Abstract
In PC12 neuroendocrine cells, synaptic-like microvesicles (SLMV) are thought to be formed by two pathways. One pathway sorts the proteins to SLMV directly from the plasma membrane (or a specialized domain thereof) in an adaptor protein complex 2-dependent, brefeldin A (BFA)-insensitive manner. Another pathway operates via an endosomal intermediate, involves adaptor protein complex 3, and is BFA sensitive. We have previously shown that when expressed in PC12 cells, HRP-P-selectin chimeras are directed to SLMV mostly via the endosomal, BFA-sensitive route. We have now found that two endosomal intermediates are involved in targeting of HRP-P-selectin chimeras to SLMV. The first intermediate is the early, transferrin-positive, epidermal growth factor-positive endosome, from which exit to SLMV is controlled by the targeting determinants YGVF and KCPL, located within the cytoplasmic domain of P-selectin. The second intermediate is the late, transferrin-negative, epidermal growth factor-positive late endosome, from where HRP-P-selectin chimeras are sorted to SLMV in a YGVF- and DPSP-dependent manner. Both sorting steps, early endosomes to SLMV and late endosomes to SLMV, are affected by BFA. In addition, analysis of double mutants with alanine substitutions of KCPL and YGVF or KCPL and DPSP indicated that chimeras pass sequentially through these intermediates en route both to lysosomes and to SLMV. We conclude that a third site of formation for SLMV, the late endosomes, exists in PC12 cells.
INTRODUCTION
Neurons and neuroendocrine cells possess two types of regulated secretory organelles (RSO): secretory granules and synaptic vesicles (SV) in neurons and secretory granules and synaptic-like microvesicles (SLMV) in neuroendocrine cells. These organelles store and release their content upon external stimulation (for review, see De Camilli and Jahn, 1990; Kelly, 1991). After regulated exocytosis, membrane proteins of both types undergo recycling, thereby maintaining the integrity of RSO pools. In addition to regulated traffic, there are constitutive pathways for delivery of newly synthesized RSO membrane proteins to the appropriate organelle. These biosynthetic pathways are fundamentally different for secretory granules and SV/SLMV. Whereas the secretory granules are well known to be formed from the trans-Golgi network (TGN) (for review, see Arvan and Castle, 1998; Tooze, 1998), the newly synthesized SV/SLMV membrane proteins first reach the plasma membrane via the constitutive pathway before being sorted to SV/SLMV (Cutler and Cramer, 1990; Regnier-Vigouroux et al., 1991; Bauerfeind and Huttner, 1993).
There is an accumulating body of evidence that SV/SLMV can be generated by two pathways: directly from part of the plasma membrane (or an invagination thereof) and/or via an early endosomal intermediate. The first route was found to be adaptor protein complex 2 (AP-2), clathrin, and dynamin dependent and brefeldin A (BFA) insensitive (Takei et al., 1996; Shupliakov et al., 1997; Schmidt and Huttner, 1998; Shi et al., 1998). The second route requires AP-3 and the small GTPase ADP ribosylation factor 1 (ARF1) (Faundez et al., 1997; Faundez et al., 1998) as well as being BFA sensitive (Shi et al., 1998). The two pathways of SV/SLMV formation may coexist in the same cell (Shi et al., 1998; Zakharenko et al., 1999). In addition, recent data have documented that at least in neuroendocrine PC12 cells, different proteins use these two pathways to SLMV to differing extents (Shi et al., 1998; Blagoveshchenskaya et al., 1999a,b; Zakharenko et al., 1999).
As the pathways and cytoplasmic machinery involved in formation of SV/SLMV are being characterized, so too are the sequence determinants (often called targeting signals) usually located within the cytoplasmic domains of transmembrane proteins, which direct them to SV/SLMV, via adaptor and coat protein binding to the appropriate targeting signal thereby triggering sorting steps in membrane traffic (for review, see Marks et al., 1997; Odorizzi et al., 1998; Bonifacino and Dell'Angelica, 1999). The first characterized SLMV targeting signal, which was identified within the cytoplasmic tail of vesicle-associated membrane protein II (VAMPII), forms an amphipathic α-helix and binds to AP-3 (Grote et al., 1995; Salem et al., 1998). However, mutations of SLMV targeting signals within VAMPII can also affect internalization of this protein from the plasma membrane (Grote and Kelly, 1996). More recently, the tyrosine-based targeting signal YGVF, Lys-768, and DPSP were all shown to mediate SLMV targeting, but not endocytosis, of HRP-P-selectin chimeras heterologously expressed in PC12 cells (Blagoveshchenskaya et al., 1999a). In addition, we have found that a di-leucine signal that binds AP-3 in vitro (Höning et al., 1998) and the related di-hydrophobic MetLeu signal are involved in promoting BFA-sensitive SLMV targeting of tyrosinase and synaptotagmin, respectively, in neuroendocrine PC12 cells (Blagoveshchenskaya et al., 1999a,b).
In addition to being localized to RSO, many synaptic vesicle proteins are found within endocytic compartments in both neurons and neuroendocrine cells (for review, see Hannah et al., 1999). In proteins in which SLMV targeting signals and internalization signals are closely related, as exemplified by VAMPII, it can be difficult to determine whether these endosomes are intermediates en route to SLMV. This problem does not arise for proteins in which these signals are separate; i.e., inactivation of the SLMV targeting signal leads to accumulation in an endosomal compartment but does not affect internalization from the plasma membrane. One good model protein for studying the endosomal intermediates involved in SLMV formation is therefore P-selectin, because when expressed in PC12 cells, it is not only efficiently targeted to SLMV via a BFA-sensitive route, but also the internalization and SLMV targeting signals within this protein are distinct (Norcott et al., 1996; Blagoveshchenskaya et al., 1999a).
P-selectin is a type I membrane protein, which was originally found in the secretory granules of endothelial cells and platelets (Bonfanti et al., 1989; McEver et al., 1989). It functions as a receptor for leukocytes (Johnston et al., 1989). When heterologously expressed in cells lacking RSO, P-selectin is constitutively delivered to the cell surface, followed by efficient endocytosis to degradative compartments in a signal-mediated manner (Green et al., 1994; Blagoveshchenskaya et al., 1998a,b; Straley et al., 1998). When expressed in cells with regulated secretory pathways, some P-selectin is transported to secretory granules directly from the TGN, whereas the rest, depending on the cell type, is delivered to the plasma membrane before being internalized and sorted to SLMV or to endosomal and lysosomal compartments (Subramaniam et al., 1993; Norcott et al., 1996; Blagoveshchenskaya et al., 1999a).
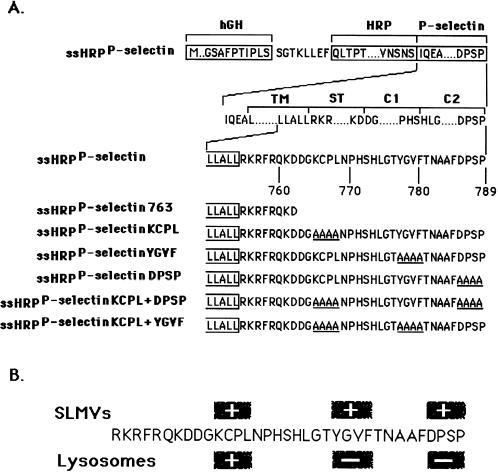
By following HRP-P-selectin chimeras expressed in PC12 cells, we have previously found that the cytoplasmic tail of P-selectin contains three SLMV targeting signals: KCPL, YGVF, and DPSP, mutations of which lead not only to a failure in SLMV delivery (Figure 1B) but also to differential accumulation within the endosomal–lysosomal system (Blagoveshchenskaya et al., 1999a). We have now characterized the endosomal intermediates through which HRP-P-selectin chimeras are delivered to SLMV. We conclude that sorting from the early transferrin (Trn)-positive, epidermal growth factor (EGF)-positive endosomes to SLMV is dependent on the cytoplasmic sequences KCPL and YGVF. Sorting to SLMV from the late Trn-negative, EGF-positive endosomes is controlled by both DPSP and YGVF. In addition, both routes are BFA sensitive.
Figure 1.
Schematic illustration of HRP-P-selectin chimeras and localization of major targeting determinants within the cytoplasmic tail of P-selectin. (A) The top line shows the position of components used for construction: hGH, human growth hormone signal sequence; P-selectin, transmembrane and cytoplasmic domain of P-selectin. The cytoplasmic domain of P-selectin was divided into the stop transfer (ST), C1, and C2 subdomains according to exon–intron boundaries (Johnston et al., 1989). The bottom part shows the full amino acid sequences of the cytoplasmic domains of the chimeras. The carboxyl-terminal end of the transmembrane domain shown is boxed. The amino acids substituted for alanine are shown to the left of the diagram and included in name of the chimera. ssHRPP-selectin763 is a chimera in which both the C1 and C2 subdomains are removed. (B) Localization of SLMV and lysosomal targeting signals within the cytoplasmic domain of P-selectin. The determinants inactivation of which reduces targeting to the level of tailless ssHRPP-selectin763, are shown within the + boxes. The determinants inactivation of which increases targeting over the level of wild-type ssHRPP-selectin, are shown within the − boxes.
MATERIALS AND METHODS
Materials and Reagents
Mouse receptor grade EGF and human iron-saturated Trn were iodinated using the modified IODO-GEN (Sigma, St. Louis, MO) procedure as described elsewhere (Wiley and Cunningham, 1982). The specific activity of iodinated proteins was 80,000–100,000 cpm/ng. Mouse monoclonal antibody against HRP (clone 2H11) was purchased from Advanced Immunochemical (Luton Beds, United Kingdom). Polyclonal affinity-purified antibody against the cytoplasmic tail of LAMP1 was a generous gift from Dr. C.R. Hopkins and S. Muxwell (Medical Research Council Laboratory for Molecular Cell Biology). Polyclonal anti-human Trn was obtained from Dako (High Wycombe, United Kingdom). Fluorescent conjugates of secondary antibodies were from Jackson ImmunoResearch (Wembley, Middlesex, United Kingdom). Micro BCA protein assay kit was purchased from Pierce. Other chemicals were purchased from Sigma (Poole, United Kingdom).
Constructs
A chimeric cDNA comprising the human growth hormone signal sequence followed by HRP and the transmembrane and cytoplasmic domains of P-selectin (see Figure 1) was constructed as described previously (Norcott et al., 1996) as was a chimera with a truncated cytoplasmic tail (ssHRPP-selectin763). The tetra-alanine substitutions and double mutants were made as described (Blagoveshchenskaya et al., 1998a,b).
Cell Culture and Transfections
The rat pheochromocytoma cell line PC12 (CCL23; American Type Culture Collection, Manassas, VA) was cultivated and transfected as described previously (Norcott et al., 1996), except 3 μg of DNA were used per transfection unless indicated otherwise. Transfected cells were analyzed 3 d after transfection. Where necessary, cells were treated with 10 μg/ml BFA for 30 min at 37°C.
Endocytosis Assay of 125I-Trn and 125I-EGF
Cells on 90-mm dishes were washed with binding media (Dulbecco's modified Eagle's medium, 20 mM HEPES, and 0.1% bovine serum albumin) and incubated with either 10 ng/ml 125I-EGF at 4°C for 1 h or 100 ng/ml 125I-Trn at 37°C for 1 h. Excess ligand was removed by three rinses with ice-cold Dulbecco's modified Eagle's medium. Those cells loaded with 125I-Trn were placed on ice and subsequently treated with 2-[N-morpholino]ethanesulfonic acid (MES) buffer I (25 mM MES, pH 4.0, 150 mM NaCl) and MES buffer II (25 mM MES, pH 7.5, 150 mM NaCl) at 4°C for 10 min each to remove any cell surface-bound ligand. The cells incubated with 125I-EGF were transferred to 37°C for 2 or 20 min to label early or late endosomes, correspondingly. Noninternalized 125I-EGF was removed by a 2.5-min wash with an acidic buffer (100 mM sodium acetate, pH 4.5, 500 mM NaCl) at 4°C. In both cases, the cells were then thoroughly washed with ice-cold binding medium and twice with homogenization buffer (HB) (320 mM sucrose, 10 mM HEPES, pH 7.3) and subjected to subcellular fractionation as detailed below.
Subcellular Fractionation and Quantitation of Data
Homogenization, preparation of postnuclear supernatant (PNS), centrifugation on 1–16% Ficoll gradients, and fractionation were performed essentially as described previously (Cramer and Cutler, 1992; Norcott et al., 1996).
To further isolate early endosomes, fractions from the initial gradients containing the peak of 125I-Trn radioactivity (fractions 5–10) were collected, diluted with HB, recentrifuged on 9-ml 3–16% Ficoll velocity gradients for 50 min at 35,000 rpm in an SW40Ti rotor (Beckman Instruments, Palo Alto, CA), and fractionated as above. Targeting data were expressed as early endosome targeting index (EE-TI), i.e., the amount of HRP activity present within the early endosomal peak (fractions 5–10; see Figure 6 C) for each chimera (HRP peak) divided by that for wild-type ssHRPP-selectin and normalized for interexperimental variations of expression level (HRP hmg) and the recovery of early endosomes, as judged by the amount of 125I-Trn within the peak (125I-Trn peak) and by that in the homogenate (125I-Trn hmg). After simplifying the original equation, the EE-TI was determined as follows:
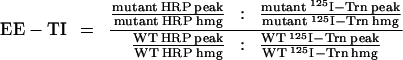 |
Figure 6.
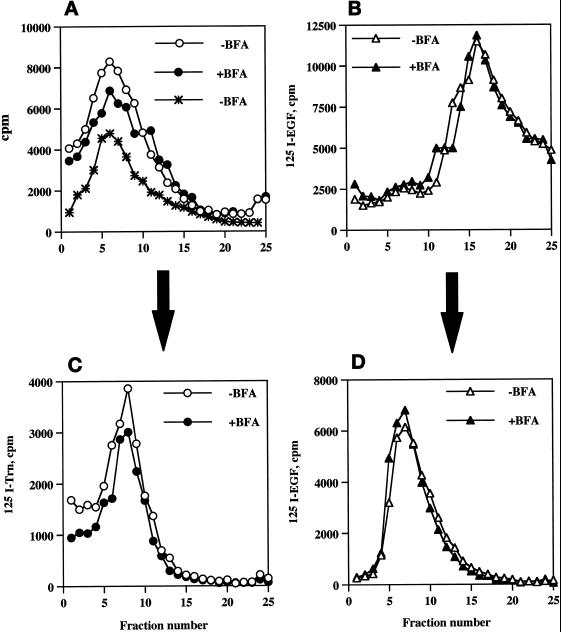
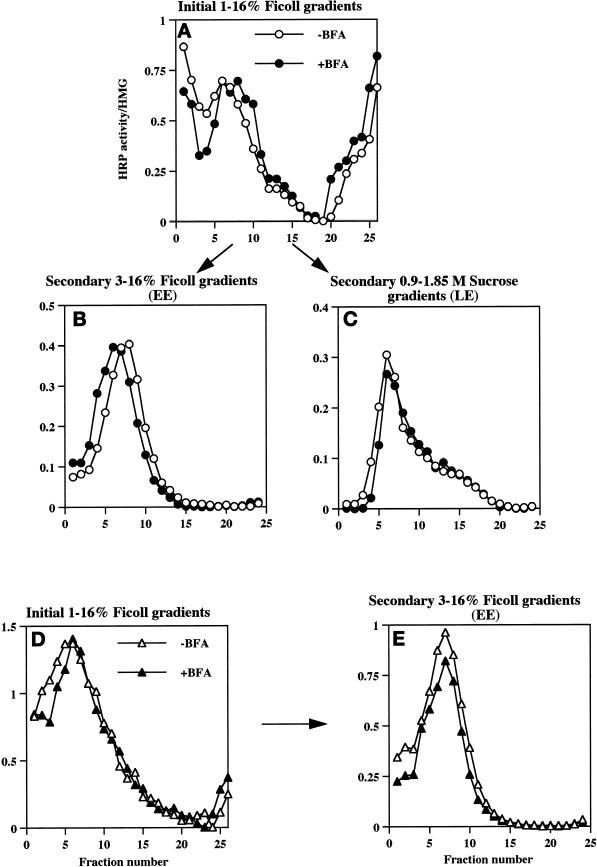
Compartmentalization of internalized 125I-Trn and 125I-EGF in the presence or absence of BFA. PC12 cells expressing wild-type ssHRPP-selectin were fed with 125I-Trn for 1 h at 37°C in the presence (●) or absence (○) of 10 μg/ml BFA added in the medium during last 30 min of incubation. Parallel dishes were incubated with 125I-EGF for 1 h on ice in the presence or absence of BFA for the last 10 min of incubation, washed, and transferred to 37°C for 20 min to label late endosomes in the presence (▴) or absence (▵) of BFA. One dish labeled with 125I-EGF for 1 h on ice was washed and allowed to internalize ligand for 2 min at 37°C with no BFA added (A, ∗). After removal of the noninternalized ligand, cells were rinsed with HB and homogenized, and a PNS was centrifuged on the 1–16% Ficoll velocity gradients (A and B). After fractionation, the peak containing 125I-Trn (fractions 5–10; A) was collected and then recentrifuged on a 3–16% Ficoll gradient (C), whereas the peak containing 125I-EGF (fractions 13–19; B) was collected and recentrifuged on a 0.9–1.85 M sucrose equilibrium gradient (D) as described in MATERIALS AND METHODS.
To isolate late endosomes, the PNS from cells labeled with 125I-EGF was fractionated using a two-step procedure consisting of a 1–16% Ficoll velocity gradient followed by an 0.9–1.85 M Sucrose equilibrium gradient as described previously (Blagoveshchenskaya et al., 1999a). To estimate the efficiency of targeting of different chimeras to late endosomal compartments, which sediment within the lower-density peak on the sucrose gradients (Blagoveshchenskaya et al., 1999a), we have calculated targeting indexes (late endosomal targeting index [LE-TI]) essentially as described above, excepting only that the amount of N-acetyl-β-d-glucosaminidase (NAGA) activity in the peak as well as that in the homogenate were used to normalize for recovery of late endosomes.
Feeding Experiments and Immunofluorescent Microscopy
PC12 cells transfected with HRP-P-selectin chimeras were plated on poly-l-lysine-coated dishes containing glass coverslips and used for experiments 3 d after transfection. To follow endocytic trafficking of HRP-P-selectin chimeras or Trn from the cell surface, cells were rinsed twice with serum-free growth medium containing 1% BSA and then were loaded with 2H11 (5 μg/ml) and/or with human iron-saturated Trn (50 μg/ml) in the same medium for 2 h at 37°C. Cells on coverslips were then washed in PBS and fixed with 4% paraformaldehyde in PBS for 20 min at room temperature. They were then permeabilized with PBS containing 0.2% saponin and 3% gelatin (permeabilization buffer) and incubated with primary antibodies either against LAMP1 or against Trn in permeabilization buffer for 45 min. After three washes with permeabilization buffer, primary antibodies were visualized with appropriate FITC- or Texas Red-conjugated secondary antibodies and viewed in an MRC-1000 confocal microscope (Bio-Rad, Hercules, CA).
Miscellaneous Methods
NAGA activity was measured as described elsewhere (Kornilova et al., 1992). HRP activity in the samples was determined in triplicate as previously described (Norcott et al., 1996). To inhibit HRP activity present on the plasma membrane, cells were treated three times with buffered ascorbate (100 mM ascorbic acid, 20 mM HEPES, pH 7.0, 70 mM NaCl, 0.015% H2O2) on ice for 10 min each. Protein concentration was determined using the Micro BCA protein assay kit (Pierce) according to the manufacturer's instructions.
RESULTS
Targeting of HRP-P-selectin Chimeras to Early Endosomes at Steady State in PC12 Cells
We have previously found that alanine substitution of Leu-768 or of a KCPL tetrapeptide within the C1 domain of the cytoplasmic tail of P-selectin dramatically reduces targeting both to SLMV and to lysosomes while causing accumulation of HRP-P-selectin chimeras within early, Trn-positive endosomes (Blagoveshchenskaya et al., 1999a). However, inactivation of other SLMV targeting signals, such as YGVF and DPSP (and, to a lesser extent, TNAAF, all of which are located within the C2 domain), resulted in rerouting of mutant chimeras to degradative compartments (Blagoveshchenskaya et al., 1999a). These data suggest that more than one endosomal compartment might be involved in SLMV formation. To identify these endosomal intermediates, we have quantitatively evaluated targeting to different endosomal populations of those chimeras with the most pronounced effects on SLMV and lysosomal targeting (Figure 1). We have previously established subcellular fractionation protocols, which allow for accurate measurement of levels of HRP chimeras in a variety of post-Golgi compartments (Blagoveshchenskaya et al., 1999a; Strasser et al., 1999).
To determine the extent to which different HRP-P-selectin chimeras accumulate within Trn-positive, EGF-positive early endosomes, PC12 cells transiently expressing the chimeras shown in Figure 1A were fed with 125I-Trn for 60 min, and the early endosomes were then isolated by a two-step subcellular fractionation procedure (see Figures 6 and 7). To avoid the potential contamination of the early endosome peak with plasma membrane (our unpublished data), the HRP activity of chimeras present on the plasma membrane was inhibited by treatment of cells with ascorbic acid in the presence of H2O2 as described in MATERIALS AND METHODS. A typical distribution of 125I-Trn and 125I-EGF internalized for 2 min at 37°C on an initial 1–16% Ficoll gradient as well as on the secondary 3–16% Ficoll gradient is shown in Figure 6, A and C, −BFA. The efficiency of targeting of each chimera to early endosomes was expressed as a targeting index (EE-TI), i.e., the amount of HRP activity present within the early endosome peak divided by that in the homogenate and then normalized for endosomal yield as judged by recovery of 125I-Trn. The EE-TI for wild-type ssHRPP-selectin was set at 1; the targeting indexes for other chimeras were normalized by the wild-type EE-TI in each experiment, to facilitate cross-comparisons.
Figure 7.
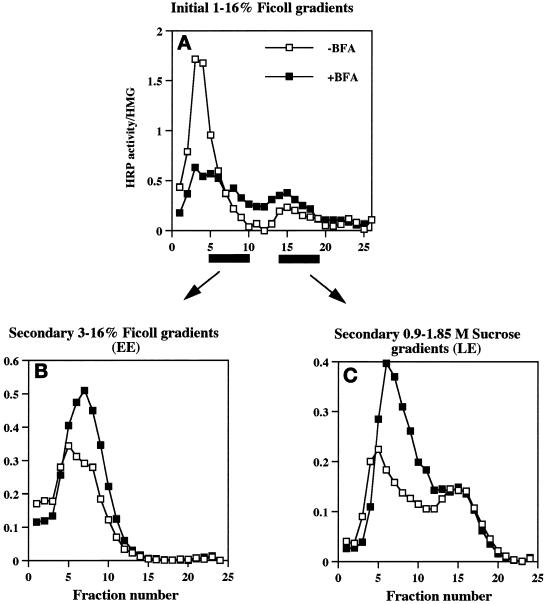
Effect of BFA on the distribution of wild-type ssHRPP-selectin along the 1–16% Ficoll initial gradients and along the secondary gradients used for isolation of early and late endosomes. PC12 cells expressing wild-type ssHRPP-selectin were fed with 125I-Trn or with 125I-EGF to monitor the position of early or late endosomes and incubated in the presence (▪) or absence (□) of BFA as described in the legend for Figure 6. After centrifugation and fractionation on 1–16% Ficoll gradients, HRP activity was determined in each fraction and divided by that in the homogenate (A). Fractions corresponding to early endosomes (5–10), as seen by distribution of 125I-Trn (Figure 6A), were pooled together and recentrifuged on 3–16% Ficoll gradients (B). Fractions corresponding to late endosomes (13–19), as judged by distribution of 125I-EGF internalized for 20 min at 37°C (Figure 6B), were collected and recentrifuged on 0.9–1.85 M sucrose equilibrium gradients (C). After fractionation, HRP activity was measured in each fraction and normalized to that in the homogenate.
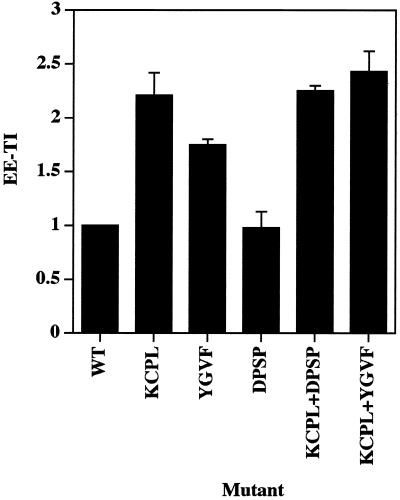
As shown in Figure 2, ssHRPP-selectinDPSP is targeted to early endosomes as efficiently as wild-type ssHRPP-selectin (EE-TI = 1), whereas ssHRPP-selectinKCPL and ssHRPP-selectinYGVF accumulated to significantly higher levels: 2.21 ± 0.21 (mean ± SE; n = 3) and 1.75 ± 0.05, respectively. These results suggest that KCPL and YGVF are both necessary for exit of HRP-P-selectin chimeras from early endosomes to SLMV, whereas DPSP promotes SLMV targeting from elsewhere along the endocytic pathway.
Figure 2.
Quantitation of targeting of HRP-P-selectin chimeras to early endosomes. PC12 cells expressing the chimera indicated were labeled with 125I-Trn, treated with ascorbate to inhibit HRP activity present on the plasma membrane, homogenized, and fractionated using 1–16% Ficoll velocity gradients followed by 3–16% Ficoll velocity gradients to isolate early endosomes. After fractionation, targeting to early endosomes was measured by calculating a targeting index (EE-TI) for each chimera as the amount of HRP activity within the peak of early endosomes normalized both by the expression level and by 125I-Trn recovery. Each bar represents the mean ± SE of three independent experiments.
Delivery of HRP-P-selectin Chimeras to Late Endosomes
According to the data of Schmidt et al. (1997), one possible SLMV precusor compartment could be a specialized plasma membrane invagination devoid of Trn receptor. However, although we have no data directly relating to this subcompartment, ssHRPP-selectinDPSP was previously shown not to accumulate on the plasma membrane but to be efficiently internalized (Blagoveshchenskaya et al., 1999a; see Figure 5; current work). Another potential compartment from which DPSP (and the other SLMV targeting signals, which function as lysosome avoidance signals) might operate is the late endosome. To ascertain whether this is the case, we quantitated targeting to this compartment. PNS from PC12 cells transiently expressing the chimera indicated (Figure 3) was fractionated on 1–16% Ficoll gradients followed by recentrifugation of a peak containing both late endosomes and dense-core granules (DCG) on 0.9–1.85 M sucrose gradients. The latter step allows for a clear separation of late endosomes from DCG and early endosomes, as seen by the distribution of HRP activity, of [3H]dopamine (a DCG marker), of 125I-EGF internalized for 20 min at 37°C and N-acetyl-β-d-glucosaminidase (the late endosomal markers), as well as of 125I-Trn (Blagoveshchenskaya et al., 1999a; see Figure 7, −BFA; this work).
Figure 5.
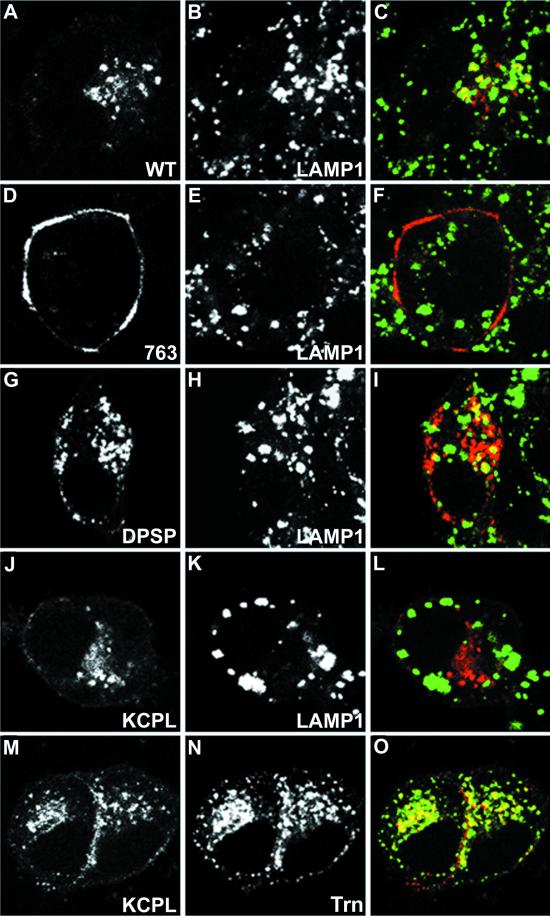
Immunofluorescent localization of internalized HRP-P-selectin chimeras and endosomal markers in PC12 cells. Cells transiently expressing wild-type ssHRPP-selectin (A–C), tailless ssHRPP-selectin763 (D–F), ssHRPP-selectinDPSP (G–I), or ssHRPP-selectinKCPL (J–O) grown on poly-l-lysine-coated coverslips were washed with serum-free medium containing 1% BSA and fed with 50 μg/ml Trn (J–O) and/or with 5 μg/ml 2H11 (A–O) for 2 h at 37°C. Cells were then fixed, permeabilized, and stained as described in MATERIALS AND METHODS. 2H11 (A, D, G, J, and M) was visualized with Texas Red-conjugated goat anti-mouse secondary antibody; LAMP1 (B, E, H, and K) was immunodetected with rabbit polyclonal anti-LAMP1 followed by FITC-conjugated goat anti-rabbit secondary antibody; and Trn (N) was detected with rabbit polyclonal anti-Trn followed by FITC-conjugated goat anti-rabbit secondary antibody. Color images show the merger of both channels.
Figure 3.
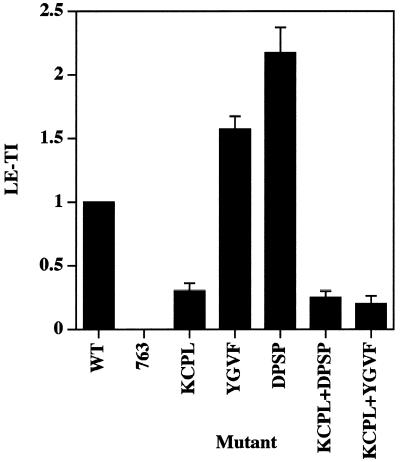
Quantitation of targeting of HRP-P-selectin chimeras to late endosomes. PC12 cells expressing the chimeras indicated were homogenized and fractionated on 1–16% Ficoll velocity gradients followed by recentrifugation on 0.9–1.85 M sucrose equilibrium gradients for separation of late endosomes. LE-TIs were then calculated by normalizing the amount of HRP activity within the endosomal peak both for organelle recovery as judged by NAGA activity and for the expression level. Each bar represents the mean ± SE of three independent experiments.
The calculation of LE-TI was carried out as described above for early endosomes, except that the amounts of NAGA activity in the late endosomal peak and in the homogenates were used to normalize for organelle recovery. LE-TI for tailless ssHRPP-selectin763, which was previously found to be incapable of internalization and to be present on the plasma membrane (Norcott et al., 1996; Blagoveshchenskaya et al., 1999a; see Figure 5; current work), was subtracted from LE-TI for all chimeras tested. In agreement with experiments measuring the amount of chimeras within the protease-rich compartments (Blagoveshchenskaya et al., 1999a), targeting to late endosomes of ssHRPP-selectinKCPL was reduced by 70%, thereby supporting the inference that KCPL mediates exit from the early Trn-positive, EGF-positive endosomes to later, degradative compartments (Figure 3). ssHRPP-selectinYGVF and ssHRPP-selectinDPSP, which exhibited increased targeting to lysosomes (Blagoveshchenskaya et al., 1999a), are also present within late endosomal compartments in higher amounts than is the wild-type chimera: 1.57 ± 0.1, 2.17 ± 0.2, and 1, respectively (Figure 3), suggesting that late endosomes might well be the second endosomal intermediate from which HRP-P-selectin chimeras are transported to SLMV in a DPSP- and YGVF-mediated manner. Interestingly, the amount of ssHRPP-selectinDPSP within late endosomes is 1.4 times higher than that of ssHRPP-selectinYGVF, in agreement with the results described above, demonstrating the partial retention of the latter chimera within the early endosomes (Figure 2). Together these data show that alanine substitution of YGVF causes this chimera to accumulate in both early and late endosomes, whereas substitution of KCPL and DPSP causes differential accumulation within early and late subcompartments, respectively.
Saturability of Endosomal Targeting of HRP-P-selectin Chimeras
A large body of data indicates that signal-dependent sorting of membrane proteins to various intracellular compartments is a saturable process, which depends on both the levels of expression of the protein of interest and those of the endogenous membrane proteins (Grote and Kelly, 1996; Marks et al., 1996; Warren et al., 1998). In all cases documented, expression of proteins at very high levels caused a reduction in rates of endocytosis, leading to an accumulation on the plasma membrane. To ascertain whether saturation attributable to overexpression is affecting signal-mediated sorting of HRP-P-selectin chimeras within the endosomal system of PC12 cells, we have quantitated the efficiency of targeting to late endosomes of those mutant HRP-P-selectin chimeras with the most profoundly altered phenotypes, i.e., ssHRPP-selectinDPSP and ssHRPP-selectinKCPL (see above), at various expression levels.
An accumulating body of evidence shows a wide phenotypic variations, e.g., in terms of the presence of RSO and individual proteins, between different clones obtained during the making of stably expressing PC12 cell lines (Borgonovo et al., 1998; Kasai et al., 1999; Pance et al., 1999). We have therefore deliberately chosen to use transient expression to circumvent this problem. In addition, transiently expressing cells transfected with varied amounts of cDNAs have previously been used successfully by others to examine the effects of expression levels on post-Golgi trafficking (Grote and Kelly, 1996; Marks et al., 1996).
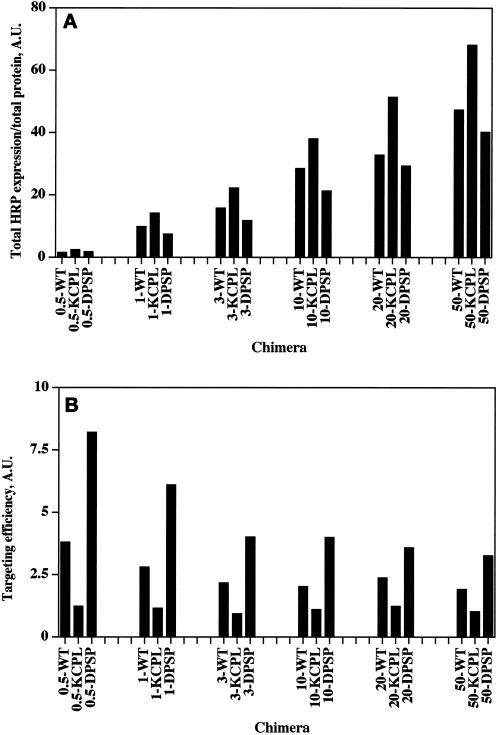
PC12 cells were transiently transfected with a range of increasing amounts of cDNAs encoding the chimeras shown in Figure 4 and assayed 3 d after transfection for total HRP expression level, which was then normalized by amount of protein. The data show that the increase in expression levels was not proportionate to the increase in the amount of cDNA used for each transfection (Figure 4A): a 2-fold increase in cDNA levels at the low end of the range (0.5–1 μg) resulted in a 5- to 6-fold increase in HRP expression level compared with a <1.5-fold increase in expression seen for a 2.5-fold increase in the amount of cDNA at the high end of the range (20–50 μg). A previous study on saturation of protein targeting identified a similar relationship between amounts of DNA and the level of protein expression (Marks et al., 1996).
Figure 4.
Effect of different levels of expression on targeting of HRP-P-selectin chimeras to late endosomes. (A) Titration of HRP expression. PC12 cells were transiently transfected by electroporation using increasing amounts of cDNA for ssHRPP-selectin (WT), ssHRPP-selectinKCPL (KCPL), or ssHRPP-selectinDPSP (DPSP). Cells were washed with HB, scraped, and homogenized. HRP activity (shown in arbitrary units [A.U.]) was measured in each homogenate and normalized to total protein within the homogenate. The name of chimera and the amount of DNA used (starting from 0.5 μg) are shown along the abscissa. (B) Efficiency of late endosomal targeting at different expression levels. PC12 cells transiently expressing ssHRPP-selectin, ssHRPP-selectinKCPL, or ssHRPP-selectinDPSP at different levels were processed by subcellular fractionation for isolation of late endosomes as described in the legend for Figure 3. The amount of HRP activity (shown in A.U. along the ordinate) within the late endosomal peak was measured, divided by that in the homogenate, and normalized for NAGA recovery. The amount of DNA used is shown before the name of the chimera. Note that the level of constitutive targeting of tailless ssHRPP-selectin763 to late endosomes, which usually comprises 25% of the level found for the wild-type chimera, has not been subtracted from the values of targeting efficiency for chimeras analyzed in this experiment.
To evaluate the effect of expression level on the efficiency of targeting of HRP-P-selectin chimeras to late endosomes, we have quantitated the amount of HRP activity in late endosomes normalized to that in the homogenates by subcellular fractionation as described above. As shown in Figure 4B, the efficiency of late endosomal targeting of two chimeras with an unaltered lysosomal targeting signal, i.e., ssHRPP-selectin and ssHRPP-selectinDPSP, declined with increasing amounts of cDNA, as did the difference in targeting efficiency between ssHRPP-selectin and ssHRPP-selectinDPSP. Since under these same conditions, the total HRP expression level continued to increase (Figure 4A), these data most likely reflect the saturation of sorting machinery by overexpression. In agreement with this conclusion, the level of ssHRPP-selectinKCPL (which has a mutated lysosomal targeting signal) within the late endosomal population remained unchanged irrespective of the amount of cDNA used.
However, even at the highest expression levels (20–50 μg of DNA), which do affect sorting to the late endosomal–lysosomal system to some extent, the targeting efficiency of ssHRPP-selectinKCPL was consistently lower, whereas that of ssHRPP-selectinDPSP was consistently higher than the corresponding value for wild-type chimera. We therefore conclude that the differential targeting that we described above to the late endosomal–lysosomal system is not an artifact resulting from overexpression of chimeras by transient transfection but is a signal-mediated process. Moreover, despite some saturation of the sorting machinery, at even the highest levels of expression our observations indicate only a reduction in the differences between targeting efficiencies of the chimeras but no changes in their respective relationship.
Differential Localization of Mutant HRP-P-selectin Chimeras within the Late Endosomal–Lysosomal System at the Light Microscopy Level
To provide independent evidence for the differential distribution of the HRP-P-selectin chimeras with the most altered sorting within the endosomal system, we determined where in the cell these chimeras are localized by indirect confocal immunofluorescent microscopy. Transiently transfected PC12 cells grown on coverslips were fed with cold Trn and/or with 2H11, fixed, permeabilized, and stained, as described in MATERIALS AND METHODS. The results of these experiments are shown in Figure 5. In agreement with our previous data (Norcott et al., 1996; Blagoveshchenskaya et al., 1999a), tailless chimera ssHRPP-selectin763 was accumulated at the plasma membrane and revealed no colocalization with LAMP1, used as a late endosomal–lysosomal marker (Figure 5, D–F). By contrast, ssHRPP-selectinDPSP (Figure 5, G–I) and wild-type ssHRPP-selectin (Figure 5, A–C) were efficiently internalized into structures, of which some are LAMP1-positive. ssHRPP-selectinKCPL, which is not targeted to lysosomes as previously shown in PC12 cells by an HRP-proteolysis assay (Blagoveshchenskaya et al., 1999a), was not seen in LAMP1-positive vesicles (Figure 5, J–L) but instead was observed on the plasma membrane and in Trn-positive puncta concentrated in the perinuclear region (Figure 5, M–O). These data indicate that ssHRPP-selectinKCPL is located in the Trn-containing early endosomal compartment and continuously recycles to the plasma membrane rather than being targeted to late endosomes and lysosomes as are ssHRPP-selectinDPSP and ssHRPP-selectin.
DPSP and YGVF Operate Downstream of the KCPL Determinant along the Endocytic Pathway
Our subcellular fractionation data showing that ssHRPP-selectinKCPL and ssHRPP-selectinYGVF accumulate in early endosomes, coupled to the observation that ssHRPP-selectinDPSP and ssHRPP-selectinYGVF exhibit increased targeting to late endosomes and lysosomes, indicate that KCPL, YGVF, and DPSP are involved in distinct sorting steps along the endocytic pathway. In principle, these determinants could operate either at sequential sorting steps along one route or on separate pathways.
To address this issue, we have constructed two double mutants: ssHRPP-selectinKCPL+DPSP and ssHRPP-selectinKCPL+YGVF (Figure 1). If both signals are operating sequentially on the same pathway, the phenotype of any double mutant should be that of the single mutant, which operates first, most likely ssHRPP-selectinKCPL. On the other hand, if there are two routes, then the efficiency of targeting to late compartments of both double mutants, although dependent on the relative strength of each signal, is likely to be higher than the basal level of ssHRPP-selectinKCPL. Quantitation of late endosomal targeting indicates that both ssHRPP-selectinKCPL+DPSP and ssHRPP-selectinKCPL+YGVF exhibited phenotypes similar to that for ssHRPP-selectinKCPL (Figure 3). In addition, all these chimeras accumulate within the early, 125I-Trn-positive endosomes in the same low amounts as seen for ssHRPP-selectinKCPL (Figure 2). These data therefore support the hypothesis that delivery to lysosomes requires two sequential sorting steps after internalization.
Because alanine substitutions of KCPL, YGVF, and DPSP on their own already dramatically abrogate SLMV targeting, we cannot use the same double-mutant approach as used above in analyzing endocytic targeting to determine whether two sequential signal-mediated steps are involved in trafficking to SLMV. However, the very close relationship between endocytic and SLMV targeting strongly suggests that those endosomal compartments through which HRP-P-selectin is sequentially moving to lysosomes might be the precusors of SLMV as well and that the same sequential signal-mediated steps might operate en route to SLMV.
Effect of BFA on the Endocytic Trafficking of 125I-Trn and 125I-EGF in PC12 Cells
We have previously documented that trafficking of HRP-P-selectin chimeras to SLMV via an endosomal intermediate is a BFA-sensitive process (Blagoveshchenskaya et al., 1999a). We have now extended this observation by determining which endosomal compartments are affected by BFA. We began by testing the effect of BFA on the distribution of the endosomal markers Trn and EGF, used in this study to label the endosomes, in PC12 cells. Cells transiently expressing wild-type ssHRPP-selectin were allowed to endocytose either 125I-Trn or 125I-EGF in the presence or absence of 10 μg/ml BFA as described in MATERIALS AND METHODS, and the PNS from these cells was subjected to subcellular fractionation on 1–16% Ficoll gradients.
After treatment with BFA, we observed an 18% fall of 125I-Trn radioactivity within the peak of early endosomes (Figure 6A, fractions 5–10), whereas the distribution of 125I-EGF within the late endosomes (Figure 6B, fractions 13–19; Blagoveshchenskaya et al., 1999a) was not affected (Figure 6B). Similar results were obtained after recentrifugation of the peaks corresponding to early or late endosomes on secondary gradients (specifically designed for further isolation of these compartments to quantitate targeting of HRP chimeras; see above, Figure 6, C and D). These data therefore indicate that BFA treatment has only a minor effect on the endocytic trafficking of 125I-Trn and does not affect that of 125I-EGF, as seen by subcellular fractionation, thus providing an appropriate context within which to determine those BFA-sensitive endosomal populations through which HRP-P-selectin chimeras are transported en route to SLMV.
BFA Treatment Leads to the Accumulation of ssHRPP-selectin within Both Early and Late Endosomes
To determine whether BFA affects exit of P-selectin from both early and late endosomes, PC12 cells transiently expressing ssHRPP-selectin were incubated in the presence or absence of 10 μg/ml BFA, and PNS obtained from these cells was fractionated on 1–16% Ficoll gradients. As shown in Figure 7A, the majority of HRP activity within the SLMV peak (fractions 3–4; Norcott et al., 1996) is redistributed into those denser fractions containing early and late endosomes. To accurately determine the proportion of chimera that accumulates within either endosomal population after BFA treatment, the fractions enriched in early endosomes (5–10), as seen by the distribution of 125I-Trn internalized for 60 min and of 125I-EGF internalized for 2 min at 37°C (Figure 6A), or in late endosomes (13–19), as judged by the distribution of 125I-EGF internalized for 20 min at 37°C (Figure 6B), were pooled and then recentrifuged on secondary 3–16% Ficoll or 0.9–1.85 M sucrose gradients, respectively.
Figure 7 shows that incubation of cells with BFA resulted in a 1.5-fold increase of HRP activity within early endosomes (Figure 7B) and in a 1.7-fold increase within late endosomes (Figure 7C). These data therefore indicate that abrogation of SLMV targeting of ssHRPP-selectin by BFA treatment causes the retention of this chimera within both the early and late endosomes. This suggests that both these compartments are most likely the ultimate precusors of SLMV for HRP-P-selectin chimeras within PC12 cells.
Effect of BFA on Endosomal Trafficking of ssHRPP-selectinYGVF and ssHRPP-selectinKCPL
We have also analyzed the endosomal trafficking of the mutant chimeras in the presence of BFA to establish whether the endosomal compartments in which wild-type ssHRPP-selectin accumulates after BFA treatment are those in which the chimeras with mutated SLMV targeting signals are retained (Figures 2 and 3). If this is the case, then the mutant chimeras should show no BFA-dependent increase in the levels of HRP activity present within the early or late endosomes. Accordingly, PC12 cells expressing either ssHRPP-selectinYGVF or ssHRPP-selectinKCPL were incubated in the presence or absence of BFA and processed by two-step subcellular fractionation as described above. The results indicate that for both chimeras the distribution of HRP activity in the presence of BFA was identical to that in contol, untreated cells, as seen on the initial 1–16% Ficoll gradients as well as on the secondary gradients for isolation of early or late endosomes (Figure 8). These data therefore strongly support the notion that those early and late endosomal populations from which HRP-P-selectin is sorted to SLMV in a BFA-sensitive manner are most likely those endosomes where the mutant chimeras with inactivated SLMV targeting signals accumulate and where these signals operate.
Figure 8.
Effect of BFA on the distribution of ssHRPP-selectinYGVF and ssHRPP-selectinKCPL along the endocytic pathway. PC12 cells expressing either ssHRPP-selectinYGVF (A–C) or ssHRPP-selectinKCPL (D and E) were incubated in the presence (filled symbols) or absence (open symbols) of BFA, homogenized, and fractionated on the 1–16% Ficoll gradients. Fractions corresponding to early or late endosomes for ssHRPP-selectinYGVF were collected separately and recentrifuged on 3–16% Ficoll gradients (B) or 0.9–1.85 M sucrose gradients (C), respectively. The peak of early endosomes for ssHRPP-selectinKCPL was pooled (D) and recentrifuged on a 3–16% Ficoll gradient (E). In all cases, HRP activity was measured in each fraction across the gradient and divided by that in the homogenate. One representative experiment of two is shown.
DISCUSSION
We have previously shown that SLMV targeting of HRP-P-selectin chimeras expressed in PC12 cells is controlled by multiple targeting determinants located within the cytoplasmic domain. Mutations of these sequences lead to differential accumulation of chimeras within the endosomal–lysosomal system (Blagoveshchenskaya et al., 1999a). In the present study we have used the HRP-P-selectin chimeras to investigate in detail the signal dependence of endocytic traffic in PC12 cells and to determine which endosomal intermediates are involved in SLMV formation. We have based this analysis on the assumption that by determining the compartment in which a loss-of-function targeting mutant that fails to reach the SLMV accumulates we can identify SLMV precursors. In addition, because the budding of SLMV from endosomes has been shown to be BFA dependent (Faundez et al., 1997; Shi et al., 1998; Blagoveshchenskaya et al., 1999a), we have determined whether those compartments identified as precursors by analyzing the SLMV targeting mutants are identical to those as revealed by the effects of this fungal metabolite.
Both by immunofluorescence microscopy and by quantitating the efficiency of targeting of chimeras with mutated SLMV targeting signals to different endosomal compartments using subcellular fractionation, we have found that alanine substitution of KCPL or of the tyrosine-based signal YGVF resulted in a significant accumulation of chimeras (ssHRPP-selectinKCPL and ssHRPP-selectinYGVF) within early Trn-positive, EGF-positive endosomes (Figures 2 and 5). However, substitution of YGVF or DPSP caused the accumulation of chimeras (ssHRPP-selectinYGVF and ssHRPP-selectinDPSP) within late endosomes (Figure 3). This increased accumulation within late endosomes was not a spillover induced by overexpression, because an even more pronounced increase in the targeting efficiency to late endosomes for ssHRPP-selectinDPSP compared with that for wild-type chimera was observed at expression levels well below (0.5 μg of DNA) saturating levels (Figure 4). It should be noted that although KCPL and DPSP operate at distinct stages of the endocytic pathway, YGVF exerts its effects more broadly, causing an accumulation in both early and late endosomes; albeit to a lesser extent at either location than those mutants that act at a single site. However, as we have previously established, mutation of YGVF, which acts at both putative SLMV budding sites, gives a more dramatic reduction in SLMV targeting than either of the two mutants that only block one route (Blagoveshchenskaya et al., 1999a).
One complication in interpreting the behavior of ssHRPP-selectinDPSP or ssHRPP-selectinYGVF arises from the discovery that these mutants do not solely accumulate within the late endosome but also are found within the lysosomes in comparable amounts (Blagoveshchenskaya et al., 1999a). This difference in the effects of ablating SLMV targeting from early versus later stages of the endosomal system is in agreement with the point of view that sorting from early endosomes to late endosomes and lysosomes is a signal-mediated step, whereas late endosome-to-lysosome trafficking occurs by a default pathway (for review, see Mellman, 1996). One alternative interpretation of this phenomenon is that lysosomes might also be SLMV precusors. However, we would argue that despite increasing evidence that lysosomes are not simply a dead end within the cell, but rather dynamic organelles, which can undergo fusion with other lysosomes (Ward et al., 1997) or with late endosomes (Futter et al., 1996; Bright et al., 1997; Mullock et al., 1998), until now there have been no available data to suggest that lysosomes are the origin of any other organelles or are involved in recycling to the cell surface in significant levels.
To provide independent evidence of whether the late endosomes are involved in sorting of HRP-P-selectin chimeras to SLMV as well as early endosomes, we exploited the sensitivity of SLMV trafficking of ssHRPP-selectin to BFA. This macrocyclic antibiotic had previously been shown to be an inhibitor of the ARF1/AP-3-dependent formation of SLMV from endosomes (Faundez et al., 1997; Faundez et al., 1998). We have found that, although not causing a significant redistribution of endosomal markers, BFA treatment resulted in the redistribution of ssHRPP-selectin from the peak corresponding to SLMV into those containing the early and late endosomes, as monitored by 125I-Trn and 125I-EGF on 1–16% Ficoll gradients, respectively (Figures 6 and 7). Quantification revealed that the total increment of the amount of HRP activity within both the early and late endosomal peaks was 80% (Figure 7), which is in good agreement with the 88% reduction of that within the SLMV peak after BFA treatment (Blagoveshchenskaya et al., 1999a).
Importantly, under these conditions no rise in the amount of HRP activity was detected within the lysosomes (the last five fractions on 1–16% Ficoll gradients; Figure 7). This finding indicates that when SLMV biogenesis is reversibly blocked for a short time, the accumulation of wild-type chimera is restricted to the late endosomes and is not found in the lysosomes, in contrast to the steady-state localization of chimeras with inactivated SLMV targeting signals, found both in late endosomes and lysosomes. This argues in favor of the late endosomes alone rather than together with the lysosomes as the SLMV precursor.
Taken together, our data on differential accumulation of chimeras with inactivated SLMV targeting signals within the two endosomal compartments, coupled with those on targeting to these compartments in the presence of BFA, lead us to conclude that both the early EGF-positive, Trn-positive early endosomes and EGF-positive, Trn-negative late endosomes are the precusors of SLMV. Although there is a large body of data, including those of the current work, consistent with the view that the early endosome is a site from which SLMV can bud (for review, see Hannah et al., 1999), the finding that SLMV can also bud from the late endosomes is entirely novel. In addition, the analysis of targeting of the double mutants ssHRPP-selectinKCPL+YGVF and ssHRPP-selectinKCPL+DPSP indicated that these chimeras exhibited the same phenotype as ssHRPP-selectinKCPL in terms of lysosomal targeting, strongly suggesting that P-selectin travels both to SLMV and to lysosomes via the same two subsequent endosomal intermediates (Figure 9).
Figure 9.
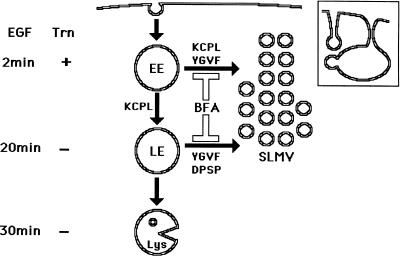
Schematic model for trafficking of HRP-P-selectin chimeras to SLMV in PC12 cells. HRP-P-selectin present on the plasma membrane is internalized into early Trn-positive, EGF-positive endosomes (EE) from which budding of SLMV occurs in a KCPL- and YGVF-dependent and BFA-sensitive manner. From the same endosomes, the remaining chimera is sorted to late, Trn-negative, EGF-positive endosomes (LE) in a KCPL-dependent, BFA-insensitive manner. From this compartment, further trafficking of P-selectin can occur in one of the two directions. One route leads to SLMV, a process that is controlled by YGVF and DPSP and is BFA sensitive. The remaining protein within LE is delivered to lysosomes (Lys) for degradation. Another potential SLMV precusor, represented by invaginations of the plasma membrane, from which budding of SLMV is BFA insensitive (12% for HRP-P-selectin vs. 88% that is BFA sensitive) and which does not involve an endosomal intermediate, is shown in the inset.
Sorting of HRP-P-selectin chimeras to SLMV from both early and late endosomes is affected by BFA, suggesting a possible involvement of coat proteins at both donor sites. Apart from the already documented use of ARF1/AP-3 in SLMV formation from the early endosomes (Faundez et al., 1997; Faundez et al., 1998), other BFA-sensitive and endosomally recruited coats might also be involved, thereby accounting for how such different BFA-sensitive SLMV targeting signals are operating. One potential candidate may be COPI, which is thought to be required for early-to-late endosomal trafficking (Whitney et al., 1995; Aniento et al., 1996). It is not yet known whether the machinery used for SLMV budding from early endosomes is also used in budding from late endosomes. Given that the SLMV which bud from late endosomes are presumably of similar (if not identical) composition to those from early endosomes, it is reasonable to suggest that the same machinery might be involved in both sorting steps. However, the finding that SLMV budding from the plasma membrane requires different, AP-2/clathrin-associated, machinery (Shi et al., 1998), coupled with data indicating that different SLMV targeting sequences are used in exit from the late as against the early endosome (this work), suggests that the machinery operating at these two endosomal populations may be different.
The pathway of SLMV biogenesis via the early endosomal intermediate is readily reconciled with models of membrane traffic in which the bulk of internalized proteins are nonselectively delivered to a common early endosome from which sorting occurs to direct the proteins to a variety of destinations (for review, see Mellman, 1996). By contrast, examples of the late endosome as a sorting station (especially en route to any final compartment) are few. These include recycling of mannose 6-phosphate receptor and furin from the plasma membrane via the early endosomes and then late endosomes to the TGN (Dahms et al., 1989; Mallet and Maxfield, 1999) and the formation of α-granules from mature multivesicular bodies in megakaryocytes and platelets (Heijnen et al., 1998). Given the origin of P-selectin in hemopoietic cells, the latter observation may be related to our current findings.
Glucose transporter 4 (GLUT4)-containing secretory vesicles, which are transferred to the cell surface after insulin stimulation, may also arise from two or more intracellular pools in series, including that of late endosomes (Malide et al., 1997), although which sorting signals within GLUT4 mediate this step remain so far unknown. However, it is not yet resolved whether the early Trn-positive endosomes are one of the multiple precusors for GLUT4-containing vesicles (Aledo et al., 1997; Malide et al., 1997; Wei et al., 1998). Our data showing the existence of two endosomal intermediates en route to SLMV are in agreement with the model of multiple intracellular pools responsible for GLUT4 sequestration (Holman et al., 1994; Verhey et al., 1995). The model of a common sorting endosome as the sole precusor for specialized organelles (Mellman, 1996) may therefore need to be modified to one in which endosomes of different composition can function as donor compartments.
Lichtenstein et al. (1998) also observed two endosomal intermediates, through which VAMP-tagged chimeras traffic to SLMV. However, both populations of these endosomes were found to contain Trn, implying that the SLMV precusor was most likely to be the endosomal tubules (Lichtenstein et al., 1998). These findings were later supported by detailed electron microscopy study (de Wit et al., 1999). One possible interpretation of these and our data is that different sites along the endocytic pathway could be used for SLMV budding, reflecting the gradual maturation and sorting processes carried out by this complex and plastic organelle. If so, then the presence of a strong lysosomal targeting signal in the cytoplasmic tail of P-selectin (Green et al., 1994; Blagoveshchenskaya et al., 1998a,b, 1999a) could lead to a larger proportion of P-selectin entering SLMV from a later compartment along the endocytic pathway than is the case for VAMPII or synaptophysin, which have no such signal and are not targeted to lysosomes in substantial amounts (de Wit et al., 1999).
One of the intriguing results of the current study is that in addition to individual signals that operate exclusively either from early (KCPL) or from late endosomes (DPSP), P-selectin uses the same signal, YGVF, to promote targeting to SLMV from both endosomal populations. In principle, YGVF could be needed for sorting of chimeras en route to SLMV from a postendosomal intermediate, e.g., that derived by forming of endosomal tubules. However, if this were so, we should have been able to monitor the appearance of ssHRPP-selectinYGVF in different fractions from those corresponding to early or late endosomes; clearly this was not the case (Figure 8). Alternatively, YGVF might facilitate the lateral redistribution of P-selectin within the plane of the perimeter endosomal membrane, allowing the concentration of protein within the endosomal subdomain from which SLMV bud in a KCPL- or DPSP-dependent manner. Validation or disproof of this hypothesis requires further investigation, probably at the electron microscopic level.
ACKNOWLEDGMENTS
We thank Drs. M. Hannah, M. Arribas, L. Turner, and D. Savery for critical reading of the manuscript. This work was supported by a Medical Research Council program grant to D.F.C.
Abbreviations used:
- AP
adaptor protein complex
- ARF1
adenosine ribosylation factor 1
- BFA
brefeldin A
- DCG
dense-core granules
- EE-TI
early endosomal targeting index
- EGF
epidermal growth factor
- GLUT
glucose transporter
- HB
homogenization buffer
- LE-TI
late endosomal targeting index
- MES
2-[N-morpholino]ethanesulfonic acid
- NAGA
N-acetyl-β-d-glucosaminidase
- PNS
postnuclear supernatant
- RSO
regulated secretory organelles
- SLMV
synaptic-like microvesicles
- SV
synaptic vesicles
- TGN
trans-Golgi network
- Trn
transferrin
- VAMP
vesicle-associated membrane protein
REFERENCES
- Aledo JC, Lavoie L, Volchuk A, Keller SR, Klip A, Hundal HS. Identification and characterization of two distinct intracellular GLUT4 pools in rat skeletal muscle: evidence for an endosomal and an insulin-sensitive GLUT4 compartment. Biochem J. 1997;325:727–732. doi: 10.1042/bj3250727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal β-COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerfeind R, Huttner WB. Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr Opin Cell Biol. 1993;5:628–635. doi: 10.1016/0955-0674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Hewitt EW, Cutler DF. A balance of opposing signals within the cytoplasmic tail controls the lysosomal targeting of P-selectin. J Biol Chem. 1998a;273:27896–27903. doi: 10.1074/jbc.273.43.27896. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Hewitt EW, Cutler DF. A complex web of signal-dependent trafficking underlies the triorganellar distribution of P-selectin in neuroendocrine PC12 cells. J Cell Biol. 1999a;145:1419–1433. doi: 10.1083/jcb.145.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Hewitt EW, Cutler DF. Di-leucine signals mediate targeting of tyrosinase and synaptotagmin to synaptic-like microvesicles within PC12 cells. Mol Biol Cell. 1999b;19:3979–3990. doi: 10.1091/mbc.10.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Norcott JP, Cutler DF. Lysosomal targeting of P-selectin is mediated by a novel sequence within its cytoplasmic tail. J Biol Chem. 1998b;273:2729–2737. doi: 10.1074/jbc.273.5.2729. [DOI] [PubMed] [Google Scholar]
- Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP-140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–1112. [PubMed] [Google Scholar]
- Bonifacino JS, Dell'Angelica ES. Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgonovo B, Racchetti G, Malosio M, Benfante R, Podini P, Rosa P, Meldolesi J. Neurosecretion competence, an independently regulated trait of the neurosecretory cell phenotype. J Biol Chem. 1998;273:34683–34686. doi: 10.1074/jbc.273.52.34683. [DOI] [PubMed] [Google Scholar]
- Bright NA, Reaves BJ, Mullock BM, Luzio JP. Dense core lysosomes can fuse with late endosomes and are reformed from the resultant hybrid organelles. J Cell Sci. 1997;110:2027–2040. doi: 10.1242/jcs.110.17.2027. [DOI] [PubMed] [Google Scholar]
- Cramer LP, Cutler DF. Sorting between secretory pathways. In: Magee AI, Wileman T, editors. Protein Traffic: A Practical Approach. Oxford, United Kingdom: Oxford University Press; 1992. pp. 57–85. [Google Scholar]
- Cutler DF, Cramer LP. Sorting during transport to the surface of PC12 cells: divergence of synaptic vesicles and secretory granule proteins. J Cell Biol. 1990;110:721–730. doi: 10.1083/jcb.110.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms NM, Lobel P, Kornfeld S. Mannose 6-phosphate receptors and lysosomal enzyme targeting. J Biol Chem. 1989;264:12115–12118. [PubMed] [Google Scholar]
- De Camilli P, Jahn R. Pathways to regulated exocytosis in neurons. Annu Rev Physiol. 1990;52:625–645. doi: 10.1146/annurev.ph.52.030190.003205. [DOI] [PubMed] [Google Scholar]
- de Wit H, Lichtenstein Y, Geuze HJ, Kelly RB, van der Sluijs P, Klumperman J. Synaptic vesicles form by budding from tubular extensions of sorting endosomes in PC12 cells. Mol Biol Cell. 1999;10:4163–4176. doi: 10.1091/mbc.10.12.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faundez V, Horng J-T, Kelly RB. ARF1 is required for synaptic vesicles budding in PC12 cells. J Cell Biol. 1997;138:505–515. doi: 10.1083/jcb.138.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faundez V, Horng J-T, Kelly RB. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell. 1998;93:423–432. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Setiadi H, McEver RP, Kelly RB. The cytoplasmic domain of P-selectin contains a sorting determinant that mediates rapid degradation in lysosomes. J Cell Biol. 1994;124:435–448. doi: 10.1083/jcb.124.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E, Hao JC, Bennet MK, Kelly RB. A targeting signal in VAMP regulating transport to synaptic vesicles. Cell. 1995;81:581–589. doi: 10.1016/0092-8674(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Grote E, Kelly RB. Endocytosis of VAMPII is facilitated by a synaptic vesicle targeting signal. J Cell Biol. 1996;132:537–547. doi: 10.1083/jcb.132.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah MJ, Schmidt AA, Huttner WB. Synaptic vesicle biogenesis. Annu Rev Cell Dev Biol. 1999;15:733–798. doi: 10.1146/annurev.cellbio.15.1.733. [DOI] [PubMed] [Google Scholar]
- Heijnen HFG, Debili N, Vainchencker W, Breton-Gorius J, Geuze HJ, Sixma JJ. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91:2313–2325. [PubMed] [Google Scholar]
- Holman GD, Leggio LL, Cushman SW. Insulin-stimulated GLUT4 glucose transporter recycling. A problem in membrane protein subcellular trafficking through multiple pools. J Biol Chem. 1994;269:17516–17524. [PubMed] [Google Scholar]
- Höning S, Sandoval IV, von Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GI, Cook RG, McEver RP. Cloning of GMP-140, a granular membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989;56:1033–1044. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- Kasai H, Kishimoto T, Liu T-T, Miyashita Y, Podini P, Grohovaz F, Meldolesi J. Multiple and diverse forms of regulated exocytosis in wild-type and defective PC12 cells. Proc Natl Acad Sci USA. 1999;96:945–949. doi: 10.1073/pnas.96.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RB. Secretory granule and synaptic vesicle formation. Curr Opin Cell Biol. 1991;3:654–660. doi: 10.1016/0955-0674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- Kornilova ES, Taverna D, Hoeck W, Hynes NE. Surface expression of erB-2 protein is post-transcriptionally regulated in mammary epithelial cells by epidermal growth factor and by the culture density. Oncogene. 1992;7:511–519. [PubMed] [Google Scholar]
- Lichtenstein Y, Desnos C, Faundez V, Kelly RB, Clift-O'Grady L. Vesiculation and sorting from PC12-derived endosomes in vitro. Proc Natl Acad Sci USA. 1998;95:11223–11228. doi: 10.1073/pnas.95.19.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malide D, Dwyer NK, Blanchette-Mackie EJ, Cushman SW. Immunocytochemical evidence that GLUT4 resides in a specialized translocation post-endosomal VAMP2-positive compartment in rat adipose cells in the absence of insulin. J Histochem Cytochem. 1997;45:1083–1096. doi: 10.1177/002215549704500806. [DOI] [PubMed] [Google Scholar]
- Mallet WG, Maxfield FR. Chimeric forms of furin and TGN38 are transported from the plasma membrane to the trans-Golgi network via distinct endosomal pathways. J Cell Biol. 1999;146:345–359. doi: 10.1083/jcb.146.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Ohno H, Kirchhausen T, Bonifacino JS. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- Marks MS, Woodruff L, Ohno H, Bonifacino JS. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet α-granule membrane protein, is also synthesized by vascuolar endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Mullock BM, Bright NA, Fearon CW, Gray SR, Luzio JP. Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent. J Cell Biol. 1998;140:591–601. doi: 10.1083/jcb.140.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcott JP, Solari R, Cutler DF. Targeting of P-selectin to two regulated secretory organelles in PC12 cells. J Cell Biol. 1996;134:1229–1240. doi: 10.1083/jcb.134.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Cowles CR, Emr SD. The AP-3 complex: a coat of many colors. Trends Cell Biol. 1998;8:282–288. doi: 10.1016/s0962-8924(98)01295-1. [DOI] [PubMed] [Google Scholar]
- Pance A, Morgan K, Guest PC, Bowers K, Dean GE, Cutler DF, Jackson AP. A PC12 variant lacking regulated secretory organelles: aberrant protein targeting and evidence for a factor inhibiting neuroendocrine gene expression. J Neurochem. 1999;73:21–30. doi: 10.1046/j.1471-4159.1999.0730021.x. [DOI] [PubMed] [Google Scholar]
- Regnier-Vigouroux A, Tooze SA, Huttner WB. Newly synthesized synaptophysin is transported to synaptic-like microvesicles via constitutive secretory vesicles and the plasma membrane. EMBO J. 1991;10:3589–3601. doi: 10.1002/j.1460-2075.1991.tb04925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem N, Faundez V, Horng J-T, Kelly RB. A v-SNARE participates in synaptic vesicle formation mediated by the AP3 adaptor complex. Nat Neurosci. 1998;1:551–556. doi: 10.1038/2787. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hannah MJ, Huttner WB. Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J Cell Biol. 1997;137:445–458. doi: 10.1083/jcb.137.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Huttner WB. Biogenesis of synaptic-like microvesicles in perforated PC12 cells. Methods. 1998;16:160–169. doi: 10.1006/meth.1998.0663. [DOI] [PubMed] [Google Scholar]
- Shi G, Faundez V, Roos J, Dell'Angelica EC, Kelly RB. Neuroendocrine synaptic vesicles are formed in vitro by both clathrin-dependent and clathrin-independent pathways. J Cell Biol. 1998;143:947–955. doi: 10.1083/jcb.143.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, Brodin L. Synaptic vesicles endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- Straley KS, Daugherty BL, Aeder SE, Hockenson AL, Kim K, Green SA. An atypical sorting determinant in the cytoplasmic domain of P-selectin mediates endosomal sorting. Mol Biol Cell. 1998;9:1683–1694. doi: 10.1091/mbc.9.7.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser JE, Arribas M, Blagoveshchenskaya AD, Cutler DF. Secretagogue-triggered transfer of membrane proteins from neuroendocrine secretory granules to synaptic-like microvesicles. Mol Biol Cell. 1999;10:2619–2630. doi: 10.1091/mbc.10.8.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam M, Koedam JA, Wagner DD. Divergent fates of P- and E-selectins after their expression on the plasma membrane. Mol Biol Cell. 1993;4:791–801. doi: 10.1091/mbc.4.8.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, De Camilli P. The synaptic vesicle cycle: a single vesicle budding step involving clathrin and dynamin. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA. Biogenesis of secretory granules in the trans-Golgi network of neuroendocrine and endocrine cells. Biochim Biophys Acta. 1998;1404:231–244. doi: 10.1016/S0167-4889(98)00059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey KJ, Yeh JI, Birnbaum MJ. Distinct signals in the GLUT4 glucose transporter for internalization and for targeting to insulin-responsive compartment. J Cell Biol. 1995;130:1071–1079. doi: 10.1083/jcb.130.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DM, Leslie JD, Kaplan J. Homotypic lysosome fusion in macrophages: analysis using an in vitro assay. J Cell Biol. 1997;139:665–673. doi: 10.1083/jcb.139.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RA, Green FA, Stenberg PE, Enns CA. Distinct saturable pathways for the endocytosis of different tyrosine motifs. J Biol Chem. 1998;273:17056–17063. doi: 10.1074/jbc.273.27.17056. [DOI] [PubMed] [Google Scholar]
- Wei ML, Bonzelius F, Scully RM, Kelly RB, Herman GA. GLUT4 and transferrin receptor are differentially sorted along the endocytic pathway in CHO cells. J Cell Biol. 1998;140:565–575. doi: 10.1083/jcb.140.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Wiley HS, Cunningham DD. The endocytic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J Biol Chem. 1982;257:4222–4229. [PubMed] [Google Scholar]
- Zakharenko S, Chang S, O'Donoghue M, Popov SV. Neurotransmitter secretion along growing nerve processes: comparison with synaptic vesicle exocytosis. J Cell Biol. 1999;144:507–518. doi: 10.1083/jcb.144.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]