Abstract
Pseudomonas aeruginosa keratitis is one of the most destructive diseases of the cornea. The host response to this infection is critical to the outcome. The cytokine interleukin-10 (IL-10) is thought to play an important role in modulating excessive inflammation and antimicrobial defenses. We have found that in IL-10−/− mice there is a significant decrease in bacterial load in corneas at 7 days postchallenge with P. aeruginosa. This decrease was accompanied by a reduction in neutrophil numbers in the cornea and changes in cytokine levels compared to those of wild-type mice. A characteristic increase in neovascularization in the cornea was found in the IL-10−/− mice. This increased angiogenesis correlated with an increased expression of KC, whereas the kinetics of macrophage inflammatory peptide 2 expression correlated with neutrophil numbers. This finding suggests that KC may play a role in corneal angiogenesis. The source of IL-10 in mouse corneas was identified as a subpopulation of infiltrating cells and keratocytes. This study demonstrates that IL-10 plays an important role in regulating the balance of inflammatory mediators during P. aeruginosa infection of the cornea.
Corneal ulceration as a result of bacterial infection can be a devastating condition leading to permanent, extensive vision loss. Pseudomonas aeruginosa accounts for up to 70% of all cases of contact lens-related bacterial keratitis (20). Bacterial keratitis can be extremely difficult to treat despite newly developed antibiotics and other modes of therapy. Although therapy may succeed in eliminating the bacterial load, blindness can still be a sequela as a result of corneal scarring. The host inflammatory response, orchestrated by cytokines and chemokines, has been implicated as an important contributor to corneal damage during infection (14, 17, 23).
Interleukin-10 (IL-10) is a cytokine thought to play an important role in host responses to microbial infection, having an immunoregulatory role in modulating excessive inflammation and antimicrobial defenses. IL-10 appears relatively late in immune responses (2), and its induction is mediated by specific bacterial products and can be specific to the strain of bacteria (24). The actions of IL-10 may be mediated through the regulation of other cytokines and chemokines and may have direct effects on inflammatory cell function (2). Cytokines reported to be regulated by IL-10 include gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-6 (2). IL-10 has also been implicated in the regulation of angiogenesis via the regulation of TNF-α, vascular endothelial growth factor (VEGF) (27), and CXC chemokines, which are also reported to be involved in blood vessel formation (1). The effects of IL-10 are pathogen specific. For example, while IL-10-deficient mice die rapidly from Toxoplasma or Trypanosoma cruzi infections (13, 15), they show increased resistance to candidiasis (31) and mycobacterial infections (21). A role for IL-10 in responses to P. aeruginosa infection was first reported by Fauntleroy et al. (11). In models of P. aeruginosa infection, IL-10 has been reported to be an important regulator of the host response with a complex role. During chronic P. aeruginosa pneumonia, a deficiency of IL-10 was found to exacerbate lung damage (5), and recent studies of transient P. aeruginosa challenge of the lungs in IL-10−/− mice have found that, although the infection is cleared, IL-10 deficiency contributes to prolonged inflammatory responses (6). Reduced IL-10 levels have also been reported to facilitate the clearance of P. aeruginosa from patients with acute pneumonia (28).
In the eye, the role of IL-10 is not well understood. Its actions in herpes simplex virus (HSV) keratitis have been investigated; it was shown that the administration of IL-10 markedly reduced the severity of the inflammation (30). The role of IL-10 during endotoxin-induced uveitis has also been explored. Under those conditions, IL-10 was found to either suppress or exacerbate ocular inflammation in a dose-dependent manner (22). However, the role of IL-10 during P. aeruginosa keratitis has yet to be characterized. The availability of mice with a targeted disruption of the IL-10 gene provides a unique tool for elucidating IL-10's role in ocular infection. Examination of the changes induced in the cornea during P. aeruginosa infection in the absence of IL-10 may lead to a better understanding of the mechanisms of the host response during bacterial keratitis. This is essential to the development of novel adjunct therapies and prophylactic measures to improve patient outcome in this blinding and increasingly common disease.
MATERIALS AND METHODS
Bacterial cultures.
Stock cultures of P. aeruginosa 6206 and 6294 stored in 30% glycerol at −70°C were inoculated into 10 ml of tryptone soy broth (Oxoid Ltd., Sydney, Australia). These strains of bacteria are well-characterized corneal isolates from cases of microbial keratitis (12) which lead to distinct corneal pathologies in a mouse model (7). Fleiszig et al. (12) have demonstrated that strain 6206 is toxic to mammalian cells but that strain 6294 is an invasive strain and is engulfed by mammalian cells. Strain 6206 was used in all experiments described in this study except for the IL-10 ELISA, where infection with strain 6294 was also analyzed. Cultures were prepared as previously described (7) and suspended in phosphate-buffered saline (PBS) to a concentration of 4 × 108 CFU/ml. The bacterial concentration was adjusted turbidimetrically, and the dose was confirmed retrospectively by counting viable cells.
Infection of animals.
IL-10 gene knockout mice generated on a C57BL/6 background and C57BL/6 wild-type control mouse breeding stocks were obtained from Jackson Laboratories (Bar Harbor, Maine) and housed under specific-pathogen-free conditions at the Biological Resources Center, University of New South Wales, Sydney, Australia. Inbred 6- to 8-week-old mice were challenged with P. aeruginosa as previously described (7). The mice were examined for signs of systemic disease prior to the commencement of experiments, and only healthy mice were used for experiments. Mice were anesthetized with 2,2,2-tribromoethanol (Avertin 125 mg; kg of body weight, intraperitoneally), the corneal surfaces of their left eyes were incised with a sterile 27-gauge needle, and 5 μl of the bacterial suspension (2.0 × 106 CFU) of either strain 6206 or strain 6294 was pipetted directly onto the wounded corneas. The right eye of each animal served as a control and was scratched but not infected. The animals were monitored during each experiment, and the Animal Care and Ethics Committee, Universities of Sydney and New South Wales, Sydney, Australia, approved all protocols for animal use. A minimum of five mice per time point and five mice for each control were used. All experiments were repeated on three occasions.
Clinical examination of the animals.
Mice were examined prior to bacterial challenge, immediately subsequent to bacterial challenge, and at intervals during the experiment by a masked observer. The animals were anesthetized for examination as described above, and the corneas were examined at a ×48 magnification under white light with an FS2 photo slit lamp biomicroscope (Topcon Corporation, Tokyo, Japan). At 1 and 7 days postchallenge, following the white light examination, 1% sodium fluorescein was instilled and the corneas were viewed under UV light. Grades of severity of corneal damage were determined and ranged from 0 to 5, with 0 signifying no disease, 1 signifying slight opacity partially covering the cornea, 2 signifying slight opacity fully covering the cornea, 3 signifying dense opacity partially covering the cornea, 4 signifying dense opacity fully covering the cornea, and 5 signifying corneal perforation. The scores were analyzed using the Kruskal-Wallis one-way analysis of variance.
Histological examination of corneas.
Mice were sacrificed at 1 day and 7 days postchallenge. The eyes were immediately enucleated, fixed in neutral buffered formalin, and embedded in paraffin. Five-micrometer-thick sections were cut and stained with hematoxylin and eosin for histopathological examination.
Quantitation of viable bacteria.
Corneas were removed at 1 day and 7 days postchallenge and homogenized in 1 ml of sterile PBS at a pH of 7.4 with a handheld Ultra-Tarrax T-8 dispersing tool (IKA, Rawang, Malaysia). To quantitate viable bacteria, a 100-μl aliquot was serially diluted 1:10 in sterile PBS. Triplicate aliquots (20 μl) of each dilution, including the original homogenate, were plated onto nutrient agar (Oxoid). Plates were incubated for 24 h at 37°C before CFU were counted. The mean number of CFU (± the standard deviation) is expressed as a log10 value. Data were examined statistically using an unpaired Student's t test.
Myeloperoxidase assays.
Myeloperoxidase activity, which is proportional to the number of polymorphonuclear leukocytes (PMN) present, was determined by a method modified from the work of Bradley et al. (3). Corneas were removed from infected mice at 0 h, 1 day, and 7 days postchallenge and were individually homogenized in 1 ml of PBS as described above. Hexadecyltrimethylammonium bromide was added to a final concentration of 0.5% (wt/vol). Samples were sonicated (three times for 10 s each time) on ice and subjected to three freeze-thaw cycles prior to centrifugation at 8,000 × g for 20 min in a refrigerated microcentrifuge. Ten-microliter aliquots of the resulting supernatants were pipetted in triplicate into a flat-bottomed microtiter plate, and the reaction was started by the addition of 90 μl of PBS containing 0.0167 g of o-dianisidine dihydrochloride per 100 ml and 0.002% (vol/vol) H2O2. The change in absorbance at 3 min was determined at 460 nm with a plate reader and compared to a standard curve on the same plate. The standard curve was prepared from purified myeloperoxidase (Sigma, St. Louis, Mo.). Results were expressed as relative units of myeloperoxidase activity per cornea. Data were examined statistically using an unpaired Student's t test.
ELISA.
For macrophage inflammatory peptide 2 (MIP-2), KC, VEGF, and IL-6 ELISAs, corneas were homogenized in 1.0 ml of sterile PBS as described above. For IL-10, TNF-α, and IFN-γ ELISAs, two corneas were homogenized in 500 μl of sterile PBS. All homogenates were immediately frozen at −70°C until they were required for assay. MIP-2-, KC-, and VEGF-paired antibodies for ELISA were purchased from R&D Systems (Minneapolis, Minn.) and used according to the manufacturer's directions, and supplied standards were used to generate a standard curve. ELISAs for IL-10, IL-6, TNF-α, and IFN-γ were carried out with Pharmingen OptEIA ELISA kits (Becton Dickinson, Sydney, Australia) according to the manufacturer's directions. Absorbances were converted to picograms of each cytokine per cornea. Data were examined statistically using an unpaired Student's t test.
Reverse transcription-PCR (RT-PCR).
Infected and control whole eyes were collected at 1 and 7 days postchallenge from wild-type and IL-10−/− mice. Eyes were homogenized in cell lysis buffer, and the total RNA was isolated with an SV RNA isolation kit (Promega, Madison, Wis.). Total RNA was reversed transcribed by using the reverse transcriptase system (Promega).
A total volume of 25 μl containing Taq polymerase (Sigma) and specific primers derived from the mouse IL-10 sequence (GenBank accession number M37897), 1.5 mM MgCl2, 100 μM each deoxynucleoside triphosphate, and reaction buffer was used. The primers used to amplify the mIL-10 gene were 5′CTTGCACTACCAAAGCCACA3′ (sense) and 5′AAGTGTGGCCAGCCTTAGAA3′ (antisense). The IFN-γ primers were 5′GAAAAGGAGTCGCTGCTGCTGAT3′ (sense) and 5′CGCAATCACAGTCTTGGCTA3′ (antisense). G3PDH was used as an internal standard or housekeeping gene. These primers were derived from the mouse G3PDH sequence (GenBank accession number MUSEC11995). The cycling conditions used were initial denaturation at 95°C for 3 min; 27 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 30 s; and a final extension at 72°C for 10 min. Control PCR without reverse transcriptase during RT was performed to confirm the absence of DNA contamination in the RNA samples. Twenty-microliter aliquots of final PCR products were analyzed by electrophoresis with 1.2% agarose gels and ethidium bromide. The bands were visualized under UV transillumination.
Immunohistochemical staining for IL-10.
Eyes for use in immunohistochemistry were fixed in Histochoice (Ameresco, Solon, Ohio) and embedded in paraffin (26). Sections were cut at a thickness of 5 μm and placed on glass slides coated with 3-aminopropyl triethoxy silane. The sections were dewaxed and rehydrated through a graded series of ethanols. Sections were stained for IL-10 by a method modified from that of Whiteland et al. (32). Controls for nonspecific binding were sections incubated with an irrelevant antibody of the same isotype and sections not incubated with the primary antibody. Briefly, endogenous peroxidase was blocked with 3% H2O2 and 0.02% sodium azide (Sigma) in PBS containing 0.1% saponin (PBS-S; Sigma) for 30 min and then sections were washed in PBS-S. Nonspecific binding sites were blocked for 30 min at room temperature with 5% (vol/vol) heat-inactivated fetal calf serum and 2% (wt/vol) bovine serum albumin in PBS-S. Sections were incubated with goat anti-mouse IL-10 antibody (1:100; R&D Systems, Bioscientific) in blocking buffer overnight at 4°C. Sections were then washed in PBS-S and incubated with biotinylated anti-goat immunoglobulin G (1:100; Vector Laboratories, Burlingame, Calif.) in PBS-S containing 1% (vol/vol) heat-inactivated fetal calf serum for 30 min at room temperature followed by avidin-conjugated horseradish peroxidase (Dako, Glostrup, Denmark) according to the manufacturer's directions. The slides were finally developed with 3,3′ diaminobenzidine (Dako). Sections were counterstained with half-strength Whitlock's hematoxylin.
RESULTS
Clinical observations.
Average macroscopic scores generated from the observations of two independent masked observers were not significantly different at 24 h postchallenge. The median score for the wild-type mice at this time was 3 (interquartile percentile, 3) and for the IL-10−/− mice was 2 (interquartile percentile, 1). At 7 days postchallenge, the macroscopic ocular response was significantly less severe in the IL-10−/− mice (median score of 2, interquartile percentile of 2) than that of the wild-type mice (median score of 5, interquartile percentile of 3.75) at 7 days postchallenge (P = 0.003).
At 24 h postchallenge the responses of the corneas of the wild-type and IL-10−/− mice were similar. Cellular infiltration and edema of the cornea were generalized, extending to the periphery. Epithelial loss was extensive when the corneas were viewed after instillation of fluorescein (data not shown). A moderate-to-severe anterior chamber response was noted for both types of mice and was indicated by the presence of cells, flare, and hypopyon. Some wild-type animals showed thinning of the central cornea, and 15% had progressed to perforation, which was not observed in the IL-10−/− mice.
At 7 days postchallenge the wild-type mice showed a severe response (Fig. 1E). There were dense peripheral infiltrates and severe edema. A severe anterior chamber response was observed. All animals showed endophthalmitis and extensive epithelial loss. Fifty percent of the wild-type corneas had progressed to perforation. In contrast, the IL-10−/− mice showed progression towards resolution of the infection (Fig. 1F). Diffuse infiltrates were observed throughout the corneas, with some dense focal infiltration remaining, usually in the central cornea. Reepithelialization had taken place in the majority of mice, and a reduced anterior chamber response was noted. A characteristic feature of the response of IL-10−/− mice at this time was an extensive neovascularization which extended over approximately 30% of the diameter of the cornea (Fig. 1F).
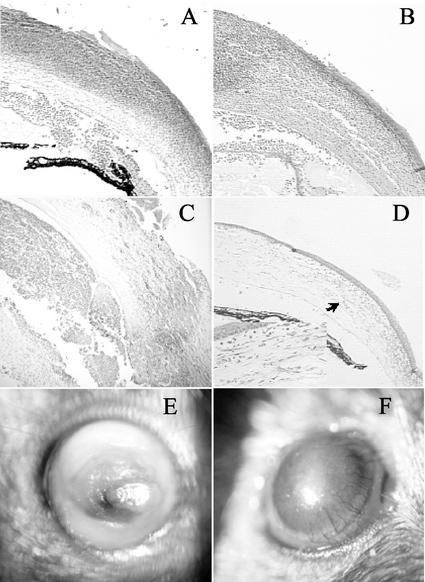
FIG. 1.
Histological and clinical examination of mouse corneas infected with P. aeruginosa. Histological sections are stained with hematoxylin and eosin. The magnification of all sections is ×200, except for the inset in panel D, which is at ×400. (A) Wild-type mouse at 24 h postchallenge; (B) IL-10−/− mouse at 24 h postchallenge; (C) wild-type mouse at 7 days postchallenge; (D) IL-10−/− mouse at 7 days postchallenge (the arrow indicates the area shown at higher magnification in the inset); (E) wild-type mouse at 7 days postchallenge; (F) IL-10−/− mouse at 7 days postchallenge showing increased vascularization.
Histology.
Histological examination of the corneas of wild-type and IL-10−/− mice at 24 h postchallenge showed a generalized inflammatory infiltrate which was composed predominantly of neutrophils. Full-thickness epithelial defects and corneal opacity were observed in both strains of mice. Both wild-type mice and IL-10−/− mice displayed hypopyon, predominantly neutrophilic (Fig. 1A and B).
At 7 days postchallenge, wild-type mice showed dense infiltration of neutrophils in the periphery of the cornea, an extensive epithelial defect, and severe edema. The anterior chambers contained large numbers of inflammatory cells (Fig. 1C). In contrast, at 7 days postchallenge, IL-10−/− mice showed diffuse infiltration of the cornea, predominantly by neutrophils, and reduced cells in the anterior chamber compared to findings at 24 h postchallenge. Reepithelialization of the cornea was evident (Fig. 1D), and neovascularization extended over approximately 30% of the corneal diameter (Fig. 1D, including inset).
Bacterial and PMN counts.
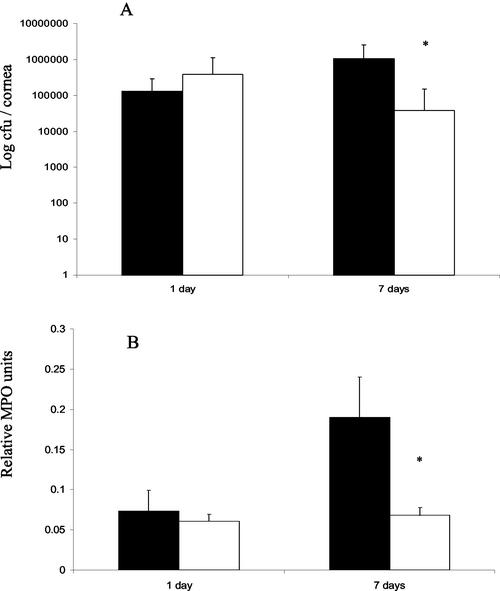
Counts of viable bacteria from corneas (n = 20) (Fig. 2A) correlated well with histological changes observed (Fig. 1). At 24 h postchallenge, bacterial counts in the corneas of IL-10−/− mice were no different from those of wild-type mice. However, at 7 days postchallenge, the bacterial loads in corneas of IL-10−/− mice were significantly reduced (P = 0.004) compared to those of wild-type mice (Fig. 2A). Estimation of the relative numbers of neutrophils in the corneas was performed with a myeloperoxidase assay, which showed a significant difference between IL-10−/− and wild-type mice only at 7 days postchallenge (Fig. 2B) (P < 0.05); IL-10−/− mice showed an approximately twofold reduction in myeloperoxidase activity at this time point.
FIG. 2.
(A) Average numbers of viable P. aeruginosa cells in corneal tissue at 1 and 7 days postchallenge as determined by direct plate counting. The mean number of CFU (± the standard deviation) is expressed as a log10 value. At 7 days postchallenge, the bacterial loads in corneas of IL-10−/− mice were significantly reduced (P = 0.004) compared to those of wild-type mice. (B) Relative myeloperoxidase (MPO) activity per cornea at 1 and 7 days postchallenge with P. aeruginosa. Black bars indicate data for wild-type mice. White bars indicate data for IL-10−/− mice. *, P < 0.05.
Corneal cytokine and chemokine levels.
Levels of the chemokines MIP-2 and KC and the cytokines IL-10, TNF-α, IFN-γ, IL-6, and VEGF were investigated using a cytokine-specific ELISA.
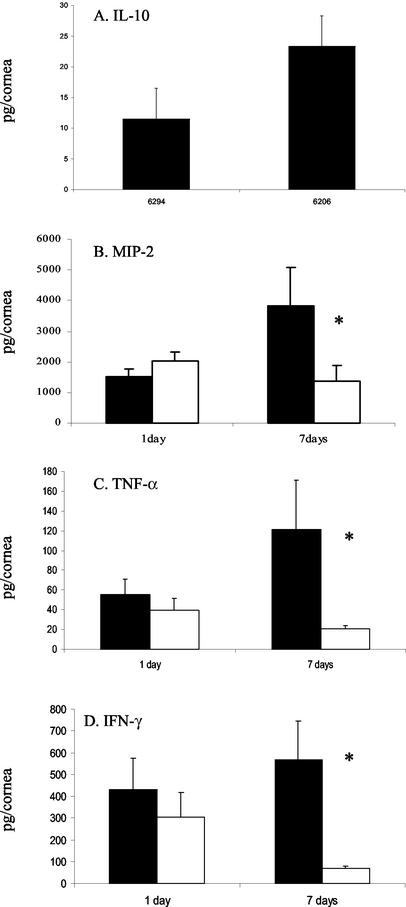
Levels of IL-10 in the corneas of mice infected with P. aeruginosa strains 6206 and 6294 were determined. IL-10 was not detected at any time point in the IL-10−/− mice. In the wild-type mice, IL-10 levels were below the limits of detection for the assay at 24 h postchallenge and in control corneas. At 7 days postchallenge, IL-10 was detected in the infected wild-type corneas (Fig. 3A). The levels of IL-10 resulting from infection with strain 6206 were approximately twofold higher than levels of IL-10 from corneas infected with strain 6294 (Fig. 3A).
FIG. 3.
Concentrations of cytokines in wild-type and IL-10−/− corneas during infection with P. aeruginosa as determined by ELISA. (A to D) Concentrations of IL-10 (A), MIP-2 (B), TNF-α (C), and IFN-γ (D) in wild-type mouse corneas 7 days after challenge with P. aeruginosa strain 6294 or 6206. Black bars indicate data for wild-type mice. White bars indicate data for IL-10−/− mice. *, P < 0.05.
There was no difference in levels of the chemokine MIP-2 in the corneas of IL-10−/− and wild-type mice at 24 h postchallenge. At 7 days postchallenge, levels of MIP-2 were approximately threefold lower (Fig. 3B) (P < 0.05) in the IL-10−/− corneas than in the corneas of wild-type mice. The MIP-2 expression pattern correlated with myeloperoxidase activity (Fig. 2B and 3B).
Levels of TNF-α were not different in the corneas of IL-10−/− and wild-type mice at 24 h postchallenge; however, at 7 days postchallenge, levels of TNF-α were approximately sixfold lower (Fig. 3C) (P < 0.05) in the IL-10−/− corneas than in the corneas of wild-type mice. The pattern of TNF-α expression also correlated with that for myeloperoxidase activity in the corneas (Fig. 2B and 3C).
Levels of IFN-γ were not different in the corneas of IL-10−/− and wild-type mice at 24 h postchallenge. Similarly, at 7 days postchallenge, levels of IFN-γ were approximately sixfold less (Fig. 3D) (P < 0.05) in the corneas of IL-10−/− mice than in those of wild-type mice. The pattern of IFN-γ expression correlated with that for myeloperoxidase activity in the corneas (Fig. 2B and 3D).
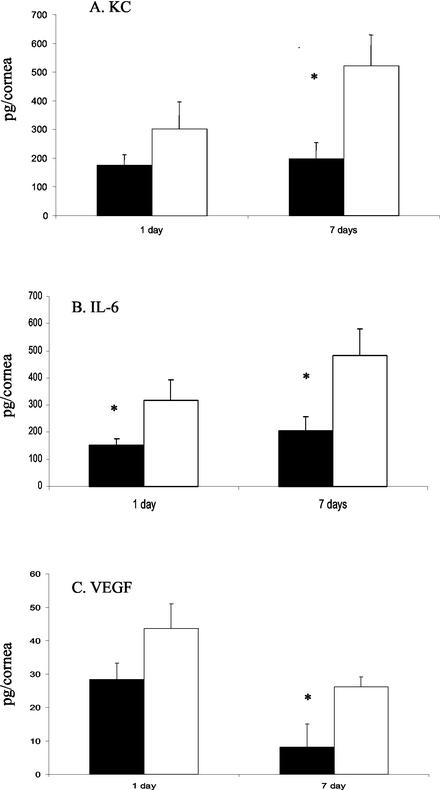
Levels of the chemokine KC were no different in the corneas of IL-10−/− and wild-type mice at 24 h postchallenge; however, at 7 days postchallenge, levels of KC were approximately 2.5-fold higher (Fig. 4A) (P < 0.05) in the corneas of IL-10−/− mice than in the corneas of wild-type mice.
FIG. 4.
Concentrations of cytokines in wild-type and IL-10−/− corneas during infection with P. aeruginosa as determined by ELISA. (A to C) Concentrations of KC (A), IL-6 (B), and VEGF (C) in mouse corneas after challenge with P. aeruginosa. Black bars indicate data for wild-type mice. White bars indicate data for IL-10−/− mice. *, P < 0.05.
IL-6 expression was approximately twofold higher in the corneas of IL-10−/− mice than in wild-type mice at 24 h postchallenge (Fig. 4B) (P < 0.05), and at 7 days postchallenge, levels of IL-6 were approximately 2.5-fold higher (Fig. 4B) (P < 0.05) in the IL-10−/− mice than in the wild-type mice.
There was no difference in the levels of VEGF in the corneas of IL-10−/− and wild-type mice at 24 h postchallenge. At 7 days postchallenge, levels of VEGF were approximately 2.5-fold higher (Fig. 4C) (P < 0.05) in the corneas of IL-10−/− mice than in the corneas of wild-type mice.
RT-PCR for IL-10 and IFN-γ expression.
The absence of IL-10 mRNA production in the IL-10−/− mice at any time point was confirmed by RT-PCR amplification. IL-10 production also was examined in the wild-type mice. A large PCR fragment with a length of 950 bp was generated to confirm production of IL-10 only at 7 days postchallenge in the wild-type mice (data not shown). IFN-γ produced an amplicon of 293 bp, which confirmed the ELISA data (Fig. 3D) showing the PCR product to be present in both wild-type and IL-10−/− mice at 24 h postchallenge and the level of IFN-γ transcripts to be reduced in the IL-10−/− compared to the level in the wild-type mice at 7 days postchallenge (data not shown).
Localization of IL-10 expression.
Immunohistochemical staining for IL-10 in wild-type corneas at 7 days postchallenge with P. aeruginosa 6206 showed that a subpopulation of infiltrating inflammatory cells and keratocytes within the stroma of the infected cornea was positive for IL-10 staining (data not shown). IL-10 was not detected immunohistochemically in the IL-10−/− corneas at any time point or in the wild-type corneas 24 h postchallenge. Sections to which the control antibody instead of the IL-10 antibody was applied showed no positive staining.
DISCUSSION
In this study we have found that the absence of IL-10 results in a significant decrease in bacterial load in corneas at 7 days postchallenge with P. aeruginosa 6206 (Fig. 2A). This decrease is accompanied by a reduction in the number of PMN in the cornea and changes in cytokine levels compared to those of wild-type mice. A characteristic increase in neovascularization in the cornea was also noted in the IL-10−/− mice at this time point (Fig. 1D and F).
Decreased pathogen loads and accelerated clearance of pathogens have also been reported for a number of IL-10−/− infection models (4, 10, 15, 21, 31). The lowering of the bacterial burden and the earlier clearance of pathogens were in some cases associated with reduced tissue damage (10), as was found here during P. aeruginosa keratitis.
IL-10 was detected at 7 days postchallenge in the wild-type mice only (Fig. 3A). This result was confirmed by RT-PCR. IL-10 was not detected during P. aeruginosa keratitis by others using an RNase protection assay (18). These differences may result from differing limits of detection or from the strains of mice and P. aeruginosa used. The infecting strain of P. aeruginosa influenced the level of IL-10 detected at 7 days postchallenge. Infection with P. aeruginosa strain 6206, a cytotoxic strain (12), gave rise to higher levels of IL-10 than did infection with P. aeruginosa strain 6294, an invasive strain (12). This finding is consistent with those of Sawa et al., who demonstrated induction of IL-10 with a cytotoxic strain of P. aeruginosa but not with an invasive strain in the lung (24). These results suggest that IL-10 is produced in response to specific bacterial products. The relatively late up regulation found in our study has been noted by others in stimulated human T-cells (2) and may reflect the role of IL-10 in dampening inflammatory responses. IL-10 in the corneas of wild-type mice was produced by a subset of the infiltrating inflammatory cells and a proportion of stromal keratocytes, a type of keratinocyte. This finding is consistent with reports that neutrophils are a source of IL-10 in the cornea during HSV keratitis (30), while keratinocytes in the skin have been reported to produce IL-10 (26).
Investigations of the expression of IFN-γ and TNF-α showed significantly lower levels in the IL-10−/− corneas than in the wild-type corneas (Fig. 3C and D). IFN-γ findings were confirmed by PCR, as IFN-γ was not detected by others using the RNase protection assay (18). This result differs from findings from other models of infection in IL-10-deficient mice (13, 25). During P. aeruginosa keratitis of the cornea, it was noted that the kinetics of TNF-α and IFN-γ production paralleled the numbers of neutrophils found in the corneal tissue. It has been shown that neutrophils are a major source of TNF-α during P. aeruginosa keratitis (8) and have also been reported to produce IFN-γ in the cornea during HSV keratitis (29, 32). These findings suggest that the reduction in IFN-γ and TNF-α may result from the reduced numbers of neutrophils present in the corneas of IL-10−/− mice at 7 days postchallenge.
The functional homologues of the CXC chemokine IL-8 in mice are MIP-2 and KC (19). In our model of P. aeruginosa keratitis, these chemokines were found to be differentially regulated in IL-10−/− mice at 7 days postchallenge (Fig. 3B and 4A). KC and MIP-2 are not functionally equivalent in the cornea, since MIP-2 plays the predominant role in neutrophil recruitment in the cornea (17, 33). The levels of KC were elevated in the IL-10−/− mice, which is consistent with the finding that resident corneal cells are the major source of KC production during P. aeruginosa keratitis (9) and HSV keratitis (33). The role of KC in corneal infection remains to be elucidated.
Angiogenesis is a complicated and highly regulated process and is mediated by a balance between proangiogenic and antiangiogenic growth factors and cytokines. IL-10 is a potent inhibitor of tumor angiogenesis (16) and of VEGF, a proangiogenic factor (27). In our model, the absence of IL-10 leads to an increase in the expression of VEGF (Fig. 4C), corresponding with increased blood vessel growth in the cornea (Fig. 1F). This finding suggests that IL-10 is an important modulator of angiogenesis during corneal infection. Here, KC correlated with increased vascularization, suggesting that KC may also play a role in corneal angiogenesis, as CXC chemokines can regulate angiogenesis (1).
This study demonstrates that IL-10 plays an important role in regulating the balance of inflammatory mediators during P. aeruginosa infection of the cornea. In the absence of IL-10, near-sterility of the cornea is achieved at the expense of more extensive vascularization. Our findings suggest that in addition to VEGF, KC may be involved in angiogenesis. The full role of this chemokine remains to be explored.
Acknowledgments
We thank Guna Karupiah for providing IL-10 gene knockout breeding pairs, Vivienne Reeve and Sitarina Widyarini for their assistance with the immunohistochemistry, and Suzanne Fleiszig for providing P. aeruginosa strains 6206 and 6294.
Financial support for this study was provided by a grant-in-aid from the Fight for Sight research division of Prevent Blindness America and the Australian Federal Government through the Co-operative Research Centers Program.
Editor: J. D. Clements
REFERENCES
- 1.Belperio, J. A., M. P. Keane, D. A. Arenberg, C. L. Addison, J. E. Ehlert, M. D. Burdick, and R. M. Strieter. 2000. CXC chemokines in angiogenesis. J. Leukoc. Biol. 68:1-8. [PubMed] [Google Scholar]
- 2.Borish, L. 1998. IL-10: evolving concepts. J. Allergy Clin. Immunol. 101:293-297. [DOI] [PubMed] [Google Scholar]
- 3.Bradley, P. P., D. A. Priebat, R. D. Christensen, and G. Rothstein. 1982. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 78:206-209. [DOI] [PubMed] [Google Scholar]
- 4.Brown, J. P., J. F. Zachary, C. Teuscher, J. J. Weis, and R. M. Wooten. 1999. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host disease. Infect. Immun. 67:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chmiel, J. F., M. W. Konstan, J. E. Knesebeck, J. B. Hilliard, T. L. Bonfield, D. V. Dawson, and M. Berger. 1999. IL-10 attenuates excessive inflammation in chronic Pseudomonas infection in mice. Am. J. Respir. Crit. Care Med. 160:2040-2047. [DOI] [PubMed] [Google Scholar]
- 6.Chmiel, J. F., M. W. Konstan, A. Saadane, J. E. Krenicky, H. Lester Kirchner, and M. Berger. 2002. Prolonged inflammatory response to acute Pseudomonas challenge in interleukin-10 knockout mice. Am. J. Respir. Crit. Care Med. 165:1176-1181. [DOI] [PubMed] [Google Scholar]
- 7.Cole, N., M. D. P. Willcox, S. M. J. Fleiszig, F. Stapleton, S. Bao, S. Tout, and A. J. Husband. 1998. Different strains of Pseudomonas aeruginosa isolated from ocular infections or inflammation display distinct corneal pathologies in an animal model. Curr. Eye Res. 17:730-735. [PubMed] [Google Scholar]
- 8.Cole, N., S. Bao, M. D. P. Willcox, and A. J. Husband. 1999. TNF-α production in the cornea in response to Pseudomonas aeruginosa challenge. Immunol. Cell Biol. 77:164-166. [DOI] [PubMed] [Google Scholar]
- 9.Cole, N., S. Bao, A. Thakur, M. D. P. Willcox, and A. J. Husband. 2000. KC production in the cornea in response to Pseudomonas aeruginosa challenge. Immunol. Cell Biol. 78:1-4. [DOI] [PubMed] [Google Scholar]
- 10.Dai, W., G. Kohler, and F. Brombacher. 1997. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J. Immunol. 158:2259-2267. [PubMed] [Google Scholar]
- 11.Fauntleroy, M. B., R. Asofsky, P. J. Baker, T. Hraba, A. Brooks, P. Stashak, and C. E. Taylor. 1993. Effects of IL-4 depletion on the antibody response to Pseudomonas aeruginosa lipopolysaccharide in mice. Immunobiology 188:379-391. [DOI] [PubMed] [Google Scholar]
- 12.Fleiszig, S. M. J., T. S. Zaidi, M. J. Preston, M. Grout, D. J. Evans, and G. B. Pier. 1996. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect. Immun. 64:2288-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazinelli, R. T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J. Immunol. 157:798-805. [PubMed] [Google Scholar]
- 14.Hazlett, L. D., X. L. Rudner, S. A. McClallan, R. P. Barrett, and S. Lighvani. 2002. Role of IL-12 and IFN-γ in Pseudomonas aeruginosa corneal infection. Investig. Ophthalmol. Vis. Sci. 43:419-424. [PubMed] [Google Scholar]
- 15.Hunter, C. A., L. A. Ellis-Neyes, S. Kanaly, G. Grunig, M. Fort, D. Rennick, and F. G. Araujo. 1997. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J. Immunol. 158:3311-3316. [PubMed] [Google Scholar]
- 16.Kawakami, T., T. Tokunaga, H. Hatanaka, T. Tsuchida, Y. Tomii, H. Osada, N. Onada, F. Morino, J. Nagata, H. Kijima, H. Yamazaki, Y. Abe, Y. Osamura, Y. Ueyama, and M. Nakamura. 2001. Interleukin-10 expression is correlated with thrombospondin expression and decreased vascular involvement in colon cancer. Int. J. Oncol. 18:487-491. [DOI] [PubMed] [Google Scholar]
- 17.Kernacki, K. A., R. P. Barrett, J. A. Hobden, and L. D. Hazlett. 2000. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. J. Immunol. 164:1037-1045. [DOI] [PubMed] [Google Scholar]
- 18.Kernacki, K. A., D. J. Goebel, M. S. Poosch, and L. D. Hazlett. 1998. Early cytokine and chemokine gene expression during Pseudomonas aeruginosa corneal infection in mice. Infect. Immun. 66:376-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, J., G. Cacalano, T. Camerato, K. Toy, M. W. Moore, and W. I. Wood. 1995. Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 155:2158-2164. [PubMed] [Google Scholar]
- 20.Liesegang, T. J. 1997. Contact lens-related microbial keratitis. I. Epidemiology. Cornea 16:125-131. [PubMed] [Google Scholar]
- 21.Murray, P. J., and R. A. Young. 1999. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect. Immun. 67:3087-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbaum, J. T., and E. Angell. 1995. Paradoxical effects of IL-10 in endotoxin-induced uveitis. J. Immunol. 155:4090-4094. [PubMed] [Google Scholar]
- 23.Rudner, X. L., K. A. Kernacki, R. P. Barrett, and L. D. Hazlett. 2000. Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J. Immunol. 164:6576-6582. [DOI] [PubMed] [Google Scholar]
- 24.Sawa, T., D. B. Corry, M. A. Gropper, M. Ohara, K. Kurahashi, and J. P. Weiner-Kronish. 1997. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J. Immunol. 159:2858-2866. [PubMed] [Google Scholar]
- 25.Sewnath, M. E., D. P. Olszyna, R. Birjmohun, F. J. W. ten Kate, D. J. Gouma, and T. van Der Poll. 2001. IL-10 deficient mice demonstrate multiple organ failure and increased mortality during Escherichia coli peritonitis despite an accelerated bacterial clearance. J. Immunol. 166:6323-6331. [DOI] [PubMed] [Google Scholar]
- 26.Shen, J., S. Bao, and V. E. Reeve. 1999. Modulation of IL-10, IL-12 and IFN-γ by UVA (320-400 nm) and UVB (280-320 nm) radiation. J. Investig. Dermatol. 113:1059-1064. [DOI] [PubMed] [Google Scholar]
- 27.Silvestre, J.-S., Z. Mallat, M. Duriez, R. Tamarat, M. F. Bureau, D. Scherman, N. Duverger, D. Branellec, A. Tedgui, and B. I. Levy. 2000. Antiangiogenic effect of IL-10 in ischemia-induced angiogenesis in mice hindlimb. Circ. Res. 87:448-452. [DOI] [PubMed] [Google Scholar]
- 28.Steinhauser, M. L., C. M. Hoagboam, S. L. Kunkel, N. W. Lukacs, R. M. Strieter, and T. J. Standiford. 1999. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J. Immunol. 162:392-399. [PubMed] [Google Scholar]
- 29.Stumpf, T. H., C. Shimeld, D. L. Easty, and T. J. Hill. 2001. Cytokine production in a murine model of recurrent herpetic stromal keratitis. Investig. Ophthalmol. Vis. Sci. 42:372-378. [PubMed] [Google Scholar]
- 30.Tumpey, T. M., H. Cheng, X.-T. Yan, J. E. Oakes, and R. N. Lausch. 1998. Chemokine synthesis in the HSV-1 infected cornea and its suppression by interleukin-10. J. Leukoc. Biol. 63:486-492. [DOI] [PubMed] [Google Scholar]
- 31.Vazquez-Torres, A., J. Jones-Carson, R. D. Wagner, T. Warner, and E. Balish. 1999. Early resistance of interleukin-10 knockout mice to acute systemic candidiasis. Infect. Immun. 67:670-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whiteland, J. L., C. Shimeld, S. M. Nicholls, D. L. Easty, N. A. Williams, and T. J. Hill. 1997. Immunohistochemical detection of cytokines in paraffin-embedded mouse tissues. J. Immunol. Methods 210:103-108. [DOI] [PubMed] [Google Scholar]
- 33.Yan, X.-T., T. M. Tumpey, S. L. Kunkel, J. E. Oakes, and R. N. Lausch. 1998. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Investig. Ophthalmol. Vis. Sci. 39:1854-1862. [PubMed] [Google Scholar]