Abstract
The ability of Salmonella enterica serovar Typhimurium to traverse the intestinal mucosa of a host is an important step in its ability to initiate gastrointestinal disease. The majority of the genes required for this invasive characteristic are encoded on Salmonella pathogenicity island 1 (SPI1), and their expression is controlled by the transcriptional activator HilA, a member of the OmpR/ToxR family of proteins. A variety of genes (hilC, hilD, fis, sirA/barA, csrAB, phoB, fadD, envZ/ompR, fliZ, hilE, ams, lon, pag, and hha) have been identified that exert positive or negative effects on hilA expression, although the mechanisms by which these gene products function remain relatively unclear. Recent work indicates that the small DNA-binding protein, Hha, has a significant role in repressing hilA transcription and the invasive phenotype, particularly in response to osmolarity signals. We have characterized the Salmonella-specific gene, hilE, and found that it plays an important regulatory role in hilA transcription and invasion gene expression. Mutation of hilE causes derepression of hilA transcription, and overexpression of hilE superrepresses hilA expression and the invasive phenotype. Bacterial two-hybrid experiments indicate that the HilE protein interacts with HilD, suggesting a possible mechanism for HilE negative regulation of hilA gene expression and the Salmonella invasive phenotype. Finally, we have found that the hilE gene resides on a region of the serovar Typhimurium chromosome that has many characteristics of a pathogenicity island.
Salmonella enterica serovar Typhimurium is a gram-negative bacterium that primarily causes enteropathogenic infections that range from self-limiting gastroenteritis of the small intestine to systemic disease of the host lymphatic system. Pathogenic salmonellae first enter the host environment after the ingestion of contaminated food or water. Subsequently, the bacteria travel into the small intestine, where they invade the specialized M cells of the epithelium of Peyer's patches (10, 33, 50) and the absorptive enterocytes of the epithelium (58). After epithelial invasion, serovar Typhimurium organisms move quickly to the draining regional lymph nodes of the mouse before spreading to the liver and spleen, where rapid growth results in lethality (32, 39, 58). Within hosts to which serovar Typhimurium is not adapted, the bacteria remain localized to the intestinal epithelium where their presence is associated with inducing proinflammatory and cytotoxic signal transduction pathways, as well as neutrophil migration and recruitment (9, 38, 43, 46, 61).
A critical step in the establishment of Salmonella infection is the ability of the bacteria to invade the apical surface of epithelial cells within the small intestine. The entry process occurs after the bacteria bind to the surface of the host cell and induce actin rearrangements (ruffles) on the apical membrane that engulf the bacteria (21, 24). The majority of the genetic elements required for the invasive phenotype of S. enterica serovar Typhimurium (50) localize to a 40-kb region of the chromosome at centisome 63 termed Salmonella pathogenicity island 1 (SPI1) (reviewed in reference 12). Many of these SPI1 genes encode components of a type III secretion system, a system that functions by translocating specific Salmonella proteins into the host cell targeted for bacterial entry (29). Genes, both inside and outside of SPI1, encode secreted effector proteins that are responsible for inducing the host cell cytoskeletal changes that lead to uptake of the bacteria (22, 27, 28, 35, 63).
The expression of the serovar Typhimurium invasion genes is tightly regulated by a variety of environmental signals, including oxygen levels, osmolarity, pH, and phase of growth, that are believed to modulate the Salmonella invasive phenotype within the host intestinal environment (18, 25, 36, 56). The SPI1-encoded hilA gene encodes an OmpR/ToxR-like transcriptional activator that appears to play a central role in modulating the expression of the type III secretion apparatus proteins and the secreted effector proteins in response to environmental signals (5, 6). Importantly, the expression of hilA is modulated by the same environmental conditions that regulate the invasive phenotype. In addition, overexpression of hilA confers a hyperinvasive phenotype, and overexpression of hilA also counteracts the effects of repressing signals (37). Therefore, modulation of hilA expression by environmental signals appears to be a primary method of regulating the invasive phenotype of Salmonella (5, 6, 37). Results from our laboratory and from others (5, 50) reveal that null mutations in hilA cause a dramatic attenuation of invasion of tissure culture cells and M cells of the Peyer's patches, in addition to attenuating mouse virulence after oral inoculation. These results establish that hilA is required for the transcriptional activation of essential invasion genes and therefore plays a crucial role in Salmonella invasion and virulence.
Due to the central role of hilA in invasion gene activation, many groups have searched for genes that affect hilA expression. A large number of positive effectors have been identified that include hilC/sirC/sprA (17, 52, 54); hilD (54); sirA/barA (2, 30); fis (62); csrAB (2); and phoB, fadD, and fliZ (42). Two of these genes, hilC and hilD, encode AraC-like transcriptional activators that activate hilA transcription in response to specific conditions (54) and have recently been shown to bind to the upstream regulatory sequences of hilA (55). The regulatory activities of FadD, FliZ, PhoB, and EnvZ/OmpR were also recently shown to require the hilA upstream regulatory sequences (55). Additional work has revealed that EnvZ/OmpR affected the transcription of hilC but none of the identified regulators had any significant effect on hilD transcription. It has been hypothesized that these positive regulators modulate hilD posttranscriptionally or that they modulate the activity of hilA-negative regulators (41, 49). A more complete understanding of these and other positive hilA regulators awaits further experimentation.
Negative regulators of hilA have also been identified. Transposon mutagenesis has been used to identify ams, hilE, and pag as negative modulators of hilA expression (19). Another search for negative modulators of hilA transcription was conducted by introducing a S. enterica serovar Typhimurium chromosomal gene bank into a serovar Typhimurium hilA::Tn5lacZY reporter strain and screening for a decrease in the expression of the hilA reporter. That work identified the hha gene as a negative regulator of hilA since it was found to repress both hilA expression and the Salmonella invasive phenotype (20). Further work demonstrated that purified Hha protein could bind to a hilA DNA promoter fragment.
In the present study, we have focused on characterizing the negative regulator, hilE. We have found that overexpression of the hilE gene significantly represses hilA expression and a hilE mutant significantly overexpresses hilA. Correlative effects are observed on the invasive phenotype of serovar Typhimurium. A series of experiments have been performed to determine the mechanism by which HilE represses hilA expression. Efforts to demonstrate binding of purified HilE protein to the hilA promoter were unsuccessful. However, bacterial two-hybrid studies revealed a binding interaction between the HilE and HilD proteins, suggesting that the HilE mechanism of hilA regulation is through interactions with the HilD activator. Finally, we have discovered that the hilE gene resides on a region of the serovar Typhimurium chromosome that possesses the characteristics of a pathogenicity island.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in the present study are shown in Table 1. Bacteria were routinely grown in Luria broth (LB; Gibco-BRL) containing the appropriate antibiotics added at the following concentrations: ampicillin at 100 μg/ml, kanamycin at 25 μg/ml, streptomycin at 100 μg/ml, and tetracycline at 25 μg/ml. S. enterica serovar Typhimurium strains were grown in LB (1% NaCl) or TYE broth (0% NaCl) for high-osmolarity or low-osmolarity conditions, respectively. High-oxygen-repressing conditions were created by inoculating 5 ml of LB or TYE broth with 10 μl of a stationary-phase bacterial culture. The culture was shaken at 225 rpm at 37°C until an optical density at 600 nm (OD600) of 0.1 to 0.14 (∼108 CFU/ml) was reached. Low-oxygen conditions were created by inoculating 5 ml of LB or TYE broth with 10 μl of a stationary-phase culture, followed by incubation statically overnight at 37°C until an OD600 of 0.4 to 0.5 was reached, which corresponds to about 4 × 108 to 5 × 108 CFU/ml (31, 50). Strains with the hilA-lacZY reporter plasmid pLS31, the hilE-expressing plasmid pMAB60, the hilD-expressing plasmids pJB1 or pJB3, as well as other control plasmids, were grown in the presence of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or phenotypea | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH12S | mcrA Δ(mrr-hsdRMS-mcrBC) F′ lacIq ΔM15 | Gibco-BRL |
| S. enterica serovar Typhimurium | ||
| BJ66 | orgA::Tn5lacZY in SL1344 | 31 |
| BJ70 | hilA::Tn5lacZY in SL1344; Tetr | 50 |
| BJ1714 | invF::Tn5lacZY in VV302; Tetr | 62 |
| BJ1894 | orgA::Tn5lacZY in VV302; Tetr | This work |
| BJ2121 | hilE2::Tn5 in SL1344; Kanr | This work |
| BJ2390 | hilE::cam in BJ1714; Cmr | This work |
| BJ2462 | hilE::cam in SL1344; Cmr | This work |
| BJ2492 | hilA::Tn5lacZY-080 hilE::cam in SL1344; Tetr Cmr | This work |
| EE658 | hilA::Tn5lacZY-080 in SL1344; Tetr | 6 |
| TF76 | hilE2::Tn5 in BJ70; Tetr Kanr | This work |
| VV302 | ΔhilA-523 in SL1344 | 5 |
| Plasmids | ||
| pACYC184 | Cmr Tetr | 8 |
| pJB1 | pZC320 vector encoding serovar Typhimurium hilD from its own promoter; Ampr | 62 |
| pJB3 | pZC320 vector encoding serovar Typhimurium hilD driven by the lac promoter; Ampr | Jones lab |
| pLS31 | pRW50 vector encoding −497 to +420 of hilA fused to lacZY; Tetr | 54 |
| pMAB59 | pGEM-T vector carrying hilE; Ampr | This work |
| pMAB60 | pACYC derivative carrying hilE; Cmr | This work |
| pMAB62 | pZC320 carrying hilE; Ampr | This work |
| pMAB70 | pACYC184 vector in which the tet gene has been deleted; Cmr | This work |
| pMMB66EH | IncQ lacIqbla (Ampr) | 23 |
| pMRP9-1 | GFP-expressing plasmid; Cmr | E. P. Greenberg |
| pTF140 | pMMB66EH vector carrying hilE; Ampr | This work |
| pZC320 | mini-F; Ampr | 57 |
Tetr, tetracycline resistant; Kanr, kanamycin resistant; Ampr, ampicillin resistant; Cmr, chloramphenicol resistant.
Plasmid constructions.
A plasmid carrying a functional hilE gene was obtained by amplifying the hilE gene from the Salmonella chromosome with PCR by using the primers hilE8 and hilE9 (see Table 2 for primer sequences) and cloning the 553-bp PCR product into pGEM-T (Promega) to create plasmid pMAB59. The hilE gene was subcloned into the cloning vectors pMMB66EH (23) and pACYC184 (8) to create the low-copy-number hilE plasmids pTF140 and pMAB60, respectively, and into the single-copy vector pZC320 (57) to produce pMAB62. The control vector pMAB70 was created by cutting pACYC184 with BspMI and EcoRV, thereby deleting the tet gene from the original vector. The hilD-expressing plasmid pJB1 contains 1,039 bp upstream of the putative hilD translation initiation codon and the entire 930 bp encompassing the hilD coding region (62). The hilD-expressing plasmid pJB3 was created by PCR amplifying hilD, including the upstream ribosome-binding site, from S. enterica serovar Typhimurium. This PCR fragment was then cloned into the single-copy vector pZC320 so that hilD can be expressed from the lac promoter (7).
TABLE 2.
PCR primers used in this study
| Primer | Sequence |
|---|---|
| hilE3B | 5′-AAGCTTCTTCAATACCGTCCAGTT-3′ |
| hilE5′ | 5′-GGATCCTTTGCGGATTACTGCCGTT-3′ |
| hilE8 | 5′-GGATCCATACAGAGACACCAACGAAATG-3′ |
| hilE9 | 5′-CGGCCGGTCCTCATCGCCACAGCG-3′ |
| hilE5W′ | 5′-GTTATAGCAGATTGTCGGTATTTAATCTGGTATACAGAGACACCAACGAACATATGAATATCCTCCTTA-3′ |
| hilE3W′ | 5′-ATTTCGCTATACAGCATCGCCCACTGCGAGTCCGCAAGCTTGTTTTGTCCGTGTAGGCTGGAGCTGCTTC-3′ |
| hns5W′ | 5′-TCTATTATTAGCTCAACAAACCACCCCAATATAAGTTTGAGATTACTACACATATGAATATCCTCCTTA-3′ |
| hns3W′ | 5′-GGCAAAAAAAATCCCGCCAGCGGCGGGATTTTAAGCATCCAGGAAGTAAAGTCTAGGCTGGAGCTGCTTC-3′ |
Plasmid cloning vectors used in the bacterial two-hybrid experiments were pDP804 and pMS604 as described by Dmitrova et al. (15). Derivatives of these plasmids, containing HilE or HilD protein fusion constructs, were made as follows. A set of PCR primers were synthesized with which to amplify the hilE gene so that when it was cloned into pDP804 it formed a fusion protein with LexA1-87408, and another set of primers was used to amplify the hilD gene so that when cloned into pMS604 it formed a fusion protein with LexA1-87. These plasmid constructs were sequenced prior to use, confirmed to have the desired fusion protein sequence, and then introduced into the Escherichia coli reporter strain SU202 for use in the experiment.
Restriction digestions, DNA ligations, bacterial electroporations, and PCR amplifications reactions were done by using standard conditions and according to standard protocols.
Computer mapping studies and DNA sequencing.
The sequence information for S. enterica serovar Typhimurium was obtained from the Washington University School of Medicine genome sequence database (http://genome.wustl.edu/gsc/bacterial/salmonella.shtml), and the genome sequence information for S. enterica serovar Typhi came from the database maintained at the Sanger Centre (http://www.sanger.ac.uk/Projects/S_typhi/blast_server.shtml). BLAST searches (4) were conducted on sequences found at the National Center for Biotechnology Information database (http://www.ncbi.nlm). Fluorescence automated sequencing (Perkin-Elmer and the University of Iowa DNA Facility) was used to sequence hilE to verify that mutations had not been introduced into the gene sequences during the PCR amplification and cloning process.
β-Galactosidase assays.
β-Galactosidase assays were conducted on bacterial cultures by the method of Miller et al. (44).
P22-mediated transductions.
Antibiotic resistant gene insertions were moved between strains by transduction with P22 HT int− as previously described (14). Transductants were selected on LB agar containing the appropriate antibiotic and 10 mM EGTA to prevent reinfection by P22. Transductants were purified twice on LB EGTA agar prior to use of the colonies.
Tissue culture conditions and cell invasion assays.
HEp-2 tissue culture cells (47) were maintained in RPMI 1640 medium containing 10% fetal bovine serum. The cells were passaged every 2 to 4 days as needed. Invasion assays were conducted, with bacteria grown in various growth conditions, by using previously described protocols (31, 50).
Modified invasion assay and confocal imaging.
Strains expressing green fluorescent protein (GFP) were used to infect HEp-2 cells on coverslips at a multiplicity of infection of 100. After 60 min of infection, the tissue culture medium was replaced with medium containing 100 μg of gentamicin/ml and then incubated for 90 min. Next, the HEp-2 cells were extensively washed with 1× phosphate-buffered saline (PBS) and fixed to the coverslips by 4% formaldehyde treatment, washed with 1× PBS, permeabilized with 0.2% Triton X-100, and stained with rhodamine phalloidin (Molecular Probes) at a 1:500 dilution. The coverslips were washed and placed cell-side down into 3 μl of VectaShield anti-quench medium (Vector Laboratories, Inc.) on a microscope slide. A Bio-Rad MRC-600 confocal scanning laser microscope was employed to visualize the rhodamine stained HEp-2 cells and GFP-expressing bacteria and confocal images are presented as a composite of 10 to 15 sections taken in the x-y plane at 1-nm sections throughout the HEp-2 cells. In three independent experiments, the bacteria within about 100 to 150 HEp-2 cells were counted for each strain in each growth condition.
Creation of defined chromosomal mutations in the hilE genes.
Three hilE::Tn5 serovar Typhimurium mutants were previously obtained and identified by our laboratory (19). However, to eliminate the possibility of Tn5 transposon effects, a defined Typhimurium hilE mutant was constructed by using the procedure described by Datsenko and Wanner (13). Briefly, PCR primers were synthesized with 50 bp of homology to the 5′ and 3′ ends of the hilE gene. In addition, the hilE5W′ primer was synthesized so that it carried priming site 2 of pKD3 (13), and the hilE3W′ primer was synthesized so that it carried priming site 1 of pKD3 (see Table 2 for primer sequences). PCR amplification was performed with these primers by using plasmid pKD3 as the template, and the expected 1.1-kb fragment was obtained. The linear PCR fragment was purified and electroporated into SL1344 carrying pKD46 and mutants were selected on L cam plates at 37°C. Several chloramphenicol-resistant (Cmr), ampicillin-sensitive (Amps)colonies were purified and found by PCR to have the transformed fragment recombined into the hilE gene on the chromosome. Subsequently, the Cmr gene within the hilE chromosomal sequences was excised by introduction of plasmid pCP20 (temperature-sensitive replicon, Ampr), which expresses the flp recombinase gene after thermal induction. Colonies that were Cms and Amps were shown to have excised the Cmr gene by PCR, and therefore these colonies carried a complete deletion of the hilE gene in the chromosome.
RESULTS
Overexpression or mutation of hilE affects the expression of a hilA::Tn5lacZY reporter and the ability of Salmonella to invade HEp-2 cells in inducing conditions.
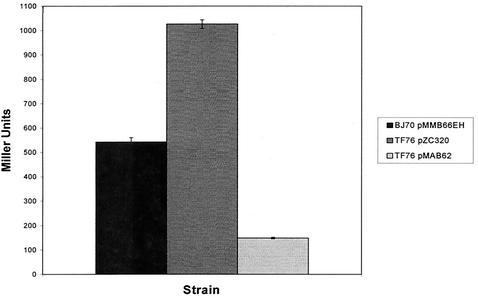
Previous work from our laboratory identified mutations in a new gene, designated hilE, that resulted in upregulation of a hilA reporter (19). In an effort to more thoroughly characterize the role of hilE in regulating Salmonella invasion gene expression, we performed experiments to measure the effect of the hilE gene on hilA expression and on the invasiveness of S. enterica serovar Typhimurium. The expression of a hilA::Tn5lacZY reporter was compared in three different strains: BJ70 (hilA::Tn5lacZY), TF76 (hilA::Tn5lacZY, hilE::Tn5) and TF76 pMAB62. Plasmid pMAB62 is a single-copy plasmid that expresses the hilE gene from the lac promoter. As shown in Fig. 1, BJ70 expressed 543 ± 17 U of β-galactosidase activity after growth in low-oxygen, high-osmolarity conditions, whereas strain TF76 expressed 1,027 ± 18 U after growth in the same conditions. Expression of hilE from the single-copy plasmid, in the absence of a functional chromosomal copy of hilE, resulted in 148 ± 3 U of β-galactosidase expression. Therefore, after growth under conditions that induce hilA the absence of hilE led to a 1.9-fold increase in hilA expression, whereas single-copy hilE expression from the lac promoter repressed hilA expression ∼3.7-fold compared to the parent strain and ∼6.9-fold compared to the hilE mutant strain.
FIG. 1.
Overexpression of hilE represses a hilA::Tn5lacZY reporter construct in S. enterica serovar Typhimurium. Strains were grown statically in low-oxygen, high-osmolarity conditions to an OD600 of ∼0.5 before we quantitated the β-galactosidase activity from each strain. Strain BJ70 pMMB66EH is the parent strain that carries the hilA::Tn5lacZY reporter. Strain TF76 is an isogenic strain of BJ70 that carries a mutation in the hilE gene. Plasmid pMAB62 is the single-copy mini-F plasmid that expresses the hilE gene from the lac promoter.
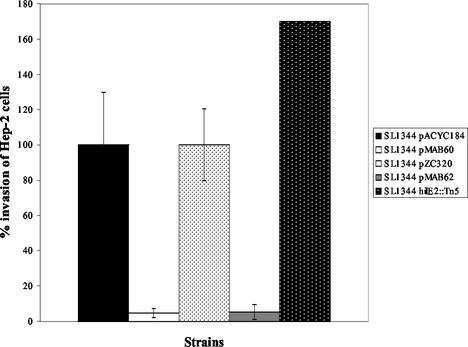
Next, experiments were performed to determine whether the effects of the hilE mutation on the ability of serovar Typhimurium to invade HEp-2 tissue culture cells would be similar to those observed on hilA::Tn5lacZY reporter expression. The invasiveness of SL1344 carrying either empty pACYC184 vector or empty pZC320, SL1344 pMAB60, SL1344 pMAB62, and SL1344 hilE2::Tn5, were assessed in a HEp-2 tissue culture cell invasion assay after growth of the bacterial strains under inducing conditions. SL1344 pZC320 and SL1344 pACYC184 are the invasive parental controls, and the invasiveness of these strains was arbitrarily set at 100% (Fig. 2). The invasiveness of SL1344 pMAB60, which carries the hilE gene on a low-copy vector, was reduced to 4.5% (∼22-fold reduction in invasion) and the invasiveness of strain SL1344 pMAB62, which carries the hilE gene on a single-copy mini-F vector, was reduced to 5.3% (∼19-fold reduction in invasion). As a control, the invasiveness of the hilE deletion strain, BJ2121, was measured and found to increase 1.7-fold to 170%, which was consistent with previous findings from our laboratory. These results establish that relatively small changes in hilE expression have significant effects on both the expression of the hilA gene and the invasive phenotype of serovar Typhimurium for tissue culture cells. These results suggest that hilE exerts a negative effect on the expression of SPI1 gene expression by reducing the levels of hilA expression which, in turn, downregulates the expression of hilA-dependent invasion genes.
FIG. 2.
Overexpression of hilE represses Salmonella tissue culture invasion after growth in inducing conditions. SL1344 is the invasive parent strain, and its invasiveness with either vector (pACYC184) or (pZC320) was compared to SL1344/pMAB60, which overexpresses hilE from the medium-copy plasmid, pACYC184, or SL1344/pMAB62, which overexpresses hilE from the single-copy plasmid, pZC320. Strains were grown in low-oxygen, high-osmolarity conditions before we assayed their ability to invade HEp-2 tissue culture cells. Invasion numbers are presented as a percentage of wild-type invasion, with wild-type invasion standardized to 100%. The SL1344 hilE2::Tn5 mutant was included as a control to show the effect of mutating the chromosomal hilE gene on Salmonella invasion.
Mutation of hilE leads to significant derepression of hilA::Tn5lacZY expression and HEp-2 cell invasion in noninducing environmental conditions.
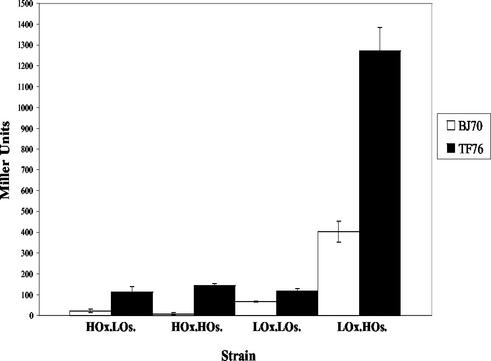
Although the experiments described above demonstrated that a hilE mutation derepressed both hilA expression and invasion after growth in inducing conditions, we were also interested in determining what effect a mutation in hilE would have on hilA expression and invasion after growth in different repressing growth conditions. The expression of β-galactosidase from the parent strain BJ70 (hilA::Tn5lacZY) and the hilE derivative TF76 (hilA::Tn5lacZY) were compared after growth in the following repressing conditions: high oxygen and low osmolarity, high oxygen and high osmolarity, and low oxygen and low osmolarity. In addition, β-galactosidase was quantitated from each strain after growth in low-oxygen, high-osmolarity conditions as a control. Under conditions in which both oxygen and osmolarity were noninducing, the hilA::Tn5lacZY reporter in BJ70 gave 22 ± 7 U, and the mutation in hilE (TF76) increased hilA::Tn5lacZY expression 113 ±27 U (a 5.1-fold increase) (Fig. 3). When osmolarity was inducing but oxygen was noninducing, the parent strain gave 9.0 ± 6 U of activity, and hilA::lacZY expression in the hilE mutant increased 12.8-fold to 145 ± 8 U. Growth of BJ70 in the noninducing condition of low oxygen and low osmolarity gave 68 ± 3 U of activity, and the β-galactosidase units from the hilE mutant (TF76) were 1.7-fold higher at 118 ± 14 U. Expression of the lacZ reporter under inducing conditons (low oxygen and high osmolarity) gave 403 ± 50 U for strain BJ70 and 1,272 ± 109 U for strain TF76, an induction of 3.2-fold. These data indicate that the hilE mutation derepresses hilA expression in all three repressing conditions examined, but the derepression is most significant in response to high-oxygen conditions, when hilA expression was derepressed 5.1- and 12.8-fold, depending on whether the osmolarity was low (repressing) or high (inducing).
FIG. 3.
Mutation of hilE derepresses hilA::Tn5lacZY expression in repressing environmental conditions. Cultures of BJ70 (hilA::Tn5lacZY) (□) or TF76 (hilA::Tn5lacZY, hilE2::Tn5) (▪) were incubated in either high-oxygen, low-osmolarity (HOx.LOs.); high-oxygen, high-osmolarity (HOx.HOs.); low-oxygen, low-osmolarity (LOx.LOs.); or low-oxygen, high-osmolarity (LOx.HOs.) conditions before we quantitated levels of β-galactosidase from each culture. These experiments were performed three times.
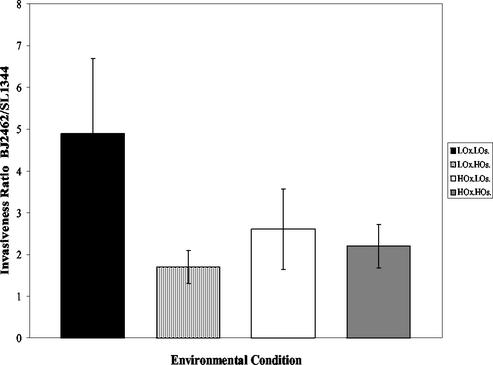
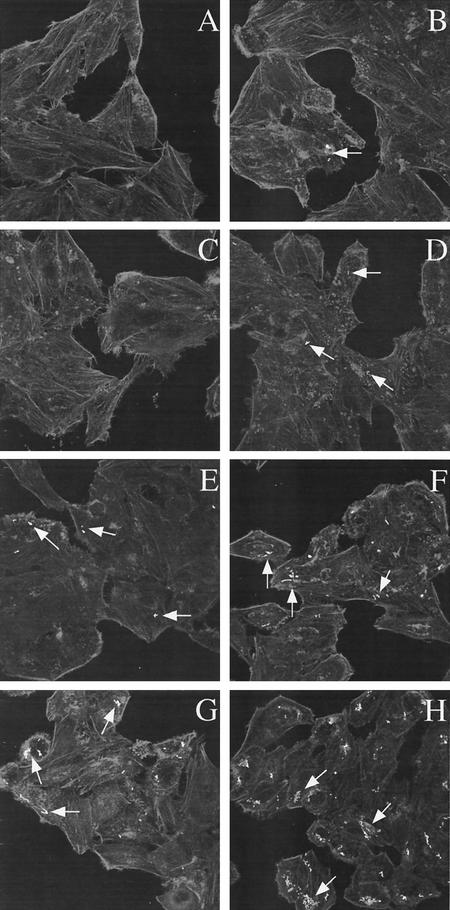
Since the hilE mutation significantly increased hilA expression in environmental conditions that were normally repressing, we examined whether the hilE mutation would increase tissue culture invasiveness after growth in repressing conditions. The invasiveness of SL1344 (parent strain) and BJ2462 (hilE) for HEp-2 tissue culture cells was compared after growth in conditions of low oxygen and low osmolarity, low oxygen and high osmolarity, high oxygen and low osmolarity, and high oxygen and high osmolarity. The invasiveness of each strain was quantitated, and the ratio of BJ2462 to SL1344 invasiveness was determined for each condition as shown in Fig. 4. Although the hilE mutant strain was more invasive for HEp-2 cells under all conditions tested, the increase in invasiveness of strain BJ2462 was not as much as we expected based upon the increase in hilA expression that we observed. Since the tissue culture invasion assay is a measure of both cellular entry and survival, we performed invasion assays with Salmonella that contained the GFP-expressing plasmid pMRP9-1, so that we could directly enumerate the bacteria that were within the tissue culture cells. Examination of the cells indicated that there were apparent differences in the number of cells containing the hilE mutant strain, as well as the numbers of bacteria within individual cells (Fig. 5). Virtually no bacteria could be detected in cells infected with SL1344 grown in conditions of high oxygen and high osmolarity or of high oxygen and low osmolarity (Fig. 5A and C), whereas it was possible to find clusters of bacteria within cells infected with BJ2462 grown in the same conditions (Fig. 5B and D). By counting 100 to 150 HEp-2 cells per environmental condition, we were able to determine that there was a 6.2-fold increase in the number of internalized BJ2462 compared to SL1344 when the bacteria were grown under high-oxygen, high-osmolarity conditions. The difference between BJ2462 and SL1344 was 15-fold when we compared bacteria that were grown under high-oxygen, low-osmolarity conditions. In low-oxygen, low-osmolarity conditions, small groups of bacteria were observed in cells infected with SL1344 (Fig. 5E), a result similar to that seen with BJ2462 grown in either high-oxygen, high-osmolarity conditions or high-oxygen, low-osmolarity conditions. In contrast, many clusters of bacteria were observed (∼6.3-fold more) for BJ2462 after growth in low-oxygen, low-osmolarity conditions (Fig. 5F). After growth in low-oxygen, high-osmolarity conditions, the difference in internalized bacteria between SL1344 and BJ2462 was ∼6.4-fold (Fig. 5G and H). The discrepancies between the invasiveness determined for strains by the gentamicin assay and that by the microscopic assay are puzzling and interesting and are currently under investigation in the laboratory.
FIG. 4.
S. enterica serovar Typhimurium with a hilE mutation is significantly more invasive in repressing environmental conditions than the wild-type invasive strain. The tissue culture invasiveness of the parent strain SL1344 and the hilE mutant BJ2462 were compared after growth in low oxygen and low osmolarity (LOx.LOs.); low oxygen and high osmolarity (LOx.HOs.), high oxygen and low osmolarity (HOx.LOs.), and high oxygen and high osmolarity (HOx.HOs.). In each experiment, the hilE mutant was more invasive than the wild-type strain, and the data are represented as a ratio of the percent invasion of BJ2462 to the percent invasion of SL1344 for each of the growth conditions shown.
FIG. 5.
Comparison of the invasiveness of strains SL1344 and BJ2462 for HEp-2 cells by using fluorescence confocal microscopy. The tissue culture invasiveness of the parent strain SL1344 and the hilE mutant BJ2462 were compared in a modified invasion assay as described in Materials and Methods after growth in high oxygen and high osmolarity (A and B), high oxygen and low osmolarity (C and D), low oxygen and low osmolarity (E and F), and low oxygen and high osmolarity (G and H). Confocal microscopy was employed to visualize the rhodamine-stained HEp-2 cells and GFP expression. In three independent experiments, the bacteria within ca. 100 to 150 HEp-2 cells were counted for each strain in each growth condition. Cells infected with SL1344 are shown in panels A, C, E, and G, and cells infected with BJ2462 are shown in panels B, D, F, and H.
Identification of the mechanism by which hilE regulates hilA expression.
In an effort to identify the mechanism by which hilE exerts its regulatory effect, we first analyzed the hilE nucleotide sequence and the HilE protein sequence by using a variety of computer programs to identify homologues and to characterize any motifs that would suggest how hilE functions. These searches failed to reveal any similarity to other gene or protein sequences present in the database except in Salmonella serovars. Next, since we had recently demonstrated that another negative regulator of hilA, Hha, bound to hilA promoter sequences (20), we tested whether purified HilE might also bind to hilA promoter sequences. Unfortunately, those efforts failed to demonstrate binding of HilE to hilA promoter sequences (data not shown).
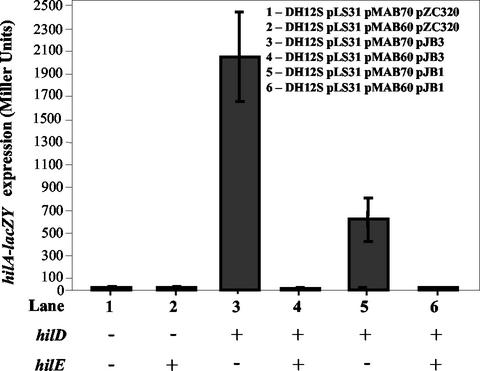
Another possible mechanism of action for the HilE repressor is by regulating transcription of the HilD activator protein. We examined this possibility by transforming plasmids encoding hilE, hilD, and/or hilA-lacZY into E. coli DH12S and measuring the levels of β-galactosidase expression from the hilA-lacZY reporter. As expected, the hilA-lacZY reporter was not expressed at significant levels in E. coli DH12S (27.0 ± 6.7 Miller units; Fig. 6, lane 1) (54). When plasmids encoding the hilD gene under the control of the lac promoter or its own promoter were introduced into this strain, high-level induction of the hilA-lacZY reporter was observed (2,051.1 ± 397.2 U [Fig. 6, lane 3] and 625.4 ± 191.1 U [Fig. 6, lane 5], respectively). However, upon introduction of pMAB60, a plasmid encoding hilE, expression of hilA-lacZY was completely repressed (Fig. 6, lanes 4 and 6) whether hilD was expressed from the lac promoter or its own promoter. This result indicates that HilE represses hilA transcription by a mechanism other than modulation of hilD transcription since HilE was able to fully repress hilA even when the HilD activator was expressed from the lac promoter.
FIG. 6.
The hilE regulator is able to repress the expression of a hilA::lacZY reporter within E. coli DH12S independently of hilD transcription. Lane 1, DH12S/pLS31/pMAB70/pZC320 (hilA::lacZY hilE hilD); lane 2: DH12S/pLS31/pMAB60/pZC320 (hilA::lacZY hilE+ hilD); lane 3, DH12S/pLS31/pMAB70/pJB3 (hilA::lacZY hilE hilD+); lane 4, DH12S/pLS31/pMAB60/pJB3 (hilA::lacZY hilE+ hilD+); lane 5, DH12S/pLS31/pMAB70/pJB1 (hilA::lacZY hilE hilD+); lane 6, DH12S/pLS31/pMAB60/pJB1 (hilA::lacZY hilE+ hilD+). pJB3 expresses hilD under the control of the lac promoter, whereas pJB1 expresses hilD under the control of its own promoter.
Two-hybrid analysis indicates that HilE and HilD interact with each other.
A system has been developed that is based upon LexA protein binding to sites upstream of the sulA gene (15). The system relies on a wild-type LexA DNA-binding domain and a mutant LexA-binding domain that, when brought together by heterologous protein-protein interactions, dimerize and bind to an altered DNA-binding site upstream of a sulA-lacZY reporter. Protein-protein binding results in repression of the sulA promoter and a significant reduction in β-galactosidase production from the sulA-lacZY reporter. Plasmids were constructed, as described in Materials and Methods, that encode a LexA1-87408-HilE fusion protein or a LexA1-87WT-HilD fusion protein. The E. coli SU202 sulA-lacZY reporter strain was transformed with both the plasmid encoding the LexA1-87408-HilE fusion protein and the LexA1-87WT-HilD fusion protein. In addition, the appropriate empty vectors were transformed into the reporter strain as controls. As seen in Table 3, the reporter strain constitutively expressed high levels of β-galactosidase (2,502 ± 47 U). When either the LexA′-HilE plasmid or the LexA-HilD plasmid was present in E. coli SU202 with only a control vector plasmid, there was no reduction in β-galactosidase activity, and in fact a slight increase in expression was observed in some of the experiments. However, when both the LexA′-HilE plasmid and the LexA-HilD plasmid were present in the E. coli reporter strain there was a 4.8-fold reduction in sulA-lacZY reporter activity. This reduction in β-galactosidase activity is indicative of a protein-protein interaction between HilE and HilD that leads to dimerization of the LexA DNA-binding proteins and repression of the sulA promoter. This system has also been used to demonstrate an interaction between the regulatory proteins FimZ and FimW that control the expression of Salmonella type 1 fimbriae (59). The interaction between FimZ and FimW was found to repress sulA-lacZY expression by ∼12-fold, indicating a stronger interaction between FimZ and FimW than between HilE and HilD.
TABLE 3.
Repression of the sulA-lacZY reporter caused by dimerization of the LexA1-87408-HilE fusion protein and the LexA1-87WT fusion protein
| Strain | Mean β-galactosidase activity from the sulA-lacZY reporter ± SD | Fold repression of β-galactosidase from sulA-lacZY (wild type vs experimental) |
|---|---|---|
| SU202 (sulA-lacZY) | 2,503 ± 47 | 1 |
| SU202/pLexA′-HilE+ pDP804 | 2,694 ± 102 | 0.93 |
| SU202/pLexA-HilD+pMS604 | 2,789 ± 98 | 0.90 |
| SU202/pLexA′-HilD pLexA-HilD | 523 ± 56 | 4.8 |
The hilE gene can repress invF expression in a hilA-independent manner.
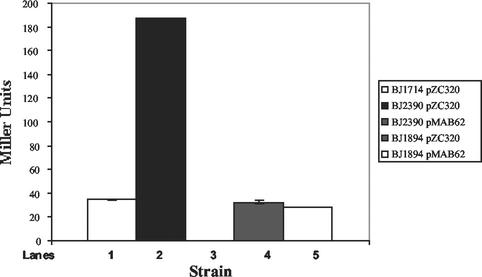
Two transcriptional regulators, hilA and invF, both encoded within SPI1, are required for activation of genes required for Salmonella invasion (5, 34). Previous work has shown that invF expression, and a subset of invasion proteins, can be activated in the absence of a functional hilA gene (16, 52). This activation appears to be HilD dependent (40). Since we have evidence that HilE may regulate invasion gene expression by binding to the HilD protein, we were interested in determining whether hilE could repress invF transcriptional activation in the absence of hilA. Such a finding would provide additional evidence that hilE regulates HilD activity. We previously constructed a Typhimurium strain that has the genotype ΔhilA invF::Tn5lacZY that was designated BJ1714. A derivative of BJ1714 was made that lacks a functional hilE gene, designated BJ2390. Both strains were grown in inducing low-oxygen, high-osmolarity conditions, and the invF::Tn5lacZY reporter was quantitated. The parent strain BJ1714 expressed the invF reporter at low levels (34.5 ± 0.2 U) and expression increased to 187.2 ± 0.6 U when a hilE mutation was present, an increase of 5.4-fold (Fig. 7). Introduction of a hilE-expressing plasmid, pMAB62, resulted in undetectable levels of β-galactosidase from the invF::Tn5lacZY reporter, a repression of >187-fold. As controls for the experiment, BJ1894/pZC320 and BJ1894/pMAB62 strains were constructed that lack hilA and carry an orgA::Tn5lacZY fusion. The orgA gene has been shown to require a functional hilA gene for expression, and these strains should be unregulated by hilE in the absence of hilA. As expected, hilE had no effect at the orgA promoter in the absence of hilA as the wild-type and hilE overexpressing strains had 32.3 ± 1.3 and 27.9 ± 0.2 U of activity, respectively. These results indicate that hilE is capable of repressing the hilA-independent activation of invF transcription, presumably by modulating HilD activity.
FIG. 7.
The hilE regulator exerts a negative influence on invF transcription in a hilA-independent manner. Strains BJ1714/pZC320 (ΔhilA invF::Tn5lacZY), BJ2390/pZC320 (ΔhilA invF::Tn5lacZY hilE::cam), BJ2390/pMAB62 (ΔhilA invF::Tn5lacZY hilE::cam hilE+), BJ1894/pZC320 (ΔhilA orgA::Tn5lacZY), and BJ1894/pMAB62 (ΔhilA orgA::Tn5lacZY, hilE+) were grown in low-oxygen, high-osmolarity growth conditions to an OD600 of ∼0.45, and then the β-galactosidase activity was quantitated for each strain.
The hilE gene resides near centisome 98 of the Salmonella chromosome on sequences that have the characteristics of a pathogenicity island.
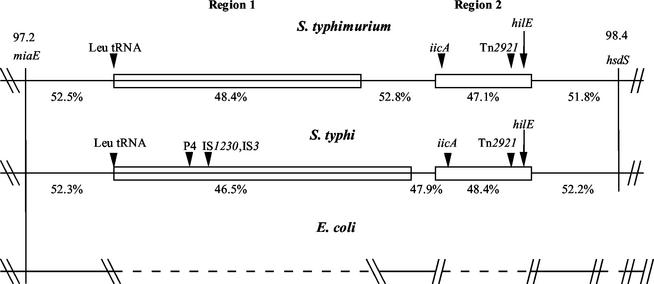
In an effort to gain understanding about the origins of the hilE regulator, the gene was mapped on the chromosomes of serovar Typhimurium and serovar Typhi by using the genomic databases available at the Washington University School of Medicine (http://genome.wustl.edu/gsc/bacterial/salmonella.shtml) and the Sanger Centre (http://www.sanger.ac.uk), respectively. It was hoped that information gleaned from neighboring genes might be used to gain additional information into the function of hilE. Mapping efforts placed the hilE gene at centisome 98 between the miaE and hsdS genes, which are separated by >40 kb (Fig. 8) (53). Analysis of this region revealed that a large portion of the sequence is found only in serovars of Salmonella. Due to this observation, we analyzed this region of the chromosome by using criteria that have been used by others to identify pathogenicity islands. The ∼40-kb region of DNA between miaE and hsdS contains two large segments of DNA that are specific for Salmonella serovars (Fig. 8). Comparison of region 1 in serovar Typhimurium (∼26 kb in size) to the same region in serovar Typhi (∼35 kb in size) revealed that these sequences bear no significant homology to one another, which was not completely surprising since their sizes differ substantially. In contrast, region 2 of both serovars (∼10 kb in size for each strain) is virtually identical (99% homologous) to each other. The hilE gene is situated near the end of region 2, nearer to the hsdS gene. One criterion that has been used for the identification of pathogenicity islands in Salmonella strains is the absence of a homologous sequence in the closely related strain, E. coli (45). We were able to establish that both regions 1 and 2 are absent in this region of the E. coli genome, since sequences that flank each side of Salmonella region 1 and Salmonella region 2 are contiguous in the E. coli chromosome. Another criterion for pathogenicity islands is whether the sequence of interest has a percent G+C content substantially different from the average G+C content of the entire chromosome, which is 52 to 54% for Salmonella strains (48). Analysis of the Typhimurium region 1 found the sequence to have a 48.4% G+C content and analysis of the Typhi region 1 revealed a 46.5% G+C content. Region 2 has percent G+C contents of 48.1% for Typhimurium and 48.4% for Typhi. A third criterion for pathogenicity islands is the presence of insertion sequence (IS) element remnants or tRNA genes that were used for the recombination of these islands into the genome (3, 26). In region 1, both serovar Typhimurium and serovar Typhi contain a Leu tRNA gene at the left end of the insert region. Serovar Typhi also contains additional IS1230 and P4 phage sequences in region 1 that may have been used for the insertion of this DNA. In region 2, both genomes contain the remnants of a Tn2921 element within the insert. Analysis of DNA sequences of region 1 and region 2, as well as the intervening sequences, revealed the presence of a large number of putative open reading frames with no known functions, in addition to some genes with known functions. The Typhi region 1 contains the fimbria-encoding sef genes previously identified within S. enterica serovar Enteriditis (11, 60). In addition to hilE, region 2 in both Typhimurium and Typhi encodes the iicA gene (for induced intracellularly A gene), which has been shown to be induced upon Salmonella internalization into host cells (51). hilE and iicA are separated by ∼6 kb of DNA on the chromosome.
FIG. 8.
Comparison of serovar Typhimurium, serovar Typhi, and E. coli chromosomal regions surrounding the hilE gene at centisome 98 on the chromosome. Regions 1 and 2 depicted in the figure are aligned for serovar Typhimurium, serovar Typhi, and E. coli. The percent G+C for each region, the positions of known genes, and the positions of the tRNA and IS elements within each region are indicated. The open boxes indicate similar sequences for region 2, and the region 1 sequences for each strain have a different pattern to note the lack of sequence similarity.
DISCUSSION
We have added additional detail here to the developing picture of Salmonella invasion gene regulation. Previous work from our laboratory has identified several negative regulatory elements that act, directly or indirectly, on hilA transcription, including hha (20) and ams, pag, and hilE (19). Other laboratories have identified a variety of positive activators, including hilC/sirC/sprA (17, 52, 54); hilD (54); sirA/barA (2, 30); fis (62); csrAB (1); and phoB, fadD, and fliZ (42). Experimental evidence is beginning to focus our understanding of how this large number of regulators function together to effectively control hilA expression in response to environmental stimuli. Recently, it was found that controlled overexpression of hilD overcomes the effects of all repressing conditions, as well as mutations in many positive effectors of hilA (42). In addition, mutations in many of the positive activators (i.e., fadD, fliA, envZ, and hilC) no longer affect hilA expression in the absence of hilD, although mutations in these genes do not signficantly affect hilD transcription. These results suggest that many of the positive activators function by affecting hilD activity posttranscriptionally. Consistent with this observation, Lucas et al. (42) noted that csrA and ams have been identified as regulators of hilA expression. Altier et al. have shown that overexpression or mutation of csrA results in reduced levels of hilD transcript, presumably by destabilization of mRNA (1). RNase E, which is encoded by ams, exerts a negative influence on hilA expression, and it is possible that it exerts its regulatory effect by selectively degrading mRNA of specific genes such as hilD and/or hilA. Fis, another identified activator of hilA, may be an exception to this proposed mechanism of regulation as preliminary data from our laboratory indicates that Fis footprints specific sequences on the hilA promoter (unpublished data).
Work on negative regulation of hilA is also beginning to yield new information. The work described here has provided clear evidence that the hilE gene is an important negative regulator of hilA expression. Our experiments reveal that overexpression of hilE from a single-copy plasmid significantly decreases hilA expression and HEp-2 invasion both in inducing and in repressing growth conditions. Conversely, deletion of the hilE gene in the chromosome leads to a significant increase in hilA expression in inducing and repressing growth conditions, particularly in high oxygen. The increase in hilA expression translates into increased invasiveness in repressing growth conditions, although the increases in invasion are not as large as we had expected. One possible explanation for this inconsistency may be that the hilE mutation makes serovar Typhimurium more susceptible to killing by the intracellular environment of cells. Future experiments will address this possibility. The hha gene has been recently identified as a negative modulator of hilA (20). Hha is a small nucleoid associated DNA-binding protein that has been shown to regulate hemolysin expression in E. coli by binding to the DNA. In addition, work from our laboratory demonstrated that purified Hha protein is able to bind to DNA upstream of the hilA gene (20). Genetic experiments have shown that a strain with a hha mutation is significantly, but not completely, derepressed for hilA expression under repressing environmental conditions, especially low osmolarity. Importantly, we have demonstrated that hilA derepression, in the hilE mutant, is most profound in high-oxygen repressing conditions. These results suggest that HilE and Hha respond to different environmental conditions to provide overlapping control of hilA transcription and expression of the invasive phenotype.
An important finding has come from our efforts to identify the mechanism of HilE repression of hilA. We were unable to demonstrate that purified HilE could bind to the hilA promoter in gel shift mobility assays. We performed genetic experiments to determine whether HilE acted by repressing hilD transcription. However, HilE was able to repress HilD-mediated activation of hilA whether hilD was transcribed from its own promoter or from the lac promoter, suggesting that HilE was not repressing hilD transcription as part of its mechanism to regulate hilA. Finally, we have found that HilE and HilD bind to each other in a two-hybrid assay. A consistent observation with this finding, also described in this report, is that HilE represses hilA-independent activation of invF. A hilA-independent activation pathway of invF and genes that invF regulates has been described (16, 52). Other workers have published work that suggests that HilD mediates this activation pathway (40). Our observation that HilE is also involved in regulating this pathway is consistent with the idea that HilE mediates its regulatory effects through protein-protein interactions with HilD.
Another aspect of hilE worth further investigation is that it appears to be a Salmonella specific regulator, similar to hilC and hilD, since we were unable to find any homologs for hilE in gene libraries. In contrast to the positive regulators of hilA, hilE is not encoded within SPI1 and instead appears to be encoded on a new island of DNA at centisome 98 with many genes of unknown function. In addition, the iicA gene (51) resides ca. 6 kb from the hilE gene. Although no specific function has been ascribed to iicA, the gene has been shown to be induced by intracellular conditions. One possibility is that, in addition to regulating invasion gene expression, hilE may play a role in iicA expression, which would provide a plausible explanation for the phenotype of the hilE mutant in the tissue culture invasion assay.
A model for invasion gene regulation through the hilA transcriptional activator has been proposed (41). Many of the positive activators of hilA expression are now believed to function by posttranscriptional modification of hilD, although the activity of the Fis protein may be more direct (62). HilC and HilD have recently been shown to bind to the hilA promoter, and it is believed that the binding of one or both of these proteins displaces repressors of hilA expression (55), although both proteins are members of the AraC family of transcriptional activators. Recent work from our lab has identified the Hha protein as a repressor of hilA that possesses the ability to bind to the hilA promoter DNA, which is consistent with the proposed model (20). In this report we have further characterized another gene, hilE, that possesses the ability to negatively regulate hilA expression. Our results indicate that HilE functions not by binding to the hilA promoter but by binding to HilD. This interaction suggests that hilA repression, at least by HilE, occurs by inhibition of HilD activity. Future work will be aimed at characterizing in detail the precise role of HilE and its interactions with HilD in the regulation of hilA and the S. enterica serovar Typhimurium invasive phenotype.
Acknowledgments
M.A.B was supported by a predoctoral fellowship on NIH training grant 5T32 GM08629, and T.F.F. was supported by a predoctoral fellowship on NIH training grant 5T32 AI07511. R.L.W. was supported by NIH postdoctoral training grant HL07638. This study was supported by National Institutes of Health grant AI38268 to B.D.J.
We thank Jennifer Boddicker for careful review of the manuscript.
Editor: V. J. DiRita
REFERENCES
- 1.Altier, C., M. Suyemoto, and S. D. Lawhon. 2000. Regulation of Salmonella enterica serovar Typhimurium invasion genes by csrA. Infect. Immun. 68:6790-6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altier, C., M. Suyemoto, A. I. Ruiz, K. D. Burnham, and R. Maurer. 2000. Characterization of two novel regulatory genes affecting Salmonella invasion gene expression. Mol. Microbiol. 35:1872-1882. [DOI] [PubMed] [Google Scholar]
- 3.Altmeyer, R. M., J. K. McNern, J. C. Bossio, I. Rosenshine, B. B. Finlay, and J. E. Galán. 1993. Cloning and molecular characterization of a gene involved in Salmonella adherence and invasion of cultured epithelial cells. Mol. Microbiol. 7:89-98. [DOI] [PubMed] [Google Scholar]
- 4.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 5.Bajaj, V., C. Hwang, and C. A. Lee. 1995. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol. Microbiol. 18:715-727. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj, V., R. L. Lucas, C. Hwang, and C. A. Lee. 1996. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol. Microbiol. 22:703-714. [DOI] [PubMed] [Google Scholar]
- 7.Boddicker, J. D., B. M. Knosp, and B. D. Jones. 2003. Transcription of the Salmonella invasion gene activator, hilA, requires HilD activation in the absence of negative regulators. J. Bacteriol. 185:525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L. M., K. Kaniga, and J. E. Galán. 1996. Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 21:1101-1115. [DOI] [PubMed] [Google Scholar]
- 10.Clark, M. A., M. A. Jepson, N. L. Simmons, and B. H. Hirst. 1994. Preferential interaction of Salmonella typhimurium with mouse Peyer's patch M cells. Res. Microbiol. 145:543-552. [DOI] [PubMed] [Google Scholar]
- 11.Clouthier, S. C., K. H. Muller, J. L. Doran, S. K. Collinson, and W. W. Kay. 1993. Characterization of three fimbrial genes. sefABC, of Salmonella enteritidis. J. Bacteriol. 175:2523-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darwin, K. H., and V. L. Miller. 1999. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin. Microbiol. Rev. 12:405-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis, R. W., D. Botstein, and J. R. Roth. 1980. Advanced bacterial genetics: a manual for genetic engineering. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 15.Dmitrova, M., G. Younes-Cauet, P. Oertel-Buchheit, D. Porte, M. Schnarr, and M. Granger-Schnarr. 1998. A new LexA-based genetic system for monitoring and analyzing protein heterodimerization in Escherichia coli. Mol. Gen. Genet. 257:205-212. [DOI] [PubMed] [Google Scholar]
- 16.Eichelberg, K., and J. E. Galán. 1999. Differential regulation of Salmonella typhimurium type III secreted proteins by pathogenicity island 1 (SPI-1)-encoded transcriptional activators InvF and hilA. Infect. Immun. 67:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichelberg, K., W. D. Hardt, and J. E. Galán. 1999. Characterization of SprA, an AraC-like transcriptional regulator encoded within the Salmonella typhimurium pathogenicity island 1. Mol. Microbiol. 33:139-152. [DOI] [PubMed] [Google Scholar]
- 18.Ernst, R. K., D. M. Dombroski, and J. M. Merrick. 1990. Anaerobiosis, type 1 fimbriae, and growth phase are factors that affect invasion of HEp-2 cells by Salmonella typhimurium. Infect. Immun. 58:2014-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahlen, T. F., N. Mathur, and B. D. Jones. 2000. Identification and characterization of mutants with increased expression of hilA, the invasion gene transcriptional activator of Salmonella typhimurium. FEMS Immunol. Med. Microbiol. 28:25-35. [DOI] [PubMed] [Google Scholar]
- 20.Fahlen, T. F., R. W. Wilson, J. D. Boddicker, and B. D. Jones. 2001. Hha is a negative modulator of hilA transcription, the Salmonella typhimurium invasion gene transcriptional activator. J. Bacteriol. 183:6620-6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis, C. L., T. A. Ryan, B. D. Jones, S. J. Smith, and S. Falkow. 1993. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364:639-642. [DOI] [PubMed] [Google Scholar]
- 22.Fu, Y., and J. E. Galán. 1998. The Salmonella typhimurium tyrosine phosphatase SptP is translocated into host cells and disrupts the actin cytoskeleton. Mol. Microbiol. 27:359-368. [DOI] [PubMed] [Google Scholar]
- 23.Fürste, J. P., W. Pansegrau, R. Frank, H. Blocker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 24.Galán, J. E. 1998. Interactions of Salmonella with host cells: encounters of the closest kind. Proc. Natl. Acad. Sci. USA 95:14006-14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galán, J. E., and R. Curtiss III. 1990. Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect. Immun. 58:1879-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groisman, E. A., and H. Ochman. 1996. Pathogenicity islands: bacterial evolution in quantum leaps. Cell 87:791-794. [DOI] [PubMed] [Google Scholar]
- 27.Hardt, W. D., L. M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galán. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93:815-826. [DOI] [PubMed] [Google Scholar]
- 28.Hong, K. H., and V. L. Miller. 1998. Identification of a novel Salmonella invasion locus homologous to Shigella ipgDE. J. Bacteriol. 180:1793-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston, C., D. A. Pegues, C. J. Hueck, A. Lee, and S. I. Miller. 1996. Transcriptional activation of Salmonella typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol. Microbiol. 22:715-727. [DOI] [PubMed] [Google Scholar]
- 31.Jones, B. D., and S. Falkow. 1994. Identification and characterization of a Salmonella typhimurium oxygen-regulated gene required for bacterial internalization. Infect. Immun. 62:3745-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones, B. D., and S. Falkow. 1996. Typhoid fever: host immune response and Salmonella virulence determinants. Annu. Rev. Immunol. 14:533-561. [DOI] [PubMed] [Google Scholar]
- 33.Jones, B. D., N. Ghori, and S. Falkow. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaniga, K., J. C. Bossio, and J. E. Galán. 1994. The Salmonella typhimurium invasion genes invF and invG encode homologues of the AraC and PulD family of proteins. Mol. Microbiol. 13:555-568. [DOI] [PubMed] [Google Scholar]
- 35.Kaniga, K., J. Uralil, J. B. Bliska, and J. E. Galán. 1996. A secreted protein tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. Mol. Microbiol. 21:633-641. [DOI] [PubMed] [Google Scholar]
- 36.Lee, C. A., and S. Falkow. 1990. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc. Natl. Acad. Sci. USA 87:4304-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, C. A., B. D. Jones, and S. Falkow. 1992. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc. Natl. Acad. Sci. USA 89:1847-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, C. A., M. Silva, A. M. Siber, A. J. Kelly, E. Galyov, and B. A. McCormick. 2000. A secreted Salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc. Natl. Acad. Sci. USA 97:12283-12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine, M. M., J. Galen, E. Barry, F. Noriega, S. Chatfield, M. Sztein, G. Dougan, and C. Tacket. 1996. Attenuated Salmonella as live oral vaccines against typhoid fever and as live vectors. J. Biotechnol. 44:193-196. [DOI] [PubMed] [Google Scholar]
- 40.Lostroh, C. P., and C. A. Lee. 2001. The HilA box and sequences outside it determine the magnitude of HilA-dependent activation of PprgH from Salmonella pathogenicity island 1. J. Bacteriol. 183:4876-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucas, R. L., and C. A. Lee. 2001. Roles of hilC and hilD in regulation of hilA expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 183:2733-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucas, R. L., C. P. Lostroh, C. C. DiRusso, M. P. Spector, B. L. Wanner, and C. A. Lee. 2000. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:1872-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCormick, B. A., P. M. Hofman, J. Kim, D. K. Carnes, S. I. Miller, and J. L. Madara. 1995. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J. Cell Biol. 131:1599-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 45.Mills, D. M., V. Bajaj, and C. A. Lee. 1995. A 40-kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol. Microbiol. 15:749-759. [DOI] [PubMed] [Google Scholar]
- 46.Monack, D. M., B. Raupach, A. E. Hromockyj, and S. Falkow. 1996. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. USA 93:9833-9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore, A. E., L. Sabachewsky, and H. W. Toolan. 1955. Culture characteristics of four permanent lines of human cancer cells. Cancer Res. 15:598. [PubMed] [Google Scholar]
- 48.Muto, A., and S. Osawa. 1987. The guanine and cytosine content of genomic DNA and bacterial evolution. Proc. Natl. Acad. Sci. USA 84:166-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olekhnovich, I. N., and R. J. Kadner. 2002. DNA-binding activities of the HilC and HilD virulence regulatory proteins of Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:4148-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Penheiter, K. L., N. Mathur, D. Giles, T. Fahlen, and B. D. Jones. 1997. Non-invasive Salmonella typhimurium mutants are avirulent because of an inability to enter and destroy M cells of ileal Peyer's patches. Mol. Microbiol. 24:697-709. [DOI] [PubMed] [Google Scholar]
- 51.Pfeifer, C. G., S. L. Marcus, M. O. Steele, L. A. Knodler, and B. B. Finlay. 1999. Salmonella typhimurium virulence genes are induced upon bacterial invasion into phagocytic and nonphagocytic cells. Infect. Immun. 67:5690-5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rakeman, J. L., H. R. Bonifield, and S. I. Miller. 1999. A HilA-independent pathway to Salmonella typhimurium invasion gene transcription. J. Bacteriol. 181:3096-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanderson, K. E., A. Hessel, and K. E. Rudd. 1995. Genetic map of Salmonella typhimurium, edition VIII. Microbiol. Rev. 59:241-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schechter, L. M., S. M. Damrauer, and C. A. Lee. 1999. Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol. Microbiol. 32:629-642. [DOI] [PubMed] [Google Scholar]
- 55.Schechter, L. M., and C. A. Lee. 2001. AraC/XylS family members, HilC and HilD, directly bind and derepress the Salmonella typhimurium hilA promoter. Mol. Microbiol. 40:1289-1299. [DOI] [PubMed] [Google Scholar]
- 56.Schiemann, D. A., and S. R. Shope. 1991. Anaerobic growth of Salmonella typhimurium results in increased uptake by Henle 407 epithelial and mouse peritoneal cells in vitro and repression of a major outer membrane protein. Infect. Immun. 59:437-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi, J., and D. P. Biek. 1995. A versatile low-copy-number cloning vector derived from plasmid F. Gene 164:55-58. [DOI] [PubMed] [Google Scholar]
- 58.Takeuchi, A. 1967. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am. J. Pathol. 50:109-136. [PMC free article] [PubMed] [Google Scholar]
- 59.Tinker, J. K., L. S. Hancox, and S. Clegg. 2001. FimW is a negative regulator affecting type 1 fimbrial expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 183:435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turcotte, C., and M. J. Woodward. 1993. Cloning, DNA nucleotide sequence, and distribution of the gene encoding the SEF14 fimbrial antigen of Salmonella enteritidis. J. Gen. Microbiol. 139:1477-1485. [DOI] [PubMed] [Google Scholar]
- 61.Wallis, T. S., and E. E. Galyov. 2000. Molecular basis of Salmonella-induced enteritis. Mol. Microbiol. 36:997-1005. [DOI] [PubMed] [Google Scholar]
- 62.Wilson, R. L., S. J. Libby, A. M. Freet, J. D. Boddicker, T. F. Fahlen, and B. D. Jones. 2001. Fis, a DNA nucleoid-associated protein, is involved in Salmonella typhimurium SPI-1 invasion gene expression. Mol. Microbiol. 39:79-88. [DOI] [PubMed] [Google Scholar]
- 63.Wood, M. W., R. Rosqvist, P. B. Mullan, M. H. Edwards, and E. E. Galyov. 1996. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol. Microbiol. 22:327-338. [DOI] [PubMed] [Google Scholar]