Abstract
Listeria monocytogenes, isolated from outbreaks in either human or nonhuman primate populations, was administered orally at doses ranging from 106 to 1010 CFU. Four of 10 treated animals delivered stillborn infants. L. monocytogenes was isolated from fetal tissue, and the pathology was consistent with L. monocytogenes infection as the cause of pregnancy loss. For all pregnancies resulting in stillbirths, L. monocytogenes was isolated from maternal feces, indicating that L. monocytogenes had survived and had probably colonized the gastrointestinal tract. Antibodies and antigen-specific lymphocyte proliferation against Listeria increased in animals that had stillbirths.
Listeriosis resulting from exposure to food containing the bacterium L. monocytogenes causes serious disease, with case fatality rates between 20 and 40% (33). Listeriosis is especially serious in susceptible populations such as immunocompromised persons and pregnant women (11, 14, 16, 20, 24, 26, 29, 32, 35). For healthy nonpregnant adults, listeriosis has a relatively low incidence, presumably due to its low infectivity in immunocompetent individuals.
Pregnancy-related listeriosis primarily affects the fetus or neonate. The maternal reaction to the presence of Listeria infection is generally an influenza-like episode with fever, backache, and perhaps diarrhea (7, 11, 13, 24, 29). The effect of fetal Listeria infection is dependent on the point in gestation time when infection occurs. First-trimester infection leads to spontaneous abortion, whereas second- and third-trimester infections lead to preterm birth followed by neonatal illness or fetal death with preterm delivery of a stillborn (7, 11, 13).
The rhesus monkey (Macaca mulatta), with a reproductive cycle and placenta comparable to those of humans (31), is widely used as an experimental model for human reproduction and development. As with humans, exposure to L. monocytogenes in pregnant nonhuman primates may result in abortions, stillbirths, or neonatal deaths (4, 27; J. Paul-Murphy, J. E. Markovits, I. Wesley, and J. A. Roberts, Lab. Anim. Sci. 40:547 [abstr.], 1990). For humans and nonhuman primates, the pathogenesis and morphological findings associated with stillbirths due to L. monocytogenes are essentially the same (1, 4, 28, 37).
Despite several epidemiological studies confirming the relationship between L. monocytogenes and specific foods (soft cheeses, undercooked chicken, paté, etc.) (2, 30), an infectious dose has not been established for healthy or susceptible human populations due to the delay between exposure and the onset of symptoms. The severe ramifications of the disease in high-risk human populations such as pregnant women precludes the use of humans in volunteer feeding studies. Recently, a draft risk assessment of L. monocytogenes in ready-to-eat foods (36) reviewed human epidemiological and animal study data. The risk assessment concluded that mouse studies provide the only acceptable data for developing dose-response information at this time and acknowledged the difficulty with the use of this model because of its differences from human listeriosis (36). The quality and accuracy of the data used to develop dose-response models have a great effect on the predicted infectious dose (21). Thus, the better the animal model, the more accurate the predictions of the dose-response model and the subsequent risk assessment for humans will be.
Farber et al. (12) fed L. monocytogenes to healthy adult (nonpregnant) cynomolgus monkeys (Macaca fascicularis) and found fecal shedding of L. monocytogenes but no adverse health effects for doses below 109 CFU. At a dose of 109 CFU, symptoms included septicemia, irritability, loss of appetite, and occasional diarrhea. In addition, reports from several primate research centers implicated listeriosis as a cause of spontaneous abortions, stillbirths, and neonatal deaths in macaques housed in outdoor compounds (27; Paul-Murphy et al., Lab. Anim. Sci. abstract). Interestingly, there are no published reports of, nor has the Yerkes Center ever detected, cases of spontaneous listeriosis occurring in macaques housed indoors, probably because the monkeys receive less exposure to L. monocytogenes in a controlled indoor environment.
The purpose of this study was to establish the pregnant rhesus monkey as an experimental model for listeriosis in pregnant humans. The results of our study indicate that pregnant rhesus monkeys are susceptible to infection by L. monocytogenes. There were no outward signs of illness among any of the animals, whether the pregnancy resulted in a stillbirth or a normal birth. Although L. monocytogenes was not detected in blood samples obtained 4 days postexposure, L. monocytogenes was detected in fecal samples from all animals whose pregnancies resulted in stillbirths.
The vehicle, the strain of L. monocytogenes, and the number of L. monocytogenes organisms administered to the animal were varied in the experiments to determine the best experimental protocol. The outcomes of the different treatment protocols are described in the following paragraphs.
Animals.
Ten pregnant rhesus monkeys (M. mulatta) with no previous history of listeriosis were selected from the Yerkes National Primate Research Center's timed-breeding colony. Pregnancies were confirmed at gestation day (gd) 30 and allowed to proceed normally until the day of Listeria exposure. At that time, the pregnant animal was sedated with ketamine (10 mg/kg of body weight) and given L. monocytogenes by nasogastric intubation. The animals were observed daily for any signs of illness, such as diarrhea or a change in eating behavior or degree of activity. The animal work was done in full compliance with all federal regulations, including the Animal Welfare Act. The Yerkes Center is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
Experimental design and treatments.
The experimental design was based on a phase I clinical trial commonly used in human pharmacological studies (15). However, rather than starting with a relatively low dose and increasing the dose with each positive outcome, our treatments began with a relatively high dose. When a stillbirth occurred, the next treatment dose was decreased, thereby conserving a larger number of animals for the lower-dose groups.
The normal gestation period for rhesus monkeys is 165 ± 14 days (mean ± standard deviation), and the last third of the pregnancy begins at approximately gd 110. Because humans are known to be susceptible to L. monocytogenes during the last trimester, gd 110 and 127 were chosen as the days for treatment. Two animals treated at gd 127 had normal birth outcomes. Because adverse pregnancy outcome resulted from treatment at gd 110 and not at gd 127, all further treatments were administered at gd 110 (±4).
L. monocytogenes strains and inoculum preparation.
Five strains of L. monocytogenes (serotype 1/2a or 4b) associated with human or primate clinical cases were chosen for the challenge study (Table 1). Cultures were maintained frozen in broth at −20°C until use. Prior to use, cultures were activated by three successive transfers in tryptic soy broth (Difco Laboratories, Detroit, Mich.) at 30°C for 24 h. Cultures were harvested by centrifugation, washed three times, and resuspended in sterile serological saline. Mixtures of strains were prepared by combining equal quantities of the cell suspensions. Skim milk, half-and-half, and whipping cream were purchased from a local store and sterilized by autoclaving at 121°C for 15 min before the mixture was diluted. One milliliter of strain mixture or individual culture was mixed with 9 ml of skim milk, half-and-half, or whipping cream to obtain the desired cell populations. The number of L. monocytogenes cells in the strain mixture or individual culture was determined by serially diluting the cell suspension in saline and by surface plating the suspension on tryptic soy agar (Difco) in duplicate. The plates were incubated at 30°C for 24 h before colony enumeration. The cell populations obtained were used to confirm the numbers of CFU of L. monocytogenes administered to animals. Fetal tissues from all adverse pregnancy outcomes were confirmed positive for L. monocytogenes according to the USDA method described by Cook (5), which includes both qualitative and quantitative detection methods. Briefly, the qualitative method includes two enrichments in nonselective and selective media followed by selective plating on modified Oxford agar plates (Difco), and the quantitative method uses direct plating on modified Oxford agar plates. In both methods, presumptively positive isolates were confirmed by morphological and biochemical methods prior to pulsed-field gel electrophoresis (PFGE) analysis.
TABLE 1.
Strains of L. monocytogenes used in treatments
| Strain | Source | Serotype |
|---|---|---|
| G3982 | Human clinical isolate associated with listeriosis outbreak linked to Mexican-style-cheese consumption | 4b |
| H7550 | Human clinical isolate associated with listeriosis outbreak linked to hot dog consumption | 4b |
| Scott A | Human clinical isolate | 4b |
| 12443 | Monkey clinical isolate | 1/2a |
| 12375 | Monkey clinical isolate | 4b |
Vehicle.
Listeriosis outbreaks have been linked to a variety of foods, including dairy products (for a review, see reference 36). Several outbreaks of salmonellosis suggest that low infective doses are associated with high-fat-content vehicles, such as chocolate (6, 8, 18), cheese (9, 19), and paprika-powdered potato chips (23). In developing our treatment strategy, three different vehicles were used: skim milk (0.25% milk fat), half-and-half (11% milk fat), and whipping cream (30% milk fat). Three of four stillbirths occurred in animals receiving L. monocytogenes in whipping cream (Table 2). Due to limited numbers of available animals, a decision was made to use whipping cream as the vehicle for all remaining animals.
TABLE 2.
Summary of outcomes and conditions for administration of L. monocytogenes to pregnant monkeys
| Animal no. | Day of treatment (gd) | Vehicle | Dose (CFU) | Strain of L. monocytogenes [serotype(s)] | Birth outcome | Day at delivery (gd) |
|---|---|---|---|---|---|---|
| RPp4 | 110 | Whipping cream | 1.2 × 108 | 5-strain mixture (1/2a and 4b) | Stillborn | 145 |
| RMt2 | 112 | Half-and-half | 1.1 × 108 | 5-strain mixture (1/2a and 4b) | Stillborn | 140 |
| RWh3 | 112 | Skim milk | 7.1 × 107 | 5-strain mixture (1/2a and 4b) | Premature (LBW)a | 147 |
| RVd3 | 112 | Whipping cream | 3.7 × 1010 | Scott A (4b) | Stillborn | 167 |
| RKq4 | 106 | Whipping cream | 5.7 × 108 | Scott A (4b) | Normal | 166 |
| RKz4 | 108 | Whipping cream | 2.8 × 107 | H7550 (4b) | Normal | 166 |
| RIr2 | 111 | Whipping cream | 9.1 × 106 | G3982 (4b) | Normal | 169 |
| RGj3 | 127 | Half-and-half | 7.9 × 106 | G3982 (4b) | Normal | 172 |
| RJy4 | 106 | Whipping cream | 7.2 × 106 | 12443 (1/2a) | Stillborn | 147 |
| RJi2 | 127 | Half-and-half | 1.1 × 106 | 12443 (1/2a) | Normal | 170 |
LBW, low birth weight.
Pregnancy outcomes.
Of the treated pregnant monkeys, four pregnancies resulted in stillborn infants, one pregnancy resulted in a premature, low-birth-weight infant, and five pregnancies resulted in normal infants (Table 2). No overt signs of illness or changes in routine behaviors were noted for any of the pregnant animals. For animals with adverse pregnancy outcomes, there were no indications of an abnormal pregnancy until the animal showed signs of labor and the stillbirth was imminent.
The length of gestation was generally shorter for animals with adverse pregnancy outcomes (average, 150 gd) than for those with normal outcomes (average, 165 gd), with one exception. Three animals delivered stillborn infants at approximately 144 gd, but one animal went full term before delivering a stillborn. For pregnancies resulting in live births, the average length of gestation was 165 days, but one animal gave birth to a live, premature infant at gd 147 (Table 2).
Tissue collection and analysis.
Blood samples were collected before treatment, at 4 days posttreatment, at the time of delivery, and at 1 month postdelivery. Tissue samples were collected from all stillborn infants for culture and histologic examination. When available, samples were also collected from the placentas.
Dose.
Actual cell numbers were confirmed after treatment by plating the cells on the day of treatment, incubating the plates for 24 h, and determining the counts (Table 2). The doses resulting in stillbirths ranged from 7.2 × 106 CFU for strain 12443 to 3.7 × 1010 CFU for the Scott A strain. Four strains (G3982, 12443, Scott A, and H7550) were tested by administering each strain individually to a pregnant monkey. Only animals treated with Scott A at 3.7 × 1010 CFU and with strain 12443 at 7.2 × 106 CFU had stillbirths (Table 2). Strain H7550 was tested individually because it had been recently found to be responsible for a human listeriosis outbreak associated with the consumption of hot dogs (3). Although the results were not conclusive, the strain associated with hot dog consumption was not isolated from tissue samples of stillborn infants when administered in a mixture of strains nor did it produce a stillbirth in the single case when administered alone.
Routine hematology.
There were no unexpected differences in hematology between animals that had normal births and animals that had stillbirths. Although animals with stillbirths had white blood cell counts that were significantly higher (P < 0.05) than those of animals with normal births at 1 month postdelivery (data not shown), the values were within the normal range for rhesus monkeys (22). All other parameters examined (number of platelets, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and numbers of neutrophils, lymphocytes, monocytes, and eosinophils) were within the normal reported ranges for rhesus monkeys, with the exception that animals with natural deliveries had red blood cell counts that were below the normal range, likely due to blood loss during the birthing process (22).
Fecal shedding.
Fecal samples were collected at specific intervals after treatment to determine the shedding of L. monocytogenes. Samples were obtained daily for 1 week, every other day for the second week, and once per week for two additional weeks. After collection, fecal samples were either immediately cultured or refrigerated for not more than 48 h until enumeration. Pregnant animals that subsequently had a stillbirth generally shed L. monocytogenes in higher numbers and for a longer period of time than did animals having normal births (Table 3).
TABLE 3.
Fecal shedding of L. monocytogenes after treatment of pregnant animals
| Birth outcome (no. of animals) | No. of samples positive/total samples collecteda | Avg % of shedding days (range) | Avg CFU of bacteria/g of feces (range) |
|---|---|---|---|
| Normal (6) | 9/60 | 15 (0-30) | 2.9 × 103 (NDb-1.6 × 104) |
| Stillborn (4) | 21/40 | 52 (30-80) | 2.6 × 104 (4.2 × 102-7.5 × 104) |
Includes all detectable cultures, including those positive by enrichment.
ND, none detected.
Although there were not enough animals for statistical analysis of fecal shedding according to the vehicle used, animals receiving L. monocytogenes in whipping cream shed up to 18 times more L. monocytogenes per gram of feces than those receiving half-and-half. This suggests that L. monocytogenes may be better able to colonize the gastrointestinal tract when delivered to animals in a higher-fat vehicle. Sprong et al. (34) determined that in rats a diet high in fat inhibits colonization of L. monocytogenes in the gut; however, in their study, L. monocytogenes was administered in a saline solution. This approach is different from our study, where L. monocytogenes was administered in a high-fat vehicle to animals on a normal monkey diet. A study is needed to directly test whether the fat content of the vehicle is important in the ability of L. monocytogenes to survive and colonize the gastrointestinal tract.
Humoral immune response.
Plasma was collected by centrifugation of heparinized venous blood and frozen at −20°C until tested. L. monocytogenes strain 12443 from a stationary-phase culture was washed three times in phosphate-buffered saline (PBS) and resuspended to an estimated concentration of 108 CFU/ml, based on the optical density at 550 nm. Fifty microliters of bacterial cell suspension was added to each well of Immulon 2 MicroElisa plates (Thermo LabSystems, Franklin, Mass.), and liquid was removed with a vacuum evaporator-concentrator (Heto Corporation, Allerod, Denmark). Nonspecific binding to MicroElisa plates was blocked for 2 h at room temperature by adding 100 μl of PBS containing 5% heat-inactivated fetal bovine serum to each well. The plates were washed two times with PBS-0.05% Tween 20 and stored at −80°C until use. Antibody titers were determined by incubating the plates with twofold serial dilutions of plasma (100 μl/well). Plasma dilutions were made in PBS containing 5% heat-inactivated fetal bovine serum and 0.05% Tween 20. The plates were incubated at 4°C overnight or for 2 h at room temperature and then washed with PBS containing 0.1% Tween 20. Bound antibody was detected by incubating the plates for 2 h at room temperature with 100 μl of a 1/2,000 dilution of peroxidase-conjugated goat anti-rhesus immunoglobulin G (heavy plus light chains) (Southern Biotechnology, Birmingham, Ala.), as previously reported (38). Titers were normalized to a baseline value derived from the absorbance obtained with a 1:100 dilution of pooled normal rhesus monkey serum (Sigma, St. Louis, Mo.) at 450 nm.
Preinfection antibody titers ranged from 800 to 1,600, with no change in titers at 4 days postinfection regardless of pregnancy outcome (Table 4). At the time of delivery or 30 days postdelivery, all animals with stillbirths had antibody titers that were between 8- and 32-fold higher than the titers of preinfection samples collected immediately prior to infection or at 4 days after infection. Animals with normal births had antibody titers no more than fourfold greater than those measured preinfection (Table 4).
TABLE 4.
Titers of antibody against L. monocytogenes in sera from monkeys before and after infection
| Birth outcome and infectious dose (CFU) | Antibody titer at:
|
|||
|---|---|---|---|---|
| Preinfectiona | Day 4 postinfection | Delivery | Recovery | |
| Normal deliveries | ||||
| 7.1 × 107 | 800 | 800 | 800 | 800 |
| 7.9 × 106 | 1,600 | 1,600 | 1,600 | 1,600 |
| 1.1 × 106 | 800 | 800 | 800 | 800 |
| 9.1 × 106 | 400 | 400 | 800 | NDb |
| 2.8 × 107 | 800 | 800 | 800 | 800 |
| 5.7 × 108 | 200 | 200 | 800 | 400 |
| Avg ± SD | 800 ± 500 | 800 ± 500 | 900 ± 300 | 900 ± 400 |
| Stillbirths | ||||
| 1.1 × 108 | 1,600 | 1,600 | 12,800c | 6,400 |
| 1.2 × 108 | 1,600 | 1,600 | >25,600c | 25,600 |
| 7.2 × 106 | 800 | 800 | >25,600c | >25,600 |
| 3.7 × 1010 | 1,600 | 1,600 | >25,600c | 25,600 |
| Avg ± SD | 1,400 ± 400 | 1,400 ± 400 | >22,400 ± 6,400 | >20,800 ± 9,600 |
Preinfection sample drawn immediately prior to infection.
ND, not determined for mother; titer for newborn, 400.
Sample drawn at time of stillbirth.
In passive-transfer studies using the mouse listeriosis model, Mackaness (25) demonstrated that there was no protective role for antibodies. More recent reports indicate that neutralizing monoclonal antibodies produced against purified listeriolysin O can protect mice against a potentially lethal infection (10). The lack of protection associated with serum transfers in Mackaness' original studies (25) may be related to the relatively weak anti-Listeria antibody response in mice. In contrast, in the rhesus model, a vigorous anti-Listeria antibody response was associated with stillbirth. An invasive Listeria infection with spreading to the placenta and fetus is clearly a prerequisite for stillbirth. It is likely that the increased antibody response is a result of the invasion of L. monocytogenes beyond the gastrointestinal tract, resulting in more extensive exposure to the immune system. The correlation of the antibody response with a negative outcome (stillbirth) does not suggest a protective role with respect to the fetus. A potential role for antibody in protection of the mother cannot be excluded given the lack of clinical disease.
Cellular immune response.
Peripheral blood mononuclear cells were isolated on Ficoll-Hypaque gradients, washed, and resuspended to a concentration of 5 × 106/ml in RPMI 1640 medium (Sigma) with glutamine plus 10% heat-inactivated fetal bovine serum and antibiotics. Cells were cultured for 72 h with heat-killed L. monocytogenes and assessed for [3H]thymidine incorporation, as previously described (38).
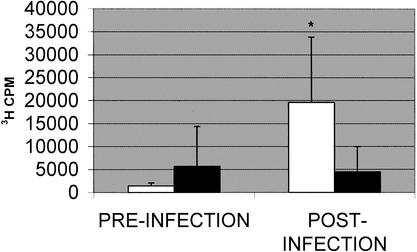
Lymphocytes isolated from peripheral blood responded to heat-killed L. monocytogenes strain 12443 differently depending on the outcome of the infection with respect to stillbirth (Fig. 1). Preinfection, or baseline, average proliferation responses were similar in animals delivering stillborn infants and in those delivering normal infants. Although there was considerable variation in the magnitude of proliferation among individual animals in both the stillbirth and normal-outcome groups, the observed significance level associated with the difference in the means between the two groups postinfection was at a P value of <0.06. Despite the individual variation in postinfection samples, the responses measured in samples from animals with stillbirths were significantly higher than their preinfection levels (P < 0.04).
FIG. 1.
Antigen-induced proliferation of peripheral blood lymphocytes. Proliferative response to heat-killed L. monocytogenes strain 12443 was measured by [3H]thymidine incorporation after 72 h of culture. Bars represent the mean responses ± standard errors of results for animals with normal pregnancy outcomes (filled bars; number of samples, 5) or stillbirths (empty bars; number of samples, 4). Proliferation responses were measured in samples collected immediately prior to infection and approximately 30 days after stillbirth or normal outcome of pregnancy. An asterisk indicates a result that is significantly different from the preinfection level at a P value of <0.05.
Antigen-induced lymphocyte proliferation also correlates with stillbirth; however, it does not appear to be a more sensitive or specific biomarker of invasive listeriosis than antibody response in this small number of animals. The occurrence of an increased proliferative response in those animals with stillbirths does indicate an activation of cell-mediated immune components. At the exposure levels used in this study, neither antibody response nor proliferation of lymphocytes was capable of indicating any immune system changes in animals with normal birth outcomes.
PFGE.
The five strains used in the initial mixture of L. monocytogenes were analyzed by PFGE according to the methods of the Centers for Disease Control and Prevention described by Graves and Swaminathan (17), and each strain was distinguishable from the others by using PFGE. When administered as a single strain, L. monocytogenes isolates from feces from the mother, placental tissue, and fetal tissue (liver, brain, and blood) were identical to the treatment strain.
Statistical analysis.
Statistical differences in hematology and immunology between animals with normal births and those with stillbirths were determined by using the Student's t test (Microsoft Excel 2000). A P value of <0.05 was used to determine significant differences.
In conclusion, the results of our study establish the pregnant rhesus monkey as a model for human listeriosis. When pregnant rhesus monkeys are exposed to L. monocytogenes at the beginning of the third trimester, they have an increased risk of delivering a stillborn infant with pathology similar to that of humans, including acute inflammation, placentitis, fetal liver necrosis, and isolation of Listeria from the placental and fetal tissues. Animals that have become infected with L. monocytogenes, as evidenced by increased antibody response, have stillbirths. This model can be useful for assessing mechanisms of infection and disease, dose response, and intervening therapies for L. monocytogenes infection.
Acknowledgments
This work was supported by FDA grant FDU001622-01 and the Yerkes Center base grant RR0016 from NIH.
We gratefully acknowledge the Centers for Disease Control and Prevention's Listeria Reference Laboratory for providing the human clinical strains of Listeria.
Editor: J. D. Clements
REFERENCES
- 1.Anderson, D. C., and H. M. McClure. 1993. Listeriosis, p. 135-141. In T. C. Jones, U. Mohr, and L. D. Hunt (ed.), Nonhuman primates, vol. I. Monograph on pathology of laboratory animals. Springer-Verlag, New York, N.Y.
- 2.Bula, C. J., J. Bille, and M. P. Glauser. 1995. An epidemic of food-borne listeriosis in western Switzerland: description of 57 cases involving adults. Clin. Infect. Dis. 20:66-72. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1998. Multistate outbreak of listeriosis—United States, 1998. Morb. Mortal. Wkly. Rep. 47:1085-1086. [PubMed] [Google Scholar]
- 4.Chalifoux, L. V., and E. M. Hajema. 1981. Septicemia and meningoencephalitis caused by Listeria monocytogenes in neonatal Macaca fascicularis. J. Med. Primatol. 10:336-339. [DOI] [PubMed] [Google Scholar]
- 5.Cook, L. V. 1998. Isolation and identification of Listeria monocytogenes from red meat, poultry, egg and environmental samples, Sect. 8.1-8.9. In B. P. Dey and C. P. Lattuada (ed.), USDA/FSIS microbiology laboratory guide book. Food Safety and Inspection Service, U.S. Department of Agriculture, Washington, D.C.
- 6.Craven, P. C., D. C. Mackel, W. B. Baine, W. H. Barker, and E. J. Gangarosa. 1975. International outbreak of Salmonella eastbourne infection traced to contaminated chocolate. Lancet i:788-792. [DOI] [PubMed] [Google Scholar]
- 7.Cruikshank, D. P., and J. C. Warenski. 1989. First-trimester maternal Listeria monocytogenes sepsis and chorioamnionitis with normal neonatal outcome. Obstet. Gynecol. 73:469-471. [PubMed] [Google Scholar]
- 8.D'Aoust, Y. E., B. J. Aris, P. Thisdele, A. Durante, N. Brisson, D. Dragon, G. Lachapelle, M. Johnston, and R. Laidley. 1975. Salmonella eastbourne outbreak associated with chocolate. J. Inst. Can. Sci. Technol. Aliment. 8:181-184. [Google Scholar]
- 9.D'Aoust, Y. E. 1985. Infective dose of Salmonella typhimurium in cheddar cheese. Am. J. Epidemiol. 122:717-720. [DOI] [PubMed] [Google Scholar]
- 10.Edelson, B. T., and E. R. Unanue. 2001. Intracellular antibody neutralizes Listeria growth. Immunity 14:503-512. [DOI] [PubMed] [Google Scholar]
- 11.Enocksson, E., B. Wretlind, G. Sterner, and B. Anzen. 1992. Listeriosis during pregnancy and in neonates. Scand. J. Infect. Dis. 71(Suppl.):89-94. [PubMed] [Google Scholar]
- 12.Farber, J. M., E. Daley, F. Coates, N. Beausoleil, and J. Fournier. 1991. Feeding trials of Listeria monocytogenes with a nonhuman primate model. J. Clin. Microbiol. 29:2606-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frederiksen, B., and S. Samuelsson. 1991. Feto-maternal listeriosis in Denmark 1981-1988. J. Infect. 24:277-287. [DOI] [PubMed] [Google Scholar]
- 15.Geller, N. L. 1990. Design of phase I and II trials in cancer. Drug Infect. J. 24:341-349. [Google Scholar]
- 16.Gellin, B. G., C. V. Broome, W. F. Bibb, R. E. Weaver, S. Gaventa, L. Mascola, and the Listeriosis Study Group. 1991. The epidemiology of listeriosis in the United States—1986. Am. J. Epidemiol. 131:392-401. [DOI] [PubMed] [Google Scholar]
- 17.Graves, L. M., and B. Swaminathan. 2001. PulseNet standardized protocol for subtyping Listeria monocytogenes by macrorestriction and pulsed-field gel electrophoresis. Int. J. Food Microbiol. 65:55-62. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood, M. H., and W. L. Hooper. 1983. Chocolate bars contaminated with Salmonella napoli: an infectivity study. BMJ 286:1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedberg, C. W., K. L. MacDonald, and M. T. Osterholm. 1994. Changing epidemiology of food-borne diseases: a Minnesota perspective. Clin. Infect. Dis. 18:671-682. [DOI] [PubMed] [Google Scholar]
- 20.Hitchens, A. D. 1996. Assessment of alimentary exposure to Listeria monocytogenes. Int. J. Food Microbiol. 30:71-85. [DOI] [PubMed] [Google Scholar]
- 21.Holcomb, D. L., M. A. Smith, G. O. Ware, Y. C. Hung, R. E. Brackett, and M. P. Doyle. 1999. Comparison of dose-response models for use with food-borne pathogens. Risk Anal. 19:1091-1100. [DOI] [PubMed] [Google Scholar]
- 22.Lee, G. R., M. Foerster, J. Lukens, F. Paraskevas, J. P. Greer, and G. M. Rodgers (ed.). 1999. Wintrobe's clinical hematology, 10th ed., p. 2752-2753. Lippincott, Williams & Wilkins, Baltimore, Md.
- 23.Lehmacher, A., J. Bockemuhl, and S. Aleksic. 1995. Nationwide outbreak of human salmonellosis in Germany due to contaminated paprika and paprika-powdered potato chips. Epidemiol. Infect. 115:501-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linnan, M. J., L. Mascola, X. D. Lou, V. Goulet, S. May, C. Salminen, D. W. Hird, M. L. Yonekura, P. Hayes, R. Weaver, A. Audurier, B. D. Plikaytis, S. L. Fannin, A. Kleks, and C. V. Broome. 1988. Epidemic listeriosis associated with Mexican-style cheese. N. Engl. J. Med. 319:823-828. [DOI] [PubMed] [Google Scholar]
- 25.Mackaness, G. B. 1971. Resistance to intracellular infection. J. Infect. Dis. 123:439-445. [DOI] [PubMed] [Google Scholar]
- 26.Matyunas, N. J. 1987. The Polar Bar ice cream recall—report on exposure in pregnant women. Vet. Hum. Toxicol. 29:469. [Google Scholar]
- 27.McClure, H. M., A. R. Brodie, D. C. Anderson, and R. B. Swenson. 1986. Bacterial infections of nonhuman primates, p. 531-556. In K. Benirshke (ed.), Primates, the road to self-sustaining populations. Springer-Verlag, New York, N.Y.
- 28.McClure, H. M., and L. M. Strozier. 1975. Perinatal listeric septicemia in a Celebese black ape. J. Am. Vet. Med. Assoc. 167:637-638. [PubMed] [Google Scholar]
- 29.McLauchlin, J. 1990. Human listeriosis in Britain, 1967-85, a summary of 722 cases. Epidemiol. Infect. 104:181-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinner, R. W., A. Schuchat, B. Swaminathan, P. S. Hayes, K. A. Deaver, R. E. Weaver, B. D. Plikaytis, M. Reeves, C. V. Broom, J. D. Wenger, and the Listeria Study Group. 1992. Role of foods in sporadic listeriosis. II. Microbiologic and epidemiologic investigation. JAMA 267:2046-2050. [PubMed] [Google Scholar]
- 31.Ramsey, E. M. 1982. The placenta: human and animal, p. 65-98. Praeger Publishers, New York, N.Y.
- 32.Rocourt, J. 1994. Listeria monocytogenes: the state of the science. Dairy Food Environ. Sanitat. 14:70-82. [Google Scholar]
- 33.Schuchat, A., K. A. Deaver, J. D. Wenger, B. D. Plikaytis, L. Mascola, R. W. Pinner, A. L. Reingold, C. V. Broome, and the Listeria Study Group. 1992. Role of foods in sporadic listeriosis. I. Case-control study of dietary risk factors. JAMA 267:2041-2045. [PubMed] [Google Scholar]
- 34.Sprong, R. C., M. F. Hulstein, and R. Van der Meer. 1999. High intake of milk fat inhibits intestinal colonization of Listeria but not of Salmonella in rats. J. Nutr. 129:1382-1389. [DOI] [PubMed] [Google Scholar]
- 35.Tappero, J. W., A. Schuchat, K. A. Deaver, L. Mascola, and J. D. Wenger. 1995. Reduction in the incidence of human listeriosis in the United States. JAMA 273:1118-1122. [DOI] [PubMed] [Google Scholar]
- 36.U.S. Food and Drug Administration/Center for Food Safety and Applied Nutrition, USDA/Food Safety and Inspection Service and the Centers for Disease Control and Prevention. 2001. Draft assessment of the relative risk to public health from foodborne Listeria monocytogenes among selected categories of ready-to-eat foods. Technical report. [Online.] http://www.foodsafety.gov/∼dms/lmrisksu.html.
- 37.Vetesi, F., A. Balsai, and F. Kemenes. 1972. Abortion in Gray's monkey (Cercopithicus mona) associated with Listeria monocytogenes. Acta Microbiol. Acad. Sci. Hung. 19:441-443. [PubMed] [Google Scholar]
- 38.Williams, K. M., E. C. Bigley III, and R. B. Raybourne. 2000. Identification of murine B-cell and T-cell epitopes of Escherichia coli outer membrane protein F with synthetic polypeptides. Infect. Immun. 68:2535-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]