Abstract
Congenital Plasmodium falciparum malaria in newborns is uncommon in sub-Saharan Africa. A significant number of infants, however, become infected or exposed to malarial antigens either in utero or at delivery and have the potential to produce antimalarial antibodies and memory cells before their first natural infection. In Yaounde, Cameroon, parasite-specific immunoglobulin M (IgM) was detected in 14% of cord blood samples. The IgM antibodies reacted with a wide range of asexual-stage antigens, with each newborn having its own unique pattern of IgM reactivity. PCR-based detection and genotyping of cord blood parasites found that the prevalence, total number of parasite genotypes, and complexity of infection were higher in newborns who had produced antimalarial IgM than those who had not. Maternal placental malaria and anemia were associated with the production of P. falciparum-specific IgM by the fetus. The effect of early immune priming on acquisition of immunity by infants is unknown and merits further investigation, since a significant proportion of Cameroonian newborns developed a humoral response to malaria before birth.
In sub-Saharan Africa, congenital malaria is rare because few newborns develop clinical disease during the first few weeks of life (16). However, finding anti-Plasmodium falciparum immunoglobulin M (IgM) and IgE antibodies in cord blood of African newborns is not uncommon (1, 4, 5, 7, 8), as studies have reported parasite-specific IgM in up to 25.4% of cord blood samples (8). The presence of P. falciparum IgM in cord blood suggests that the fetus was infected in utero and B-cell activation occurred. Alternatively, malarial antigens, perhaps as immune complexes, could have crossed the placental barrier and stimulated the response (6, 12). The specificity of antimalarial antibodies produced by the fetus is unknown but may be limited, as the antigenic repertoire of fetal B cells is reduced compared to that of adults (11, 24). As a result, it is possible that a fetus may be able to produce antibodies to only a limited number of malarial antigens.
It is important to determine the extent of in utero antigenic recognition because early immune priming may have a direct impact on the development of immunity to malaria when infants become infected later in life. Recently (2002), King et al. detected memory B cells specific for the C-terminal region of MSP-119, a determinant involved in immune protection, in the cord blood of Kenyan newborns (14). Thus, exposure to malaria in utero or at the time of delivery could result in the induction of protection prior to primary natural infection.
The present study sought to characterize the antigenic specificity of fetal antimalarial IgM antibodies and identify malaria-related factors associated with in utero immune priming. In addition, PCR-based genotyping was used to determine the number and complexity of parasite genotypes circulating in cord blood of newborns who had or had not produced antimalarial IgM in utero.
MATERIALS AND METHODS
Study population.
Approval for the study was obtained from the National Ethical Committee, Ministry of Health, Cameroon, and the Institutional Review Board, Georgetown University. This study is covered by Single Project Assurance S-934. Samples were collected between December 1997 and November 1998 at the Central Maternity Hospital, Yaounde, Cameroon. The purpose of the study was explained to each woman. After verbal informed consent was obtained, information relevant to the pregnancy was recorded, including maternal age, number of pregnancies, and date of last menstrual period. A total of 202 women participated. Following delivery, infant birth weight was recorded, as well as pregnancy outcome, i.e., abortion (nonliving infant <28 weeks), premature birth (living infant <37 weeks), stillbirth (nonliving infant ≥28 weeks), or full term. Low birth weight was defined as <2,500 g. Approximately 5 ml of heparinized maternal venous blood, 3 ml of maternal placental blood, 5 ml of fetal cord blood, and a piece (2 by 2 by 2 cm) of the midsection of the placenta were collected. A capillary tube was filled with venous peripheral blood, and the packed cell volume was determined. Plasma and red blood cells (RBC) were separated and stored at −20°C. A piece of the placenta was placed in 10% buffered formalin for histological examination.
Microscopic detection of P. falciparum parasites.
Thick and thin blood smears of maternal venous and placental blood and placental impression smears were prepared and stained with Diff-Quick (Baxter Scientific, Inc., Deerfield, Ill.), and parasitemia was calculated as the number of infected RBC (IRBC) per 2,000 total RBC. In addition, histological slides of the placenta were examined for parasites. A woman was considered to be malaria positive if parasites were detected in the peripheral blood smear, the placental impression smear, or placental histosections.
Enzyme-linked immunosorbent assay (ELISA) for anti-P. falciparum IgM antibodies.
Cord samples were depleted of IgG by incubating 10 μl of plasma with 40 μl of a 50% slurry of protein G-Sepharose (Sigma, St. Louis, Mo.) overnight at 4°C. Following centrifugation, plasma was collected, diluted, and used in the ELISA.
Nunc-Immuno Maxisorp ELISA plates were coated overnight at 4°C with 100 μl of an extract containing either 105 P. falciparum IRBC (from in vitro culture) or normal RBC (NRBC) in 0.1 M carbonate buffer (pH 9.5). Wells were washed with phosphate-buffered saline-0.05% Tween 20 (PBS-Tw) and blocked with 10% milk in PBS-Tw for 1 h at room temperature (RT), and 100 μl of plasma diluted 1:100 in 1% milk PBS-Tw was added for 1 h. Following washing, 100 μl of alkaline phosphatase-conjugated anti-human IgM antibody (Southern Biotechnology Associate, Birmingham, Ala.) was added for 1 h at RT. After washing, 100 μl of p-nitrophenyl phosphate substrate (Sigma) in diethanolamine buffer was added and optical densities (OD) at 405 and 630 nm were determined 45 to 60 min later. Negative controls included U.S. human serum and a pool of ELISA-negative Cameroonian cord plasma. The level of anti-P. falciparum IgM was calculated by subtracting the mean OD of NRBC from the OD of IRBC. If the value was twice as high as that of the negative control, the sample was considered positive for anti-P. falciparum IgM.
IgM-specific indirect immunofluorescent antibody (IFA) assay.
Smears of IRBC were fixed in acetone and incubated with plasma diluted 1:100 for 30 min at RT, followed by a 1:500 dilution of monoclonal antibody specific for human μ chain (clone MB-11; Sigma). Anti-mouse Ig coupled to fluorescein isothiocyanate (Sigma) was then applied for 30 min. Slides were mounted in 50% glycerol in barbital buffer (pH 9.0) and examined. A pool of plasma containing anti-P. falciparum IgM and two negative controls (U.S. plasma and pooled anti-P. falciparum IgM-negative cord plasma) were used.
Determination of the specificity of antimalarial IgM antibodies by Western blotting.
Aliquots containing 2 × 106 P. falciparum IRBC or NRBC in reducing sample buffer were applied to sodium dodecyl sulfate-10% polyacrylamide gels. After electrophoretic separation, the proteins were transferred to nitrocellulose membranes (0.45-μm pore size; Bio-Rad, Hercules, Calif.). Blots were blocked with 10% milk PBS-Tw overnight at 4°C, incubated for 2 h at RT with a 1:100 dilution of IgG-depleted plasma, and then incubated with 1:1,000-diluted alkaline phosphatase-labeled monoclonal antibody clone MB-11 for 1 h. Bands were visualized by using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside-Nitro Blue Tetrazolium membrane phosphatase substrate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.).
Detection and genotyping of P. falciparum by PCR.
DNA was extracted from 200 μl of packed maternal placental or fetal cord RBC as described by Snounou et al. (19, 20). The presence of the gene for the small subunit of rRNA of P. falciparum was detected by using a nested PCR (19, 20). Samples containing malarial DNA were subjected to genotype analysis for allelic variation in MSP-1 Block 2 by the nested PCR method described by Branch et al. (3).
Statistical analysis.
All statistical analyses were conducted with SAS software (Version 8.2; SAS Institute). Methods of analysis for comparisons of parametrically and nonparametrically distributed variables are described in Results. A Poisson regression model was used to compare the number of parasite genotypes, and a logistic regression model was used to identify risk factors associated with immune priming.
RESULTS
Detection of anti-P. falciparum IgM antibodies in cord blood.
A total of 207 cord blood samples were collected from the 202 deliveries. They were screened in the IgM-specific ELISA, and 24 (11.6%) were positive for antimalarial IgM. Since high-affinity IgG antibodies can interfere with the detection of IgM, the samples were depleted of IgG and rescreened. Three additional samples with low levels of antimalarial IgM were detected. The 27 IgM-positive cord blood samples were from 24 singletons and three sets of monoplacental twins. Therefore, 14% (30 of 215) of the babies were born with antimalarial IgM.
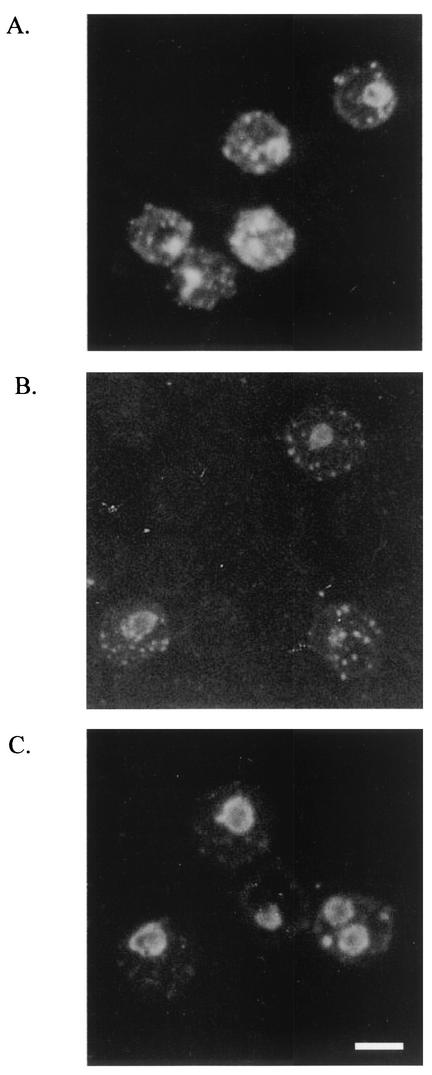
To confirm that the 27 ELISA-positive cord blood samples contained antimalarial IgM, they were tested for parasite reactivity by IFA assay. All 27 samples had IgM antibodies that bound to asexual-stage parasites (Fig. 1). Approximately 40% of the samples had antibodies that detected antigens in early- and late-stage trophozoites and within Mauer's clefts, whereas the other 60% detected antigens localized primarily within late-stage parasites only (Fig. 1B and C).
FIG. 1.
IFA patterns produced by cord blood IgM antibodies. (A) Pattern produced by adult Cameroonian IgG (positive control). (B) About 40% of plasma samples detected antigens in early- and late-stage trophozoites and within Mauer's clefts. (C) About 60% of the plasma samples detected antigens primarily within late-stage parasites. Bar, 5 μm.
Antigenic specificity of antimalarial IgM antibodies produced by the fetus.
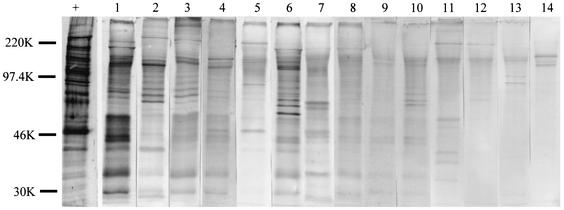
By Western blot analysis, IgM antibodies in the 27 newborns were found to identify a wide range of malarial antigens. Results from 14 of the samples are shown in Fig. 2. For comparison, the pattern produced by adult Cameroonian IgG is shown in the lane marked by a plus sign. Cord blood IgM detected 3 to >30 antigen bands (Fig. 2), with ∼45% of the samples identifying more than 10 bands. The antigenic specificity of IgM in the samples was unique for each newborn. Samples on the left of the figure had the highest IgM levels by ELISA and detected 15 or more bands. Samples 13 and 14, on the far right, had the lowest IgM levels, but each sample recognized a distinct set of three to six bands. None of the malarial antigens was immunodominant. When the samples were tested on NRBC, only zero to three bands were seen, demonstrating that the majority of the bands in Fig. 2 are malarial antigens (data not shown). Clearly, a fetus can produce antibodies to a wide repertoire of malarial antigens but the pattern is different for each newborn.
FIG. 2.
Patterns of reactivity of cord blood IgM with P. falciparum antigens. P. falciparum IRBC (2 × 106) were added to each lane. The lane marked with a plus sign was incubated with pooled adult Cameroonian plasma, followed by anti-human IgG. Lanes 1 to 14 were treated with cord plasma from 14 different newborns, followed by anti-human IgM. Samples on the left had larger amounts of IgM (as shown by ELISA) than those on the right. The results show that each newborn had a unique set of malaria-specific IgM antibodies. The values on the left are molecular sizes in kilodaltons.
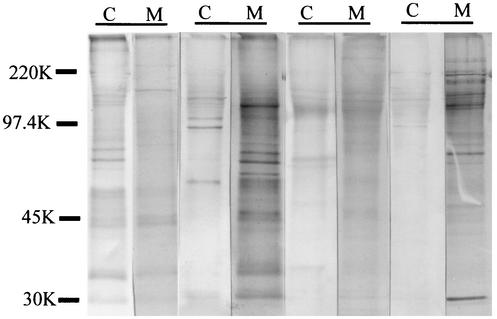
To verify that the IgM antibodies were produced by the fetus and did not come from the mother because of admixing of blood at delivery, the antimalarial specificity of the IgM of newborns was compared with that of the IgM of their mothers (Fig. 3). The results showed that the IgM of 21 of 23 mother-newborn pairs detected different sets of antigens. Two of the pairs had identical patterns, making it impossible to determine if admixing had occurred or if the mother and fetus produced antibodies to the same antigens. These results show that the majority, if not all, of the bands in Fig. 2 represent antigens that induce humoral responses in utero.
FIG. 3.
Comparison of antimalarial IgM antibodies in maternal and cord blood samples. Four pairs of fetal cord (C) and maternal (M) peripheral plasma samples were incubated with blots of IRBC, followed by anti-human IgM. The results demonstrate that fetal and maternal blood contained IgM antibodies with different antimalarial specificities. In interpreting the blots, care was taken to ensure that the patterns were definitely distinct between the pairs and did not result from differences in amounts of antibody. The values on the left are molecular sizes in kilodaltons.
PCR detection and genotyping of cord blood parasites.
Since Cameroonian cord blood samples are routinely negative for malarial parasites by microscopy (18; personal observation based on >600 samples), PCR was used to detect P. falciparum DNA in cord blood. Cryopreserved cord erythrocytes from 26 of the 27 IgM+ newborns and 41 IgM− newborns (i.e., a representative subset of the larger population) were analyzed. The results showed that 48% of the IgM+ newborns were PCR positive for P. falciparum, compared to only 19.5% of the IgM− newborns (P = 0.028 [Fisher's exact test]).
The above-described parasites were genotyped for Block 2 MSP-1 alleles to estimate the number of genotypes present in the blood of newborns with and without antimalarial IgM. On the basis of a Poisson regression model, 2.0 ± 0.72 (mean ± standard deviation) times as many genotypes were present in the cord blood of the IgM+ group as in that of the IgM− group (P = 0.0495). In the IgM+ group, the mean number of genotypes was 1.2 ± 0.34 (range, 0 to 5), with 50% (10 of 20) of the samples having parasites with one or more K1 alleles, 30% having parasites with one or more MAD20 alleles, and 10% having parasites with the RO33 allele. In contrast, in the IgM− group, the mean number of genotypes was only 0.62 ± 0.18 (range, 0 to 2), with 38% (8 of 21) of the samples having parasites with K1 alleles, 5% having parasites with MAD30 alleles, and 10% having parasites with the RO33 allele. A significant difference in the frequencies of MSP-1 Block 2 alleles was observed between the two groups (P = 0.01 [Fisher's exact test]). Thus, the prevalence, total number of different parasite genotypes, and complexity of infection differed between newborns who produced antimalarial IgM in utero and those who did not.
Risk factors associated with in utero B-cell priming.
Clinical information about mothers of infants in the IgM+ and IgM− groups was used to identify factors associated with in utero B-cell priming. Maternal age, primigravidae, length of gestation, peripheral parasitemia, level of placental parasitemia, and having twins were not significant (Table 1). However, the prevalence of placental malaria (40.7% in the IgM+ group, compared to 18.6% in the IgM− group [P = 0.04]) and maternal anemia (71.4 versus 35.8% [P = 0.008]) was significantly higher in the IgM+ group. Thus, the presence or absence of placental malaria and maternal anemia was associated with in utero production of P. falciparum-specific IgM by the fetus.
TABLE 1.
Assessment of factors associated with in utero B-cell priming
| Parameter | Cord blood
|
P value* | |
|---|---|---|---|
| IgM+ | IgM− | ||
| Mean maternal age (yr) ± SD | 26.9 ± 7.2 | 26.3 ± 6.4 | 0.649a |
| % Primigravidae | 40.7 | 32.7 | 0.696b |
| Anemia (% with packed cell volume of <30%) | 71.4 | 35.3 | 0.008b |
| % with placental malaria | 40.7 | 18.6 | 0.044b |
| Mean gestation time (wk) | 38.0 ± 3.7 | 37.8 ± 4.5 | 0.787a |
| Mean % placental parasitemia ± SE | 3.5 ± 8.5 | 1.1 ± 4.9 | 0.083c |
| Mean % peripheral parasitemia ± SE | 0.62 ± 2.7 | 0.27 ± 1.4 | 0.099c |
| % of women in each group placental parasitemia level: | 0.293d | ||
| Negative | 59.3 | 81.7 | |
| ≤1% | 11.1 | 7.4 | |
| 1.1-10% | 14.8 | 6.3 | |
| 10.1-20% | 3.7 | 1.7 | |
| >20% | 11.1 | 2.9 | |
| % Having twins | 11.1 | 5.7 | 0.537b |
Student's t test.
Fisher's exact test.
Wilcoxon rank-sum test.
Chi-square test.
There was no significant difference in delivery outcomes (i.e., proportion of abortions, premature deliveries, stillbirths, and full-term deliveries), mean infant birth weight, or percentage of low birth weight infants between the newborns with and without antimalarial IgM in their cord blood.
DISCUSSION
Although it is clear that a fetus can mount an acquired immune response to P. falciparum antigens prior to birth (1, 4, 5, 8), there is much to be learned about the frequency and impact of in utero exposure. Results of the present study suggest that ∼14% of newborns in Yaounde, Cameroon, were exposed in utero and produced malaria-specific IgM. IgM antibodies in these newborns reacted with a wide range of asexual-stage antigens, with each newborn having its own unique pattern of IgM reactivity. Some infants produced antibodies to >25 antigens, whereas others responded to a limited number (Fig. 2). IFA patterns showed that the antigens were located within intracellular parasites, as well as within Mauer's clefts, i.e., vesicles that transport antigens from the parasite to the erythrocyte surface (Fig. 1B). These results demonstrate that the fetal B-cell response was not limited to a restricted set of immunodominant epitopes. Further studies are needed to determine if the fetus recognizes vaccine candidate antigens, but such is likely to be the case, as memory B cells that secrete IgG in response to the C terminus of MSP-119 have been detected in cord blood samples from Kenyan newborns (14). Thus, in utero priming may have a positive effect in helping protect infants while they acquire immunity to malaria early in life.
Few studies have assessed risk factors associated with in utero priming (4, 14). Results from our study showed that Cameroonian mothers who delivered newborns with antimalarial IgM were more likely to be anemic (P = 0.008) and had placental malaria at the time of delivery (P = 0.04) (Table 1). In addition, they tended to have higher mean levels of placental and peripheral parasitemia, although these differences did not reach statistical significance (P = 0.08 and 0.10, respectively). An association between placental malaria and in utero production of malaria-specific IgM has been reported previously by Chizzolini et al. (4). They examined histological sections of placentas from seven mothers whose newborns were cord blood positive for malarial IgM and found some evidence of malaria-related histological changes in six of the placentas (4). Although the sample size is small, it appears that the presence of parasites, perhaps for an extended period of time, may alter the materno-fetal barrier and increase the risk of in utero exposure.
It is unclear how malarial parasites or antigens cross the placental barrier and enter the circulation of the fetus or why anemia might enhance this exchange (Table 1). Recent studies have shown that during normal pregnancies, maternal erythrocytes and leukocytes can cross the placental barrier and enter the circulation of the fetus (12, 13, 22), although the frequency and number of cells exchanged are low (15, 21, 25). It is likely that placental malaria increases the risk of transfer of maternal cells, including IRBC and antigen-presenting cells. For example, placental malaria stimulates the infiltration of mononuclear cells (and other antigen-presenting cells), induces an alteration in the cytokine balance, and causes pathological changes in trophoblasts (9, 10, 17, 23). One additional consequence of placental malaria is maternal anemia. Anemia per se places stress on the placenta, often causing an increase in placental weight (2), by increasing cellular proliferation, remodeling of placental architecture, and angiogenesis. Thus, it is easy to envision that placental malaria increases the likelihood of bidirectional materno-fetal exchange. In the present study, we found no evidence that in utero exposure had a negative effect on the fetus but more extensive studies are needed to confirm this observation.
In this study, 14% of Cameroonian newborns produced antimalarial IgM antibodies to a variety of malarial antigens in utero. In addition, parasite DNA was detected in cord blood of 19.5% of newborns who were IgM negative, suggesting that they were exposed to malarial antigens at the end of gestation or during delivery. These perinatally exposed newborns could also begin producing antibodies during the first few weeks of life and therefore mount a secondary immune response the first time they are bitten by an infected mosquito. Clearly, in utero immune priming and perinatal exposure are not uncommon events. Further studies are needed to determine how these processes affect the frequency and severity of malaria during the first year of life.
Acknowledgments
This work was supported by National Institutes of Health grants AI35839 (sample collection) and AI43888 (sample analysis).
We thank A. H. Johnson and O. Branch for fruitful discussions, members of the University of Yaounde medical team, technical and administrative staff at the Biotechnology Center, and all of the women who consented to participate in the study.
Editor: J. M. Mansfield
REFERENCES
- 1.Achidi, E. A., and L. S. Salimonu. 1997. Malaria parasitaemia and immunoglobulin levels in paired maternal-cord sera from southwestern Nigeria. Afr. J. Med. Sci. 26:167-170. [PubMed] [Google Scholar]
- 2.Beischer, N. A., M. Holsman, and W. H. Kitchen. 1968. Relationship of various forms of anemia to placental weight. Am. J. Obstet. Gynecol. 101:801-809. [DOI] [PubMed] [Google Scholar]
- 3.Branch, O. H., S. Takala, S. Kariuki, B. L. Nahlen, M. Kolczak, W. Hawley, and A. A. Lal. 2001. Plasmodium falciparum genotypes, low complexity of infection, and resistance to subsequent malaria in participants in the Asembo Bay Cohort Project. Infect. Immun. 69:7783-7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chizzolini, C., F. Trottein, F. X. Bernard, and M. H. Kaufmann. 1991. Isotypic analysis, antigen specificity, and inhibitory function of maternally transmitted Plasmodium falciparum-specific antibodies in Gabonese newborns. Am. J. Trop. Med. Hyg. 45:57-64. [DOI] [PubMed] [Google Scholar]
- 5.Deloron, P., B. Dubois, and J. Y. Le Hesran, D. Riche, N. Fievet, M. Cornet, P. Ringwald, and M. Cot. 1997. Isotypic analysis of maternally transmitted Plasmodium falciparum-specific antibodies in Cameroon, and relationship with risk of P. falciparum infection. Clin. Exp. Immunol. 110:212-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desowitz, R. S. 1988. Prenatal immune priming in malaria: antigen-specific blastogenesis of cord blood lymphocytes from neonates born in a setting of holoendemic malaria. Ann. Trop. Med. Parasitol. 82:121-125. [DOI] [PubMed] [Google Scholar]
- 7.Desowitz, R. S., J. Elm, and M. P. Alpers. 1992. Prenatal immune hypersensitization to malaria: Plasmodium falciparum-specific IgE antibody in paired maternal and cord sera from Papua New Guinea. P. N. G. Med. J. 35:303-305. [PubMed] [Google Scholar]
- 8.Egwunyenga, O. A., J. A. Ajayi, and D. D. Duhlinska-Popova. 1995. Transplacental passage of Plasmodium falciparum and seroevaluation of newborns in northern Nigeria. J. Commun. Dis. 27:77-83. [PubMed] [Google Scholar]
- 9.Fried, M., R. O. Muga, A. O. Misore, and P. E. Duffy. 1998. Malaria elicits type 1 cytokines in the human placenta: IFN-γ and TNF-α associated with pregnancy outcomes. J. Immunol. 160:2523-2530. [PubMed] [Google Scholar]
- 10.Garin, Y. J., P. Blot, P. Walter, J. M. Pinon, and A. Vernes. 1985. Malarial infection of the placenta: parasitologic, clinical, and immunologic aspects. Arch. Fr. Pediatr. 42(Suppl. 2):917-920. [PubMed] [Google Scholar]
- 11.Halista, S. M., L. A. Johnson-Robinson, A. E. El-Mohandes, A. Lees, J. J. Mond, and I. M. Katona. 1998. Characterization of early activation events in cord blood B cells after stimulation with T cell-independent activators. Pediatr. Res. 43:496-503. [DOI] [PubMed] [Google Scholar]
- 12.Jakobsen, P. H., F. N. Rasheed, J. N. Bulmer, M. Theisen, R. G. Ridley, and B. M. Greenwood. 1998. Inflammatory reactions in placental blood of Plasmodium falciparum-infected women and high concentrations of soluble E-selectin and a circulating P. falciparum protein in the cord sera. Immunology 93:264-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King, C. L., I. Malhotra, P. Mungai, A. Wamachi, J. Kioko, J. H. Ouma, and J. W. Kazura. 1998. B cell sensitization to helminthic infection develops in utero in humans. J. Immunol. 160:3578-3584. [PubMed] [Google Scholar]
- 14.King, C. L., I. Malhotra, A. Wamachi, J. Kioko, P. Mungai, S. A. Wahab, D. Koech, P. Zimmerman, J. Ouma, and J. W. Kazura. 2002. Acquired immune responses to Plasmodium falciparum merozoite surface protein-1 in the human fetus. J. Immunol. 168:356-364. [DOI] [PubMed] [Google Scholar]
- 15.Lo, Y. M., E. S. Lo, N. Watson, L. Noakes, I. L. Sargent, B. Thilaganathan, and J. S. Wainscoat. 1996. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood 88:4390-4395. [PubMed] [Google Scholar]
- 16.McGregor, I. 1984. Epidemiology, malaria and pregnancy. Am. J. Trop. Med. Hyg. 33:517-525. [DOI] [PubMed] [Google Scholar]
- 17.Moore, J. M., B. L. Nahlen, A. Misore, A. A. Lal, and V. Udhayakumar. 1999. Immunity to placental malaria. I. Elevated production of interferon-gamma by placental blood mononuclear cells is associated with protection in an area with high transmission of malaria. J. Infect. Dis. 179:1218-1225. [DOI] [PubMed] [Google Scholar]
- 18.Rasheed, F. N., J. N. Bulmer, A. De Francisco, M. F. Jawla, P. H. Jakobsen, A. Jepson, and B. M. Greenwood. 1995. Relationships between maternal malaria and malarial immune responses in mothers and neonates. Parasite Immunol. 17:1-10. [DOI] [PubMed] [Google Scholar]
- 19.Snounou, G., S. Viriyakosol, W. Jarra, S. Thaithong, and K. N. Brown. 1993. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol. Biochem. Parasitol. 58:283-292. [DOI] [PubMed] [Google Scholar]
- 20.Snounou, G., S. Viriyakosol, and X. P. Zhu, W. Jarra, L. Pinheiro, V. E. do Rosario, S. Thaithong, and K. N. Brown. 1993. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 61:315-320. [DOI] [PubMed] [Google Scholar]
- 21.Socie, G., E. Gluckman, E. Carosella, Y. Brossard, C. Lafon, and O. Brison. 1994. Search for maternal cells in human umbilical cord blood by polymerase chain reaction amplification of two minisatellite sequences. Blood 83:340-344. [PubMed] [Google Scholar]
- 22.Tobian, A. A., R. K. Mehlotra, I. Malhotra, A. Wamachi, P. Mungai, D. Koech, J. Ouma, P. Zimmerman, and C. L. King. 2000. Frequent umbilical cord-blood and maternal-blood infections with Plasmodium falciparum, P. malariae, and P. ovale in Kenya. J. Infect. Dis. 182:558-563. [DOI] [PubMed] [Google Scholar]
- 23.Walter, P., J. F. Garin, P. Blot, and E. Philippe. 1981. The placenta and malaria: a morphologic, parasitologic and clinical study. J. Gynecol. Obstet. Biol. Reprod. 10:535-542. [PubMed] [Google Scholar]
- 24.Youinou, P., C. Jamin, and P. M. Lydyard. 1999. CD5 expression in human B-cell populations. Immunol. Today 20:312-316. [DOI] [PubMed] [Google Scholar]
- 25.Zarou, D. M., H. C. Lichtman, and L. M. Hellman. 1964. The transmission of chromium-51 tagged maternal erythrocytes from mother to fetus. Am. J. Obstet. Gynecol. 88:565-571. [DOI] [PubMed] [Google Scholar]