and heme-albumin, whereas other constituents of the cell surface are responsible for most of the heme binding under the tested conditions.
Streptococcal iron acquisition (sia) transporter.
We initiated reverse genetics to identify streptococcal transporters that may be involved in iron acquisition from hemoproteins. A search of the streptococcal genome database and subsequent sequence analysis identified a three-gene transporter (spy1795, spy1794, and spy1793) with significant homology to a bacterial PBT system involved in iron complex uptake (Table 3). The transporter designated siaABC (for streptococcal iron acquisition) is distinct from the transporter for metal cations recently identified in S. pyogenes (27). The predicted protein sequence of SiaA (spy1795) shows homology to periplasmic binding proteins. The sequence of the first 20 amino acids has the characteristics of a lipoprotein signal with the LVAC sequence that conforms to the consensus sequence recognized by signal peptidase II, LX1X2C (where X1 is typically A, S, V, Q, or T, and X2 is typically G or A) (63). In addition, a region within the N terminus of SiaA contains the signature sequence of binding proteins from the iron-complex family (82-PDIELIASLKPTWILSPNS-101) (66). The deduced amino acid sequences of SiaB (spy1794) and SiaC (spy1793) have high homologies to permeases and ATPases (respectively) of heme- or siderophore-PBT systems. siaB encodes a very hydrophobic protein with a sequence in its C terminus (222-KTCNLLILDDQVIRHLGID ATALRLGISLVAVL LASVATS-261) that conforms to the conserved signature of membrane permeases of the siderophore and heme family (52). siaC codes to a hydrophilic ATP-binding protein, with Walker A (40-GANGSGKST-49) and Walker B (167-LDEPT-171) motifs and the typical ABC signature (144-LSGG-147).
TABLE 3.
Characteristics of the sia locus
| sia protein (mass [kDa], pI)a | Protein typeb | Homologue (organism) | DNA GenBank accession no. | % Similarity/ % identity | Function |
|---|---|---|---|---|---|
| Shr (144, 8.5) | Hemoprotein receptor | ||||
| Spy1796 (32, 8.4) | Unknown | NAe | |||
| SiaA (33, 5.8) | Binding protein | SirA (Staphylococcus aureus) | AF079518 | 37/18 | Siderophorec |
| HmuT (Corynebacterium diphtheriae) | AF109162 | 43/22 | Heme | ||
| HutB (Vibrio cholerae) | AF016580.1 | 35/17 | Heme | ||
| SiaB (36, 9.8) | Membrane | FhuG (Bacillus subtilis) | AJ223978 | 56/35 | Ferrichromec |
| Permease | HemU (Yersinia enterocolitica) | X77867 | 54/31 | Heme | |
| HutC (Vibrio cholerae) | AF016580.1 | 54/30 | Heme | ||
| SirB (Staphylococcus aureus) | AF079518 | 53/28 | Siderophorec | ||
| HutD (Vibrio cholerae) | AF016580.1 | 46/24 | Heme | ||
| SiaC (32, 6.4) | ATP binding | HmuV (Yersinia pestis) | U60647 | 53/32 | Heme |
| HemV (Yersinia enterocolitica) | X77867 | 49/28 | Heme | ||
| Spy1791 (66, 9) | Permease and ATP binding | CydD (H. influenzae) | U32749 | 48/28 | Cytochromec,d |
| HlyB (Escherichia coli) | AB011549 | 46/24 | Hemolysine | ||
| Spy1790 (63, 8) | Permease and ATP binding | CydC (Haemophilus influenzae) | U32795 | 46/26 | Cytochromec,d |
| LktB (Mannheimia haemolytica) | M24197 | 44/24 | Leukotoxin | ||
| APXIB (Actinobacillus pleuropneumoniae) | X68595 | 45/24 | RTX toxin | ||
| Spy1789 (22, 9.6) | Membrane | ||||
| Spy1788 (26, 10.3) | Membrane | CbiQ2 (Thermoanaerobacter tengcongensis) | AE013114 | Cobaltc | |
| Spy1787 (54, 6) | ATP binding | MTH454 (Methanobacterium thermautotrophicus) | AE000829 | Coenzyme M reductasec | |
| Cobaltc |
Calculated molecular masses and pI values are given in parentheses.
Based on sequence homology.
Putative.
Required for cytochrome synthesis.
NA, not applicable.
The siaABC transporter is part of a conserved 10-gene cluster.
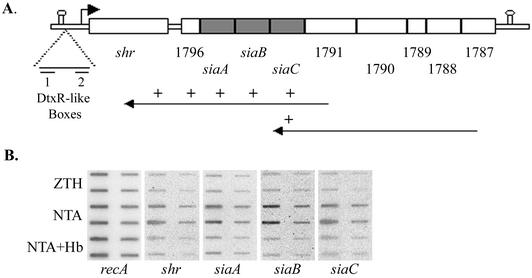
Sequence analysis suggests that siaABC genes are present as genes three through five in a 10-gene operon (Fig. 3A). The first gene, spy1798, later named shr, is separated from the second by 200 bp, whereas the other genes are very close or overlap. Potential promoter signals are found about 40 bp upstream of shr. A conserved ribosomal binding site is present in the immediate vicinity of all of the genes. Putative transcriptional termination signals are located about 400 bp upstream of shr (with ΔG of −11 kcal/mol) and 200 bp downstream of the last gene, spy1787 (ΔG of −20 kcal/mol) (Fig. 3A).
FIG. 3.
S. pyogenes iron acquisition (sia) locus. (A) The sia operon consists of 10 genes flanked by predicted transcription terminators (represented by stem-loops); the numbers below indicate the SF370 gene number, and the small arrow shows the location of the putative shr promoter. The long arrows represent the cDNAs produced for the RT-PCR analysis and the “+” symbols represent sia sequences found on the cDNAs. (B) RNA slot blots with RNA extracted from mid-log-phase SF370 grown in ZTH, ZTH and NTA (NTA), or ZTH with NTA and hemoglobin (NTA+Hb). We applied 2 and 0.4 μg of RNA from each sample to the membrane in duplicates.
S. pyogenes strains are known for their allelic variations and diversity in gene content. Primers generated on the basis of the sequence of shr, spy1796, siaC, and spy1787 were used in a series of PCRs to determine the presence and structure of the sia locus in SF370, JRS4, NZ131, and nine clinical isolates (see Materials and Methods and Table 2). The presence and organization of the sia locus was confirmed in all tested strains. Sequence analysis of the operon in the three streptococcal strains whose genome is available (SF370, MGAS8232, and MGAS315) revealed that the operon sequence is highly conserved. The amino acid residues of Shr, Spy1796, and SiaA are 99.3% identical in the three strains; 99.1% identity was found for SiaB, 98.6% identity was found for SiaC, 98.6% identity was found for Spy1791, 99.1% identity was found for Spy1790, 99.0% identity was found for Spy1789, 99.1% identity was found for Spy1788, and 96.9% identity was found for Spy1787. Spy1796 and SiaA overlap in SF370 and JRS4 (unpublished data) but appear to be in frame in the published sequences of MGAS8232 and MGAS315. It has yet to be determined whether this observation results from a sequencing error or represents a difference between the strains.
The sia locus is expressed as an iron-regulated operon.
The expression from the sia locus was investigated by RT-PCR analysis (Fig. 3A). RNA was extracted from cultures grown on iron-depleted medium, and two cDNA molecules were produced by using primers specific to the complementary strands of spy1791 and spy1787. Subsequent PCR analysis with primers for the coding regions of shr, spy1796, siaA, siaB, siaC, and spy1791 was used to determine the transcripts encoded by each of the cDNA products (see Materials and Methods and Table 2). This analysis revealed that cDNA produced with spy1791 primer codes to all genes upstream of spy1791 and that the cDNA produced with the spy1787 primer encompasses five genes and is extended to siaC. PCR performed directly on the mRNA without previous reverse transcription reaction did not result in any products, indicating that the RNA was not contaminated with chromosomal DNA. These observations suggest that the genes encoded by the sia locus are transcriptionally linked.
Negative regulation via an iron-responsive repressor is a common characteristic of iron uptake systems. We investigated the expression from the sia locus in response to iron by RNA slot blot analysis. The amounts of shr and each of the siaABC transcripts produced in cells grown in complete medium (ZTH) were compared to those produced in cells grown on iron-restricted medium (6 mM NTA) and to those grown on iron-restricted medium supplemented with hemoglobin (6 mM NTA + 8 μM Hb; Fig. 3B). All membranes were probed with a recA-specific probe to ensure that equal amounts of mRNA from each sample were loaded. Steady-state levels of transcripts from the sia locus were three to four times higher when cells were grown in iron-depleted medium than when cells were grown in complete medium. The addition of hemoglobin to the medium resulted in reduction in the NTA-induced expression from the operon, suggesting that the negative regulation of the operon is specific to iron.
SiaA is a hemoglobin-binding protein that is associated with the cell membrane.
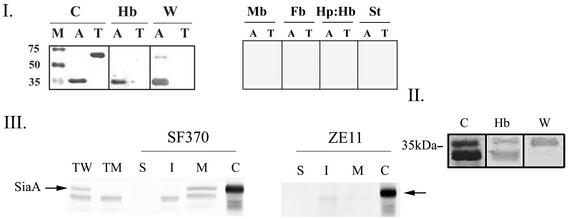
SiaA-N-terminal fusion to the His tag Xpress epitope (Invitrogen) was cloned under the control of PBAD, the arabinose promoter (23). The recombinant protein (rSiaA) was expressed and purified from E. coli extracts. A protein band in the predicted size of 35 kDa (SiaA with the addition of the epitope) that reacted with the anti-Xpress antibodies was observed, confirming the production and purification of rSiaA (Fig. 4I). In the absence of an outer membrane, substrate-binding proteins may function as surface receptors; the ability of C. diphtheria HmuT to bind heme and hemoglobin supports this notion (11). We sought to determine whether rSiaA, the binding protein of the siaABC transporter, could directly bind hemoglobin. A solid-phase binding assay with purified rSiaA fixed to a membrane demonstrated that it could bind hemoglobin (Fig. 4I). Transferrin was included as a control, as was biotinylated fibrinogen. Human transferrin did not bind hemoglobin, demonstrating that hemoglobin binding is specific to rSiaA, and rSiaA did not bind biotinylated fibrinogen or streptavidin alone (Fig. 4I). This finding indicates that rSiaA specifically binds hemoglobin and does not recognize the biotin group nor does it bind proteins nonspecifically. In addition to hemoglobin, other heme compounds can support growth of S. pyogenes in iron-depleted medium. Using the same binding assay, we determined that SiaA could bind neither myoglobin nor hemoglobin-haptoglobin complexes, indicating that the utilization of these heme compounds requires other proteins (Fig. 4I).
FIG. 4.
(I) Purified recombinant SiaA (rSiaA) binds hemoglobin in vitro. Purified rSiaA (His-Xpress-SiaA, A) and human transferrin (T) were fractionated by SDS-PAGE under seminative conditions. A Coomassie blue-stained gel (C), Western blot analysis with anti-express antibodies (W, 1:5,000 dilution), and binding blots to biotinylated hemoglobin (Hb), myoglobin (Mb), fibrinogen (Fb), haptoglobin-hemoglobin (Hp:Hb), or streptavidin (St)-conjugated alkaline phosphatase are presented. M, molecular weight marker. (II) rSiaA binding to hemoglobin is independent of the His-Xpress epitope. The results here are the same as in panel A, except the rSiaA was digested with enterokinase prior to analysis. Both the digested and the undigested rSiaA can be seen in the Coomassie blue stain and the hemoglobin blot. (III) SiaA is associated with the S. pyogenes envelope. Total protein extracts prepared from the wild-type SF370 (TW) and the mutant ZE11 (TM) were analyzed by Western blot analysis with anti-rSiaA serum (1:5,000 dilution). Proteins from various cell fractions of SF370 and ZE11 strains are included: S, surface proteins; I, intracellular proteins; and M, membrane proteins. Total protein extracts from whole S. pyogenes SF370/pCPSiaA cells expressing the native SiaA from P23 promoter is included as a positive control (C). The arrows indicate SiaA.
Histidine residues are common heme axial ligands in hemoproteins. Histidine residues conserved among heme/hemoprotein receptors were demonstrated to function in heme transport in the Yersinia enterocolitica HemR receptor (3); thus, we were concerned that the His-Xpress epitope could have contributed to hemoglobin binding. The His-Xpress epitope was removed by using enterokinase digest as indicated by the production of a predominant protein band smaller than rSiaA that did not react with the anti-Xpress antibodies (Fig. 4II). A less-abundant protein band representing intact rSiaA was also observed, indicating that the digest was not complete. Subsequent hemoglobin-binding assays showed that both rSiaA and rSiaA without the epitope bound hemoglobin, demonstrating that the binding ability of rSiaA is inherent to the native SiaA protein (Fig. 4II).
Sequence analysis suggests that the mature SiaA carries a lipid modification, which is likely to anchor it to the cell membrane. The in vivo production of SiaA and its cellular location were determined by using cell fractionation and Western blot analysis with anti-rSiaA antiserum. Proteins were prepared from different fractions of S. pyogenes cells, by using lysin, a bacteriophage muralytic enzyme (42). S. pyogenes cells expressing the native SiaA protein in trans from the P23 promoter (46) were used as a positive control. The rSiaA antiserum reacted with a protein band in the expected size found in both SF370 cells (TW, Fig. 4III) and in the positive control, SF370/pCPSiaA (C, Fig. 4III). A lower-molecular-weight band that cross-reacts with anti-SiaA was also found in the streptococcal protein preparations. In agreement with the predicted lipid modification, SiaA protein was found only in the membrane fraction of SF370 (M, Fig. 4III). As expected from the RNA analysis, iron restriction induced the production of SiaA (data not shown).
Shr is a hemoprotein binding receptor.
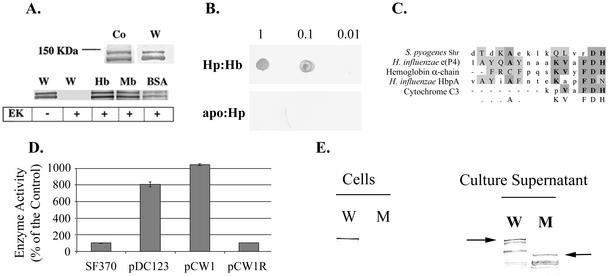
The first gene in the cluster, shr (for streptococcal hemoprotein receptor [spy1798]), encodes a large hydrophilic protein that has no significant homologues in other bacteria but shares partial homology with eukaryotic receptors such as Toll and G-protein-dependent receptors (gi 15675635 [GenBank]). Additional sequence analysis identified a domain with leucine-rich repeats and a heme-binding motif described by Reidl and Mekalanos (47) that is shared by both prokaryotic and eukaryotic heme-binding proteins (Fig. 5C).
FIG. 5.
(A) Purified rShr (His-Xpress-Shr) was fractionated by SDS-PAGE under seminative conditions. Coomassie blue-stained gel (Co), Western blot analysis with anti-Xpress antibodies (W, 1:5,000 dilution) and binding blots to biotinylated hemoglobin (Hb), myoglobin (Mb), and heme-BSA (BSA) are shown. The “+” sign indicates digestion of rShr with enterokinase prior to analysis. (B) rShr was spotted onto a membrane (1, 0.1, and 0.01 μg/spot) and reacted with biotinylated haptoglobin-hemoglobin (Hp:Hb) or apo-haptoglobin (apo:Hp). (C) Shr heme-binding motif. (D) PhoZ enzyme activity in cell-free culture supernatant of S. pyogenes harboring Shr-PhoZ fusion. Enzyme activity is expressed as a percentage of the background activity measured in SF370. Activity in cultures of cells harboring PhoZ expressed with its native leader peptide (pDC123, positive control), Shr-PhoZ fusion (pCW1), and Shr-PhoZ fusion in a reverse orientation (pCW1R, negative control) are shown. The mean and standard deviation of at least two independent experiments, each conducted in duplicates, are shown. (E) Western blot analysis of proteins prepared from SF370 (W) and ZE11 (M) strains with anti-rShr serum (1:5,000 dilution). Proteins prepared from cells and cell-free culture supernatant are included. The arrows point to the full-length Shr in wild-type and mutant strains.
The shr gene was cloned into pBAD-His vector (Invitrogen), and the recombinant protein (rShr) was expressed and purified from E. coli extracts. SDS-PAGE and Western blot analysis with the anti-Xpress antibodies confirmed the production of Shr (Fig. 5A). Partial degradation during protein purification was apparent, producing a smaller band. This cleavage took place at the C terminus of rShr, since the resulting bands reacted with the anti-Xpress antibodies. The His tag Xpress epitope was removed by enterokinase (as indicated by Western analysis), and the binding of Shr to biotinylated hemoproteins was investigated. Shr directly bound hemoglobin, myoglobin, heme albumin, and the hemoglobin-haptoglobin complex but not apo-haptoglobin or fibrinogen (Fig. 5A and B and data not shown). Together, these observations suggest that Shr specifically recognizes heme-containing proteins.
Amino acid sequence analysis suggested that Shr contains a leader peptide sequence. To determine whether Shr has a functional leader peptide, we constructed a translational fusion between the 5′ shr region and the alkaline phosphatase gene (phoZ) of Enterococcus faecalis (7) and tested for the secretion of the fusion protein. The construct carrying the fusion protein (plasmid pCW1) was introduced into S. pyogenes SF370 strain, and the alkaline phosphatase activity was measured by using a colorimetric detection assay. The PhoZ activity in the culture supernatant of cells harboring pCW1 was 10 times higher than the activity observed in cells that do not carry PhoZ or in cells that carry the fusion in the reverse orientation (pCW1R), indicating that Shr has a functional leader peptide (Fig. 5D).
Western blot analysis demonstrated the in vivo production of Shr (Fig. 5E). A protein band of the expected size (∼140 kDa) that reacted with the anti-Shr antibodies was found both in the total cell extract and free in the culture supernatant. In addition to the full-length Shr, partial degradation was apparent in the supernatant, producing a banding pattern that is similar to that obtained when Shr was purified from E. coli extract, implying that the protein is cleaved by common proteases. To estimate the cell lysis occurring in the assayed cultures, we used the enzyme GusA carried by plasmid pNZ276 (15b). Since GusA does not have a leader peptide, it may be used as a cytoplasmic marker, and β-glucuronidase activity in the culture supernatant may be proportional to cell lysis. Enzyme activity associated with whole cells was compared to that found in the corresponding cell-free supernatant of S. pyogenes cultures. In cells that harbor pNZ276, β-glucuronidase activity associated with whole cells was 24 ± 13 U, which is about 11 times the background level (2.25 ± 0.35 U). In the culture supernatant, β-glucuronidase activity was 0.45 ± 0.49 U, which is comparable to the background level (0.65 ± 0.07 U). Based on difference in the activity found between whole cells and culture supernatant, we estimate that about 2% of the cells in the culture may have lysed. Two percent may represent an underestimate of the actual cell lysis, since it is possible some of the GusA activity may have been lost due to the presence of proteases. However, as the amounts of soluble Shr appears comparable to the amount of cell associated Shr (Fig. 5E), it seems reasonable that the presence of Shr in the supernatant results from secretion rather then cell lysis. Treatment of whole cells with proteinase K prior to protein extraction and Western blot analysis resulted in the disappearance of the Shr band from the total protein extract (data not shown). The susceptibility of Shr in intact cells to protease digest indicates that it is associated with the cell surface. The hydrophobic region found in the C terminus of Shr may help anchor the protein to the cell membrane.
Inactivation of the sia operon results in decreased iron uptake and hemoglobin binding to the surface.
To further characterize the role of the sia locus, an insertion duplication mutation was constructed in the shr gene (strain ZE11, Fig. 6A). The structure of the chromosomal mutation was verified by PCR and Southern blot analysis. A chromosomal fragment that reacted with the shr specific probe was produced in the wild-type strain (W, Fig. 6B). In agreement with the integration of the pCB2 plasmid into the shr gene, the corresponding fragment in the mutant strain was larger and reacted with the aad9-specific probe (M, Fig. 6B and data not shown). Western blot analysis with Shr antiserum revealed the production of a truncated Shr that is found predominantly in the supernatant (Fig. 5E).
RT-PCR analysis indicated that genes in the sia gene cluster are transcriptionally linked (Fig. 3A). We investigated the effect of the shr::pCB2 mutation on the expression from the sia operon by RNA slot blot analysis. The amounts of 3′ shr and siaA mRNAs produced in the wild-type cells grown in iron-rich and iron-depleted media were compared to those produced in the mutant cells. All membranes were probed with a recA-specific probe as a control for the mRNA amounts. A dramatic decrease in the production of the 3′ portion of the shr transcript was observed in ZE11, indicating that pCB2 integration into the shr gene does not allow transcription into the 3′ end of shr. Accordingly, almost no siaA mRNA was observed in the mutant (Fig. 6C) and expression of siaB and spy1791 was significantly reduced as well (data not shown). These observations indicate that, under the tested conditions, the shr promoter provides most of the transcription of the sia operon. This conclusion was further supported by Western blot analysis with anti-SiaA antibodies, which demonstrated that SiaA protein was not detected in the mutant strain, although the lower-molecular-weight cross-reacting band is observed (TM and ZE11 panel, Fig. 4III).
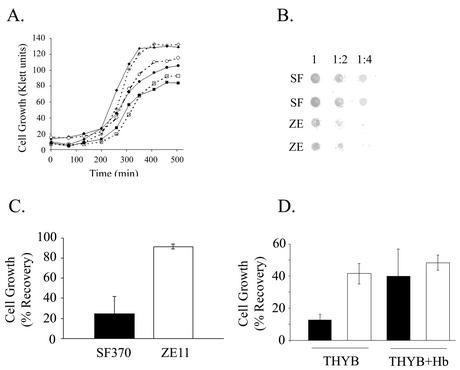
The growth of ZE11 was compared to that of the parent strain in complete medium, iron-restricted medium, and iron-restricted medium supplemented with hemoglobin. The mutant growth rate was similar to that of the wild type under all conditions (Fig. 7A). In addition, no difference in the ability of the mutant to use whole blood, heme-albumin, or ferric citrate was observed (data not shown), indicating that iron acquisition from hemoproteins in S. pyogenes is complex and involves multiple pathways.
FIG. 7.
Analysis of strain ZE11. (A) Growth of the wild-type strain SF370 (solid shapes) and the mutant strain ZE11 (open shapes). Cells were used to inoculate (1:100 dilution) fresh ZTH medium (diamonds), NTA containing medium (squares), and NTA-medium supplemented with 8 μM hemoglobin (circles). (B) Binding of biotinylated hemoglobin to cells of SF370 (SF) and ZE11 (ZE). The hemoglobin-binding assay was done as described in Fig. 2A. (C) Sensitivity to streptonigrin of the wild-type and the mutant strains. Cells were used to inoculate (1:1,000 dilution) fresh ZTH medium and medium containing 1.6 μM streptonigrin. The culture optical density (OD600) after overnight incubation (17 h) was determined. The data are presented as the percent growth of each strain in ZTH. The results are from seven independent experiments; the error bars indicate the standard deviation (P < 0.01, t test). (D) Sensitivity of SF370 and ZE11 to hydrogen peroxide. Overnight cultures were used to inoculate THYB or THYB containing 15 μM hemoglobin. Cells in mid-log phase were challenged with 5 mM hydrogen peroxide for 30 min. Viable counts were determined by plating samples taken before and after the challenge, and cell recovery was expressed as the percentage. The data represent results obtained from at least three experiments and plating was done in triplicate. Error bars represent the standard error of the mean (P < 0.01 for SF370 and ZE11 in THYB and P < 0.02 for SF370 in THYB and SF370 in THYB+Hb according to the Student t test).
Since both Shr and SiaA proteins bind hemoglobin in vitro, we tested the ability of the mutant strain to bind hemoglobin. In agreement with the loss of SiaA and the production of a truncated Shr, ZE11 cells exhibited 40% ± 8% (n = 6, P < 0.01) reduction in hemoglobin binding (Fig. 7B). Therefore, the sia operon contributes to the in vivo binding of hemoglobin to the surface of S. pyogenes, but additional proteins are likely to be involved.
The antibiotic streptonigrin interacts with the intracellular iron pool, causing the formation of reactive oxygen species. The toxicity of streptonigrin is proportional to the intracellular iron pool (71), and mutants in iron uptake systems are more resistant to the drug (4, 53). We compared the sensitivity of the shr mutant to that of the wild-type strain. Complete medium (TH) or medium containing increasing concentrations of streptonigrin were inoculated with S. pyogenes, and the culture growth after overnight incubation was determined. ZE11 cells were able to grow in higher concentrations of streptonigrin than the wild-type SF370, indicating that the mutant is more resistant to streptonigrin. Figure 7C shows that with 1.6 μM streptonigrin the growth of the wild type was dramatically inhibited (the culture reached 20% ± 14% of the growth on TH), whereas growth of the mutant was not significantly affected (88% ± 8%). These results suggest that the intracellular iron concentration in the sia mutant is lower than that of the wild type.
The susceptibility ZE11 to hydrogen peroxide stress was compared to that of the wild-type strain. Cells grown in THY to their mid-logarithmic phase were treated with 5 mM hydrogen peroxide for 30 min, and the bacterial recovery was determined. ZE11 recovery was higher than that of the wild-type strain (42 and 13%, respectively). This observation is also consistent with a decrease in intracellular iron content in the ZE11 mutant, since iron enhances oxidative stress. It was previously reported that the addition of hemoglobin to the culture medium resulted in a small growth stimulation of strain HSC5 in the presence of methyl violgen, a phenomenon attributed to the intrinsic peroxidase activity of hemoglobin (27a). We investigated the effect of the presence of hemoglobin on the cell susceptibility to hydrogen peroxide. Hemoglobin in the culture medium increased the recovery of SF370 from 13 to 40%, whereas no such stimulation was observed in the ZE11 mutant. This result is in agreement with the observed reduction in hemoglobin binding by ZE11 and suggests that the sia locus may be involved in hemoglobin-dependent resistance to hydrogen peroxide.
DISCUSSION
The growth of most organisms requires iron, which serves as a catalyst for electron transfer and is an essential component of many important enzymes. The use of heme and hemoproteins as a source of iron is ubiquitous in bacteria; however, the bacterial mechanisms involved are not fully understood. S. pyogenes is an obligate parasite of the human body that requires iron for growth and can make use of hemoglobin, myoglobin, heme-albumin, and the hemoglobin-haptoglobin complex (13, 14, 18). S. pyogenes cannot use transferrin and lactoferrin, the ferric carriers in the serum and secretions (14). Therefore, it is likely that heme compounds are an important source of iron for Streptococcus during infection. The means by which S. pyogenes obtains iron from hemoproteins or transports any form of iron complex have not been characterized. The present study describes a molecular genetic investigation of iron acquisition from hemoproteins in S. pyogenes.
The study of S. pyogenes strain SF370 (M1 serotype) shows that it, like JRS4 (M6 serotype), can use heme compounds to support its needs for iron (Fig. 1). We have also demonstrated for the first time that whole blood can support S. pyogenes growth in iron-depleted medium; this observation is consistent with the hemolytic nature of this pathogen and suggests that hemolysis is used by S. pyogenes to obtain iron. Iron restriction was reported to induce hemolytic activity in S. pyogenes (22), and recent analysis of SF370 revealed that it induced the expression of SLS and other genes encoding putative hemolysins (60). The optimal heme concentration required when provided by whole blood or by heme-albumin is similar (6 to 8 μM), whereas it is about 10-fold higher when provided in the form of hemoglobin solution (Fig. 1). It is possible that in solution hemoglobin is oxidized to different forms (oxyhemoglobin and methemoglobin [19]) affecting its utilization by S. pyogenes, whereas in the blood hemoglobin is kept in the reduced form because of the presence of methemoglobin reductase.
Cell-binding assays revealed that S. pyogenes binds heme-containing proteins such as hemoglobin and heme-albumin to its surface (Fig. 2A and data not shown). Proteins mediate hemoprotein binding as was shown by the protease sensitivity assays (Fig. 2C). Streptococcal binding of hemoproteins is probably the first step in iron capture by the bacteria. The increase in bacterial recovery after hydrogen peroxide challenge observed in the presence of hemoglobin (Fig. 7D) suggests that binding to hemoglobin and possibly other hemoproteins may serve a protective role as well, since S. pyogenes produces hydrogen peroxide during aerobic growth (21) and may encounter it during infection.
S. pyogenes can also bind free heme (Fig. 2B). Hyaluronic acid may contribute to heme binding to S. pyogenes surface due to the electrostatic interactions between the polyanionic polysaccharide and the cationic heme (20); this macromolecule can also bind human hemopexin, a heme-binding glycoprotein found in the serum (25). Bacterial protein receptors for hemoproteins can often bind free heme as well (19, 70), which may also be the case for S. pyogenes. This assumption is supported by our observation that heme can compete, although not efficiently, with hemoglobin binding to the surface (data not shown). However, resistance to protease treatment suggests that heme binding to a nonprotein constituent is the dominant mechanism.
Using a genomic approach, we identified the siaABC transporter that shares significant homology with transporters of heme and siderophores. The siaABC transporter is located in the middle of a 10-gene cluster, which was confirmed by RT-PCR and ZE11 mutant analysis to be expressed as an operon (Fig. 3A and 6C). The loss of SiaA expression in strain ZE11 (TM and ZE11 panel in Fig. 4III) and the dramatic decrease in the transcription of the sia genes (Fig. 6C) suggests that most of the transcription in the sia locus initiates from the promoter of the first gene, shr. This region contains a possible −35 box (GTGATA) that corresponds to that of the closely related species L. lactis (-TG-T-) (69), a 16-bp spacer, and a putative −10 box (TATAAA).
RNA analysis shows that, as in many iron acquisition systems, iron restriction induces expression from the sia operon, although low constitutive expression is observed when the cells are growing in complete medium. The ability of hemoglobin to repress the sia expression back to the background level indicates that the regulation is specific for iron. Putative sequence elements that may participate in the iron regulation of the operon are found in the upstream region of shr. These sequences have imperfect inverted repeats sharing 12 and 13 of 19 bp with the consensus sequence of the DtxR-repressor binding box (55). One of the binding boxes overlaps the −10 sequence of the inferred shr promoter mentioned above (Fig. 3A). Homologues to both Fur and DtxR (spy0187 and spy0450, respectively) can be identified in the genome of S. pyogenes, but the presence of DtxR-like binding sequences implies that a DtxR homologue participates in the sia regulation. A second iron complex transporter found in SF370 genome (spy0383, spy0384, spy0385, and spy0386) is induced by iron restriction in cells growing at 37°C (unpublished data) or 40°C (60). Interestingly, regulation of this transporter is probably mediated by a Fur homologue, as evidenced by the presence of a potential Fur-box in the spy0383 promoter region. Iron regulation is only poorly understood in S. pyogenes; additional work is required to reveal the physiological role of the different repressors and the regulatory network in which they are involved.
We demonstrated that SiaA, the transporter binding protein, is associated with the cell envelope and can produce a stable complex with hemoglobin (Fig. 4I). In addition, the sia operon codes for Shr, a surface-associated protein that binds in vitro to a variety of heme-compounds (Fig. 5A and B). Cells of strain ZE11 bind significantly less hemoglobin (Fig. 7B), indicating that the sia operon contributes to hemoglobin binding in vivo. The increased resistance to streptonigrin of the ZE11 mutant strain (Fig. 7C) suggests that this strain takes up less iron. Streptonigrin toxicity is proportional to the internal iron concentration (71); consequently, mutants in iron uptake pathways are typically more resistant to this antibiotic (4, 53). Together, the decrease in binding and the increased resistance to streptonigrin argue that the sia operon is involved in iron capture from hemoproteins. ZE11 was also more resistant to hydrogen peroxide when grown on THYB (Fig. 7D). This observation supports reduced iron uptake in this strain, since intracellular iron can augment oxidative stress. The addition of hemoglobin to the medium did not enhance ZE11 resistance to hydrogen peroxide as it did for SF370; this observation suggests that the sia operon may mediate hemoglobin-dependent resistance to hydrogen peroxide, possibly by binding hemoglobin (which has intrinsic peroxidase activity) to the cell surface, or by some other mechanism that may utilize the heme molecule.
It is likely, however, that S. pyogenes SF370 contains hemoprotein utilization machinery in addition to the sia locus, since ZE11 was able to use heme compounds similarly to the wild-type strain (Fig. 7A) and since binding to hemoglobin or hemoglobin-haptoglobin was not eliminated (Fig. 7B). Redundancy in hemoprotein utilization systems was observed in other pathogenic bacteria, including C. diphtheriae (54), S. pneumoniae (4), and strains of Neisseria (33, 61) and Haemophilus (48). The second iron complex transporter found in the genome of SF370 (spy0383, spy0384, spy0385, and spy0386) is a likely candidate for an additional heme uptake system.
To the best of our knowledge, HmuT and SiaA are the only substrate-binding proteins in gram-positive bacteria that were demonstrated to bind hemoglobin. The ability of SiaA to directly bind hemoglobin corresponds to the model proposed by Drazek et al., which suggests that in gram-positive bacteria, binding proteins function as surface receptors for heme and hemoglobin (11). Schneider and Hantke previously proposed a similar model for siderophore uptake systems (56). However, unlike the situation in siderophore uptake, binding proteins involved in iron-heme capture from hemoproteins do not interact directly with their ligand; rather, they must extract it from a high-affinity protein carrier. Therefore, the function of such binding proteins in gram-positive bacteria is conceptually similar to that of outer membrane heme-hemoprotein receptors. Invariant histidine residues, conserved glutamic acid residues, and a highly conserved receptor domain containing two amino acid motifs (FRAP and NPNL) were identified in outer membrane receptors engaged in heme acquisition (3, 59). SiaA does not share sequence homology with hemoglobin-heme outer membrane receptors, nor does it carry the sequence motifs mentioned above; additional work is required to determine how SiaA recognizes and binds hemoglobin.
The identification of Shr is intriguing, since this protein that binds hemoglobin, hemoglobin-haptoglobin, myoglobin, and heme-albumin does not share homology with any of the known heme or hemoprotein receptors. Shr did not bind apo-haptoglobin, fibrinogen, or streptavidin, indicating that Shr specifically recognizes hemoproteins. Although Shr lacks overall homology to hemoprotein receptors, a heme-binding motif is found in the carboxy terminus of Shr (Fig. 5C). This motif is shared by several heme-binding proteins, including H. influenzae eP4 and HbpA, which are required for heme acquisition, and the heme-containing proteins hemoglobin (alpha-chain) and cytochrome C3 (47). Shr may be able to recognize both the heme moiety and the protein portion of hemoproteins; this recognition may be facilitated by Shr's leucine-rich repeats. The ability to discriminate between apo-haptoglobin and hemoglobin loaded haptoglobin was also demonstrated in the P. gingivalis hemoprotein receptor HmuR (39). This property may allow the receptor to unload the apo-haptoglobin after the capture of heme.
The linkage of shr to the sia operon that is conserved among streptococcal isolates argues that Shr is also functionally related to the SiaABC transporter. One might speculate that Shr and SiaABC may compose a multicomponent iron uptake system, in which Shr functions to obtain heme or iron from hemoproteins and to deliver it to the SiaABC transporter. Only a few bacterial multicomponent systems involved in heme capture are known. These include HpuAB, the bipartite hemoglobin-haptoglobin receptor (33), two types of hemophore-dependent systems (70), the Porphyromonas Kgp and HRgpA proteases that interact with the outer membrane receptor HmuR (39), and the Hbp hemoglobin protease of E. coli (EB10), which was proposed to function as a shuttle protein of a heme uptake system (41). It is interesting that, like the hemophore HxuA and the Kgp hemoglobinase, Shr is found both on the cell surface and in the culture supernatant (9, 45). Analysis of the Shr-PhoZ fusion demonstrated that Shr has a functional leader peptide; however, the mechanism that anchors Shr to the surface remains unclear. Shr lacks the LPXTG motif commonly used to anchor proteins to the cell wall in gram-positive bacteria (37). Shr may be threaded through the membrane via its hydrophobic C terminus. Although we favor the hypothesis that shr is involved in iron acquisition, it is also possible that it may have other functions. The ability to bind hemoproteins and perhaps to acquire heme raises the possibility that it uses heme for redox sensing.
In addition to shr and siaABC, the operon codes for six other genes whose characteristics are described in Table 3. Two ATP-binding proteins (spy1791 and spy1790) with homology to hemolysin secretion permeases such as hlyB (34) and to the cytochrome biosynthesis genes cydCD (44) are found downstream of the siaABC genes. S. pyogenes is a facultative anaerobe, and early studies determined that it does not contain cytochrome-like NADH oxidase enzymes (49). The two related species E. faecalis and Lactococcus lactis code to a cydABCD gene cluster and are capable of aerobic respiration when grown in the presence of heme (12, 72). However, we could not identify any cydAB homologues in the SF370 chromosome. In conclusion, the sia locus is a conserved iron regulated operon involved in iron acquisition and hemoprotein binding and includes genes that may be involved in the acquisition and/or use of heme.
Acknowledgments
We are grateful to V. A. Fischetti for the lysine, to C. E. Rubens for plasmid pDC123, and to P. Moreillon for plasmid pOri23. We thank M. M. Farley, S. W. Satola, and the Georgia Emerging Infections Program for their help and for providing clinical isolates. We also thank K. S. McIver for scientific discussions and critical review of the manuscript.
This work was supported by The American Heart Association, Southeast Affiliate, Beginning Grant In Aid award 0060284B.
Editor: J. N. Weiser
REFERENCES
- 1.Al-Tawfiq, J. A., K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2000. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J. Infect. Dis. 181:1049-1054. [DOI] [PubMed] [Google Scholar]
- 2.Altman, P. L., and D. D. Katz. 1961. Blood and other body fluids. Federation of American Societies for Experimental Biology, Washington, D.C.
- 3.Bracken, C. S., M. T. Baer, A. Abdur-Rashid, W. Helms, and I. Stojiljkovic. 1999. Use of heme-protein complexes by the Yersinia enterocolitica HemR receptor: histidine residues are essential for receptor function. J. Bacteriol. 181:6063-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, J. S., S. M. Gilliland, and D. W. Holden. 2001. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 40:572-585. [DOI] [PubMed] [Google Scholar]
- 5.Brown, J. S., A. D. Ogunniyi, M. C. Woodrow, D. W. Holden, and J. C. Paton. 2001. Immunization with components of two iron uptake ABC transporters protects mice against systemic Streptococcus pneumoniae infection. Infect. Immun. 69:6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnette, W. N. 1981. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112:195-203. [DOI] [PubMed] [Google Scholar]
- 7.Chaffin, D. O., and C. E. Rubens. 1998. Blue/white screening of recombinant plasmids in gram-positive bacteria by interruption of alkaline phosphatase gene (phoZ) expression. Gene 219:91-99. [DOI] [PubMed] [Google Scholar]
- 8.Cope, L. D., S. E. Thomas, Z. Hrkal, and E. J. Hansen. 1998. Binding of heme-hemopexin complexes by soluble HxuA protein allows utilization of this complexed heme by Haemophilus influenzae. Infect. Immun. 66:4511-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cope, L. D., S. E. Thomas, J. L. Latimer, C. A. Slaughter, U. Muller-Eberhard, and E. J. Hansen. 1994. The 100-kDa haem:haemopexin-binding protein of Haemophilus influenzae: structure and localization. Mol. Microbiol. 13:863-873. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drazek, E. S., C. A. Hammack, and M. P. Schmitt. 2000. Corynebacterium diphtheriae genes required for acquisition of iron from haemin and haemoglobin are homologous to ABC haemin transporters. Mol. Microbiol. 36:68-84. [DOI] [PubMed] [Google Scholar]
- 12.Duwat, P., S. Sourice, B. Cesselin, G. Lamberet, K. Vido, P. Gaudu, Y. Le Loir, F. Violet, P. Loubiere, and A. Gruss. 2001. Respiration capacity of the fermenting bacterium Lactococcus lactis and its positive effects on growth and survival. J. Bacteriol. 183:4509-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eichenbaum, Z., B. D. Green, and J. R. Scott. 1996. Iron starvation causes release from the group A Streptococcus of the ADP-ribosylating protein called plasmin receptor or surface glyceraldehyde-3-phosphate dehydrogenase. Infect. Immun. 64:1956-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichenbaum, Z., E. Muller, S. A. Morse, and J. R. Scott. 1996. Acquisition of iron from host proteins by the group A Streptococcus. Infect. Immun. 64:5428-5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichenbaum, Z., and J. R. Scott. 1997. Use of Tn917 to generate insertion mutations in the group A Streptococcus. Gene 186:213-217. [DOI] [PubMed] [Google Scholar]
- 15a.Eichenbaum, Z., M. J. Federle, D. Marra, W. M. de Vos, O. P. Kuipers, M. Kleerebezem, and J. R. Scott. 1998. Use of the lactococcal nisA promoter to regulate gene expression in gram-positive bacteria: comparison of induction level and promoter strength. Infect. Immun. 64:2763-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis, R. T., Jr., and R. R. Becker. 1984. Specific indication of hemoproteins in polyacrylamide gels using a double-staining process. Anal. Biochem. 136:509-514. [DOI] [PubMed] [Google Scholar]
- 18.Francis, R. T., Jr., J. W. Booth, and R. R. Becker. 1985. Uptake of iron from hemoglobin and the haptoglobin-hemoglobin complex by hemolytic bacteria. Int. J. Biochem. 17:767-773. [DOI] [PubMed] [Google Scholar]
- 19.Genco, C. A., and D. W. Dixon. 2001. Emerging strategies in microbial haem capture. Mol. Microbiol. 39:1-11. [DOI] [PubMed] [Google Scholar]
- 20.Ghinea, N. 1986. Cationic heme undecapeptide as stain for detecting glycosaminoglycans on cellulose acetate strips. Anal. Biochem. 155:78-82. [DOI] [PubMed] [Google Scholar]
- 21.Gibson, C. M., T. C. Mallett, A. Claiborne, and M. G. Caparon. 2000. Contribution of NADH oxidase to aerobic metabolism of Streptococcus pyogenes. J. Bacteriol. 182:448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths, B. B., and O. McClain. 1988. The role of iron in the growth and hemolysin (streptolysin S) production in Streptococcus pyogenes. J. Basic Microbiol. 28:427-436. [DOI] [PubMed] [Google Scholar]
- 23.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson, D. P., and S. M. Payne. 1994. Vibrio cholerae iron transport systems: roles of heme and siderophore iron transport in virulence and identification of a gene associated with multiple iron transport systems. Infect. Immun. 62:5120-5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hrkal, Z., K. Kuzelova, U. Muller-Eberhard, and R. Stern. 1996. Hyaluronan-binding properties of human serum hemopexin. FEBS Lett. 383:72-74. [DOI] [PubMed] [Google Scholar]
- 26.Husmann, L. K., D. L. Yung, S. K. Hollingshead, and J. R. Scott. 1997. Role of putative virulence factors of Streptococcus pyogenes in mouse models of long-term throat colonization and pneumonia. Infect. Immun. 65:1422-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janulczyk, R., J. Pallon, and L. Bjorck. 1999. Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificity for metal cations. Mol. Microbiol. 34:596-606. [DOI] [PubMed] [Google Scholar]
- 27a.King, K. Y., J. A. Horenstein, and M. G. Caparon. 2000. Aerotolerance and peroxide resistance in peroxidase and PerR mutants of Streptococcus pyogenes. J. Bacteriol. 182:5290-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koster, W. 2001. ABC transporter-mediated uptake of iron, siderophores, heme and vitamin B12. Res. Microbiol. 152:291-301. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 30.Letoffe, S., J. M. Ghigo, and C. Wandersman. 1994. Iron acquisition from heme and hemoglobin by a Serratia marcescens extracellular protein. Proc. Natl. Acad. Sci. USA 91:9876-9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Letoffe, S., V. Redeker, and C. Wandersman. 1998. Isolation and characterization of an extracellular haem-binding protein from Pseudomonas aeruginosa that shares function and sequence similarities with the Serratia marcescens HasA haemophore. Mol. Microbiol. 28:1223-1234. [DOI] [PubMed] [Google Scholar]
- 32.Lewis, J. P., J. A. Dawson, J. C. Hannis, D. Muddiman, and F. L. Macrina. 1999. Hemoglobinase activity of the lysine gingipain protease (Kgp) of Porphyromonas gingivalis W83. J. Bacteriol. 181:4905-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis, L. A., E. Gray, Y. P. Wang, B. A. Roe, and D. W. Dyer. 1997. Molecular characterization of hpuAB, the haemoglobin-haptoglobin- utilization operon of Neisseria meningitidis. Mol. Microbiol. 23:737-749. [DOI] [PubMed] [Google Scholar]
- 34.Makino, K., K. Ishii, T. Yasunaga, M. Hattori, K. Yokoyama, C. H. Yutsudo, Y. Kubota, Y. Yamaichi, T. Iida, K. Yamamoto, T. Honda, C. G. Han, E. Ohtsubo, M. Kasamatsu, T. Hayashi, S. Kuhara, and H. Shinagawa. 1998. Complete nucleotide sequences of 93-kb and 3.3-kb plasmids of an enterohemorrhagic Escherichia coli O157:H7 derived from Sakai outbreak. DNA Res. 5:1-9. [DOI] [PubMed] [Google Scholar]
- 35.Mazoy, R., and M. L. Lemos. 1996. Identification of heme-binding proteins in the cell membranes of Vibrio anguillarum. FEMS Microbiol. Lett. 135:265-270. [DOI] [PubMed] [Google Scholar]
- 36.McIver, K. S., A. S. Heath, and J. R. Scott. 1995. Regulation of virulence by environmental signals in group A streptococci: influence of osmolarity, temperature, gas exchange, and iron limitation on emm transcription. Infect. Immun. 63:4540-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishina, Y., S. Miyoshi, A. Nagase, and S. Shinoda. 1992. Significant role of an exocellular protease in utilization of heme by Vibrio vulnificus. Infect. Immun. 60:2128-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olczak, T., D. W. Dixon, and C. A. Genco. 2001. Binding specificity of the Porphyromonas gingivalis heme and hemoglobin receptor HmuR, gingipain K, and gingipain R1 for heme, porphyrins, and metalloporphyrins. J. Bacteriol. 183:5599-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otto, B. R., S. J. van Dooren, C. M. Dozois, J. Luirink, and B. Oudega. 2002. Escherichia coli hemoglobin protease autotransporter contributes to synergistic abscess formation and heme-dependent growth of Bacteroides fragilis. Infect. Immun. 70:5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otto, B. R., S. J. van Dooren, J. H. Nuijens, J. Luirink, and B. Oudega. 1998. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J. Exp. Med. 188:1091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pancholi, V., and V. A. Fischetti. 1992. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate dehydrogenase with multiple binding activity. J. Exp. Med. 176:415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez-Casal, J., M. G. Caparon, and J. R. Scott. 1991. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J. Bacteriol. 173:2617-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poole, R. K., L. Hatch, M. W. Cleeter, F. Gibson, G. B. Cox, and G. Wu. 1993. Cytochrome bd biosynthesis in Escherichia coli: the sequences of the cydC and cydD genes suggest that they encode the components of an ABC membrane transporter. Mol. Microbiol. 10:421-430. [PubMed] [Google Scholar]
- 45.Potempa, J., R. Pike, and J. Travis. 1995. The multiple forms of trypsin-like activity present in various strains of Porphyromonas gingivalis are due to the presence of either Arg-gingipain or Lys-gingipain. Infect. Immun. 63:1176-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Que, Y. A., J. A. Haefliger, P. Francioli, and P. Moreillon. 2000. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect. Immun. 68:3516-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reidl, J., and J. J. Mekalanos. 1996. Lipoprotein e(P4) is essential for hemin uptake by Haemophilus influenzae. J. Exp. Med. 183:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren, Z., H. Jin, D. J. Morton, and T. L. Stull. 1998. hgpB, a gene encoding a second Haemophilus influenzae hemoglobin- and hemoglobin-haptoglobin-binding protein. Infect. Immun. 66:4733-4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ritchey, T. W., and H. W. Seely, Jr. 1976. Distribution of cytochrome-like respiration in streptococci. J. Gen. Microbiol. 93:195-203. [DOI] [PubMed] [Google Scholar]
- 50.Rossi, M. S., J. D. Fetherston, S. Letoffe, E. Carniel, R. D. Perry, and J. M. Ghigo. 2001. Identification and characterization of the hemophore-dependent heme acquisition system of Yersinia pestis. Infect. Immun. 69:6707-6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sambrook, J., T. Maniatis, and E. F. Fritsch. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 52.Saurin, W., W. Koster, and E. Dassa. 1994. Bacterial binding protein-dependent permeases: characterization of distinctive signatures for functionally related integral cytoplasmic membrane proteins. Mol. Microbiol. 12:993-1004. [DOI] [PubMed] [Google Scholar]
- 53.Schmitt, M. P. 1997. Utilization of host iron sources by Corynebacterium diphtheriae: identification of a gene whose product is homologous to eukaryotic heme oxygenases and is required for acquisition of iron from heme and hemoglobin. J. Bacteriol. 179:838-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitt, M. P., and E. S. Drazek. 2001. Construction and consequences of directed mutations affecting the hemin receptor in pathogenic Corynebacterium species. J. Bacteriol. 183:1476-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmitt, M. P., and R. K. Holmes. 1994. Cloning, sequence, and footprint analysis of two promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. J. Bacteriol. 176:1141-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider, R., and K. Hantke. 1993. Iron-hydroxamate uptake systems in Bacillus subtilis: identification of a lipoprotein as part of a binding protein-dependent transport system. Mol. Microbiol. 8:111-121. [DOI] [PubMed] [Google Scholar]
- 57.Scott, J. R., P. C. Guenthner, L. M. Malone, and V. A. Fischetti. 1986. Conversion of an M-group A Streptococcus to M+ by transfer of a plasmid containing an M6 gene. J. Exp. Med. 164:1641-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simon, D., and J. J. Ferretti. 1991. Electrotransformation of Streptococcus pyogenes with plasmid and linear DNA. FEMS Microbiol. Lett. 66:219-224. [DOI] [PubMed] [Google Scholar]
- 59.Simpson, W., T. Olczak, and C. A. Genco. 2000. Characterization and expression of HmuR, a TonB-dependent hemoglobin receptor of Porphyromonas gingivalis. J. Bacteriol. 182:5737-5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smoot, L. M., J. C. Smoot, M. R. Graham, G. A. Somerville, D. E. Sturdevant, C. A. Migliaccio, G. L. Sylva, and J. M. Musser. 2001. Global differential gene expression in response to growth temperature alteration in group A Streptococcus. Proc. Natl. Acad. Sci. USA 21:10416-10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stojiljkovic, I., V. Hwa, L. de Saint Martin, P. O'Gaora, X. Nassif, F. Heffron, and M. So. 1995. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol. Microbiol. 15:531-541. [DOI] [PubMed] [Google Scholar]
- 62.Stojiljkovic, I., J. Larson, V. Hwa, S. Anic, and M. So. 1996. HmbR outer membrane receptors of pathogenic Neisseria spp.: iron-regulated, hemoglobin-binding proteins with a high level of primary structure conservation. J. Bacteriol. 178:4670-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sutcliffe, I. C., and R. R. Russell. 1995. Lipoproteins of gram-positive bacteria. J. Bacteriol. 177:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tai, S. S., C. J. Lee, and R. E. Winter. 1993. Hemin utilization is related to virulence of Streptococcus pneumoniae. Infect. Immun. 61:5401-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tai, S. S., T. R. Wang, and C. J. Lee. 1997. Characterization of hemin binding activity of Streptococcus pneumoniae. Infect. Immun. 65:1083-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tam, R., and M. H. Saier, Jr. 1993. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol. Rev. 57:320-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomas, P. S. 1983. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 100:255-266. [DOI] [PubMed] [Google Scholar]
- 68.Torres, A. G., P. Redford, R. A. Welch, and S. M. Payne. 2001. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 69:6179-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van de Guchte, M., J. Kok, and G. Venema. 1992. Gene expression in Lactococcus lactis. FEMS Microbiol. Rev. 8:73-92. [DOI] [PubMed] [Google Scholar]
- 70.Wandersman, C., and I. Stojiljkovic. 2000. Bacterial heme sources: the role of heme, hemoprotein receptors and hemophores. Curr. Opin. Microbiol. 3:215-220. [DOI] [PubMed] [Google Scholar]
- 71.White, J. R., and H. N. Yeowell. 1982. Iron enhances the bactericidal action of streptonigrin. Biochem. Biophys. Res. Commun. 106:407-411. [DOI] [PubMed] [Google Scholar]
- 72.Winstedt, L., L. Frankenberg, L. Hederstedt, and C. von Wachenfeldt. 2000. Enterococcus faecalis V583 contains a cytochrome bd-type respiratory oxidase. J. Bacteriol. 182:3863-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]