Abstract
Fourteen human carriage Listeria monocytogenes isolates were compared to sporadic and epidemic-associated human strains in order to ascertain the pathogenic behavior of these unrecognized asymptomatic strains. Experimental infection of 14-day-old chick embryos revealed that the majority of the carriage strains were attenuated for virulence. Of the 10 attenuated carriage strains, 5 were affected in their invasion capacities in vitro. Western blot analysis with antibody directed against InlA, the surface protein implicated in the internalization in host cells, allowed correlation between the ability of the carriage strains to enter Caco-2 cells and InlA expression. Indeed, these five carriage strains produced truncated forms of InlA. Four of the five truncated forms of InlA had an apparent molecular mass of 47 kDa. In order to assess the existence of a genetic lineage, partial sequences of inlA gene of these four strains were compared and revealed that they had a high degree of sequence conservation at the gene (99.86%) and amino acid (100%) levels. Comparison of their nucleotide sequences with that of the corresponding segment of inlA from EGD-e and Scott A strains, taken as epidemic references, showed more divergence. Taken together, these observations suggest the presence of specific traits that characterize L. monocytogenes strains isolated during asymptomatic carriage. Some of these traits could provide some explanations about the determinants that make them unable to cause systemic human infection.
Infections of humans with the gram-positive facultative intracellular bacterium Listeria monocytogenes may cause severe diseases, such as sepsis or meningitis, mainly in newborn children, in the elderly, and in immunocompromised persons (54). It is one of the most well-studied bacterial pathogens. There is a fairly good understanding of the pathways followed by Listeria that lead to the infection of the different cell types. After the ingestion of contaminated food, Listeria disseminates from the intestinal lumen to the central nervous system and the fetoplacental unit. L. monocytogenes induces its own uptake even by normally nonphagocytic host cells, especially with the help of the cell surface proteins, internalin A (InlA) and InlB. InlA promotes the infection of human enterocyte-like cell lines through interaction with the human cellular receptor E-cadherin (hEcad) (36). The nature of the amino acid at position 16 of E-cadherin is crucial for the InlA-E-cadherin interaction and is species specific (30). For example, the chicken hEcad homolog but not the mouse E-cadherin (mEcad) is a receptor for InlA (31). InlB shows a much broader host cell spectrum and promotes invasion by activation of phosphatidylinositol 3-kinase (19). Two other proteins, gC1q-R/p32 and a Met receptor tyrosine kinase, have been identified as host cell receptors recognized by InlB (1, 50). After the uptake stage, other virulence factors, including the proteins listeriolysin O and Pi-PLC (for phosphatidylinositol-specific phospholipase C), are necessary to escape from the phagocytic vacuole, and the proteins ActA and Pc-PLC (for phosphatidylcholine-specific phospholipase C), which are necessary for intracellular actin-based mobility and cell-to-cell spread (8).
Despite the fact that molecular and cellular biology studies defined a series of virulence factors involved in the bacterial pathogenesis, some aspects of the human listeriosis have not been not elucidated. For example, L. monocytogenes is sometimes able to infect immunocompetent adults, and these listeriosis cases were reported particularly during epidemic outbreaks (16, 26, 48). Conversely, the pathogen can be carried in the intestine of between 1 and 6% of the general population, without declaration of any symptoms (17, 44). Heterogeneity in the virulence potential of the L. monocytogenes strains could explain these occurrences of epidemic or sporadic forms of listeriosis and the occurrence of both fecal and oral carriage states. Concerning the first cited example, some especially virulent strains, referred to as epidemic-associated strains, could be responsible for a high infection rate even in healthy persons. Herd and Kocks (18) considered the possibility of genetic loci encoding additional pathogenic traits into epidemic-associated strains in contrast to sporadic strains. In the same way, we hypothesize that the human carriage strains could be enclosed in a group with specific traits influencing, for example, the course or the outcome of an infection.
Most of our knowledge about the L. monocytogenes carriage strains comes from epidemiologic investigations and so concern human standards rather than bacterial features. These studies have focused only on the rate of healthy human or animal carriers. Factors such as sex (49) and categories of people such as pregnant women (23, 33), patients with diarrhea (37), workers handling or not handling L. monocytogenes (12, 25, 43), or those who have contact with animals (24, 34) may have no impact on the rate of asymptomatic fecal carriage. However, some of these data are the subject of debate since, in contrast, in some environments (e.g., slaughterhouses and the surroundings of patients with listeriosis) more people are found to carry L. monocytogenes in their intestinal tracts (49). Periods in which L. monocytogenes is excreted in the feces appear to be rather short (24, 25, 45). Usually, excretion is not accompanied by gastrointestinal or other clinical symptoms (49).
Few studies have been focused on the characteristics of carriage strains, but recently, in a previous study, we reported the pathogenic behavior of two human fecal carriage strains, H1 and H2 (41). Strain H2 should be considered pathogenic with regard to its virulence in chick embryos and its invasion efficiency within Caco-2 cells. In contrast, strain H1 showed attenuated virulence toward chick embryos and could not be translocated through the intestinal wall. This phenotype could be explained, in part, by a mutation in inlA of strain H1. A similar mutation was previously reported by Jonquieres et al. (22) for the human reference carriage strain LO28. We report here on the pathogenic behavior of 14 carriage L. monocytogenes strains, which were compared to the levels of virulence of 20 sporadic and epidemic-associated L. monocytogenes strains in order to determine a “carriage phenotype.”
MATERIALS AND METHODS
Bacterial strains and growth media.
L. monocytogenes strains used are listed in Table 1. Prior to initiation of the present study, a total of 2,500 oral, genital, or fecal samples from healthy patients free of signs or symptoms of listeriosis were collected between January 1991 and November 1997 in Philippe le Bon Hospital (Beaune, France). From these 2,500 samples, 14 asymptomatic carriage L. monocytogenes strains were recovered by conventional techniques (45). Among these carriage strains, the virulence potential of two of these carriage strains, isolates H1 and H2, was assessed (41). Among isolates collected from patients with listeriosis, all sporadic strains and H36 epidemic strain were provided by hospitals in Dijon, Beaune, Chalon/Saône, Strasbourg, and Rennes (France). L. monocytogenes strain Scott A (obtained by the Institut Pasteur Collection, Paris, France), LO28 and EGD-e (kindly provided by P. Cossart, Institut Pasteur, Paris, France) were added as reference strains for comparative analysis.
TABLE 1.
Characteristics of L. monocytogenes strains used in this study
| L. monocytogenes human isolate | Origin (city, country, yr) | Serogroup | Activitya
|
||
|---|---|---|---|---|---|
| Hemolytic | Pi-PLC | Pc-PLC | |||
| Fecal isolates | |||||
| H1 | Healthy pregnant carrier (Beaune, France, 1991) | 1 | +++ | +++ | +++ |
| H2 | Healthy 3-year-old child (Beaune, France, 1992) | 4 | +++ | ++ | ++ |
| H6 | Healthy 30-year-old woman (Beaune, France, 1991) | ++ | ++ | ++ | |
| H11 | Healthy 35-year-old man (Beaune, France, 1992) | 1 | +++ | +++ | +++ |
| H17 | Healthy 10-year-old child (Beaune, France, 1992) | + | PA | ++ | |
| H27 | Healthy 2-year-old child (Beaune, France, 1994) | 4 | +++ | +++ | ++ |
| H28 | Healthy 7-year-old child (Beaune, France, 1994) | 1 | ++ | ++ | ++ |
| H31 | Healthy 55-year-old man (Beaune, France, 1995) | 1 | ++ | +++ | +++ |
| H32 | Healthy 19-year-old woman (Beaune, France, 1996) | 1 | ++ | ++ | +++ |
| H34 | Healthy 8-year-old child (Beaune, France, 1997) | 1 | +++ | + | +++ |
| H35 | Healthy 11-year-old child (Beaune, France, 1997) | 1 | + | ++ | ++ |
| H38 | Healthy carrier (Beaune, France, date not communicated) | + | ++ | ++ | |
| LO28 | Healthy pregnant carrier (Spain) | 1/2c | +++ | +++ | +++ |
| Oral isolate H12 | Healthy 39-year-old man (Beaune, France, 1991) | 4 | + | ++ | ++ |
| Sporadic patient isolates | |||||
| H3 | Blood culture (Chalon/Saône, France, 1991) | 1 | ++ | +++ | ++ |
| H5 | Cephalorachid liquids (Chalon/Saône, France, 1991) | 1 | + | +++ | ++ |
| H7 | Genital swab (Beaune, France, 1992) | 1 | ++ | + | + |
| H8 | Blood culture (Chalon/Saône, France, 1992) | 1 | ++ | +++ | + |
| H9 | Blood culture (Dijon, France, 1992) | 1 | ++ | ++ | + |
| H10 | Peripheral swab (Chalon/Saône, France, 1991) | 4 | ++ | +++ | ++ |
| H13 | Blood culture and cephalorachid liquids (Chalon/Saône, France, 1991) | 4 | ++ | +++ | ++ |
| H14 | Cephalorachid liquids (Rennes, France, 1992) | 4 | ++ | + | + |
| H15 | Blood culture (Rennes, France, 1992) | 1 | +++ | +++ | +++ |
| H16 | Blood culture (Rennes, France, 1992) | 1 | +++ | ++ | +++ |
| H18 | Blood culture (Strasbourg, France, 1992) | 1/2b | ++ | +++ | ++ |
| H19 | Blood culture (Strasbourg, France, 1992) | 4b | + | PA | ++ |
| H21 | Blood culture (Strasbourg, France, 1992) | 4 | + | ++ | ++ |
| H22 | Blood culture (Strasbourg, France, 1992) | 4b | ++ | ++ | + |
| H23 | Liver (Strasbourg, France, 1992) | 1 | ++ | ++ | ++ |
| H24 | Placenta (Strasbourg, France, 1992) | 4b | + | PA | + |
| H25 | Placenta (Strasbourg, France, 1992) | 1/2a | + | +++ | ++ |
| Epidemic-associated strains | |||||
| Scott A | Massachusetts milk outbreak (United States, 1983) | 4b | + | + | ++ |
| EGD-e | Rabbit outbreak (England, 1924) | 1/2a | + | ++ | ++ |
| H36 | Epoisse cheese outbreak (Epoisses, France, 1999) | 4b | ++ | ++ | ++ |
Symbols: +++, strong activity; ++, moderate activity; +, weak activity. PA, positive activity on ALOA agar plates.
Overnight static cultures of Listeria cells grown at 37°C in brain heart infusion (BHI) broth (bioMérieux, Marcy l'Etoile, France) were used for the chicken embryo bioassays and cell culture assays. For Western blot analysis, protein samples were derived from cultures grown at 37°C in MCDB 202 (Cryo Bio-Systems, l'Aigle, France) supplemented with glucose at 50 mg liter−1, 10% yeast nitrogen base without amino acids (YNB; Difco Laboratories, Detroit, Mich.), and 1% trace elements (Cryo Bio-Systems). Viable counts were performed on acriflavine (10 mg liter−1; Sigma)-ceftazidime (50 mg liter−1; Glaxo-Wellcome, Marly Le Roi, France) BHI agar (ACA) plates incubated 48 h at 37°C.
Biochemical and physiological methods.
The test strains were spot inoculated (109 bacteria/spot) onto appropriate agar plates for the observation of three distinct enzymatic activities: the ability to induce zones of hemolysis around the colonies (hemolytic activity) was tested on Bacto Columbia blood agar base EH medium (mBAP; Difco Laboratories) supplemented with 5% (vol/vol) saline-washed horse red blood cells, as described elsewhere (14). Pc-PLC activity characterized by the development of zones of opacity due to degraded egg yolk lecithin around the colonies was evaluated by a standard procedure by inoculating bacteria onto BHI agar supplemented with 5% (vol/vol) fresh egg yolk (7). The test strains were examined for Pi-PLC activity as described by Notermans et al. (40): a turbid halo (the insoluble diacylglycerol) around the colonies was obtained after the colonies grown on TY agar (1% tryptone, 0.5% yeast extract, 1% NaCl, 1% agar) at 37°C had been covered with 1% l-α-phosphatidylinositol substrate (Sigma) in 0.7% agarose. ALOA agar plates (AES Laboratoires) were used for the detection of Pi-PLC activity of strains that were negative on TY agar.
Virulence in chick embryos.
Fertile hens eggs were purchased from IFFA-CREDO (l'Arbresle, France). Eggs were incubated for 14 days as described by Buncic and Avery (3) at 37.5°C in a rotary egg incubator (S84 model; Grumbach, Amboise, France). Strains of L. monocytogenes were grown in BHI at 37°C to an optical density at 560 nm of 1.0 and were harvested by centrifugation at 6,000 × g for 10 min at room temperature. The cell pellets were gently suspended in phosphate-buffered saline (PBS; Dulbecco phosphate-buffered saline with 1 mg of glucose ml−1 and 36 mg of sodium pyruvate liter−1; Invitrogen, Life Technologies, Ltd., Paisley, Scotland) at pH 7.2 to obtain an initial cell density of 3 × 107 to 3 × 108 CFU ml−1. Serial dilutions of the cell suspensions were prepared in PBS, and embryos were inoculated with 0.1 ml of a 10−5 dilution via the chorioallantoic membrane after an artificial hole was bored through the shell as described previously by Buncic et al. (4). The inoculum dose was confirmed by enumeration of the viable count on ACA plates. At least five embryos were used per strain tested. Negative controls consisting of eggs inoculated with sterile PBS were added in each experiment to verify the viability of the chick embryos.
Inoculated eggs were incubated at 37.5°C horizontally, and the vitality of the embryos was monitored daily for 6 days by using transillumination (CL TH model; Grumbach, Amboise, France); death was recognized by a loss of all visible blood vessel structure, a gelling of the suspending liquids, and an absence of any reflexive movement of the embryo. The calculation of the mean time to death (MTD) was made on the basis of the mean date of death of the five eggs (38). Calculation of the mortality rate completed the appreciation of the level of virulence for each strain tested.
Culture cell line.
The human colon carcinoma cell line Caco-2 was obtained from the European Collection of Cell Cultures (ECACC no. 86010202, Salisbury, Witshire, United Kingdom). The Caco-2 cell monolayers were used between passages 40 and 50. The cells were routinely grown in 25-cm2 plastic tissue culture flasks (Greiner) at 37°C in a humidified atmosphere of 5% (vol/vol) CO2 air. The culture medium used for growth of the cell line was Dulbecco modified Eagle medium (DMEM) supplemented with 10% (vol/vol) fetal calf serum, 2 mM l-glutamine, 1% (vol/vol) nonessential amino acids, and antibiotics (100 U of penicillin ml−1 and 100 μg of streptomycin ml−1). All reagents were purchased from Invitrogen (Life Technologies).
Adhesion and invasion assays.
The ability of L. monocytogenes test strains to adhere to and invade Caco-2 cells was determined by a previously described procedure (47, 53). Semiconfluent cell monolayers were treated with trypsin and adjusted on 24-well tissue-culture plates (Greiner) to obtain almost early confluent monolayers after 3 days of incubation at 37°C (106 cells well−1). Cells were incubated in medium without antibiotics for 12 h and were washed with fresh medium just before use. After overnight growth in BHI, the listerial cells were centrifuged (6,000 × g for 10 min at room temperature) and resuspended in DMEM before infection of the Caco-2 monolayers. The Caco-2 monolayers were infected with 300 μl of the bacterial suspension (multiplicity of infection of 10 bacteria cell−1). Adhesion of L. monocytogenes cells to Caco-2 cells was allowed to occur for 30 min at 37°C. The cell monolayers were then washed five times with PBS to remove nonadherent bacteria. Adherent bacteria were harvested after lysis of the cell monolayers with 0.5 ml of Triton X-100 (1% in cold PBS) for 10 min at 4°C. The CFU values for viable bacteria were determined by plating suitable dilutions of the lysates onto ACA plates. The plates were subsequently incubated for 48 h at 37°C. For invasion assay, monolayers were infected with bacteria for 2 h at 37°C. After incubation, nonadherent bacteria were removed from the monolayers by washing them three times with PBS. Cell monolayers were then covered with fresh DMEM containing gentamicin at a bactericidal concentration (100 μg ml−1) to kill extracellular bacteria. After a 1.5-h contact time at 37°C, the rate of bacterial entry into the Caco-2 cells was determined by plate count, after washing of the Caco-2 cells with 0.5 ml of Triton X-100 (1% in cold PBS) to lyse the cells. It was considered that counts obtained 3.5 h after onset of infection represented the number of bacteria that had been internalized. Adhesion and invasion assays were done in duplicate in three independent experiments (n = 6).
Plaque forming in Caco-2 cells.
The plaque formation assay was performed as described by Sokolovic et al. (51) with the following modifications. The early confluent cell monolayers were prepared as outlined above in six-well tissue culture plates (Greiner). After overnight growth in BHI, the listerial cells were centrifuged (6,000 × g for 10 min at room temperature) and serially diluted in PBS. Caco-2 cells were infected with 0.1 ml of the 10−3, 10−4, and 10−5 dilutions, respectively, in three distinct wells. After a 2-h contact time at 37°C, nonadherent bacteria were removed from the monolayers by washing them three times with PBS and by overlaying them with 2 ml of DMEM containing 0.8% agarose, 20% fetal calf serum, 2 mM l-glutamine, and 10 μg of gentamicin ml−1. After 24 h of incubation at 37°C, a second overlay containing 0.8% agarose in DMEM, 0.01% neutral red, and 15 μg of gentamicin ml−1 was added to each well, and plaque formation was observed 24 h later.
SDS-polyacrylamide gel electrophoresis (PAGE) and Western blot analysis.
Proteins from sodium dodecyl sulfate (SDS) extracts were prepared by using the technique of Kocks et al. (27). Bacterial cultures were harvested by centrifugation (6,000 × g, 10 min at room temperature). The cell pellets were washed in 10 mM Tris-HCl (pH 8), centrifuged, and immediately resuspended in 1% (vol/vol) SDS in 10 mM Tris-HCl (pH 8). They were then incubated for 15 min with gentle shaking (1,000 rotations min−1). The suspensions were then centrifuged, and the bacterial surface proteins in the supernatants were precipitated with 16% (vol/vol) trichloroacetic acid and allowed to stand at 4°C overnight.
All precipitated protein fractions were washed twice in 90% acetone, suspended in Laemmli sample buffer (28), and heated at 95°C for 7 min prior to SDS-PAGE and Western blot analysis. SDS-PAGE was done by the method of Laemmli with 12.5% polyacrylamide gels (28). After electrophoresis, proteins were transferred to nitrocellulose sheets (Protean; Schleicher & Schuell) by using the semidry electroblotting method. Membrane hybridizations were performed with diluted (1:1,000) mouse antibodies directed against InlA (L7.7) (35). For immunodetection of the hybridized proteins, bound primary antibodies were revealed with an anti-mouse immunoglobulin-horseradish peroxidase conjugate (diluted at 1:1,000; Sigma). Peroxidase activity was detected by using an enhanced chemiluminescence kit (Amersham Pharmacia).
DNA sequencing.
For inlA from L. monocytogenes strains that produced truncated forms of InlA (LO28, H17, H32, and H34) and from reference strain Scott A, sequencing was performed on DNA fragments generated by PCR (positions 799 to 1531 of the 2,400 bp of inlA). The primers were seq01 (5′-AATCTAGCACCACGTTCGGG-3′) and seq02 (5′-TGTGACCTTCTTTTACGGGC-3′), and they generated a 733-bp gene fragment. Genome Express sequenced the PCR products in both orientations. The nucleotide sequences were deposited in the GenBank database. Accession numbers for partial sequences for inlA gene for strains LO28, H17, H32, and H34 and Scott A are AY166686, AY126441, AY126442, AY126443, and AY166685, respectively. These sequences were compared to the corresponding segment of inlA from the strain H1 (AF468816 [41]) and EGD-e (LMO00433 [15]).
RESULTS
Virulence of L. monocytogenes strains toward chick embryos.
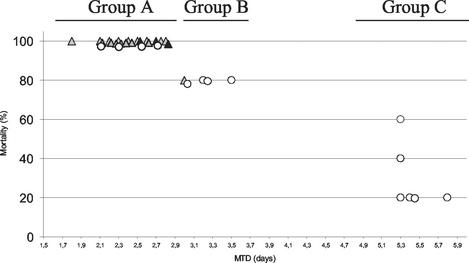
The chicken embryo model was chosen to study the virulence of L. monocytogenes strains isolated from epidemic or sporadic cases of listeriosis and from healthy patients free of signs or symptoms of listeriosis (carriage isolates) (Table 1). The inoculum dose, determined by plate count, ranged from 1.5 to 2.5 log10 CFU per egg. Inoculation of 14-day-old chick embryos via the chorioallantoic fluids distinguished highly virulent strains (group A), weakly attenuated virulent strains (group B) and strongly attenuated virulent strains (group C) (Fig. 1). Each strain was characterized by the percent mortality and the MTD.
FIG. 1.
Dot blot of mortality rate and MTD of chick embryos inoculated with 14 human asymptomatic carriage strains (○), 17 human sporadic strains (shaded triangle), and 3 epidemic-associated strains of L. monocytogenes (▴). For each strain, ca. 1.5 to 2.5 log10 CFU were inoculated into five 14-day-old chick embryos, for which the mortality was monitored daily for 6 days. Groups A, B, and C represent the three levels of virulence obtained from the chick embryos assay.
The most virulent strains against chick embryos were characterized by a 100% mortality rate with an MTD of <3 days. This group, group A, included all three epidemic strains tested (EGD-e [100%, 2.5 days], H36 [100%, 2.7 days], and Scott A [100%, 2.8 days]) and most of the sporadic strains. Four human asymptomatic carriage strains (H2 [100%, 2.1 days], H38 [100%, 2.3 days], H12 [100%, 2.6 days], and H27 [100%, 2.7 days]) showing levels of virulence similar to those of the epidemic and sporadic strains in the 14-day-old chick embryos also belonged to group A.
Group B isolates induced death in 80% of the embryos by 3 days after inoculation. The sporadic strain H5 and four human asymptomatic carriage strains (H28 [80%, 3 days], H31 [80%, 3.2 days], H35 [80%, 3.2 days], and LO28 [80%, 3.5 days]) belong to this weakly attenuated virulent group.
The last group, group C, was characterized by mortality rates ranging from 20 to 60% with the highest MTD values (>5 days). On the 14 human asymptomatic carriage strains, 6 belong to this least-virulent group of L. monocytogenes strains (H6 [60%, 5.3 days], H32 [40%, 5.3 days], H11 [20%, 5.3 days], H17 [20%, 5.4 days], H34 [20%, 5.4 days], and H1 [20%, 5.8 days]). Considering the high occurrence of human asymptomatic carriage strains exhibiting low virulence for the chicken embryo, we studied the behavior of these carriage strains in in vitro assays in an attempt to clarify the reasons behind the lack of infection in chicken embryos and in humans.
Enzymatic potential for virulence of asymptomatic carriage strains.
Virulence-associated enzymatic activities (hemolytic and phospholipase activities) (Table 1) demonstrated that all human carriage L. monocytogenes strains had activities similar to those of sporadic or epidemic-associated strains. No Pi-PLC activity was detected for H17, H19, and H24 strains with TY agar. However, additional tests for these three human sporadic and carriage strains performed with ALOA agar plates allowed the detection of Pi-PLC activity. Then, on the basis of all of these levels of enzymatic activities, the human carriage strain had the potential to be as virulent than sporadic and epidemic-associated strains.
Efficiency of human L. monocytogenes asymptomatic carriage strains in infecting Caco-2 cells.
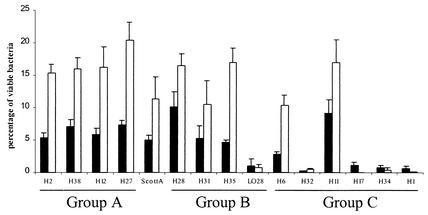
The ability of L. monocytogenes carriage strains to adhere to and enter into intestinal epithelial cells was evaluated by using an in vitro model with human enterocyte-like Caco-2 cells. The results obtained for each strain are summarized in Fig. 2. The kinetics of Caco-2 cell infection by Scott A was added as a reference epidemic strain. To take into account the differences in the initial listerial cell numbers between strains, the results are expressed as percentages of listerial adhesion to Caco-2 cells and of listerial entry into Caco-2 cells.
FIG. 2.
Listerial adhesion to and entry into Caco-2 cells. For adhesion (▪) and entry (□) rates, the number of viable bacteria was determined after 30 min and 3.5 h of infection, respectively, by cell lysis and inoculation onto ACA plates by using appropriate dilutions. Groups A, B, and C represent the three levels of virulence obtained from the chick embryos. The results are expressed as the mean percentage (of initial inoculum) of viable recovered bacteria per well from two experiments analyzed in duplicate. Vertical bars depict the standard deviation.
The most-virulent strains for chick embryos (group A) were efficient in infecting Caco-2 cells and developed higher levels of adhesion and entry than the epidemic strain Scott A. The rates of adhesion for H2, H38, H12, and H27 ranged from 5.4 to 7.3%, and the rates of entry ranged from 15.3 to 20.4%. The rates of adhesion and entry were 5 and 11.3%, respectively, for Scott A.
In group B, the kinetics of H28, H31, and H35 were similar to those of group A strains, with efficient capacities of adhesion and entry. In contrast, strain LO28 showed low adhesion and entry values (1 and 0.75%, respectively, of deposited bacteria).
Among the carriage strains with strongly attenuated virulence toward chick embryos (group C) two strains, H6 and H11, showed results similar to those of group A strains, and the four other attenuated virulence strains (H32, H17, H34, and H1) showed results that were closer to the behavior of strain LO28 than to the behavior of L. monocytogenes Scott A.
Furthermore, the plaque-forming assay allowed us to verify that there was no problem concerning the efficiency of the human carriage strains in the cell-to-cell spread process. Indeed, all L. monocytogenes tested were able to continue the infectious cycle and to form plaque of dissemination in the Caco-2 monolayers (data not shown).
We had previously observed that both LO28 and H1 were unable to enter Caco-2 efficiently and that they produced a truncated form of InlA (41), a surface protein that has been implicated in adhesion mechanisms. Considering that some other carriage strains seem to have similar behaviors toward chick embryos and Caco-2 cells, this led us to examine the synthesis of the surface protein InlA in the human asymptomatic carriage strains.
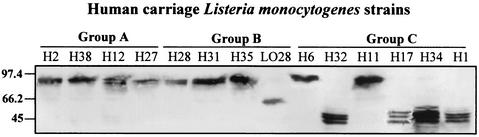
Analyses of L. monocytogenes human asymptomatic carriage strains surface protein InlA required for entry into epithelial cells.
The presence of the surface protein InlA in the human asymptomatic carriage strains was analyzed in SDS extracts by using specific monoclonal antibodies L7.7 (35) (Fig. 3). In the same way, the results obtained for each strain are summarized in Fig. 3. Although InlA was produced with the expected molecular mass of 80 kDa in the strains that were efficient in entering Caco-2 cells, strains that showed low adhesion and entry values produced truncated forms of InlA with apparent molecular masses of 63 kDa for LO28 and of 47 kDa for H17, H32, H34, and H1. Western blots performed with 10 randomly selected sporadic and epidemic virulent strains allowed us to verify that these virulent strains produced the functional 80-kDa protein InlA (data not shown).
FIG. 3.
Western blot analysis of Internalin in SDS-extract fraction of MCDB-grown cells from human carriage L. monocytogenes strains. Proteins were harvested at early stationary phase. Groups A, B, and C represent the three levels of virulence obtained from the chick embryos assay. Loadings correspond to 25 ml of initial bacterial culture. Internalin was detected with MAb L7.7 at a final dilution of 1/1,000. Molecular mass markers are indicated in kilodaltons to the left of the panel.
Comparative sequence analysis of inlA gene for the L. monocytogenes strains that produced truncated InlA.
Nucleotide sequence analysis of the partial sequence of inlA gene from strains LO28, H17, H32, H34, Scott A, and the previously published sequences for H1, EGD-e showed significant conservation (>95%) among these strains (Table 2).
TABLE 2.
Nucleotide sequence identities between partial sequences of inlA genes ( positions 799 to 1531 bp) and deduced theoretical molecular masses of InlA of L. monocytogenes strains H1, H17, H32, H34, and LO28 and the epidemic reference strains Scott A (serotype 4b) and EGD-e (serotype 1/2a)
| Strain (serotype) | % Nucleotide sequence identity (no. of nucleotide differences) (n = 733)
|
Theoretical molecular mass (kDa) of InlAa | ||
|---|---|---|---|---|
| Scott A | EGD-e LO28 | H1, H32, and H34 | ||
| Scott A (4b) | 80 | |||
| EGD-e (1/2a) | 95.91 (30) | 100.00 | 80 | |
| LO28 | 95.91 (30) | 100.00 | 63 (1636∧1637insA)b | |
| H1, H32, and H34 | 97.27 (20) | 98.23 (13) | 100.00 | 47 (1414 C→T) |
| H17 | 97.14 (21) | 98.09 (14) | 99.86 (1) | 47 (1414 C→T) |
The nucleotide change implicated in synthesis of a truncated form of InlA is given in parentheses. The position of the first translated codon is denoted as +1.
Results are from the partial sequences of inlA from Jonquieres et al. (22).
First of all, the mutation detected for strain H1 in a previous study consisting in the substitution of a cytosine for a thymidine at position 1414 was also found for strains H17, H32, and H34. This point mutation created a nonsense codon in the coding sequence, resulting in the production of proteins with a theoretical molecular mass of 47 kDa. The inlA gene partial sequence was identical for strains H1, H32, and H34. One additional nucleotide difference was observed between strains H1, H32, H34, and strain H17, but it did not affect the amino acid sequence of the InlA protein. inlA partial sequence (positions 799 to 1531) was identical for strains EGD-e and LO28 but, according to the results of Jonquieres et al. (22), an additional deletion of an adenine at position 1637 led to the creation of a nonsense codon for position 1729. This nonsense codon generated an open reading frame encoding the 63-kDa protein. The sequence of the inlA gene of L. monocytogenes strain Scott A indicated a greater divergence with identity percentages that were lower than those observed for the other strains. Most of the changes in nucleotides were silent mutations, but this polymorphism was sometimes responsible for changes in the deduced InlA amino-acid partial sequence. Finally, seven amino acids differed in the coding sequences obtained from the partial sequences of inlA of strains Scott A and EGD-e, three amino acids differed between those of Scott A and strains H1, H17, H32, and H34, and three amino acids differed between those of EGD-e and carriage strains unable to produce an InlA of 80 kDa.
DISCUSSION
This study was designed to evaluate whether some of the L. monocytogenes strains isolated from healthy patients free of signs or symptoms of listeriosis could have an attenuated ability to infect the host cells. We tested this hypothesis by using the following approaches: (i) we compared the level of virulence of 14 human carriage strains, 17 sporadic strains, and 3 epidemic-associated strains; (ii) we evaluated, for the 14 human asymptomatic carriage strains, both the adhesion and entry abilities into Caco-2 cells; and (iii) we evaluated the production of the surface protein InlA that was found to mediate invasion of enterocytes and crossing of the intestinal barrier (32).
Considering the critical role of the polymorphism identified between species in the E-cadherin amino acid sequence for InlA-mediated entry (30), the choice of the in vivo model is important to reproduce some aspects of the human listeriosis. Whereas in the mouse and in the rat bacterial translocation across the intestinal barrier is low because their E-cadherin is not a receptor for InlA (32), previous reports from our laboratory and other investigators have demonstrated that the chicken embryo model appears to be a reliable alternative for the assessment of the pathogenicity of Listeria spp. (39, 41, 52). Inoculation of 14-day-old chick embryos via the chorioallantoic membrane was also used to evaluate the pathogenic potential of 14 human asymptomatic carriage strains in comparison to those of sporadic or epidemic-associated strains of L. monocytogenes.
Interestingly, most of the human asymptomatic carriage strains were attenuated for virulence toward the chicken embryo. Indeed, 10 of the 14 human carriage strains belong to the less-virulent groups of strains (groups B and C). These findings suggest that, according to their virulent phenotype, this type of human carriage strain could constitute a particular population of L. monocytogenes strains with specific traits.
In order to characterize further these human asymptomatic carriage strains, we assessed their ability to infect human epithelial cells in vitro by using the Caco-2 cell line. As previously described for strains H1 and LO28 (22, 41), some of the strains that exhibited the lowest virulence for chicken embryo (H17, H32, and H34) seemed to have a reduced capacity to invade Caco-2 cells, and these same three strains with reduced capacity to invade Caco-2 cells produced a truncated form of InlA. For all of the strains tested, there was a good relationship between their invasiveness into Caco-2 cells and the production of truncated forms of the surface internalization protein InlA, suggesting that the production of a truncated form of InlA affects its functions in vitro. Whether or not the truncated form of InlA could be one of the factors that sentences some strains to the carriage state in vivo is a challenging issue.
Similar production of truncated forms of virulence factors has been already described in particular for ActA by Chakraborty et al. (5), Sokolovic et al. (51), and Jacquet et al. (20). By comparing the molecular mass of the proteins ActA, Ami, InlB, and listeriolysin O between strains isolated from clinical human cases or food, Jacquet et al. (20) observed that attenuated or nonvirulent strains could be associated with truncated forms of ActA, which could be considered as a good marker of L. monocytogenes virulence in humans. Considering the critical role of InlA in crossing the intestinal barrier in vivo (32) and the inability of strains with strongly truncated forms of InlA to enter host cells, we could suggest in the same way that analysis of InlA could be useful to identify virulence attenuated L. monocytogenes strains.
The inlA genes in strains H1, H32, and H34 had identical nucleotide sequences, and strain H17 had one additional nucleotide change. Taking into account that these partial sequences showed between 13 and 22 nucleotide differences in comparison to that of the corresponding segment of inlA from EGD-e and Scott A strains, these results suggest that H1, H17, H32, and H34 could be descended from a common ancestor and may maintain their identities over the years (these strains were isolated independently between 1991 and 1997). Polymorphisms in virulent genes and in the deduced amino acid sequence have been already observed, notably by Jeffers et al. (21) by using ribotyping and PCR-restriction fragment length polymorphism typing of the genes actA and hly. These studies have shown that distinct clonal groups obtained from this typing appeared to differ in their pathogenic potential. Considering the differences observed in the deduced amino acid sequences of InlA between strains Scott A and EGD-e, it would be interesting to determine whether these changes are silent or influence the pathogenic process.
Carriage strains that produced the entire form of InlA but that belong to groups B and C (H6, H11, H28, H31, and H35) were able to enter and to disseminate within Caco-2 cells efficiently. Similarly, in comparison with the sporadic and epidemic L. monocytogenes strains, these carriage strains had the virulence-associated enzymatic activities (i.e., hemolytic and phospholipase activities) required for pathogenicity. Taken together, these results suggest that thus far such carriage strains should be considered potentially pathogenic until some other factor(s) involved in the reduction of virulence in other steps of listeriosis is discovered.
The strains isolated at the carriage status and belonging to the most-virulent group A, group A (i.e., H2, H38, H12, and H27), seem to be at least as virulent as the sporadic or epidemic strains for the chicken embryo. Moreover, our results confirmed the absence of correlation between epidemic prevalence and increased virulence as has been previously described with other animal models or cell culture tests (2, 11, 18, 29, 51). Indeed, no significant differences of level of virulence were observed between the sporadic and epidemic-associated strains.
The reason why no symptoms of human listeriosis were observed with these four human carriage strains is unclear. Considering the inoculation of L. monocytogenes via chorioallantoic fluids in the present study, we can propose that these carriage strains are better equipped to survive and multiply within the chorioallantoic fluids of the chicken embryo than within the gastrointestinal tract. Furthermore, the initial dose of ingested bacteria and factors that diminished the dose in the human tract could be implicated. Resistance of the carriage strains to several sources of stress (i.e., the low-pH environment of the stomach, the antimicrobial environment of the mucosal surfaces, and the presence of bile and enzymes in the gastric fluids) has to be evaluated since significantly different behaviors have been already described for strains of clinical or meat origin (9, 10, 13).
Factors extrinsic to the bacterium could explain the discrepancies between human and chicken embryo virulence for these four human asymptomatic carriage strains. First, resident microbiota of human gastointestinal tract exert a barrier effect against invasion of enteropathogens, whereas this microbial interference did not exist in chick embryos. The nature of the receptor, E-cadherin, is also critical. In fact, oral inoculation of mice results in poor L. monocytogenes infection because the nature of the sixteenth amino acid of murine E-cadherin is different from that of human E-cadherin and results in low interaction with internalin (32). In the same way, human carriers with the germ line E-cadherin mutation could be protected from infection, resulting in an abrogation of InlA-dependent entry into human cells. Indeed, several authors have already described the existence of germ line E-cadherin mutations in general population as in families fulfilling the criteria for gastric and colon cancer (6, 42, 46). To date, 28 truncating and missense mutations have been characterized along the protein and, notably, around the calcium-binding site considered to be potentially involved in the interaction with InlA (32, 36).
Acknowledgments
We gratefully acknowledge P. Cossart (Institut Pasteur, Paris, France) for kindly providing antibodies directed against InlA (35).
This work was supported by the Ministère de la Recherche et de l'Enseignement.
Editor: A. D. O'Brien
REFERENCES
- 1.Braun, L., B. Ghebrehiwet, and P. Cossart. 2000. gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J. 19:1458-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brosch, R., B. Catimel, G. Milon, E. Buchrieser, E. Vindel, and J. Rocourt. 1993. Virulence heterogeneity of Listeria monocytogenes strains from various sources (food, human, animal) in immunocompetent mice and its association with typing characteristics. J. Food Prot. 56:296-301. [DOI] [PubMed] [Google Scholar]
- 3.Buncic, S., and S. M. Avery. 1996. Relationship between variations in pathogenicity and lag phase at 37°C of Listeria monocytogenes previously stored at 4°C. Lett. Appl. Microbiol. 23:18-22. [DOI] [PubMed] [Google Scholar]
- 4.Buncic, S., S. M. Avery, and A. R. Rogers. 1996. Listeriolysin O production and pathogenicity of non-growing Listeria monocytogenes stored at refrigeration temperature. Int. J. Food Microbiol. 31:133-147. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty, T., F. Ebel, J. Wehland, J. Dufrenne, and S. Notermans. 1994. Naturally occurring virulence-attenuated isolates of Listeria monocytogenes capable of inducing long-term protection against infection by virulent strains of homologous and heterologous serotypes. FEMS Immunol. Med. Microbiol. 10:1-10. [DOI] [PubMed] [Google Scholar]
- 6.Chun, Y. S., N. M. Lindor, T. C. Smyrk, B. T. Petersen, L. J. Burgart, P. J. Guilford, and J. H. Donohue. 2001. Germline E-cadherin gene mutations. Cancer 92:181-187. [DOI] [PubMed] [Google Scholar]
- 7.Coffey, A., F. M. Rombouts, and T. Abee. 1996. Influence of environmental parameters on phosphatidylcholine phospholipase C production in Listeria monocytogenes: a convenient method to differentiate L. monocytogenes from other Listeria species. Appl. Environ. Microbiol. 62:1252-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cossart, P., and M. Lecuit. 1998. Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands, and signaling. EMBO J. 17:3797-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotter, P. D., C. G. Gahan, and C. Hill. 2001. A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Mol. Microbiol. 40:465-475. [DOI] [PubMed] [Google Scholar]
- 10.Czuprynski, C. J., N. G. Faith, and H. Steinberg. 2002. Ability of the Listeria monocytogenes strain Scott A to cause systemic infection in mice infected by the intragastric route. Appl. Environ. Microbiol. 68:2893-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Corral, F., R. L. Buchanan, M. M. Bencivengo, and P. H. Cooke. 1990. Quantitative comparison of selected virulence associated characteristics in food and clinical isolates of Listeria. J. Food Prot. 53:1003-1009. [DOI] [PubMed] [Google Scholar]
- 12.Durst, J., and M. Zimanyi. 1976. The Listeria monocytogenes carrier state of the staff of maternity centres in nonepidemic periods. Zentbl. Bakteriol. Orig. A 234:281-283. (In German.) [PubMed] [Google Scholar]
- 13.Dykes, G. A., and S. M. Moorhead. 2000. Survival of osmotic and acid stress by Listeria monocytogenes strains of clinical or meat origin. Int. J. Food Microbiol. 56:161-166. [DOI] [PubMed] [Google Scholar]
- 14.Fujisawa, T., and M. Mori. 1994. Evaluation of media for determining hemolytic activity and that of API Listeria system for identifying strains of Listeria monocytogenes. J. Clin. Microbiol. 32:1127-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couvé, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K.-D. Entian, H. Fsihi, F. Garcia-Del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L.-M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurupkat, E. Madueno, A. Maitournam, J. Mata Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J.-C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J.-A. Vasquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 16.Goulet, V., A. Lepoutre, J. Rocourt, P. Courtieu, P. Dehaumont, and P. Veit. 1993. Epidémie de listériose en France: bilan final et résultats de l'enquète épidémiologique. Bull. Epidemiol. Hebd. 4:13-14. [Google Scholar]
- 17.Grif, K., I. Hein, M. Wagner, E. Brandl, O. Mpamugo, J. McLauchlin, M. P. Dierich, and F. Allerberger. 2001. Prevalence and characterization of Listeria monocytogenes in the feces of healthy Austrians. Wien Klin. Wochenschr. 113:737-742. [PubMed] [Google Scholar]
- 18.Herd, M., and C. Kocks. 2001. Gene fragments distinguishing an epidemic-associated strain from a virulent prototype strain of Listeria monocytogenes belong to a distinct functional subset of genes and partially cross-hybridize with other Listeria species. Infect. Immun. 69:3972-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ireton, K., B. Payrastre, and P. Cossart. 1999. The Listeria monocytogenes protein InlB is an agonist of mammalian phosphoinositide 3-kinase. J. Biol. Chem. 274:17025-17032. [DOI] [PubMed] [Google Scholar]
- 20.Jacquet, C., E. Gouin, D. Jeannel, P. Cossart, and J. Rocourt. 2002. Expression of ActA, Ami, InlB, and listeriolysin O in Listeria monocytogenes of human and food origin. Appl. Environ. Microbiol. 68:616-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeffers, G. T., J. L. Bruce, P. L. McDonough, J. Scarlett, K. J. Boor, and M. Wiedmann. 2001. Comparative genetic characterization of Listeria monocytogenes isolates from human and animal listeriosis cases. Microbiology 147:1095-1104. [DOI] [PubMed] [Google Scholar]
- 22.Jonquieres, R., H. Bierne, J. Mengaud, and P. Cossart. 1998. The inlA gene of Listeria monocytogenes LO28 harbors a nonsense mutation resulting in release of internalin. Infect. Immun. 66:3420-3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kampelmacher, E. H., W. T. Huysinga, and L. M. van Noorle Jansen. 1972. The presence of Listeria monocytogenes in feces of pregnant women and neonates. Zentbl. Bakteriol. Orig. A 222:258-262. [PubMed] [Google Scholar]
- 24.Kampelmacher, E. H., D. E. Maas, and L. M. van Noorle Jansen. 1976. Occurrence of Listeria monocytogenes in feces of pregnant women with and without direct animal contact. Zentbl. Bakteriol. Orig. A 234:238-242. [PubMed] [Google Scholar]
- 25.Kampelmacher, E. H., and L. M. van Noorle Jansen. 1972. Further studies on the isolation of L. monocytogenes in clinically healthy individuals. Zentbl. Bakteriol. Orig. A 221:70-77. (In German.) [PubMed] [Google Scholar]
- 26.Kelly, J., S. Barnass, E. Sawicka, and A. Dean. 1999. Listeria meningitis presenting in an immunocompetent adult patient. Hosp. Med. 60:140-141. [DOI] [PubMed] [Google Scholar]
- 27.Kocks, C., E. Gouin, M. Tabouret, P. Berche, H. Ohayon, and P. Cossart. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68:521-531. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli, U. K. 1970. Cleavage of a structural protein during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 29.Lammerding, A. M., K. A. Glass, A. Gendron-Fitzpatrick, and M. P. Doyle. 1992. Determination of virulence of different strains of Listeria monocytogenes and Listeria innocua by oral inoculation of pregnant mice. Appl. Environ. Microbiol. 58:3991-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lecuit, M., S. Dramsi, C. Gottardi, M. Fedor-Chaiken, B. Gumbiner, and P. Cossart. 1999. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 18:3956-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lecuit, M., H. Ohayon, L. Braun, J. Mengaud, and P. Cossart. 1997. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect. Immun. 65:5309-5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292:1722-1725. [DOI] [PubMed] [Google Scholar]
- 33.Lwin, M. M., J. De Louvois, and D. R. Hurley. 1991. Carriage of Listeria monocytogenes in pregnant women. J. Obstet. Gynaecol. 11:41-42. [Google Scholar]
- 34.MacGowan, A. P., R. J. Marshall, I. M. MacKay, and D. S. Reeves. 1991. Listeria faecal carriage by renal transplant recipients, haemodialysis patients and patients in general practice: its relation to season, drug therapy, foreign travel, animal exposure and diet. Epidemiol. Infect. 106:157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mengaud, J., M. Lecuit, M. Lebrun, F. Nato, J. C. Mazie, and P. Cossart. 1996. Antibodies to the leucine-rich repeat region of internalin block entry of Listeria monocytogenes into cells expressing E-cadherin. Infect. Immun. 64:5430-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mengaud, J., H. Ohayon, P. Gounon, R. M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 37.Muller, H. E. 1990. Listeria isolations from feces of patients with diarrhea and from healthy food handlers. Infection 18:97-99. [DOI] [PubMed] [Google Scholar]
- 38.Norrung, B., and J. K. Andersen. 2000. Variations in virulence between different electrophoretic types of Listeria monocytogenes. Lett. Appl. Microbiol. 30:228-232. [DOI] [PubMed] [Google Scholar]
- 39.Notermans, S., J. Dufrenne, P. Teunis, and T. Chackraborty. 1998. Studies on the risk assessment of Listeria monocytogenes. J. Food Prot. 61:244-248. [DOI] [PubMed] [Google Scholar]
- 40.Notermans, S. H., J. Dufrenne, M. Leimeister-Wachter, E. Domann, and T. Chakraborty. 1991. Phosphatidylinositol-specific phospholipase C activity as a marker to distinguish between pathogenic and nonpathogenic Listeria species. Appl. Environ. Microbiol. 57:2666-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olier, M., F. Pierre, J. P. Lemaitre, C. Divies, A. Rousset, and J. Guzzo. 2002. Assessment of the pathogenic potential of two Listeria monocytogenes human faecal carriage isolates. Microbiology 148(Pt. 6):1855-1862. [DOI] [PubMed] [Google Scholar]
- 42.Oliviera, C., M. C. Bordin, N. Grehan, D. Huntsman, G. Suriano, J. C. Machado, T. Kiviluoto, L. Aaltonen, E. J. Jackson, R. Seruca, and C. Caldas. 2002. Screening E-cadherin in gastric cancer families reveals germline mutations only in hereditary diffuse gastric cancer kindred. Hum. Mutat. 19:510-517. [DOI] [PubMed] [Google Scholar]
- 43.Ortel, S. 1971. Excretion of Listeria monocytogenes in the stools of healthy persons. Zentbl. Bakteriol. Orig. A 217:41-46. (In German.) [PubMed] [Google Scholar]
- 44.Rocourt, J., C. Jacquet, and A. Reilly. 2000. Epidemiology of human listeriosis and seafoods. Int. J. Food Microbiol. 62:197-209. [DOI] [PubMed] [Google Scholar]
- 45.Rousset, A., J. P. Lemaitre, and A. Delcourt. 1994. Research of Listeria monocytogenes from different sites (faecal, genital, and oropharyngeal secretions). Med. Mal. Infect. 1994:1174-1179. [Google Scholar]
- 46.Salahshor, S., H. Hou, C. B. Diep, A. Loukola, H. Zhang, T. Liu, J. Chen, L. Iselius, C. Ru, R. A. Lothe, L. Aaltonen, X. F. Sun, and A. Lindblom. 2001. A germline E-cadherin mutation in a family gastric and colon cancer. Int. J. Mol. Med. 8:439-443. [DOI] [PubMed] [Google Scholar]
- 47.Santiago, N. I., A. Zipf, and A. K. Bhunia. 1999. Influence of temperature and growth phase on expression of a 104-kilodalton Listeria adhesion protein in Listeria monocytogenes. Appl. Environ. Microbiol. 65:2765-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlech, W. F., III, P. M. Lavigne, R. A. Bortolussi, A. C. Allen, E. V. Haldane, A. J. Wort, A. W. Hightower, S. E. Johnson, S. H. King, E. S. Nicholls, and C. V. Broome. 1983. Epidemic listeriosis-evidence for transmission by food. N. Engl. J. Med. 308:203-206. [DOI] [PubMed] [Google Scholar]
- 49.Schuchat, A., K. Deaver, P. S. Hayes, L. Graves, L. Mascola, and J. D. Wenger. 1993. Gastrointestinal carriage of Listeria monocytogenes in household contacts of patients with listeriosis. J. Infect. Dis. 167:1261-1262. [DOI] [PubMed] [Google Scholar]
- 50.Shen, Y., M. Naujokas, M. Park, and K. Ireton. 2000. InlB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103:501-510. [DOI] [PubMed] [Google Scholar]
- 51.Sokolovic, Z., S. Schuller, J. Bohne, A. Baur, U. Rdest, C. Dickneite, T. Nichterlein, and W. Goebel. 1996. Differences in virulence and in expression of PrfA and PrfA-regulated virulence genes of Listeria monocytogenes strains belonging to serogroup 4. Infect. Immun. 64:4008-4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terplan, G., and S. Steinmeyer. 1989. Investigations on the pathogenicity of Listeria spp. by experimental infection of the chick embryo. Int. J. Food Microbiol. 8:277-280. [DOI] [PubMed] [Google Scholar]
- 53.Van Langendonck, N., E. Bottreau, S. Bailly, M. Tabouret, J. Marly, P. Pardon, and P. Velge. 1998. Tissue culture assays using Caco-2 cell line differentiate virulent from non-virulent Listeria monocytogenes strains. J. Appl. Microbiol. 85:337-346. [DOI] [PubMed] [Google Scholar]
- 54.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]