Abstract
Innate immunity as a first defense is indispensable for host survival against infectious agents. We examined the roles of natural killer (NK) T cells in defense against Trypanosoma cruzi infection. The T. cruzi parasitemia and survival of CD1d-deficient mice exhibited no differences compared to wild-type littermates. NK T-cell activation induced by administering α-galactosylceramide (α-GalCer) to T. cruzi-infected mice significantly changed the parasitemia only in the late phase of infection and slightly improved survival when mice were infected intraperitoneally. The combined usage of α-GalCer and benznidazole, a commercially available drug for Chagas' disease, did not enhance the therapeutic efficacy of benznidazole. These results suggest that NK T cells do not play a pivotal role in resistance to T. cruzi infection. In addition, we found that the coadministration of α-GalCer with DNA vaccine impaired the induction of epitope-specific CD8+ T cells and undermined the DNA vaccine-induced protective immunity against T. cruzi. Our results, in contrast to previous reports demonstrating the protective roles of NK T cells against other infectious agents, suggest that these cells might even exhibit adverse effects on vaccine-mediated protective immunity.
Trypanosoma cruzi is the etiological agent that causes Chagas' disease in Central and South America (8, 22, 23, 36). As it invades and replicates inside essentially all types of cells in mammalian hosts, various immune effector cells must join to contain the devastating infection and to suppress the formation of systemic pathologies. Particularly in the acute phase, both innate immunity, including neutrophils, macrophages, and natural killer (NK) cells (3, 5, 9, 10), and acquired immunity, including CD8+ and CD4+ T cells (10, 31, 38, 39, 45, 46, 47), are orchestrated to mount the protective immune responses of the host.
NK T cells are an immune cell population that was first identified relatively recently (2, 41). They exhibit several characteristic features, including unique localization, i.e., they localize mainly in the liver, spleen, and thymus (2, 41). The discovery of a specific ligand for NK T-cell activation, α-galactosylceramide (α-GalCer), facilitated the analyses of the immunological function of NK T cells in vivo (21, 24, 25). They secrete vast amounts of cytokines in a CD1-restricted manner (15, 42) and effectively exert protective immune responses against tumors (21, 34) and infectious diseases (13, 18, 20). Both gamma interferon (IFN-γ) and interleukin-4 (IL-4) cytokines secreted by NK T cells could accelerate the induction and maintenance of effective CD8+-T-cell responses (1, 7). In addition to the strong protective immune responses exerted by the direct activation of NK T cells, their potent adjuvant effect in enhancing CD8+-T-cell-mediated immunity was demonstrated when immunogens were coadministered together with α-GalCer (14). This effect could revolutionize vaccine strategy, since CD8+ T cells are one of the most important effector cell populations for containing intracellular infectious agents (19, 30, 31, 33, 35).
However, the immunological roles of NK T cells in defense against infectious diseases were mostly elucidated in diseases that primarily cause pathologies in restricted organs, such as liver, where NK T cells are intensely localized. There are few reports concerning their function in infections resulting in systemic diseases affecting all organs. In addition, there are conflicting reports on the roles of NK T cells with regard to CD8+-T-cell induction with NK T cells reported either to enhance (14) or suppress (32, 48) CD8+-T-cell responses.
We therefore decided to try to answer these questions by elucidating the immunological function of NK T cells in systemic T. cruzi infection. It was recently demonstrated that NK T cells can limit parasitemia and augment antibody response (11, 12); however, immunological function affecting T-cell-mediated immunity has not yet been characterized. We report here that NK T cells might play only minimal roles in defense against T. cruzi infection. On the contrary, we found that they can impair the induction of CD8+-T-cell responses and abolish vaccine-induced protective immunity. Our results are in contrast to those of previous reports demonstrating the potent protective roles of NK T cells in infections (13, 18, 20) and tumors (21, 34), as well as the potent adjuvant effect of α-GalCer in enhancing T-cell-mediated immunity (14).
MATERIALS AND METHODS
Animals and parasite.
Female C57BL/6 (B6) (H-2b) and BALB/c (H-2d) mice, 5 to 8 weeks of age, were purchased from Japan SLC (Hamamatsu, Shizuoka, Japan). CD1-deficient mice, either from a BALB/c background or from a B6 background (CD1d−/−), were obtained from Luc Van Kaer at the Department of Microbiology and Immunology, Howard Hughes Medical Institute, Vanderbilt University School of Medicine (Nashville, Tenn.). Blood-form trypomastigotes of T. cruzi Tulahuen strain (28) were maintained in outbred CD1 or inbred BALB/c mice by intramuscular (i.m.) inoculation of 5,000 trypomastigotes into naive mice every 2 weeks.
Reagents.
α-GalCer was supplied by Takashi Mise and Kazuhiro Motoki at the Kirin Brewery Co. and was produced as described previously (25). The stock solution (200 μg/ml) of α-GalCer was diluted with phosphate-buffered saline to make a working solution of 10 μg/ml just before inoculation. Two hundred microliters of the working solution (2 μg of α-GalCer) was administered intraperitoneally (i.p.) at the times indicated in the figure legends. We decided to use 2 μg of α-GalCer per dose for all of our studies based on either our own experiences in tumor immunology (15, 24, 34, 42), in which the same dose had been reproducibly effective in various assays, or on the report of Duthie and Kahn (12), who demonstrated the doses of 0.5, 1, and 5 μg to be effective in assays with T. cruzi nonvirulent strain for infection. The intact biological activity of α-GalCer, after it was administered into mice, was occasionally monitored and confirmed by measuring both the IFN-γ and the IL-4 content in serum as described by Hayakawa et al. (15). Benznidazole (BNZ) was purchased from the Hoffmann-La Roche, Inc. (Sao Paulo, Brazil). The doses for BNZ administration were 100 mg/kg/day for an optimal dose and 25 mg/kg/day for a suboptimal dose. BNZ was given per os (p.o.) for seven consecutive days beginning on the day of challenge infection as described previously (27).
Cells and culture.
The C57BL/6-derived thymoma cell line EL-4 was used for antigen-presenting cells for the CD8+-T-cell cultures and assays. These cells were cultured in high-glucose Dulbecco modified Eagle medium (DMEM; Life Technologies/Gibco-BRL, Rockville, Md.) supplemented with 10% fetal calf serum, 2 g of sodium bicarbonate (Sigma, St. Louis, Mo.)/liter, 200 mg of l-arginine hydrochloride (Life Technologies/Gibco-BRL)/liter, 36 mg of l-asparagine (Life Technologies/Gibco-BRL)/liter, 2.6 g of HEPES (Sigma)/liter, 5 × 10−5 M 2-mercaptoethanol (Sigma), and antibiotics (complete DMEM). The medium used for the enzyme-linked immunospot (ELISPOT) assay, and the culture of lymphocytes was supplemented with phorbol myristate acetate-stimulated EL-4 cell culture supernatant as a source of 30 U of IL-2 (complete DMEM-IL-2)/ml.
Plasmid DNA and peptide.
pCMV-Tag epitope tagging mammalian expression vector (pCMV; Stratagene) was used to construct a T. cruzi trans-sialidase surface antigen (TSSA) gene-expressing plasmid DNA and was designated pTSSA (19). A CD8+-T-cell-inducing, H-2Kb-restricted peptide, ANYNFTLV, derived from TSSA (19) was used for immunological assay.
Routes of immunization, schedules, dosages, and challenge infection.
We used several immunization and infection protocols as described in the figure legends. For the induction of immune T cells by DNA immunization, mice were injected i.m. with 100 μg of pTSSA or control pCMV vector suspended in 50 μl of sterile phosphate-buffered saline into the right-hind-leg quadriceps once or twice at approximately 10-day intervals, depending on the immunization schedule. α-GalCer was administered i.p., and BNZ was given orally. The mice were challenged with an appropriate number of Tulahuen strain T. cruzi blood-form trypomastigotes between 11 and 14 days after the last immunization. The challenge infection was administered intravenously (i.v.), i.m., or i.p. Blood from all infected animals was obtained from the tail vein periodically, and the numbers of parasites in 5 μl of blood (parasitemia) were determined microscopically. Survival was monitored daily.
Quantification of antigen-specific T cells by ELISPOT assay.
The frequency of antigen-specific T cells was determined by ELISPOT assay for IFN-γ-secreting cells essentially as described previously (6, 29, 30, 33). Briefly, serial dilutions of splenocytes or T cells (1 × 104 to 100 × 104) were cocultured with irradiated EL-4 cells that had been pulsed with 1 μM peptide in anti-IFN-γ monoclonal antibody-coated plates for 24 to 28 h. The spots formed by IFN-γ-secreting cells were detected by using biotinylated anti-IFN-γ monoclonal antibody, followed by the addition of peroxidase-labeled streptavidin and diaminobenzidine. The developed spots were counted under a microscope and were expressed as the number of spots per 106 cells.
Statistical analysis.
Statistical analyses were performed by using the unpaired Student t test for parasitemia counts and ELISPOT assays and Dunnett one-tailed t test for the counts of parasitemia in Fig. 4. The unpaired Mann-Whitney U test or the Fisher exact test were used to determine significant differences in the survival data. P values of <0.05 were considered significant.
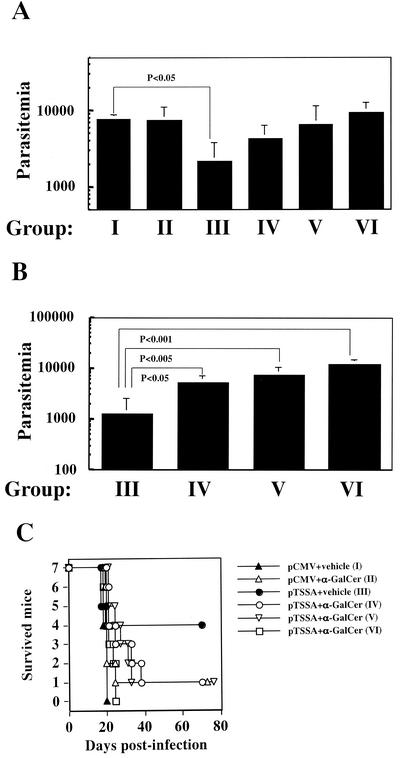
FIG. 4.
α-GalCer administration undermines DNA vaccine-induced protective immunity against T. cruzi infection. (A) C57BL/6 mice, with seven mice in each group, were divided into six groups (groups I through VI) and treated as described in Table 1. The animals were infected i.p. with 5,000 Tulahuen strain T. cruzi blood-form trypomastigotes 12 days after DNA immunization. The number of parasites in 5 μl of peripheral blood (parasitemia) 17 days postinfection was counted, and the data are indicated as the means ± the standard deviations of parasitemia of mice in each group. The P values comparing parasitemia between mice in group I and mice in group III were significant and are indicated in the figure. (B) Parasitemia at 20 days postinfection was measured, and the data are indicated as the means ± the standard deviations of parasitemia of mice in groups III, IV, V, and VI. The P values comparing parasitemia between mice in group III and mice in groups IV, V, and VI were significant and are indicated in the figure. (C) The survival curves reveal that α-GalCer administration either at the time of DNA vaccination or at the time of T. cruzi infection eventually abolished the pTSSA vaccine-induced protective immunity against T. cruzi infection. The symbols represent the survival of mice immunized with pCMV-vehicle (▴) (group I), pCMV-α-GalCer (▵) (group II), pTSSA-vehicle (•) (group III), pTSSA-α-GalCer (○) (group IV), pTSSA-α-GalCer (▿) (group V), andpTSSA-α-GalCer (□) (group VI). It was determined by using the Fisher exact test that the survival of mice in group III was significantly better (P < 0.05) than that of mice in group I. However, the survival of mice in groups II, IV, V, or VI was not significantly different compared to that of mice in group I. The data are representative one of two independent experiments.
RESULTS AND DISCUSSION
NK T cells are ineffective for controlling T. cruzi infection in the quiescent state.
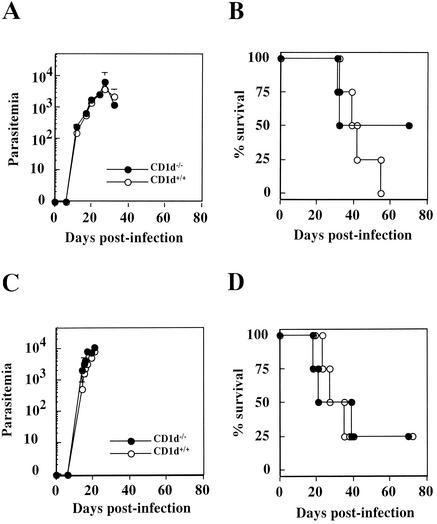
We first infected CD1d-deficient mice, which lack CD1d-restricted Vα14 NK T cells, that had either a B6 or a BALB/c background, with 10 or 20 T. cruzi blood-form trypomastigotes i.m. in order to determine whether NK T cells play a role in resistance to the infection. An infection dose of 10 or 20 per mouse is generally sublethal; however, it can lead to patent parasitemia and kill at least some infected mice. Therefore, we could determine whether the absence of NK T cells would increase or decrease the susceptibility of these mice to T. cruzi infection. Figure 1 shows that both the time course of parasitemia and the percent survival of CD1d-deficient mice from either a B6 background (Fig. 1A and B) or a BALB/c background (Fig. 1C and D) were almost identical to those of wild-type littermates; CD1d-deficient mice exhibited no statistically significant differences with respect to either indicator. These results contradict those of a previous report demonstrating that NK T-cell deficiency increased the susceptibility of mutant mice to T. cruzi infection (11). We assume that this contradiction derives from the differences in the T. cruzi strains used, since we used one of the most virulent strains, the Tulahuen strain (28), for our studies. In contrast, Duthie et al. (11) used the CL strain of T. cruzi, which was nonlethal to all B6 or BALB/c mice even when an infection dose of 5 × 104 or 1 × 105 per mouse was used. Alternatively, as for the route of infection, we infected mice i.m., whereas Duthie et al. (11) chose i.p. infection. Since we found that i.m. infection causes a more virulent course of disease in T. cruzi infection than i.p. or i.v. infection, the difference in the route of infection might have affected the disease outcome. Another possible interpretation for the discrepancy between the data of Duthie et al. and the present study might be that the CD1d deficiency is not enough to delete the NK T-cell activity completely, thus resulting in our inability to detect the alterations of T. cruzi infection in the CD1d−/− mice when we used the virulent strain of T. cruzi for infection. Duthie et al. (11) used Jα28l−/− mice, which are deficient in NK T-cell activity more thoroughly; this difference might possibly have allowed us to detect the alterations of T. cruzi Tulahuen strain infections.
FIG. 1.
A deficiency of the CD1 molecule does not alter the course of T. cruzi infection in mice. Four CD1d-deficient mice (•) and four wild-type littermates (○) were infected i.m. with 20 (C57BL/6 mice [A and B]) or 10 (BALB/c mice [C and D]) Tulahuen strain of T. cruzi blood-form trypomastigotes. The number of parasites in 5 μl of peripheral blood (parasitemia) was counted periodically (A and C); survival was monitored daily (B and D).
Duthie et al. analyzed the dynamics of NK T-cell populations both in the liver and in the spleen during the infection of T. cruzi CL strain (11). These authors found that the percentage of NK T-cell populations in the organs varied depending on the number of days postinfection. Duthie et al. observed that the intensity of NK1.1 staining of the NK T cells observed between 21 and 45 days postinfection was decreased, leading them to speculate that the NK T cells, during the infection, might gain an altered phenotype which might affect their function. Although we did not perform similar analyses for our studies during the infection of T. cruzi Tulahuen strain, there might be different dynamics for the NK T-cell populations that could be distinct from the ones noted during the T. cruzi CL infection. The similar analyses might possibly explain why we did not detect the significant differences in parasitemia and percent survival when CD1d−/− mice were infected with a more virulent strain of T. cruzi.
Since NK T cells localize primarily in restricted organs such as the liver, spleen, and thymus (2, 41), it is possible that NK T cells might not be able to exert their immunological function at distant organs where the intracellular stage form of T. cruzi replicates and proliferates. We assumed that liver infection might be exacerbated in CD1d-deficient mice due to their lack of NK T cells. To examine this possibility, we scanned 200 fields of each stained live pathological section, which was derived from either three CD1d+/+ mice or three CD1d−/− mice, and then counted the number of T. cruzi-infected hepatocytes. However, we observed no statistically significant difference in the number of infected hepatocytes as determined by the unpaired Student t test (data not shown), suggesting that the role of NK T cells in the quiescent state might not be strong enough to alter disease progression both systemically and locally in natural T. cruzi infection.
α-GalCer-activated NK T cells slightly improve the survival of mice with lethal T. cruzi infection.
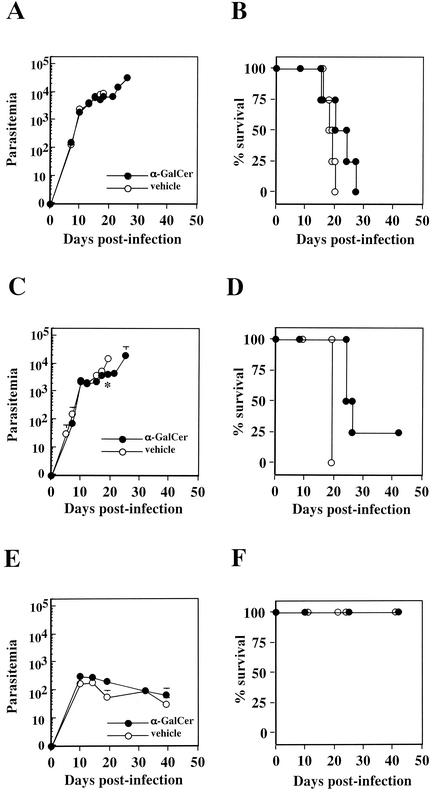
We next tested whether the activation of NK T cells by α-GalCer (21) administration confers protection against T. cruzi infection. Considering the immunological effect of cytokines, including IFN-γ and IL-4, which NK T cells secrete vastly upon α-GalCer activation (15, 42), we expected that the compound would have a therapeutic efficacy against T. cruzi infection. In accordance with this expectation, there have been several studies demonstrating the potent immunological effect of α-GalCer conferring resistance against tumors (21, 34) or infectious agents (13, 18, 20). We injected 2 μg of α-GalCer into mice four times at 4-day intervals before and after lethal T. cruzi inoculation. After mice were infected i.m. with 5,000 T. cruzi blood-form trypomastigotes 2 days after the first α-GalCer administration, parasitemia and the percent survival showed no significant improvement compared to vehicle-administered control mice throughout the infection (Fig. 2A and B). We assumed that our inability to demonstrate the immunological capacity of NK T-cell activation might be partly due to the route of infection chosen for the experiment. When mice were infected i.m., the intracellular stage of T. cruzi replicates and proliferated locally at places distant from the liver, spleen, and thymus, eventually leading to the formation of a localized tumor-like pathology. If the primarily infected foci where intracellular T. cruzi are proliferating are far from the place where the NK T cells are present, the secretion of large amounts of cytokines, which are effective against intracellular amastigotes but not against extracellular trypomastigotes (17, 37), might be useless in reducing the proliferating parasite burden. We therefore decided to change the route of infection by infecting the mice i.p. or i.v., through which T. cruzi might swiftly spread to the whole body without forming locally restricted intracellular T. cruzi replication sites. In the case of i.p. infection, mice given α-GalCer mice survived significantly longer than the vehicle-administered mice (P < 0.05), although parasitemia in the groups differed significantly only in the late stage of infection (Fig. 2C and D). Although NK T cells are reported to be capable of killing cells directly (26), secreted cytokines, such as IFN-γ, might be the main effector mechanism in the acute phase for controlling infections (13, 17, 18, 37) and might not be enough to suppress effectively the rising T. cruzi parasitemia. The importance of IFN-γ, secreted by activated NK T cells, in containing infectious agents has also been suggested for T. cruzi infection as reported elsewhere (12). Infection of mice i.v. with 5,000 parasites was no longer lethal (Fig. 2F); however, the activation of NK T cells had little impact on the improvement of parasitemia (Fig. 2E). Considering reports that have demonstrated the strong immunological capacity of NK T cells against other infections, the present results are rather surprising. One obvious interpretation for the results would be that NK T cells, even if they are activated by α-GalCer, are not so effective against systemic T. cruzi infection. An alternative interpretation of the results would be that the routes of infection and α-GalCer administration may have affected the disease outcome. Since α-GalCer was administered i.p. in all experiments, the proximity of parasite inoculation and α-GalCer administration in T. cruzi i.p. infection might effectively mount NK T-cell responses in order to suppress local parasite proliferation (Fig. 2C and D). Although we do not have any data to reject the second interpretation, we think that it is not likely because of the restricted localization of NK T-cell populations, mostly in liver, spleen, or thymus. Third, we considered the possibility that repeated injection of α-GalCer biased the host immune responses toward the Th2 type as reported elsewhere (4), consequently suppressing IFN-γ production. We therefore tested the efficacy of a one-time inoculation of α-GalCer on the day of i.m. infection; however, we observed similar results as in mice receiving repeated administration of α-GalCer, demonstrating no improvement in either parasitemia or percent survival (data not shown). Duthie et al. observed that the multiple α-GalCer administration prior to T. cruzi CL strain infection exacerbated the infection, whereas a single α-GalCer administration at day −1 led to the optimal protection (12). We were not aware of the similar phenomena during the virulent T. cruzi Tulahuen strain infection in mice, partly because the infection dose of 5,000 blood-form trypomastigotes, which was employed for our studies, has already reached to the dose for inducing the deadly infection course for host and was lethal to all naive B6 mice. In addition, we never performed a single α-GalCer administration at day −1 for our studies; therefore, it is possible that this type of treatment could result in optimal protection even when the virulent strain of T. cruzi was used in these studies. The absence of a drastic effect of NK T-cell activation on T. cruzi infection is in contrast to other infectious agents such as hepatitis B virus (18) and malaria (13) infection, against which NK T-cell activation contains the liver-restricted pathogens and suppresses the formation of pathologies.
FIG. 2.
Activation of NK T cells by administering α-GalCer during the T. cruzi infection slightly improves survival. C57BL/6 mice, four mice in each group, were administered 2 μg of α-GalCer (•) or vehicle solution (○) four times at four-day intervals. Mice were infected i.m. (A and B), i.p. (C and D), or i.v. (E and F) with 5,000 Tulahuen strain T. cruzi blood-form trypomastigotes 2 days after the first α-GalCer inoculation. The number of parasites in 5 μl of peripheral blood (parasitemia) was counted periodically (A, C, and E); survival was monitored daily (B, D, and F). ✽, P < 0.05 (in comparisons between groups of α-GalCer-treated mice and vehicle-treated mice). The percent survival of α-GalCer-administered mice was significantly different from that of vehicle-administered control mice when infection was achieved i.p. (P < 0.05) (D).
α-GalCer-activated NK T cells do not augment the therapeutic efficacy of BNZ against Chagas' disease.
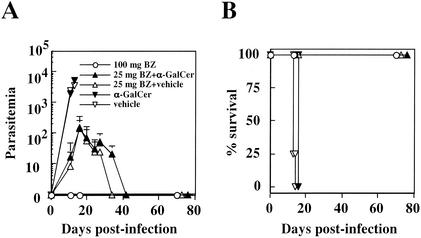
As α-GalCer administration alone was not effective in suppressing the disease progression, we decided to combine it with a conventional therapy. BNZ is one of the few commercially available therapeutic drugs against T. cruzi; however, its clinical efficacy has frequently been questioned, especially for the chronic stage of infection. Although the biochemical mechanisms of its drug action have not been fully elucidated, the involvement of cytokines in its efficacy has not yet been demonstrated (27). Despite this situation, the combined usage of BNZ with IL-12 administration demonstrates a cumulative therapeutic effect against T. cruzi (27), prompting us to test the combined usage of α-GalCer together with BNZ in curing the Chagas' disease. As shown in Fig. 3, however, we saw no significant suppression of parasitemia in mice treated with a suboptimal dose of BNZ (25 mg/kg/day for seven consecutive days) and α-GalCer (2 μg per mouse) compared to mice treated with suboptimal BNZ-vehicle solution. Parasitemia was obviously suppressed by the use of BNZ; however, the injection of α-GalCer produced no cumulative effect of further suppressing the parasitemia. This result suggests that it might be impractical to target NK T-cell activation in order to improve the efficacy of conventional therapeutics as BNZ is the mainstream compound for treating Chagas' disease.
FIG. 3.
NK T-cell activation by administering α-GalCer has no cumulative effect in curing T. cruzi infection when administered in combination with BNZ. C57BL/6 mice, with four mice in each group, were given 2 μg of α-GalCer (▾) or vehicle solution (▿) four times at 4-day intervals. The mice were infected i.m. with 5,000 Tulahuen strain T. cruzi blood-form trypomastigotes 2 days after the first α-GalCer inoculation. In addition to α-GalCer treatment, mice were treated with a suboptimal dose of BNZ (25 mg/kg/day) p.o. (▴) for seven consecutive days beginning on the day of T. cruzi infection. As controls, mice were treated with BNZ (25 mg/kg/day) p.o. and vehicle solution (▵), whereas others were treated with an optimal dose of BNZ (100 mg/kg/day) p.o. for seven consecutive days (○). The number of parasites in 5 μl of peripheral blood (parasitemia) was counted periodically (A); survival was monitored daily (B). BZ, BNZ.
α-GalCer administration either at the time of vaccination or at the time of T. cruzi infection undermines DNA vaccine-induced protective immunity.
We next tested whether NK T cells affect DNA vaccine-induced acquired protective immunity against T. cruzi infection. The DNA encodes TSSA, designated as pTSSA, which confers T-cell-dependent protective immunity in B6 mice (19). Mice were divided into six groups designated groups I through VI, with each group consisting of seven mice (Table 1). The mice were infected i.p. with 5,000 blood-form T. cruzi trypomastigotes 12 days after immunization. As shown in Fig. 4A, the parasitemia at 17 days postinfection in mice in group III was effectively suppressed by pTSSA immunization compared to that in pCMV-immunized mice in group I (P < 0.05). The effect of α-GalCer administration was not visible at this point, as the parasitemia in mice in group II, which were coadministered pCMV and α-GalCer, was not significantly different from that in mice in group I. Coadministration of α-GalCer with pTSSA vaccine in mice of groups IV, V, and VI affected the course of T. cruzi parasitemia similarly in spite of the different timing of α-GalCer inoculation. The parasitemias in mice of these three groups were not significantly suppressed any longer compared to that in the pCMV-immunized mice in group I. The reverse of the effective suppression of T. cruzi parasitemia induced by pTSSA vaccination was more prominent at 20 days postinfection (Fig. 4B), with significantly higher parasitemia demonstrated in mice in groups IV, V, and VI compared to mice in group III. The final outcome of T. cruzi infection as shown in Fig. 4C reveals that the significant improvement in survival demonstrated in mice in group III compared to the survival of pCMV-immunized mice in group I (P < 0.05) was no longer observed in mice in groups IV, V, and VI, which were coadministered pTSSA and α-GalCer. This result suggests that the coadministration of α-GalCer either at the time of DNA vaccination or at the time of T. cruzi infection abolishes the pTSSA-conferred protective immunity.
TABLE 1.
Mouse groups
| Group | DNA vaccinea | Treatment
|
|
|---|---|---|---|
| At vaccinationb | At infectionc | ||
| I | pCMV | Vehicle | Vehicle |
| II | pCMV | α-GalCer | α-GalCer |
| III | pTSSA | Vehicle | Vehicle |
| IV | pTSSA | α-GalCer | α-GalCer |
| V | pTSSA | α-GalCer | Vehicle |
| VI | pTSSA | Vehicle | α-GalCer |
The DNA constructs of pCMV and pTSSA are described in Materials and Methods and in reference 19. A total of 100 μg of DNA was injected one time i.m. into the right-hind-leg quadriceps.
α-GalCer (2 μg per mouse) or 200 μl of vehicle solution was administered i.p. once on the day of DNA immunization.
α-GalCer (2 μg per mouse) or 200 μl of vehicle solution was administered i.p. four times at 4-day intervals starting 2 days before T. cruzi infection.
In support of the results presented in Fig. 4, which suggest that the pTSSA-α-GalCer cotreatment would be detrimental to host resistance to T. cruzi infection, we obtained a preliminary data demonstrating that the mice that were passively transferred with pTSSA-α-GalCer-immunized B6 splenocytes were significantly more susceptible to T. cruzi infection than were mice that were passively transferred with pTSSA-vehicle-immunized B6 splenocytes (data not shown).
pTSSA vaccine-induced ANYNFTLV-specific CD8+-T-cell responses are impaired by the coadministration of α-GalCer.
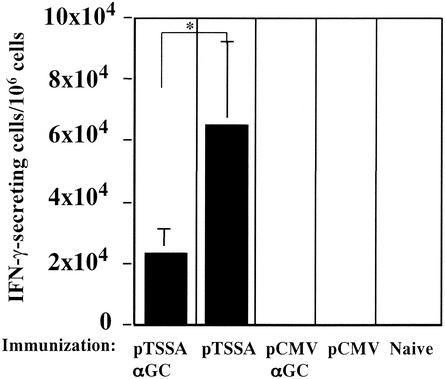
As CD8+ T cells are important for controlling the acute phase of T. cruzi infection, we evaluated the induction of epitope-specific CD8+ T cells in mice coadministered DNA and α-GalCer. Mice, with four mice in each group, were immunized twice with DNA with or without α-GalCer and were subjected to ELISPOT assay 12 days after the last immunization (Fig. 5). Appropriate control groups of mice were included in the experiment as shown in Fig. 5. The induction of ANYNFTLV-specific CD8+ T cells (19) in mice immunized with the pTSSA-α-GalCer solution was significantly lower than in mice coimmunized with pTSSA-vehicle (P < 0.05) (Fig. 5). Since α-GalCer would influence several cell populations, as well as T cells, via NK T-cell activation, as evidenced by the enormous spleen enlargement, we assumed that the relative number of T cells in the whole immune cell population was reduced, therefore undermining the CD8+-T-cell responses. Another possible explanation for the reduced CD8+-T-cell responses in DNA-α-GalCer-administered mice is that the effect of α-GalCer on T cells could be slow progressing; therefore, the CD8+-T-cell responses might not be fully enhanced only 12 days after the last α-GalCer administration. To exclude these possibilities, we performed the ELISPOT assay when the immune cells had reverted to the quiescent state more days after the final α-GalCer administration. However, the number of epitope-specific CD8+-T-cell responses in pTSSA-α-GalCer solution-immunized mice was still significantly lower than in the pTSSA-vehicle-immunized mice (P < 0.01) at 21 days postimmunization (data not shown), thus confirming the impaired induction of ANYNFTLV-specific CD8+ T cells in pTSSA-α-GalCer-immunized mice. We assume that the inability to control the parasite burden at the end of acute T. cruzi infection in pTSSA-α-GalCer-coadministered mice was partly, if not totally, due to the impaired induction of protective CD8+-T-cell responses. Duthie et al. reported that the antibody responses in NK T cell-deficient mice were quite different from the ones in wild-type mice. Although we could not reject a possibility that antibody responses to the TSSA could be negatively affected by α-GalCer administration resulting in the impairment of DNA-vaccine-induced protective immunity, our previous findings, which demonstrated the importance of T-cell-mediated protective immunity in pTSSA vaccination (19), suggested that the failure of protection in the pTSSA-α-GalCer-immunized mice were mainly due to the impairment of T-cell-mediated immunity rather than the alterations of antibody responses.
FIG. 5.
Induction of ANYNFTLV specific CD8+ T-cell responses is impaired in mice coimmunized with pTSSA/α-GalCer. C57BL/6 mice, four mice in each group, were immunized i.m. with 100 μg of pTSSA or pCMV into the right hind leg quadriceps, and administered 2 μg of α-GalCer or vehicle solution i.p. on the same day. The same immunization was performed again 8 days later. The mice were sacrificed 12 days after the second immunization, and their spleens were removed. The splenocytes from individual mice were stimulated with irradiated EL-4 cells pulsed with ANYNFTLV for one week. ELISPOT assay determined the number of ANYNFTLV-reactive CD8+ T cells with one-week stimulated immune splenocytes as effector cells and peptide pulsed or un-pulsed EL-4 cells as antigen presenting cells. The number of IFN-γ-secreting cells was counted 24 h later. The number of IFN-γ-secreting cells that appeared using peptide un-pulsed EL-4 cells was subtracted from the number appearing using peptide pulsed EL-4 cells. Data are the mean ± SD of four mice in each group. *, P < 0.05 compared with data for mice immunized with pTSSA/vehicle solution.
The results are rather surprising in one aspect, as they are obviously contradictory to previous reports demonstrating the potent adjuvant effect of α-GalCer coadministration in enhancing CD8+-T-cell-mediated protective immunity against malaria (14) and tumors (34). This contradiction might be partly due to the immunogens used for coadministrating with α-GalCer. Gonzalez-Aseguinolaza et al. used recombinant viruses (14) for immunization, whereas we used DNA for vaccination. The immunological effects induced by vaccination in various situations vary depending on the immunogens used. For example, although immunization of CD28-deficient mice with viruses can induce epitope-specific CD8+-T-cell responses as robust as those in wild-type mice (44), immunization of mice with DNA vaccine failed to induce this response (16). We therefore assume that it is perhaps not surprising to see opposite effects of immunization depending on the immunogens used in studies.
Our results, however, might not be surprising in another aspect, since NK T cells are documented to mediate the repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway (48) and to regulate immune suppression, resulting in skin cancer development (32). In these respects, it might be possible that NK T cells possess a potent capacity to suppress the induction of CD8+ T cells in some situations. NK T cells are also crucial for the induction of systemic tolerance called anterior chamber-associated immune deviation (43). In this situation, NK T-cell-derived IL-10 is essential for the differentiation of antigen-specific T regulatory cells (43). We therefore think that the activation of NK T cells by coadministering α-GalCer may induce “regulatory cells,” resulting in the impairment of DNA-vaccine induced protective immunity.
Overall, our results suggest that NK T cells might not play major roles in resolving T. cruzi infection of virulent strains. This cell population has been called a “double-edged sword” (40) and can even exert a negative impact on hosts immune defenses. In the case of T. cruzi infection, α-GalCer-activated NK T-cell-derived IFN-γ could limit the parasitemia (Fig. 2) (12) but reduce epitope-specific CD8+-T-cell responses (Fig. 5). The conflicting roles of NK T cells in host immune responses might be partly due to their capacity to secrete large amounts of IFN-γ, IL-4, and IL-10 (15, 42, 43), which have contradictory immunological functions under the current concept of cytokine dichotomy. Most importantly, NK T-cell activation is now suggested as a potent adjuvant for enhancing CD8+-T-cell-mediated protective immunity (14); however, our results caution that the effect of NK T-cell activation should be defined precisely in individual diseases.
Acknowledgments
We thank Naoki Shimada at the Department of Preventive Medicine and Public Health in Keio University School of Medicine for his help in performing statistical analyses.
This work was supported in part by grant-in-aid for scientific research 14570222 from the Ministry of Education, Science, Sports, and Culture of Japan; by the Ohyama Health Foundation to Y.M.; and also in part by a grant for the Research For The Future Program (JSPS-RFTF-97L00701).
Editor: J. M. Mansfield
REFERENCES
- 1.Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8+ T-cell homeostasis by perforin and interferon-γ. Science 290:1354-1358. [DOI] [PubMed] [Google Scholar]
- 2.Biron, C. A., and L. Brossay. 2001. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13:458-464. [DOI] [PubMed] [Google Scholar]
- 3.Brener, Z., and R. T. Gazzinelli. 1997. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int. Arch. Allergy Immunol. 114:103-110. [DOI] [PubMed] [Google Scholar]
- 4.Burdin, N., L. Brossay, and M. Kronenberg. 1999. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 29:2014-2025. [DOI] [PubMed] [Google Scholar]
- 5.Cardillo, F., J. C. Voltarelli, S. G. Reed, and J. S. Silva. 1996. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect. Immun. 64:128-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carvalho, L. H., J. C. R. Hafalla, and F. Zavala. 2001. ELISPOT assay to measure antigen-specific murine CD8+ T cell responses. J. Immunol. Methods 252:207-218. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho, L. H., G. Sano, J. C. R. Hafalla, Morrot, A., M. A. C. Lafaille, and F. Zavala. 2002. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat. Med. 8:166-170. [DOI] [PubMed] [Google Scholar]
- 8.Chagas, C. 1909. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., agente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz 1:159-218. [Google Scholar]
- 9.Chen, L., T. Watanabe, H. Watanabe, and F. Sendo. 2001. Neutrophil depletion exacerbates experimental Chagas' disease in BALB/c, but protects C57BL/6 mice through modulating the Th1/Th2 dichotomy in different directions. Eur. J. Immunol. 31:265-275. [DOI] [PubMed] [Google Scholar]
- 10.DosReis, G. A. 1997. Cell-mediated immunity in experimental Trypanosoma cruzi infection. Parasitol. Today 13:335-342. [DOI] [PubMed] [Google Scholar]
- 11.Duthie, M. S., M. Wleklinski-Lee, S. Smith, T. Nakayama, M. Taniguchi, and S. J. Kahn. 2002. During Trypanosoma cruzi infection CD1d-restricted NK T cells limit parasitemia and augment the antibody response to a glycophosphoinositol-modified surface protein. Infect. Immun. 70:36-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duthie, M. S., and S. J. Kahn. 2002. Treatment with α-Galactosylceramide before Trypanosoma cruzi infection provides protection or induces failure to thrive. J. Immunol. 168:5778-5785. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Aseguinolaza, G., C. de Oliveira, M. Tomaska, S. Hong, O. Bruna-Romero, T. Nakayama, M. Taniguchi, A. Bendelac, L. Van Kaer, Y. Koezuka, and M. Tsuji. 2000. α-galactosylceramide-activated Vα 14 natural killer T cells mediate protection against murine malaria. Proc. Natl. Acad. Sci. USA 97:8461-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Aseguinolaza, G., L. Van Kaer, C. C. Bergmann, J. M. Wilson, J. Schmieg, M. Kronenberg, T. Nakayama, M. Taniguchi, Y. Koezuka, and M. Tsuji. 2002. Natural killer T cell ligand α-galactosylceramide enhances protective immunity induced by malaria vaccines. J. Exp. Med. 195:617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayakawa, Y., K. Takeda, H. Yagita, S. Kakuta, Y. Iwakura, L. Van Kaer, I. Saiki, and K. Okumura. 2001. Critical contribution of IFN-γ and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of α-galactosylceramide. Eur. J. Immunol. 31:1720-1727. [PubMed] [Google Scholar]
- 16.Horspool, J. H., P. J. Perrin, J. B. Woodcock, J. H. Cox, C. L. King, C. H. June, D. M. Harlan, D. C. St. Louis, and K. P. Lee. 1998. Nucleic acid vaccine-induced immune responses require CD28 costimulation and are regulated by CTLA4. J. Immunol. 160:2706-2714. [PubMed] [Google Scholar]
- 17.Hunter, C. A., T. Slifer, and F. Araujo. 1996. Interleukin-12-mediated resistance to Trypanosoma cruzi is dependent on tumor necrosis factor alpha and gamma interferon. Infect. Immun. 64:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kakimi, K., L. G. Guidotti, Y. Koezuka, and F. V. Chisari. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192:921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katae, M., Y. Miyahira, K. Takeda, H. Matsuda, H. Yagita, K. Okumura, T. Takeuchi, T. Kamiyama, A. Ohwada, Y. Fukuchi, and T. Aoki. 2002. Co-administration of interleukin-12 gene with a Trypanosoma cruzi gene improves vaccine efficacy. Infect. Immun. 70:4833-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawakami, K., Y. Kinjo, S. Yara, K. Uezu, Y. Koguchi, M. Tohyama, M. Azuma, K. Takeda, S. Akira, and A. Saito. 2001. Enhanced gamma interferon production through activation of Vα14+ natural killer T cells by α-galactosylceramide in interleukin-18-deficient mice with systemic cryptococcosis. Infect. Immun. 69:6643-6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, H. Sato, E. Kondo, M. Harada, H. Koseki, T. Nakayama, Y. Tanaka, and M. Taniguchi. 1998. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc. Natl. Acad. Sci. USA 95:5690-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirchhoff, L. V. 1990. Trypanosoma species (American trypanosomiasis, Chagas disease): biology of trypanosomes, p. 2077-2084. In G. L. Mandell, R. G. Douglas, Jr., and J. E. Bennett (ed.), Principles and practice of infectious diseases, 3rd ed. Churchill Livingstone, New York, N.Y.
- 23.Kirchhoff, L. V. 1993. American trypanosomiasis (Chagas' disease): a tropical disease now in the United States. N. Engl. J. Med. 329:639-644. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura, H., K. Iwakabe, T. Yahata, S. Nishimura, A. Ohta, Y. Ohmi, M. Sato, K. Takeda, K. Okumura, L. Van Kaer, T. Kawano, M. Taniguchi, and T. Nishimura. 1999. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 189:1121-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi, E., K. Motoki, T. Uchida, H. Fukushima, and Y. Koezuka. 1995. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol. Res. 7:529-534. [PubMed] [Google Scholar]
- 26.Metelitsa, L. S., O. V. Naidenko, A. Kant, H. W. Wu, M. J. Loza, B. Perussia, M. Kronenberg, and R. C. Seeger. 2001. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J. Immunol. 167:3114-3122. [DOI] [PubMed] [Google Scholar]
- 27.Michailowsky, V., S. M. Murta, L. Carvalho-Oliveira, M. E. Pereira, L. R. Ferreira, Z. Brener, A. J. Romanha, and R. T. Gazzinelli. 1998. Interleukin-12 enhances in vivo parasiticidal effect of benznidazole during acute experimental infection with a naturally drug-resistant strain of Trypanosoma cruzi. Antimicrob. Agents Chemother. 42:2549-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyahira, Y., and J. A. Dvorak. 1994. Kinetoplastidae display naturally occurring ancillary DNA-containing structures. Mol. Biochem. Parasitol. 65:339-349. [DOI] [PubMed] [Google Scholar]
- 29.Miyahira, Y., K. Murata, D. Rodriguez, J. R. Rodriguez, M. Esteban, M. M. Rodrigues, and F. Zavala. 1995. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J. Immunol. Methods 181:45-54. [DOI] [PubMed] [Google Scholar]
- 30.Miyahira, Y., A. Garcia-Sastre, D. Rodriguez, J. R. Rodriguez, K. Murata, M. Tsuji, P. Palese, M. Esteban, F. Zavala, and R. S. Nussenzweig. 1998. Recombinant viruses expressing a human malaria antigen can elicit potentially protective immune CD8+ responses in mice. Proc. Natl. Acad. Sci. USA 95:3954-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyahira, Y., S. Kobayashi, T. Takeuchi, T. Kamiyama, T. Nara, J. Nakajima-Shimada, and T. Aoki. 1999. Induction of CD8+ T cell-mediated protective immunity against Trypanosoma cruzi. Int. Immunol. 11:133-141. [DOI] [PubMed] [Google Scholar]
- 32.Moodycliffe, A. M., D. Nghiem, G. Clydesdale, and S. E. Ullrich. 2000. Immune suppression and skin cancer development: regulation by NKT cells. Nat. Immunol. 1:521-525. [DOI] [PubMed] [Google Scholar]
- 33.Murata, K., A. Garcia-Sastre, M. Tsuji, M. Rodrigues, D. Rodriguez, J. R. Rodriguez, R. S. Nussenzweig, P. Palese, M. Esteban, and F. Zavala. 1996. Characterization of in vivo primary and secondary CD8+ T-cell responses induced by recombinant influenza and vaccinia viruses. Cell. Immunol. 173:96-107. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura, T., H. Kitamura, K. Iwakabe, T. Yahata, A. Ohta, M. Sato, K. Takeda, K. Okumura, L. Van Kaer, T. Kawano, M. Taniguchi, M. Nakui, M. Sekimoto, and T. Koda. 2000. The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d-expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int. Immunol. 12:987-994. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira-Ferreira, J., Y. Miyahira, G. T. Layton, N. Savage, M. Esteban, D. Rodriguez, J. R. Rodriguez, R. S. Nussenzweig, and F. Zavala. 2000. Immunogenicity of Ty-VLP bearing a CD8+ T cell epitope of the CS protein of P. yoelii: enhanced memory response by boosting with recombinant vaccinia virus. Vaccine 17:1863-1869. [DOI] [PubMed] [Google Scholar]
- 36.Prata, A. 2001. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 1:92-100. [DOI] [PubMed] [Google Scholar]
- 37.Revelli, S., H. Davila, M. E. Ferro, M. Romero-Piffiguer, O. Musso, J. Valenti, J. Bernabo, E. Falcoff, J. Wietzerbin, and O. Bottasso. 1992. Acute and chronic experimental Trypanosoma cruzi infection in the rat: response to systemic treatment with recombinant rat interferon-gamma. Microbiol. Immunol. 39:275-281. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues, M. M., M. Ribeirao, V. Pereira-Chioccola, L. Renia, and F. Costa. 1999. Predominance of CD4 Th1 and CD8 Tc1 cells revealed by characterization of the cellular immune response generated by immunization with a DNA vaccine containing a Trypanosoma cruzi gene. Infect. Immun. 67:3855-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rottenberg, M. E., A. Riarte, L. Sorrong, J. Altcheh, P. Petray, A. M. Ruiz, and H. Wigzell. 1995. Outcome of infection with different strains of Trypanosoma cruzi in mice lacking CD4 and/or CD8. Immunol. Lett. 45:53-60. [DOI] [PubMed] [Google Scholar]
- 40.Smyth, M. J., and D. I. Godfrey. 2000. NKT cells and tumor immunity-a double-edged sword. Nat. Immunol. 1:459-460. [DOI] [PubMed] [Google Scholar]
- 41.Smyth, M. J., N. Y. Crowe, Y. Hayakawa, K. Takeda, H. Yagita, and D. I. Godfrey. 2002. NKT cells-conductors of tumor immunity? Curr. Opin. Immunol. 14:165-171. [DOI] [PubMed] [Google Scholar]
- 42.Smyth, M. J., N. Y. Crowe, D. G. Pellicci, K. Kyparissoudis, J. M. Kelly, K. Takeda, H. Yagita, and D. I. Godfrey. 2002. Sequential production of interferon-gamma by NK1.1+ T cells and natural killer cells is essential for the antimetastatic effect of α-galactosylceramide. Blood 99:1259-1266. [DOI] [PubMed] [Google Scholar]
- 43.Sonoda, K. H., D. E. Faunce, M. Taniguchi, M. Exley, S. Balk, and J. Stein-Streilein. 2001. NK T cell-derived IL-10 is essential for the differentiation of antigen-specific T regulatory cells in systemic tolerance. J. Immunol. 166:42-50. [DOI] [PubMed] [Google Scholar]
- 44.Suresh, M., J. K. Whitmire, L. E. Harrington, C. P. Larsen, T. C. Pearson, J. D. Altman, and R. Ahmed. 2001. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J. Immunol. 167:5565-5573. [DOI] [PubMed] [Google Scholar]
- 45.Tarleton, R. L. 1990. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J. Immunol. 144:717-724. [PubMed] [Google Scholar]
- 46.Tarleton, R. L., B. H. Koller, A. Latour, and M. Postan. 1992. Susceptibility of β2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356:338-340. [DOI] [PubMed] [Google Scholar]
- 47.Tarleton, R. L., M. J. Grusby, M. Postan, and L. H. Glimcher. 1996. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int. Immunol. 8:13-22. [DOI] [PubMed] [Google Scholar]
- 48.Terabe, M., S. Matsui, N. Noben-Trauth, H. Chen, C. Watson, D. D. Donaldson, D. P. Carbone, W. E. Paul, and J. A. Berzofsky. 2000. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 1:515-520. [DOI] [PubMed] [Google Scholar]