Abstract
Mycoplasma hyopneumoniae is the etiological agent of swine enzootic pneumonia, a chronic nonfatal disease affecting pigs of all ages. The goal of this study was to design DNA vaccines by constructing plasmid pcDNA3/P42, carrying the heat shock protein gene P42 of M. hyopneumoniae, and to evaluate the immune responses elicited in BALB/c mice. The expression of P42 was first examined in transfected NIH 3T3 cells by reverse transcription-PCR to ensure that the construct was functional. The humoral and cell-mediated immune responses induced by the plasmid were further evaluated in BALB/c mice through intramuscular injection. Both immunoglobulin G1 (IgG1) and IgG2a levels were 64 times those of the control groups during the first 8 weeks. The levels of interleukin-2 (IL-2), IL-4, and gamma interferon mRNAs in the immunized animals were elevated, and the proliferation of spleen cells was also enhanced in the immunized animals. The results indicate that pcDNA3/P42 DNA immunization induces both Th1 and Th2 immune responses. In addition, antiserum from the immunized animals was found to inhibit the growth of M. hyopneumoniae. The present study reveals that DNA vaccination could be a new strategy against infection by M. hyopneumoniae and may have potential for developing vaccines for other infectious diseases as well.
Enzootic pneumonia of pigs caused by Mycoplasma hyopneumoniae is a mild and chronic respiratory disease affecting pigs of all ages (20). Pigs with primary mycoplasmal infection are predisposed to potentially fatal secondary invaders such as Pasteurella multocida and Actinobacillus pleuropneumoniae (5, 26), resulting in poor food conversion, retarded growth, and even higher mortality. Traditionally, M. hyopneumoniae infection is controlled by the use of antibiotics. However, the increased prevalence of antimicrobial-resistant strains of A. pleuropneumoniae increases the risk of reinfection within herds (13). In addition to the use of antibiotic and animal management procedures, the prevention of swine enzootic pneumonia through vaccination is needed.
The efforts to develop effective and safe vaccines against mycoplasmas have been partially successful. Vaccines against M. hyopneumoniae are usually in the form of inactivated whole cells or culture supernatant (7, 19, 21, 23), and some molecular approaches have also been tested. The recombinant Mhp1 antigens (a 124-kDa protein from M. hyopneumoniae) have been designed as a potential vaccine but provide only minimal protection (16). As a more successful example, oral immunization of mice with attenuated Salmonella enterica serovar Typhimurium aroA expressing a recombinant M. hyopneumoniae NrdF (fusion protein of ribonucleotide reductase R2 subunit with β-galactosidase) can elicit significant secretory immunoglobulin A (IgA) immune responses in the respiratory tract of mice and may provide a cost-effective alternative to the present control strategies for porcine enzootic pneumonia (8).
M. hyopneumoniae attaches only to the cilia on epithelial cells in the respiratory tract, followed by a loss of ciliary activity and damage to mucosal epithelial tissues (6). The molecular mechanism of the pathogenesis of this microorganism remains elusive. Eradication of the pathogens has been proposed to depend on both the presence of serum antibody and an increase in cell-mediated immunity during mycoplasmal infection (23). Certainly, regular recombinant vaccines usually are hampered in inducing cell-mediated immune responses and limit its potential. Genetic immunization or a DNA vaccine is a new promising approach to develop effective and safe vaccines (1, 2, 11, 25).
Since DNA vaccination has been shown to elicit both humoral and cell-mediated immune responses (11), we speculated that a DNA vaccine could be developed to control M. hyopneumoniae infection. By screening a lambda EMBL3 genomic library of M. hyopneumoniae, a few antigen genes were identified (4). One of the recombinant clones expressed a 42-kDa antigen, which was characterized as part of the 65-kDa protein gene (P65) in M. hyopneumoniae after correcting the codon usage. The P65 antigen was further demonstrated to be a heat shock protein (belonging to the HSP70 family) and monospecific antibodies against P42/P65 were purified and found to be able to block the growth of M. hyopneumoniae (4, 29). Therefore, P42 was chosen to design a DNA vaccine against M. hyopneumoniae, and its potential was evaluated in the present study through analyzing P42-specific antibodies, proliferation of splenocytes, and T cell-specific cytokine released in BALB/c mice.
MATERIALS AND METHODS
Construction of expression plasmids.
The mammalian expression vector pcDNA3 (Invitrogen) and Escherichia coli expression vector pGEX4T (Amersham Pharmarcia Biotech), were chosen to clone and express the P42 antigen gene of M. hyopneumoniae. Briefly, the 1.2-kb DNA fragment containing the P42 gene was obtained by PCR (Perkin Elmer 2400 Thermal Cycler) with the primer pair 5′-TTTTGGTTCATGGCGCTTACAA GACTT-3′ and 5′-TTTGAATTCATTTTAATCCTGCTT-3′ from the HindIII-XmaI fragment of plasmid pMH65 (4). The DNA fragment was then inserted into pcDNA3 and pGEX4T predigested with BamHI and EcoRI, obtaining plasmid pcDNA3/P42 (as a DNA vaccine candidate) and plasmid pGEX4T/P42 (to express antigen for further enzyme-linked immunosorbent assay [ELISA]), respectively.
Expression of P42 gene in transfected cells.
The mouse embryo cell line NIH 3T3 was transfected with either pcDNA3/P42 or pcDNA3 with the Lipofectamine Plus reagent (Life Technologies) according to the manufacturer's instructions. The transfected cells were harvested 48 h later and washed with phosphate-buffered saline three times. Total RNA was extracted with Trizol reagent (Life Technologies). Reverse transcription-PCR (RT-PCR) amplification was performed with the Fast-Run Moloney murine leukemia virus RT-PCR kit (Protech) containing the RNA and specific primers for the P42 gene. The reaction was carried out in a thermal cycler (Perkin-Elmer 2400) with the following profiles (30 cycles): 50°C for 30 min (one cycle), 94°C for 5 min (one cycle), and 94°C for 1 min, 61°C for 1 min, and 72°C for 1.5 min. The amplified products were analyzed with a 1% agarose gel.
Immunization of mice.
Six-week-old female BALB/c mice were obtained from the Laboratory Animal Center (Taipei, Taiwan) of the National Science Council. Groups of four mice were anesthetized and injected intramuscularly in the left hind thigh muscle with various amounts of pcDNA3/P42 (5 μg, 10 μg, and 50 μg of DNA dissolved in 100 μl of PBS). Mice immunized with 50 μg of pcDNA3 and 100 μl of PBS served as the negative controls. Each animal was boosted with the same dose 14 days after the first immunization. Serum samples were collected by tail bleeding every 2 weeks for 12 weeks and analyzed for P42-specific antibodies.
Expression and purification of P42 antigen.
E. coli strain DH5α [endA1 hsdR17(rK− mK+) supE44 thi-1 recA1 gyrA(Nalr) relA1 Δ(lacZYA-argF)U169 (ϕ80 Δlacd(lacZ)M15)] was transformed with plasmid pGEX4T/P42 and grown in Luria-Bertani (LB) medium supplemented with antibiotics (50 μg of ampicillin per ml) until late log phase (A600 reached 0.6 to 0.8). Expression of the cloned gene was induced by the addition of isopropylthiogalactopyranoside (IPTG; final concentration, 2 mM). The culture was grown for another 2 h, and the bacterial cells were broken with a B-PER bacterial protein extraction reagent (Pierce). The extracted lysates were loaded onto an immobilized glutathione column (Clontech), and the fusion proteins were further digested by thrombin (50 U/ml in PBS) on the column. After 16 h of digestion at room temperature, the P42 protein was eluted with PBS and concentrated by spin column. The P42 protein was identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and confirmed by Western blot analysis with rabbit anti-P42 antibody.
Antigen-specific ELISA analysis.
The antibodies specific to P42 were analyzed. Briefly, 96-well microtiter plates were coated with P42 protein (5 μg in 100 μl of PBS) and incubated at 37°C for 1 h. The wells were then cleaned and blocked with 100 μl of TBST (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.1% Tween 20) containing 5% (wt/vol) skim milk at 37°C for 1 h. The serum samples in duplicate were diluted with serial twofold dilutions (in an appropriate range for the particular analysis) and incubated on the plates at 37°C for 1 h. The wells were washed with TBST and incubated with 50 μl of 1:5,000 diluted goat anti-mouse IgG (or IgG1 and IgG2a) antibodies conjugated with alkaline phosphatase (Zymed Labs) at 37°C for 1 h. The wells were then washed and developed with p-nitrophenyl phosphate in 3,3′,5,5′-tetramethylbenzidine solution (Zymed Labs). After 15 min of incubation, the enzymatic reaction was stopped by adding 0.5 N HCl, and the plates were read at 405 nm with an MRX ELISA reader (Dynex Technologies). The endpoint titers of the antigen-specific IgG and subclass antibodies were defined as the last dilution giving a cutoff optical density larger than 0.1.
SDS-PAGE and Western blot analysis.
The purified P42 protein and total lysate of M. hyopneumoniae were analyzed by SDS-PAGE with the Bio-Rad minigel system (12% polyacrylamide gel). The prestained protein ladders (Bio-Rad) were used as molecular weight standards. Electrophoretic transfer onto nitrocellulose membranes (Hybond-C; Amersham Pharmacia Biotech) was done with a mini-Trans-Blot electrophoretic cell system (Bio-Rad). The membrane was blocked by incubation with 5% skim milk in TBST for 1 h at room temperature. Antiserum (diluted 1:100 in TBST) was added and incubated for 1 h at room temperature. The membrane was then washed with TBST three times. The anti-mouse IgG antibodies conjugated with alkaline phosphatase were diluted 1:1,000 in TBST and used as the secondary antibody (1 h of incubation at room temperature). Again, the membranes were washed with TBST three times and incubated with 20 ml of alkaline phosphatase buffer. The substrates (45 μl of nitroblue tetrazolium and 35 μl of 5-bromo-4-chloro-3-indolylphosphate) were added and mixed until the purple color bands appeared on the membrane.
Assay for splenocyte proliferation and cytokine secretion.
Mononuclear cells from the spleen were prepared from mice (14) and added to the wells of microtiter plates precoated with 100 μl of P42 protein solution (final concentration, 10 μg/ml) for 72 h at 37°C in a humid atmosphere of 5% CO2. During the last 16 h of incubation, 0.5 μCi of [3H]thymidine (New England Nuclear) was included, and its incorporation was determined with a liquid scintillation counter (Wallac 1409 counter; Pharmacia). The stimulation index was defined as the ratio of counts per minute of stimulated cells (by P42) versus the nonstimulated control (medium only).
The intensity of cytokine-specific mRNA was measured by the RT-PCR method (15). Total RNA was extracted from spleen cells with Trizol reagent, and RT-PCR amplification was performed. The following primers (obtained from MdBio Inc.) were used for specific PCRs: murine β-actin sense (5′-TGGAATCCTGTGGCATCCATGAAAC-3′) and antisense (5′-TAAAACGCAGCTCAGTAACAGT-CCG-3′); IFN-γ sense (5′-TGAACGCTACACACTGCATCTTGG-3′) and antisense (5′-CGACTCCTTTTCCGCTTCCTGAG-3′); IL-2 sense (5′-TCTGACACAGAGGAGTGGCTAAG) and antisense (5′-TCTGACCACAGTGAGGAATGTC-3′); and IL-4 sense (5′-ATGGGTCTCAACCCCCAGCTAGT-3′) and antisense (5′-GCTCTTT AGGCTTTCCAGGAAGTC-3′).
The PCR products were electrophoresed on a 2% agarose gel and visualized by staining with ethidium bromide (0.2 μg/ml). The level of cytokine-specific mRNA was estimated with the NIH Image software based on the corresponding β-actin signal.
Growth inhibition test.
M. hyopneumoniae strain 232 (provided by R. F. Ross, Iowa State University) was cultured in Friis medium (10) supplemented with 20% (vol/vol) porcine serum. The cultures were incubated at 37°C for 48 h. The bacterial cells were harvested by centrifugation at 25,000 × g for 15 min and resuspended to 1/10th of the original volume. The mycoplasmal cells (100 μl) were then inoculated into 3 ml of culture medium. The inhibition of mycoplasmal cell growth by antiserum from immunized mice (after a 12-week immunization) was assayed by adding the serum (100 μl of 1:100 and 1:1,000 dilutions) in the growth medium. Since M. hyopneumoniae produces only small colonies on agar, the CFU were very difficult to quantify. Therefore, the change in color changing units (CCU) with phenol red as the indicator at an optical density of 560 nm (OD560) was measured as an estimate of the growth of mycoplasmas (28). Growth medium containing antiserum from unimmunized mice was used as a negative control.
Statistical evaluation.
The significance of differences of the means between the groups was evaluated by the nonparametric Mann-Whitney U test (Mann-Whitney U test software for Excel; Analyze-It Software Inc.). All conclusions were based on significance levels of P < 0.05.
RESULTS
Construction and expression of plasmid carrying P42 gene.
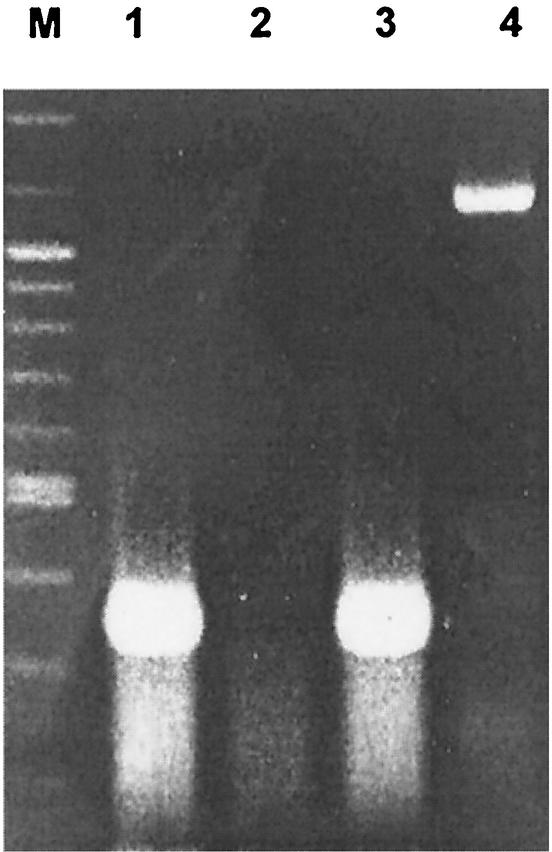
DNA vaccination is a relatively new vaccine strategy and has been reported to be able to induce both humoral and cellular immunity in many disease models (11). To investigate the potential of a DNA vaccine expressing the P42 antigen of M. hyopneumoniae in eliciting effective immune responses, the plasmid pcDNA3/P42, carrying the 42-kDa antigen gene of M. hyopneumoniae, was constructed and evaluated. To ensure that the P42 gene can indeed be transcribed in mammalian cells, the presence of P42 gene-specific mRNA was measured by RT-PCR from total RNA isolated from NIH 3T3 cells transfected with pcDNA3/P42. As shown in Fig. 1, an amplified product of approximately 1.2 kb, the same size as the P42 gene, was found in the transfected cells. DNA sequencing and sequence analysis further confirmed the identity of the amplified product. However, the expected protein was invisible on the immunoblot (data not shown) as we attempted to detect the presence of P42 in culture supernatant or the lysate from NIH 3T3 cells transfected with pcDNA3/P42.
FIG. 1.
Transcription of P42 gene in NIH 3T3 cells. Plasmid pcDNA3/P42 or pcDNA3 was transfected with Lipofectamine into NIH 3T3 cells. Production of specific mRNA was assessed 48 h later. Total RNA was purified from transfected cells and amplified by RT-PCR with P42-specific primers, with β-actin as the control. Lane M, molecular size markers: 1,500 bp, 1,200 bp, and ladders from 1,000 bp to 100 bp. Lane 1, RT-PCR for β-actin gene from NIH 3T3 cells without transfection. Lane 2, RT-PCR for P42 gene from nontransfected NIH 3T3 cells. Lane 3, RT-PCR for β-actin gene from NIH 3T3 transfected with pcDNA3/P42. Lane 4, RT-PCR for P42 gene from NIH 3T3 transfected with pcDNA3/P42.
Induction of humoral immune responses by pcDNA3/P42 vaccination.
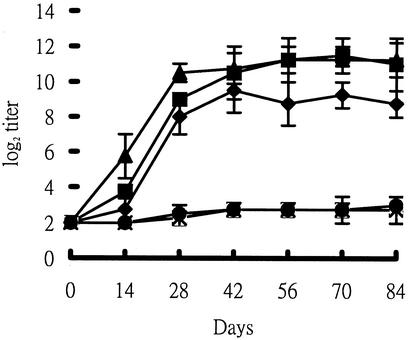
The humoral immune responses induced by pcDNA3/P42 vaccination were analyzed in mice. As shown in Fig. 2, P42-specific IgG antibodies were produced in the serum and reached a plateau (with a titer above 28) after 4 weeks. Actually, the titers of antibodies were maintained at approximately 210 even after 14 weeks in the serum of mice injected with pcDNA3/P42 (10 μg or 50 μg). The titer was a little lower (approximately 28) with 5 μg of plasmid but nondetectable with 1 μg of DNA (data not shown). The two negative control groups (injected with 50 μg of pcDNA3 or saline solution only) did not exhibit any detectable antibodies against the P42 gene product.
FIG. 2.
Induction of P42-specific IgG responses in the serum of mice immunized with pcDNA3/P42. The IgG levels in the serum were determined by ELISA at various times. The endpoint titer of P42-specific antibodies was defined as described in Materials and Methods. Means and standard deviations of log2 titers were based on measurements from four mice. Mice were injected with 50 μg of pcDNA3/P42 (▴), 10 μg of pcDNA3/P42 (▪), 5 μg of pcDNA3/P42 (♦),or 50 μg of pcDNA3 and PBS (×).
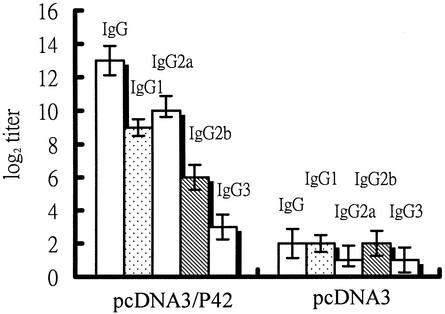
The IgG subclass was further clarified after 6 weeks of vaccination with 50 μg of pcDNA3/P42. The levels of IgG1, IgG2a, IgG2b, and IgG3 were estimated similarly by the ELISA method. As shown in Fig. 3, IgG1 and IgG2a were the dominant subclasses, with IgG2a slightly higher than IgG1. The levels of IgG2b and IgG3 were also significant compared with the control animals (injected with pcDNA3). This result reveals that the P42-specific immune response induced by plasmid pcDNA3/P42 is a mixed Th1 and Th2 type.
FIG. 3.
IgG subclass analysis of P42-specific antibodies. Mice were immunized with 50 μg of pcDNA3/P42 (or 50 μg of pcDNA3), and IgG levels in serum were analyzed by ELISA as described in Materials and Methods. Means and standard derivations were calculated from measurements of samples from four mice.
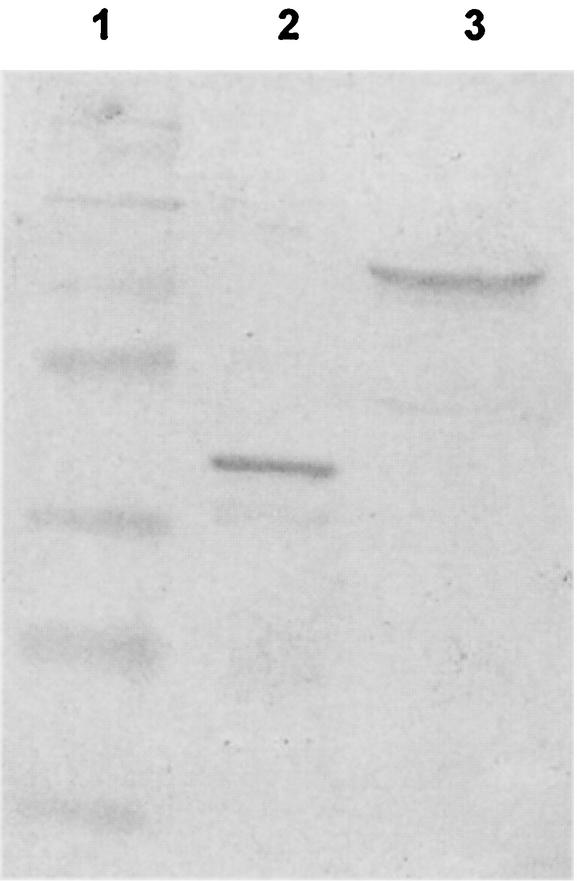
The sera from three mice injected with 50 μg of pcDNA3/P42 after a 14-weeks vaccination were pooled to detect the 42-kDa protein purified from E. coli carrying plasmid pGEX4T/P42 and the total cell lysate of M. hyopneumoniae. The results (Fig. 4) show that a single 42-kDa protein band from the transformed E. coli cells was detected and a 65-kDa band appeared from the total lysate of M. hyopneumoniae.
FIG. 4.
Western analysis of serum from mice vaccinated for 14 weeks. Sera from 12 mice injected with pcDNA3/P42 were pooled to perform Western analysis. Lane 1, molecular size markers (from the top: 175 kDa, 83 kDa, 62 kDa, 47.5 kDa, 32.5 kDa, 25 kDa, and 16.5 kDa). Lane 2, purified P42 from E. coli carrying plasmid pGEX4T/P42. Lane 3, total protein of M. hyopneumoniae.
Proliferation of mononuclear cells in the spleen.
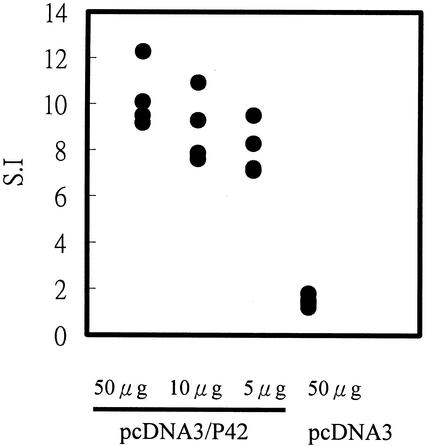
To determine whether P42-specific proliferation responses were induced in the immunized animals, the proliferation of mononuclear cells in the spleen was measured. Mononuclear cells from the spleens from individual mice were harvested 14 weeks after vaccination with pcDNA3/P42 and stimulated with purified P42 protein for 3 days. As shown in Fig. 5, P42-specific proliferation in mononuclear cell of the spleen was profound. The stimulation indexes were estimated to be 10.3, 8.9, and 8.0 in mice injected with 50 μg, 10 μg, and 5 μg pcDNA3/P42, respectively. By contrast, no proliferation of mononuclear cells was found in pcDNA3-immunized mice or nonimmunized mice.
FIG. 5.
Proliferation of mononuclear cells stimulated by DNA vaccination. Mononuclear cells were harvested from mice 14 weeks after vaccination with 50 μg of pcDNA3/P42. After stimulation for 72 h with purified P42 protein, [3H]thymidine was added and incubated for 16 h, and then the incorporated radioactivity was measured with a liquid scintillation counter. The stimulation index (S.I.) was defined as the ratio of counts per minute in spleen cells stimulated with pcDNA3/P42 versus counts per minute in spleen cells stimulated with pcDNA3.
Cytokine-specific mRNA in mononuclear cells.
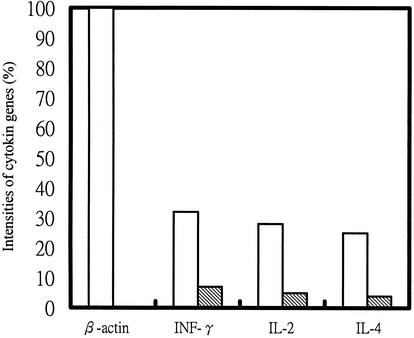
To assay cytokine-specific mRNAs released from mononuclear spleen cells 6 weeks after vaccination with pcDNA3/P42, cytokine mRNAs were analyzed by RT-PCR. β-Actin mRNA was used as a reference to judge the levels of cytokine-specific mRNAs. As shown in Fig. 6, the stimulated mononuclear cells synthesized significantly higher amounts of IFN-γ, IL-2, and IL-4 mRNAs in mice injected with pcDNA3/P42 than in mice injected with the vector pcDNA3.
FIG. 6.
Cytokine-specific messages from stimulated mononuclear cells. RNA samples were obtained from the spleen cells of mice immunized for 6 weeks. RT-PCRs were performed to estimate the levels of β-actin, IFN-γ, IL-2, and IL-4 mRNAs. The RT-PCR products were electrophoresed and stained with ethidium bromide, and the intensity was quantified with the NIH Image software. The intensities of the cytokine gene signals are presented as a percentage of the β-actin gene signals. The open and shaded bars represent mice injected with pcDNA3/P42 and pcDNA3, respectively.
Growth inhibition of M. hyopneumoniae.
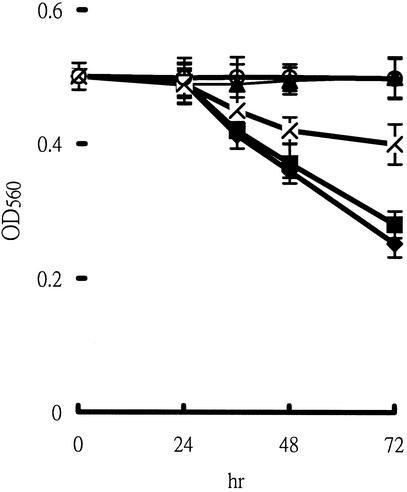
Antiserum from mice injected with pcDNA3/P42 may contain antibodies capable of inhibiting the growth of M. hyopneumoniae, and its presence may serve as a critical marker to evaluate potential vaccines. The growth of M. hyopneumoniae can be monitored by the color and optical density change (OD560) of the indicator in the growth medium. As the mycoplasmal cells grow, the red color of phenol red would gradually turn yellow. The growth inhibition test monitored the growth of M. hyopneumoniae in growth medium with various amounts of antiserum. As shown in Fig. 7, when 3 ml of culture medium contained 100 μl of antiserum at a 1:100 dilution, the OD560 remained the same, indicating no cell growth, during the 72-h growth period, while the OD dropped from 0.5 to 0.4 as the antiserum was diluted to 1:1,000. By contrast, the OD560 decreased significantly from 0.5 to 0.25 or 0.28 as M. hyopneumoniae grew in the culture medium without antiserum or with antiserum from mice injected with the vector pcDNA3, respectively. The results indicate that the growth of M. hyopneumoniae was inhibited by the antiserum and that the inhibitory effect was concentration dependent.
FIG. 7.
Growth inhibition assay of M. hyopneumoniae. The growth rate of the mycoplasmal cells was monitored by measuring the OD560. The growth-inhibitory effects of antiserum from mice immunized with (▴) pcDNA3/P42 (1:100 dilution), (×) pcDNA3/P42 (1:1,000 dilution), or (▪) pcDNA3 (1:100 dilution) and (♦) of no antiserum were tested. The open circle represents the OD560 of the growth medium without cells.
DISCUSSION
DNA vaccines with the pcDNA3 vector (driven by the cytomegalovirus promoter) have been reported to persist in muscle for over 1 year and induce effective immune responses with bacterial antigen (25). In the present study, the pcDNA3/P42 plasmid was designed and evaluated. As shown in Fig. 1, P42 mRNA was observed in the transfected NIH 3T3 cells, though the P42 protein level may not be high enough to be detected by Western blotting. The production of P42-specific antibodies (Fig. 2 and 3) and the proliferation of spleen cells in pcDNA3/P42-immunized mice (Fig. 5) further confirmed that P42 was expressed and induced significant immune responses in the immunized animals.
CpG dinucleotides have been recognized more frequently in the genomes of bacteria and viruses than of vertebrates. The unmethylated CpG dinucleotides (CpG motifs) were even found to induce murine B cells to proliferate and secrete immunoglobulins (mainly IL-6 and IgM) in vitro and in vivo (17, 18, 27). The CpG motifs in the plasmid backbone of DNA vaccines were also demonstrated to play an important role in the induction of antigen-specific immunity (17). The entire P65 sequence (GenBank accession number U50209; P65 has 1,803 nucleotides, where P42 is located at nucleotides 685 to 1803) was checked for CpG motifs by the LALIGN program (http://www.ch.embnet.org/software/LALIGN_form.html) for CpG dinucleotides flanked by two 5′ purines (optimally an ApG) and two 3′ pyrimidines (optimally a TpC or TpT). Two CpG motifs, agCGtt (nucleotides 114 to 119) and aaCGcc (nucleotides 562 to 567) were found in the P42 gene sequence, while the hexamer CpG motifs which may inhibit the immunogenicity of DNA vaccine (22) did not exist in the region. Besides CpG motifs, heat shock proteins have been shown to recognize multiple B-cell and T-cell epitopes (26) and have also been used as an adjuvant to enhance cell-mediated immune responses in DNA vaccines (3). Therefore, P42, as a heat shock protein, may contribute additional immunostimulatory effects and may induce higher immunogenicity in DNA vaccination.
DNA vaccination has been demonstrated to induce both humoral and cellular immune responses. The relevant Th type and IgG subclass distribution could be critical for protection against a particular disease. Differentiation of naïve Th cells into Th2 or Th1 cells determines whether humoral or cell-mediated immunity will be predominant (12). Upon activation, Th1 cells can activate macrophages to destroy intracellular microorganisms more efficiently and also activate B cells to produce strongly opsonizing antibodies such as IgG2a and IgG2b in mice. The main effector function of Th2 cells is to drive B cells to proliferate and produce antibodies such as IgG1 and other types. Therefore, we assayed both the IgG subclasses (IgG1, IgG2a, IgG2b, and IgG3) and cytokines messages (IL-2, IL-4, and IFN-γ) produced by T cells to examine whether pcDNA3/P42 immunization induced a Th1 or Th2 immune response in mice. The results (Fig. 3 and Fig. 6) reveal that the titer of IgG2a (through the Th1 pathway) is only slightly higher than that of IgG1 (mainly through activation of Th2 cells). The elevation of macrophage-activating effectors IFN-γ and IL-2 (secreted mainly by Th1 cells) stimulated by pcDNA3/P42 is comparable to the B-cell-activating effector molecule IL-4 (secreted by Th2 cells). Therefore, both factors indicate that pcDNA3/P42 vaccination induced a mixed Th1/Th2-type immune response in mice. However, many factors, such as the plasmid vector, delivery route, nature of the antigen, and mouse strain, may also affect the levels of effector molecules produced by helper T cells (9, 12, 24) and thus make it difficult to conclude unambiguously the route of immunization.
The present work demonstrates that the genetic vaccination approach may induce both humoral and cellular immunities and represent a potentially new approach to design vaccine against M. hyopneumoniae. The levels of serum IgG, the proliferation of splenocytes, and the levels of T-cell-specific cytokines significantly increased in mice injected with pcDNA3/P42. The pcDNA3/P42-induced antiserum was shown to be able to bind specifically the antigenic heat shock protein of M. hyopneumoniae and even to inhibit cell growth. Certainly, the immune responses of mice should not be overextrapolated, and the potential of using pcDNA3/P42 immunization for the control of swine enzootic pneumonia requires further studies in pigs.
Acknowledgments
This work was partially supported by research grants from the National Science Council ROC (NSC90-2311-B259-001 and NSC89-2317-B110-001) and Kaohsiung Veteran General Hospital (VGHNSU90-03).
Editor: J. M. Mansfield
REFERENCES
- 1.Akbari, O., N. Panjwani, S. Garcia, R. Tascon, D. Lowrie, and B. Stockinger. 1999. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J. Exp. Med. 189:169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, M. A., W. C. Lai, and S. A. Johnston. 1995. Protection against mycoplasma infection with expression-library immunization. Nature 377:632-635. [DOI] [PubMed] [Google Scholar]
- 3.Chen, C. H., T. L. Wang, C. F. Hung, Y. Yang, R. A. Young, D. M. Pardoll, and T. C. Wu. 2000. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 60:1035-1042. [PubMed] [Google Scholar]
- 4.Chou, S. Y., T. L. Chung, R. J. Chen, L. H. Ro, P. I. Tsui, and D. Shiuan. 1997. Molecular cloning and analysis of a HSP (heat shock protein)-like 42 kDa antigen gene of Mycoplasma hyopneumoniae. Biochem. Mol. Biol. Intl. 41:821-831. [DOI] [PubMed] [Google Scholar]
- 5.Ciprian, A., C. Pijoan, T. Cruz, J. Camacho, J. Tortora, G. Colmenares, R. R. Lopez, and M. de la Garcia. 1988. Mycoplasma hyopneumoniae increases the susceptibility of pigs to experimental Pasteurella multocida pneumonia. Can. J. Vet. Res. 52:434-438. [PMC free article] [PubMed] [Google Scholar]
- 6.DeBey, M., and R. F. Ross. 1994. Ciliostasis and loss of cilia induced by Mycoplasma hyopneumoniae in porcine tracheal organ culture. Infect. Immun. 62:5312-5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djordjevic, S. P., G. J. Eamens, L. F. Romails, P. J. Nicolls, V. Taylor, and J. Chin. 1997. Serum and mucosal antibody responses and protection in pigs vaccinated with Mycoplasma hyopneumoniae vaccines containing a denatured membrane antigen pool and adjuvant. Aust. Vet. J. 75:504-511. [DOI] [PubMed] [Google Scholar]
- 8.Fagan, P. K., S. P. Djordjevic, J. Chin, G. J. Eamens, and M. J. Walker. 1997. Oral immunization of mice with attenuated Salmonella typhimurium aroA expressing a recombinant Mycoplasma hyopneumoniae antigen (NrdF). Infect. Immun. 65:2502-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felguate, D. M., S. Heaney, R. G. Webster, and H. L. Robinson. 1997. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 158:2278-2284. [PubMed] [Google Scholar]
- 10.Friis, N. F. 1975. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma floculare. Nord. Vet. Med. 27:337-339. [PubMed] [Google Scholar]
- 11.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 12.Haddad, D., S. Liljeqvist, S. Stahl, P. Perlmann, K. Berzins, and N. Ahlborg. 1998. Differential induction of immunoglobulin G subclasses by immunization with DNA vectors containing or lacking a signal sequence. Immunol. Lett. 61:201-204. [DOI] [PubMed] [Google Scholar]
- 13.Hannan, P. C., B. S. Bhogal, and J. P. Fish. 1982. Tylosin tartrate and tiamutilin effects on experimental piglet pneumonia induced with pneumonia pig lung homogenate containing mycoplasmas, bacteria and viruses. Res. Vet. Sci. 33:76-88. [PubMed] [Google Scholar]
- 14.Harlow, E., and D. Lane (ed.). 1988. Antibodies, p. 220. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 15.Kawabata, S., Y. Terao, T. Fujiwara, I. Nakagawa, and S. Hamada. 1999. Targeted salivary gland immunization with plasmid DNA elicits specific salivary immunoglobulin A and G antibodies and serum immunoglobulin G antibodies in mice. Infect. Immun. 67:5863-5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King, K. W., D. H. Faulds, E. L. Rosey, and R. J. Yancey. 1996. Characterization of the gene encoding Mhp1 from Mycoplasma hyopneumoniae and examination of Mhp1's vaccine potential. Vaccine 15:25-35. [DOI] [PubMed] [Google Scholar]
- 17.Klinman, D. M., G. Yamshchikov, and Y. Ishigatsubo. 1997. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J. Immunol. 158:3635-3639. [PubMed] [Google Scholar]
- 18.Krieg, A. M., A. K. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-549. [DOI] [PubMed] [Google Scholar]
- 19.Maes, D., H. Deluyker, M. Verdonck, F. Castryck, C. Miry, B. Vrijens, W. Verbeke, J. Viaene, and A. Kruif. 1999. Effect of vaccination against Mycoplasma hyopneumoniae in pig herds with an all-in/all-out production system. Vaccine 17:1024-1034. [DOI] [PubMed] [Google Scholar]
- 20.Ross, R. F. 1992. Mycoplasmal disease, p. 537-551. In A. D. Leman, B. E. Straw, W. L. Mengeling, S. D. Allaire, and D. J. Taylor (ed.), Diseases of swine, 7th ed. Iowa State University Press, Ames, Iowa.
- 21.Ross, R. F., B. J. Zimmermann-Erickson, and T. F. Young. 1984. Characteristics of protective activity of Mycoplasma hyopneumoniae vaccine. Am. J. Vet. Res. 45:1899-1905. [PubMed] [Google Scholar]
- 22.Stratford, R., G. Gouce, L. Zhang-Barber, N. Faairweather, J. Eskola, and G. Dougan. 2001. Influence of codon usage on the immunogenicity of a DNA vaccine against tetanus. Vaccine 19:810-815. [DOI] [PubMed] [Google Scholar]
- 23.Thacker, E. L., B. J. Thacker, M. Kuhn, P. A. Hawkins, and W. R. Waters. 2000. Evaluation of local and systemic immune response induced by intramuscular injection of a Mycoplasma hyopneumoniae bacterin to pigs. Am. J. Vet. Res. 61:1384-1389. [DOI] [PubMed] [Google Scholar]
- 24.Vercammen, M., T. Scorza, K. Huygen, J. De Braekeleer, R. Diet, D. Jacobs, E. Saman, and H. Verscheren. 2000. DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infect. Immun. 68:38-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff, J. A., J. J. Ludtke, G. Acsadi, P. Williams, and A. Jani. 1992. Long-term persistence of plasmid DNA and foreign gene expression in muscle. Hum. Mol. Genet. 1:363-369. [DOI] [PubMed] [Google Scholar]
- 26.Yagihashi, T., T. Nunoya, T. Mitsui, and M. Tajiman. 1984. Effect of Mycoplasma hyopneumoniae infection on the development of Haemophilus pleuropneumoniae pneumonia in pigs. Jpn. J. Vet. Sci. 46:705-713. [DOI] [PubMed] [Google Scholar]
- 27.Yi, A. K., D. M. Klinman, T. L. Martin, S. Matson, and A. M. Krieg. 1996. Rapid immune activation by CpG motifs in bacterial DNA. J. Immunol. 157:5394-5402. [PubMed] [Google Scholar]
- 28.Zhang, Q., T. F. Young, and R. F. Ross. 1995. Identification and characterization of a Mycoplasma hyopneumoniae adhesin. Infect. Immun. 63:1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zugel, U., and S. H. Kaufmann. 1999. Role of heat shock proteins in protection from pathogenesis of infectious disease. Clin. Microbiol. Rev. 1:19-39. [DOI] [PMC free article] [PubMed] [Google Scholar]