Abstract
An intravenous mouse infection model was used to compare the virulence of Enterococcus faecalis strains, to study bacterial localization and organ histopathology, and to examine the effects of Nramp1 and gamma interferon (IFN-γ) on the course of infection. Infection of BALB/c mice with 5 × 108 CFU of E. faecalis JH2-2, MGH-2, 418, DS16C2, or OG1X revealed the following virulence ranking (from highest to lowest): MGH-2, 418, DS16C2, JH2-2, and OG1X. Discernible differences in the number of MGH-2 and JH2-2 bacteria were observed at 7 days (168 h) in the blood (P = 0.037), at 72 h in the liver (P = 0.002), and at 8 h in the spleen (P = 0.036). At these time points, the number of MGH-2 bacteria was higher in the blood and liver while the number of JH2-2 bacteria was higher in the spleen. At 72 h, livers from MGH-2-infected mice had higher numbers of coalescing aggregates of leukocytes and a greater degree of caseous necrosis than those from JH2-2-infected mice. These results indicate a correlation between the virulence of the E. faecalis strain, the number of bacteria in the liver, and the degree of histopathology of the liver at 72 h postinfection. IFN-γ was important in E. faecalis infection, since IFN-γ gene knockout mice had reduced mortality and massive coagulative necrosis was observed in wild-type mice. The contribution of Nramp1 was unclear, since Nramp1−/− mice and the respective control mice were innately resistant to E. faecalis. The mortality of mice in this model is probably due to induction of cytokine release and massive coagulative necrosis.
Enterococcus faecalis is the third leading nosocomial isolate from patients with bacteremia (11). Bacteremia with E. faecalis is a life-threatening condition that causes death in 28 to 75% of patients (1, 14, 17, 29, 31, 37, 44, 53) and has a mortality rate of 1.7 to 20% in patients who develop endocarditis (3, 14, 32, 37, 52, 53). Bloodstream infections with E. faecalis can occur due to contamination of intravenous catheters, ascending urinary tract infections following catheterization, intravenous drug abuse, or abdominal surgery (2, 4, 12, 17, 25, 26, 31, 33). Many studies have focused on demonstrating that the presence of specific virulence factors such as aggregation substance, cytolysin, surface protein EspA, and extracellular superoxide production are closely associated with E. faecalis isolates from bacteremic patients (20, 21, 30, 42). Results from these studies suggest that the presence of these virulence factors (or a subset of these factors) may augment the ability of E. faecalis to exist in the bloodstream, since fecal isolates less frequently contain these factors.
Animal studies to determine the role of virulence factors in disease (41, 45, 46) or to study antimicrobial efficacy (5, 6, 34-36) have often relied on intraperitoneal injection of mice with E. faecalis, either alone (23) or in conjunction with a virulence adjuvant such as mucin or sterile rat fecal extracts (5, 35, 46). Preliminary studies in our laboratory have shown that the use of the intraperitoneal infection model with mucin in BALB/c mice induces a peritoneal inflammatory response that results in adherence of inflammatory cells to the outer surfaces of organs and necrosis. In addition, in our experience, injection of E. faecalis with mucin resulted in deposition of the bacteria on the surfaces of organs. The deposition of bacteria and inflammation at the organ surface precluded accurate evaluation of the bacterial burden and histopathology within the organs of infected mice (unpublished data). In this study we developed a mouse intravenous infection model and compared the virulence and histopathology induced by a more virulent E. faecalis isolate with those induced by a less virulent E. faecalis isolate. This animal model was chosen because we believed that it approximated bloodstream infections in patients following contamination of intravenous catheters or intravenous drug abuse. The bacterial burden and histopathology in organs of intravenously infected mice were examined over a 10-day infection period, and statistical analyses were performed to determine whether there was a discernible difference in the numbers of bacteria in organs from mice infected with either a more versus a less mouse-virulent E. faecalis isolate.
Previous studies in our laboratory indicated that E. faecalis isolates can persist in mouse peritoneal macrophages and suggested that macrophages play a role in E. faecalis infections (15). Therefore, the intravenous mouse infection model was also used to test whether disruption of the Nramp1 allele or the gamma interferon (IFN-γ) gene altered mouse mortality.
MATERIALS AND METHODS
Bacterial strains.
E. faecalis MGH-2 (isolate from patient with bacteremia; positive for aggregation substance and cytolysin, deficient in gelatinase) was kindly supplied by Michael Cohen (Parke-Davis Pharmaceutical Research, Division of Warner-Lambert Co., Ann Arbor, Mich.) (6). E. faecalis JH2-2 (plasmid free; deficient in aggregation substance, cytolysin, and gelatinase; a derivative of clinical strain JH2) (24), OG1X (streptomycin resistant; an aggregation substance-, cytolysin-, and gelatinase-defective strain) (22), and DS16C2 (cured of pAD2; derivative of clinical isolate DS16; positive for aggregation substance, cytolysin, and gelatinase) (13) were provided by Don Clewell (University of Michigan School of Dentistry, Ann Arbor). E. faecalis 418 (aggregation substance positive, cytolysin deficient, and gelatinase positive) was isolated in our laboratory and has been described previously (15).
Mice and reagents.
BALB/c mice (10-week-old males) were purchased from Harlan Sprague-Dawley, Indianapolis, Ind. Breeding pairs of Nramp1−/− (Bcgs Lshs Itys) mice (created on a 129/svEvTac background) and 129/svEvTac (Bcgr Lshr Ityr) resistant control mice were generously provided by P. Gros (McGill University, Montreal, Canada) (51). Progeny of Nramp1−/− mice and 129/svEvTac mice were maintained in isolator units at the Colorado State University (CSU) Laboratory Animal Resource Building. IFN-γ gene knockout (GKO) mice (10) were provided by Ian Orme (CSU, Fort Collins, Colo.). Nonhemolyzed rabbit serum was purchased from Pel-Freez Biologicals, Rogers, Ark. The rabbit serum was heat inactivated at 56°C for 1 h prior to use. Brain heart infusion (BHI) broth and agar were purchased from BD Biosciences, Bedford, Mass. Dulbecco's phosphate-buffered saline (PBS) (Ca2+ and Mg2+ free) was purchased from Life Technologies, Inc. (GIBCO BRL), Rockville, Md. Somatic cell slides were purchased from Bellco Glass, Vineland, N.J. Methylene blue and heparinized microhematocrit capillary tubes were purchased from Fisher Scientific, Pittsburgh, Pa. Costar 96-well cell culture plates were purchased from Corning Inc., Acton, Mass. Test tube plugs were purchased from VWR, West Chester, Pa. Tube and pestle sets for tissue homogenization were purchased from Glas-Col Co., Terre Haute, Ind.
Intravenous infection of mice.
E. faecalis strains were grown overnight at 37°C in 5 ml of heat-inactivated, nonhemolyzed rabbit serum under stationary conditions, with the exception of E. faecalis DS16C2 and OG1X. The latter two strains were grown in BHI broth, since they clumped when grown in rabbit serum, making accurate quantitation by the Breed count method (see below) difficult. The overnight culture was diluted 1:100 (strain MGH-2 was diluted 1:50) into 100 ml of rabbit serum or BHI broth (DS16C2 and OG1X) and incubated under the conditions described above for 16 to 20 h. The culture was concentrated by centrifugation at 14,636 × g (for 15 min at 26°C). The bacterial pellet was suspended in 2 ml of PBS, pH 7.2, to a final concentration of 109 bacteria/50 μl. Bacteria were counted by the Breed count method (7). The bacterial suspension was diluted to 5 × 108/100 μl based on the Breed slide count, and 100 μl was injected intravenously (with a 28-gauge, 0.5-in. needle) into the tail vein of each mouse. The number of bacteria for injection was derived from a series of preliminary studies in our laboratory in which groups of mice were infected with a range of 106 to 109 CFU of E. faecalis. A bacterial suspension of 5 × 108 CFU was found to be optimal for inducing a reproducible, longer-term, nonacute infection with morbidity and mortality in mice. The number of bacteria injected per mouse was confirmed by broth dilution of the inoculum and viable plate counts. Mice were observed daily for morbidity and mortality, and any mice exhibiting noticeable morbidity (scruffy coats, lethargy) were euthanized immediately by CO2 asphyxiation. Groups of 10 to 15 BALB/c and IFN-γ GKO mice were used for each experiment, and infection experiments were repeated three times. Due to the low number of Nramp1−/− and 129/svEvTac progeny, infection studies with these mice consisted of two experiments using groups of 10 Nramp1−/− and 129/svEvTac mice. The total number of dead mice was tallied at 5, 10, 15, and 20 days postinfection, and the cumulative percent mortality was determined by dividing the number of dead mice by the total number of mice and multiplying by 100. The cumulative percent mortality was plotted versus number of days postinfection by using CA-Cricket Graph III software (Computer Associates International, Inc., Islandia, N.Y.).
Localization of E. faecalis in mouse organs.
E. faecalis MGH-2 and JH2-2 were grown in rabbit serum and injected intravenously into BALB/c mice as described above, except that 50 to 60 BALB/c mice were used for each experiment. Four mice from each group were euthanized at 8, 24, 72, 168 (7 days), and 240 (10 days) h postinfection by CO2 asphyxiation. Blood, heart, liver, kidneys, and spleen were removed to quantify viable bacteria. The heart, liver, kidneys, and spleen were placed in 4.5, 9, 4.5, and 4.5 ml of PBS, pH 7.2, respectively, and homogenized to completion (i.e., a homogenous cell suspension was derived) using individually sterilized pestles attached to a Sears Craftsman 2.5-hp drill. During homogenization, tissues were placed in an ice-water bath inside a biosafety cabinet. Aliquots of each homogenate were serially diluted, plated onto BHI agar, incubated overnight at 37°C, and observed for viable colonies. Counts of viable colonies were normalized by organ weight when organs were halved and processed for histopathological examination. This experiment was repeated three times to allow for statistical analysis.
Histopathology of organs.
A portion (one-half) of the heart, liver, kidneys, and spleen from four to six mice at each time point was fixed for a minimum of 24 h in 10% buffered neutral formalin (pH 7) and submitted to the Histotechnology Laboratory at the CSU Veterinary Diagnostic Laboratory. The remainder of each organ was reserved for bacterial quantitation as described above. All organs were cut by a standardized method and placed in tissue cassettes for further processing by one individual. Slides of hematoxylin-eosin-stained tissues were prepared and observed for histopathology by microscopic examination. Histopathology results are from one experiment.
Statistical analyses of bacterial burden and histopathological lesions in organs.
The number of bacteria recovered from each organ was plotted versus time postinfection as a scattergraph using SigmaPlot 4.0 (SPSS Inc., Chicago, Ill.). Histopathological lesions were graded for severity on a scale of 0 to 4. The scores were as follows: 0, rare inflammatory cells in aggregates of less than 5, scattered randomly in the parenchyma; 1, occasional small aggregates of 5 to 15 neutrophils, lymphocytes, or macrophages in the interstitium; 2, moderate numbers of cellular aggregates of 15 to 30 neutrophils, lymphocytes, or macrophages in the interstitium with no necrosis of tissue; 3, moderate to frequent numbers of multifocal to coalescing aggregates of neutrophils, macrophages, or lymphocytes with caseous necrosis of parenchyma; 4, multifocal to coalescing aggregates of neutrophils, macrophages, or lymphocytes with caseous or coagulative necrosis of parenchyma. Bacterial burdens in the organs were analyzed by nonparametric Mann-Whitney and Kruskal-Wallis ranked-order analysis by using MINITAB software (State College, Pa.) to determine the whether there was a discernible difference between MGH-2- and JH2-2-infected mice. A P value of ≤0.05 was used as an indicator of discernible differences between experimental parameters. A P value of ≤0.1 was also included in Table 1 and throughout the Results and Discussion, since this value has been used by some investigators as an indicator of discernible differences in animal studies.
TABLE 1.
Discernible differences between bacterial counts from organs of mice infected with E. faecalis MGH-2 and JH2-2
| Organ | Time point |
Pa
|
Strain with highest viable counts | |
|---|---|---|---|---|
| Kruskal-Wallis | Mann-Whitney | |||
| Blood | 7 days | 0.035 | 0.037 | MGH-2 |
| Spleen | 8 h | 0.034 | 0.036 | JH2-2 |
| Kidney | 72 h | 0.056 | 0.059 | JH2-2 |
| Liver | 72 h | 0.002 | 0.002 | MGH-2 |
| 7 days | 0.057 | 0.060 | MGH-2 | |
| Heart | NDD | NDD | NDD | |
95% confidence level, P ≤ 0.05; 90% confidence level, P ≤ 1.0. NDD, no discernible difference; P ≥ 1.0.
RESULTS
Comparison of virulence of E. faecalis isolates.
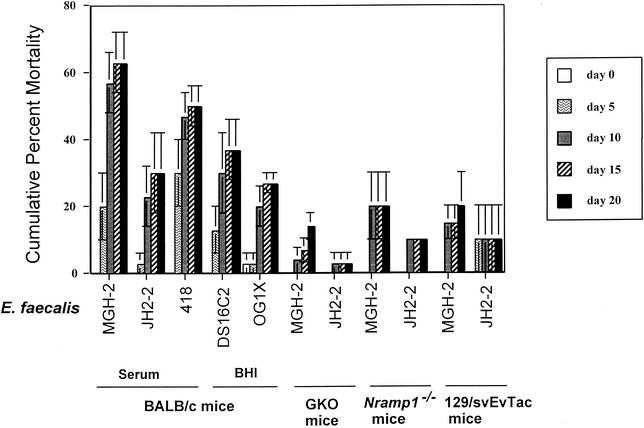
Intravenous infection of BALB/c mice with E. faecalis MGH-2, JH2-2, 418, DS16C2, or OG1X resulted in 63, 30, 50, 37, or 27% mortality over the 20-day infection period (Fig. 1). Deaths of mice occurred primarily between 7 and 10 days postinfection. Based on these initial studies, E. faecalis MGH-2 and JH2-2 were chosen for further infection studies, with MGH-2 representing the more-virulent strain and JH2-2 representing the less-virulent strain.
FIG. 1.
Mortality of BALB/c, IFN-γ GKO, Nramp1−/−, and 129/svEvTac mice after intravenous infection with E. faecalis strains. Each bar represents the cumulative percent mortality for day 0, 5, 10, 15, or 20 postinfection. The medium used for bacterial growth (rabbit serum or BHI broth) is shown below the graph. In initial infections with BALB/c mice, strains MGH-2, JH2-2, and 418 were grown in rabbit serum while E. faecalis strains DS16C2 and OG1X were grown in BHI prior to injection for the reasons given in Materials and Methods. In subsequent experiments with IFN-γ GKO, Nramp1−/−, and 129/svEvTac mice, E. faecalis MGH-2 and JH2-2 were grown in rabbit serum before injection.
Since it is well established that bacterial survival in macrophages and/or the disease course is often related to the presence of the Nramp1 protein (9, 16, 18, 19, 38, 40, 47, 48) and IFN-γ (8, 10), we infected IFN-γ GKO and Nramp1−/− mice intravenously with either a more-virulent (MGH-2) or a less-virulent (JH2-2) E. faecalis isolate and compared their mortality rates with those of their respective control mice (BALB/c and 129/svEvTac). Following infection, the mortality of the mice was observed over a 20-day infection period to determine whether loss of these genetic loci relevant to survival of bacteria in macrophages would affect the severity of disease in intravenously infected mice.
In IFN-γ GKO mice, the cumulative mortality upon infection with MGH-2 or JH2-2 was reduced from 63 or 30%, respectively, to 14 or 3.3%. Time of death was delayed in the IFN-γ GKO mice, with mouse deaths occurring at 9 days postinfection and later. Similar results were found for Nramp1−/− and 129/svEvTac mice. The cumulative percent mortality for MGH-2- and JH2-2-infected Nramp1−/− mice was 21.3 and 10%, respectively, while mortality in 129/svEvTac mice was 20 and 10%, respectively.
Organ burden in E. faecalis-infected mice.
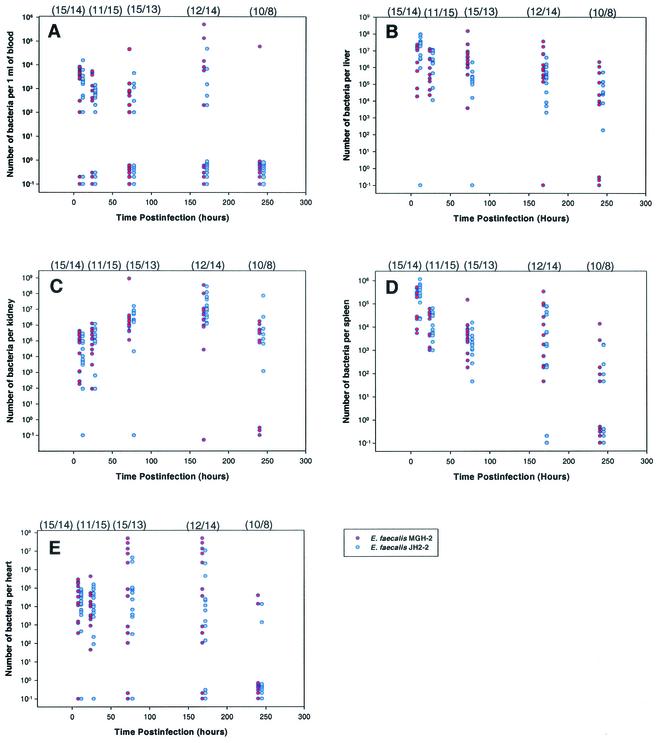
Numbers of viable bacteria in the blood, liver, heart, kidneys, and spleen following infection with either E. faecalis MGH-2 or JH2-2 were quantitated and graphed against infection time (Fig. 2). Due to variability in the numbers of recovered bacteria, the values were plotted to show bacterial counts for individual mice in specific organs. A summary of the nonparametric statistical analysis of this data is shown in Table 1. Analysis of the bacterial load in BALB/c mice revealed that at 8 h postinfection there was a discernible difference in the numbers of MGH-2 and JH2-2 bacteria recovered from the spleen (P = 0.036), with a greater recovery of JH2-2. At 72 h postinfection, there was a distinct difference (P = 0.002) in the recovery of the two strains from the liver (MGH-2 was recovered at a higher level) and a slight difference (P = 0.059) in the kidneys (higher viable counts of JH2-2). By 7 days (168 h) postinfection, the only discernible difference in the number of MGH-2 and JH2-2 bacteria was in the blood (P = 0.037), however, the number of MGH-2 bacteria was slightly higher than the number of JH2-2 bacteria (P = 0.057) in the liver. MGH-2 was present at a higher level than JH2-2 in the blood (P = 0.060). The heart was not preferentially colonized by either MGH-2 or JH2-2 over the infection period.
FIG. 2.
Scattergraph of viable bacteria recovered from organs of BALB/c mice at 8, 24, 72, 168, or 240 h postinfection. Organs from which bacteria were recovered include the blood (A), liver (B), kidney (C), spleen (D), and heart (E). Mice were infected intravenously with either E. faecalis MGH-2 (red circles) or JH2-2 (blue circles). Each circle represents the number of viable bacteria (CFU per organ or CFU per milliliter of blood) in an individual infected mouse. In some cases, where the numbers of viable bacteria in organs from different mice are almost identical, the values are not visible as individual circles. Circles below 100 on the y axis represent values below the level of detection. The numbers of mice infected with MGH-2 or JH2-2 and necropsied at each time point are given in parentheses (MGH-2/JH2-2) above the graph.
Histopathology of organs from infected mice.
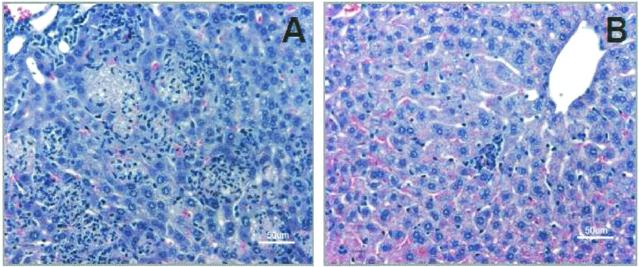
Minor histopathological lesions were observed at equivalent levels in the liver at 8 and 24 h postinfection in MGH-2- and JH2-2-infected mice (Table 2). Microscopic examination of the livers from MGH-2-infected mice at 72 h revealed moderate to frequent numbers of multifocal coalescing aggregates of leukocytes with caseous necrosis in the parenchyma (Fig. 3). Livers from mice infected with JH2-2 showed occasional small aggregates of 5 to 15 leukocytes in the interstitium of the liver; however, the number of infectious foci was reduced. These observations continued to 7 days (168 h) postinfection. Resolution of the necrotic foci in the liver was observed by 10 days (240 h) in mice infected with MGH-2, while two of five mice infected with JH2-2 had moderate to low numbers of pathological lesions. Noticeable lesions did not occur in the kidney until 24 h postinfection in mice infected with JH2-2. Mice infected with JH2-2 had minor lesions in the kidney at 24 and 72 h postinfection, while kidneys from mice infected with MGH-2 lacked histopathological lesions at this time point. However, by 7 days (168 h) postinfection, the severity of the lesions in the kidney was slightly greater in MGH-2-infected mice than in JH2-2-infected mice. At 10 days (240 h) postinfection, the lesions had almost completely resolved in the MGH-2-infected mice, while the JH2-2-infected mice had low levels of lesions in the kidney. Consistent histopathological lesions were not observed at any time in the hearts or spleens of mice infected with either MGH-2 or JH2-2. Due to the low numbers of mice whose organs were examined microscopically (one experiment; four to seven mice per time period, depending on mouse survival), these data could not be analyzed by Mann-Whitney or Kruskal-Wallis nonparametric statistics.
TABLE 2.
Histopathology scores for liver and kidney sections from mice infected with E. faecalis MGH-2 or JH2-2
| Time point | Strain (no. of mice) | No. of mice with the following histopathology scorea:
|
||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Liver | ||||||
| 8 h | MGH-2 (8) | 5 | 2 | 1 | 0 | 0 |
| JH2-2 (8) | 6 | 2 | 0 | 0 | 0 | |
| 24 h | MGH-2 (6) | 4 | 2 | 0 | 0 | 0 |
| JH2-2 (7) | 4 | 3 | 0 | 0 | 0 | |
| 72 h | MGH-2 (7) | 2 | 0 | 0 | 5 | 0 |
| JH2-2 (7) | 2 | 5 | 0 | 0 | 0 | |
| 7 days | MGH-2 (7) | 0 | 3 | 3 | 0 | 1 |
| JH2-2 (7) | 1 | 1 | 3 | 2 | 0 | |
| 10 days | MGH-2 (4) | 4 | 0 | 0 | 0 | 0 |
| JH2-2 (5) | 3 | 0 | 1 | 1 | 0 | |
| Kidney | ||||||
| 8 h | MGH-2 (8) | 8 | 0 | 0 | 0 | 0 |
| JH2-2 (8) | 8 | 0 | 0 | 0 | 0 | |
| 24 h | MGH-2 (6) | 6 | 0 | 0 | 0 | 0 |
| JH2-2 (7) | 5 | 2 | 0 | 0 | 0 | |
| 72 h | MGH-2 (7) | 7 | 0 | 0 | 0 | 0 |
| JH2-2 (7) | 2 | 5 | 0 | 0 | 0 | |
| 7 days | MGH-2 (7) | 2 | 0 | 1 | 4 | 0 |
| JH2-2 (7) | 1 | 1 | 3 | 2 | 0 | |
| 10 days | MGH-2 (4) | 3 | 1 | 0 | 0 | 0 |
| JH2-2 (5) | 2 | 1 | 1 | 1 | 0 | |
The severity of histopathology was graded as 0 to 4 for each tissue section as described in Materials and Methods.
FIG. 3.
Liver sections from BALB/c mice at 72 h after infection with E. faecalis MGH-2 (A) or JH2-2 (B). Note numerous multifocal coalescing aggregates of leukocytes and caseous necrosis in livers of mice infected with E. faecalis MGH-2. Slides were stained with hematoxylin-eosin.
DISCUSSION
An intravenous mouse model was used to compare different E. faecalis strains for virulence in BALB/c mice by measuring the percent cumulative mortality over a 20-day infection period. One obvious difference between the intravenous mouse infection model and the intraperitoneal infection model is that the latter is an acute death model, i.e., death in infected, untreated mice occurs within approximately 24 to 48 h. In the intravenous infection model, mouse mortality occurred primarily between 7 to 10 days postinfection. As with other animal models used to study the virulence of E. faecalis strains or isogenic mutants, the intravenous infection model we describe required a high dose of bacteria (5 × 108 CFU) to cause morbidity and mortality in mice. Early methods to compare E. faecalis strains for virulence in mice consisted of a mouse intraperitoneal infection model that required injection of ∼3 × 108 CFU per mouse (strain ICR) to cause acute death (within 4 to 5 h postinfection) in all infected animals (23). More recently, E. faecalis mutants have been tested for reduced virulence by injecting ICR mice intraperitoneally with the bacteria in conjunction with a 50% suspension of sterile rat fecal extract (46, 50, 54). The 50% lethal dose (LD50) of the E. faecalis strains tested in this model was 2.2 × 108 to 3.2 × 108 CFU/mouse, with the greatest mortality between 24 and 36 h. Intraperitoneal injection of mice with E. faecalis suspended in mucin has been used to induce systemic infection and to evaluate the efficacy of antimicrobial agents. In these experiments, mice were injected intraperitoneally with E. faecalis (5 to 100 LD50s, depending on the study) suspended in 5 to 20% hog gastric mucin (6, 27, 34-36, 49). These studies used CD1 or ICR mouse strains, and with the exception of one study (Cohen et al. [6]) (104 to 105 CFU/mouse), the number of bacteria injected was not reported. In addition to these models, a neutropenic mouse model was recently used to determine the effect of granulocyte colony-stimulating factor in modulating the efficacy of antibiotics (39). The LD80 of E. faecalis was 107 CFU in neutropenic Swiss Webster mice injected intraperitoneally with the bacteria in 3% mucin. From these investigations, it is apparent that a high number of E. faecalis is required to cause lethal infection in mice and that treating mice with agents to reduce their immune function, thereby mimicking disease in immunocompromised patients, could probably reduce the required dose for infection in the intravenous mouse model.
By use of the mouse intravenous infection model in this laboratory, the virulence ranking (from most to least virulent) for the E. faecalis strains tested was as follows: MGH-2, 418, DS16C2, JH2-2, and OG1X. The lack of any single virulence factor (aggregation substance, cytolysin, or gelatinase) did not affect percent mortality; however, it is interesting that MGH-2, which produces a combination of aggregation substance and cytolysin, had the highest degree of virulence (63% mortality in mice) of the strains tested. In contrast, strains 418, JH2-2, and OG1X are deficient in aggregation substance and cytolysin and were associated with reduced virulence for mice in this model. An exception to this association of known virulence factors and mouse mortality was strain DS16C2, which produces aggregation substance, cytolysin, and gelatinase but caused only 37% mortality in this model. It is noteworthy that E. faecalis strains DS16C2 and OG1X were grown in BHI broth, rather than rabbit serum, in order to reduce clumping and to allow accurate quantitation of these bacteria prior to injection. Growth in serum has been found to alter the expression of E. faecalis virulence factors, and this must be taken into consideration in evaluating the results of these experiments (28, 43). A representative virulent strain, MGH-2, and a less virulent strain, JH2-2, were used for further studies because they were readily quantitated after growth in rabbit serum.
Since previous studies in this laboratory determined that E. faecalis strains were capable of prolonged survival in mouse peritoneal macrophages, we examined the effects of lack of IFN-γ (and thus lack of macrophage activation) on mortality caused by E. faecalis strains. Transgenic mice lacking IFN-γ (IFN-γ GKO on a BALB/c background) were infected with E. faecalis MGH-2 and JH2-2 and were observed for percent cumulative mortality over a 20-day infection period. Cumulative percent mortality was significantly reduced in both MGH-2-infected IFN-γ GKO mice (4.5-fold decrease) and JH2-2-infected GKO mice (9-fold decrease) from that in infected BALB/c mice. Experiments to determine organ localization and histopathology were not performed for IFN-γ GKO mice due to a lack of available animals. Despite this limitation, the results suggest that activation of macrophages is important in the death of animals following infection with E. faecalis, and this may reflect the role of inflammatory cytokine release in causing damage during infection.
Infection of transgenic mice lacking Nramp1 with E. faecalis MGH-2 and with E. faecalis JH2-2 resulted in similar reductions in the percent cumulative mortality of infected mice (threefold reduction each for MGH-2 and JH2-2) from that for BALB/c mice. However, the cumulative percent mortality of Nramp1−/− mice infected with MGH-2 or JH2-2 was almost identical to that of the control 129/svEvTac mice. It is difficult to determine whether or not Nramp1 plays a role in death due to E. faecalis infections, since 129/svEvTac background mice have lower mortality than BALB/c mice and are similar to Nramp1−/− mice.
Since E. faecalis MGH-2 and JH2-2 differed in their abilities to cause death in BALB/c mice, the localization of these two bacterial strains and the resulting histopathology in blood and organs were investigated. The number of bacteria and severity of pathology in the liver at 72 h postinfection were the most reliable indicators of mouse mortality in this infection model. At this time point, mice infected with the more virulent MGH-2 strain had discernibly higher numbers of bacteria and more severe lesions in the liver than JH2-2-infected mice. These observations corresponded to a higher percent cumulative mortality in MGH-2-infected mice. The number of bacteria present correlated with the severity and number of histopathological lesions, suggesting that MGH-2 caused greater damage to the liver by one of the following mechanisms: (i) inducing a greater inflammatory reaction in response to bacterial infection, (ii) crossing the endothelial barrier more efficiently and therefore entering the tissues more readily and eliciting an immune response, (iii) increasing the ability of leukocytes to enter sites of infection, (iv) being less susceptible to killing by Kupffer macrophages, or (v) surviving at higher levels in the liver.
The numbers of MGH-2 and JH2-2 bacteria and the severity of lesions in the liver were similar by 7 days (168 h) postinfection. The number of bacteria in the liver remained high until 7 days (168 h) postinfection, followed by a gradual decrease in bacterial load by 10 days (240 h) postinfection. These observations suggest that during early infection, MGH-2 can multiply more rapidly or survive in the liver while JH2-2 is more readily killed in the liver. However, by 7 days (168 h) postinfection, the number of JH2-2 bacteria and the subsequent degree of histopathology approach those seen in MGH-2-infected mice.
The spleen efficiently removed both bacterial strains from the blood; however, on the first day of infection, the less virulent JH2-2 strain was present in the spleen at a higher level than the more virulent MGH-2 strain. These results suggest that JH2-2 was taken up by the spleen more readily than MGH-2 or that MGH-2 was killed more rapidly in the spleen than JH2-2. If the latter hypothesis is true, then MGH-2 was sustained or multiplied in the blood, since both JH2-2 and MGH-2 were found at approximately equivalent levels in the blood throughout the infection period.
While histopathological lesions were observed in the kidneys and heart, there was not a striking difference in the number of bacteria or the severity of lesions between MGH-2-infected and JH2-2-infected mice. Infection of the kidneys was associated with mouse mortality but was not a prognostic indicator of relative mortality, since no major differences in pathological lesions were observed between MGH-2-infected and JH2-2-infected mice. In the heart, there was more variation of bacterial counts than in other organs. Since there was not a consistent trend in histopathological lesions in the heart, it is difficult to determine whether heart colonization led to increased mouse mortality.
A high degree of variability in the number of bacteria, and thus in bacterial clearance or multiplication, was observed in the blood of individual mice infected with E. faecalis. Numbers of bacteria of both strains decreased in the blood until 7 days (168 h) postinfection, when a slight trend toward an increase in the number of MGH-2 and JH2-2 in the blood was manifest. It is noteworthy that there was a slightly higher number of the more virulent MGH-2 (versus JH2-2) bacteria in the blood at 7 days (168 h) postinfection, suggesting that MGH-2 was not controlled effectively by the liver and that spillover of the bacteria from the liver or kidneys to the blood occurred at this time point. The peak time of mortality in intravenously infected mice occurred between 7 and 10 days postinfection, and this peak time of death appears to correspond to the presence of bacteria in the blood (in the case of MGH-2) and the inflammatory response in the liver and kidneys. The histopathological results indicated that the primary cause of death in the mice was a massive coagulative necrosis that occurred primarily in the liver and kidneys.
In summary, it appears that both MGH-2 and JH2-2 are removed from the blood by the liver and spleen and are effectively controlled until day 3 (72 h) of the infection. One can speculate that during this time the more virulent strain, MGH-2, acquires the ability to survive and multiply in the liver, presumably in Kupffer macrophages. The less virulent strain, JH2-2, lags behind in its ability to survive and multiply in the liver. Between 3 and 7 days of infection, MGH-2 and JH2-2 are released from the liver into the blood, resulting in a “secondary infection” of the blood. Possibly, those bacteria that survive in the liver exhibit enhanced survival in the blood and spleen and can now effectively adhere to and colonize the kidneys and heart. The two strains infect the blood and organs equally at 7 days (168 h); however, the more virulent strain MGH-2 may cause slightly more severe histopathology in the kidneys and liver, due to unidentified factors that are expressed following multiplication in the liver. The “secondary infection” of the blood and organs at 7 days (168 h) coincides with the beginning of mouse deaths.
The hypotheses suggested in this study remain to be tested. Our results indicate that the intravenous mouse infection model is a useful tool for identifying more and less mouse-virulent E. faecalis strains and for examining the histopathology of infection in mice. This infection model should be helpful in identifying a factor(s) produced by more-virulent strains of E. faecalis that contributes to increased mortality following bacteremia.
Acknowledgments
We thank Phillipe Gros and Ian Orme for providing us with mouse strains and helpful information, Don Clewell and Michael Cohen for bacterial strains, and Joanne Turner for helpful discussions and suggestions concerning the manuscript. We also thank Mo Salman and Philip Chapman for assistance with the statistical analyses.
This work was supported by NIH grant RO1 AI43473-04.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Antalek, M. D., J. M. Mylotte, A. J. Lesse, and J. A. Sellick, Jr. 1995. Clinical and molecular epidemiology of Enterococcus faecalis bacteremia, with special reference to strains with high-level resistance to gentamicin. Clin. Infect. Dis. 20:103-109. [DOI] [PubMed] [Google Scholar]
- 2.Caballero-Granado, F. J., B. Becerril, J. M. Cisneros, L. Cuberos, I. Moreno, and J. Pachon. 2001. Case-control study of risk factors for the development of enterococcal bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 20:83-90. [DOI] [PubMed] [Google Scholar]
- 3.Caballero-Granado, F. J., B. Becerril, L. Cuberos, M. Bernabeu, J. M. Cisneros, and J. Pachon. 2001. Attributable mortality rate and duration of hospital stay associated with enterococcal bacteremia. Clin. Infect. Dis. 32:587-594. [DOI] [PubMed] [Google Scholar]
- 4.Caballero-Granado, F. J., J. M. Cisneros, R. Luque, M. Torres-Tortosa, F. Gamboa, F. Diez, J. L. Villanueva, R. Perez-Cano, J. Pasquau, D. Merino, A. Menchero, D. Mora, M. A. Lopez-Ruz, A. Vergara, et al. 1998. Comparative study of bacteremias caused by Enterococcus spp. with and without high-level resistance to gentamicin. J. Clin. Microbiol. 36:520-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chenowith, C. E., K. A. Robinson, and D. R. Schaberg. 1990. Efficacy of ampicillin versus trimethoprim-sulfamethoxazole in a mouse model of lethal enterococcal peritonitis. Antimicrob. Agents Chemother. 34:1800-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, M. A., S. L. Yoder, M. D. Huband, G. E. Roland, and C. L. Courtney. 1995. In vitro and in vivo activities of clinafloxacin, CI-990 (PD 131112), and PD 138312 versus enterococci. Antimicrob. Agents Chemother. 39:2123-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins, C. H., and P. M. Lyne (ed.). 1970. Estimating bacterial numbers, 3rd ed. University Park Press, Baltimore, Md.
- 8.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crocker, P. R., J. M. Blackwell, and D. J. Bradley. 1984. Transfer of innate resistance and susceptibility to Leishmania donovani infection in mouse radiation bone marrow chimaeras. Immunology 52:417-422. [PMC free article] [PubMed] [Google Scholar]
- 10.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 11.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Guerrero, M. L., C. Verdejo, J. Azofra, and M. de Gorgolas. 1995. Hospital-acquired infectious endocarditis not associated with cardiac surgery: an emerging problem. Clin. Infect. Dis. 20:16-23. [DOI] [PubMed] [Google Scholar]
- 13.Franke, A. E., and D. B. Clewell. 1981. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of “conjugal” transfer in the absence of a conjugative plasmid. J. Bacteriol. 145:494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrison, R. N., D. E. Fry, S. Berberich, and H. C. Polk. 1982. Enterococcal bacteremia: clinical implications and determinants of death. Ann. Surg. 196:43-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentry-Weeks, C. R., R. Karkhoff-Schweizer, A. Pikis, M. Estay, and J. M. Keith. 1999. Survival of Enterococcus faecalis in mouse peritoneal macrophages. Infect. Immun. 67:2160-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goto, Y., E. Buschman, and E. Skamene. 1989. Regulation of host resistance to Mycobacterium intracellulare in vivo and in vitro by the Bcg gene. Immunogenetics 30:218-221. [DOI] [PubMed] [Google Scholar]
- 17.Graninger, W., and R. Ragette. 1992. Nosocomial bacteremia due to Enterococcus faecalis without endocarditis. Clin. Infect. Dis. 15:49-57. [DOI] [PubMed] [Google Scholar]
- 18.Gros, P., E. Skamene, and A. Forget. 1983. Cellular mechanisms of genetically-controlled host resistance to Mycobacterium bovis (BCG). J. Immunol. 131:1966-1973. [PubMed] [Google Scholar]
- 19.Gros, P., E. Skamene, and A. Forget. 1981. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J. Immunol. 127:2417-2421. [PubMed] [Google Scholar]
- 20.Huycke, M. M., and M. S. Gilmore. 1995. Frequency of aggregation substance and cytolysin genes among enterococcal endocarditis isolates. Plasmid 34:152-156. [DOI] [PubMed] [Google Scholar]
- 21.Huycke, M. M., W. Joyce, and M. F. Wack. 1996. Augmented production of extracellular superoxide by blood isolates of Enterococcus faecalis. J. Infect. Dis. 173:743-746. [DOI] [PubMed] [Google Scholar]
- 22.Ike, Y., R. A. Craig, B. A. White, Y. Yagi, and D. B. Clewell. 1983. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc. Natl. Acad. Sci. USA 80:5369-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ike, Y., H. Hashimoto, and D. B. Clewell. 1984. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect. Immun. 45:528-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jett, B. D., M. M. Huycke, and M. S. Gilmore. 1994. Virulence of enterococci. Clin. Microbiol. Rev. 7:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karchmer, A. W. 2000. Nosocomial bloodstream infections: organisms, risk factors, and implications. Clin. Infect. Dis. 31(Suppl. 4):S139-S143. [DOI] [PubMed] [Google Scholar]
- 27.Kim, J. H., J. A. Kang, Y. G. Kim, J. W. Kim, J. H. Lee, E. C. Choi, and B. K. Kim. 1997. In vitro and in vivo antibacterial efficacies of CFC-222, a new fluoroquinolone. Antimicrob. Agents Chemother. 41:2209-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreft, B., R. Marre, U. Schramm, and R. Wirth. 1992. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect. Immun. 60:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landry, S. L., D. L. Kaiser, and R. P. Wenzel. 1989. Hospital stay and mortality attributed to nosocomial enterococcal bacteremia: a controlled study. Am. J. Infect. Control 17:323-329. [DOI] [PubMed] [Google Scholar]
- 30.Libertin, C. R., R. Dumitru, and D. S. Stein. 1992. The hemolysin/bacteriocin produced by enterococci is a marker of pathogenicity. Diagn. Microbiol. Infect. Control 15:115-120. [DOI] [PubMed] [Google Scholar]
- 31.Maki, D. G., and W. A. Agger. 1988. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine 67:248-269. [PubMed] [Google Scholar]
- 32.Megran, D. W. 1992. Enterococcal endocarditis. Clin. Infect. Dis. 15:63-71. [DOI] [PubMed] [Google Scholar]
- 33.Murray, B. E. 1990. The life and times of the Enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagarajan, R., A. A. Schabel, J. L. Occolowitz, F. T. Counter, and J. L. Ott. 1988. Synthesis and antibacterial activity of N-acyl vancomycins. J. Antibiot. 41:1430-1438. [DOI] [PubMed] [Google Scholar]
- 35.Nicas, T. I., D. L. Mullen, J. E. Flokowitsch, D. A. Preston, N. J. Snyder, R. E. Stratford, and R. D. Cooper. 1995. Activities of the semisynthetic glycopeptide LY191145 against vancomycin-resistant enterococci and other gram-positive bacteria. Antimicrob. Agents Chemother. 39:2585-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicas, T. I., D. L. Mullen, J. E. Flokowitsch, D. A. Preston, N. J. Snyder, M. J. Zweifel, S. C. Wilkie, M. J. Rodriguez, R. C. Thompson, and R. D. Cooper. 1996. Semisynthetic glycopeptide antibiotics derived from LY264826 active against vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 40:2194-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noskin, G. A., M. Till, B. K. Patterson, J. T. Clarke, and J. R. Warren. 1991. High-level gentamicin resistance in Enterococcus faecalis bacteremia. J. Infect. Dis. 164:1212-1215. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien, A. D., and E. S. Metcalf. 1982. Control of early Salmonella typhimurium growth in innately Salmonella-resistant mice does not require functional T lymphocytes. J. Immunol. 129:1349-1351. [PubMed] [Google Scholar]
- 39.Onyeji, C. O., D. P. Nicolau, C. H. Nightingale, and L. Bow. 2000. Modulation of efficacies and pharmacokinetics of antibiotics by granulocyte colony-stimulating factor in neutropenic mice with multidrug-resistant Enterococcus faecalis infection. J. Antimicrob. Chemother. 46:429-436. [DOI] [PubMed] [Google Scholar]
- 40.Plant, J. E., and A. Glynn. 1976. Genetics of resistance to infection with Salmonella typhimurium in mice. J. Infect. Dis. 133:72-78. [DOI] [PubMed] [Google Scholar]
- 41.Qin, X., K. V. Singh, G. M. Weinstock, and B. E. Murray. 2000. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect. Immun. 68:2579-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shankar, V., A. S. Baghdayan, M. M. Huycke, G. Lindahl, and M. S. Gilmore. 1999. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect. Immun. 67:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shepard, B. D., and M. S. Gilmore. 2002. Differential expression of virulence-related genes in Enterococcus faecalis in response to biological cues in serum and urine. Infect. Immun. 70:4344-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shlaes, D. M., J. Levy, and E. Wolinsky. 1981. Enterococcal bacteremia without endocarditis. Arch. Intern. Med. 16:126-129. [PubMed] [Google Scholar]
- 45.Singh, K. V., T. M. Coque, G. M. Weinstock, and B. E. Murray. 1998. In vivo testing of an Enterococcus faecalis efaA mutant and use of efaA homologs for species identification. FEMS Immunol. Med. Microbiol. 21:323-331. [DOI] [PubMed] [Google Scholar]
- 46.Singh, K. V., X. Qin, G. M. Weinstock, and B. E. Murray. 1998. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. J. Infect. Dis. 178:1416-1420. [DOI] [PubMed] [Google Scholar]
- 47.Skamene, E., P. Gros, A. Forget, P. J. Patel, and M. Nesbitt. 1984. Regulation of resistance to leprosy by chromosome 1 locus in the mouse. Immunogenetics 19:117-120. [DOI] [PubMed] [Google Scholar]
- 48.Skamene, E., E. Schurr, and P. Gros. 1998. Infection genomics: Nramp1 as a major determinant of resistance to intracellular infections. Annu. Rev. Med. 49:275-287. [DOI] [PubMed] [Google Scholar]
- 49.Song, H.-K., J.-I. Oh, Y.-Z. Kim, I.-C. Kim, and J.-H. Kwak. 1996. In-vitro and in-vivo antibacterial activity of LB10517, a novel catechol-substituted cephalosporin with a broad antibacterial spectrum. J. Antimicrob. Chemother. 37:711-726. [DOI] [PubMed] [Google Scholar]
- 50.Teng, F., L. Wang, K. V. Singh, B. E. Murray, and G. M. Weinstock. 2002. Involvement of PhoP-PhoS homologs in Enterococcus faecalis virulence. Infect. Immun. 70:1991-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vidal, S., M. L. Tremblay, G. Govoni, S. Gauthier, G. Sebastiani, D. Malo, E. Skamene, M. Olivier, S. Jothy, and P. Gros. 1995. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wells, L. D., and A. von Graevenitz. 1980. Clinical significance of enterococci in blood cultures from adult patients. Infection 8:147-151. [DOI] [PubMed] [Google Scholar]
- 53.Wells, V. D., E. S. Wong, B. E. Murray, P. E. Coudron, D. S. Williams, and S. M. Markowitz. 1992. Infections due to beta-lactamase-producing, high-level gentamicin-resistant Enterococcus faecalis. Ann. Intern. Med. 116:285-292. [DOI] [PubMed] [Google Scholar]
- 54.Xu, Y., K. V. Singh, X. Qin, B. E. Murray, and G. M. Weinstock. 2000. Analysis of a gene cluster of Enterococcus faecalis involved in polysaccharide biosynthesis. Infect. Immun. 68:815-823. [DOI] [PMC free article] [PubMed] [Google Scholar]