Abstract
Some strains of Escherichia coli related to acute cystitis or colitis produce a toxin named cytotoxic necrotizing factor 1 (CNF-1). CNF-1 mediates its effects on epithelial cells or phagocytes via the permanent activation of small GTP-binding proteins, caused by the toxin-induced deamidation of Glu63 of p21 Rho. The behavior of peripheral blood T lymphocytes during the acute phase of bacterial colitis has been poorly investigated. Our study was conducted to test whether (i) peripheral blood T lymphocytes can be activated by CNF-1 and (ii) CNF-1-activated T lymphocytes are cytotoxic against intestinal epithelial cells. Activation of T lymphocytes by CNF-1 was assessed by electrophoresis, flow cytometry, confocal microscopy, and electron microscopy studies. Assays for migration and adherence of CNF-1-treated T lymphocytes were performed in Transwell chambers with T84 intestinal epithelial cells grown on polycarbonate semipermeable filters. CNF-1 induced a decrease in the electrophoretic mobility of the GTP-binding protein Rho in treated T lymphocytes. CNF-1 provoked an increase in the content of actin stress fibers and pseudopodia in T lymphocytes. Several adherence molecules were clustered into cytoplasmic projections in CNF-1-treated T lymphocytes and adherence of such lymphocytes on the basolateral pole of T84 was increased, resulting in cytotoxicity toward epithelial cells. Such enhanced adherence in response to CNF-1 was dependent on p42-44MAP kinase activation of T lymphocytes. Taken together, these results suggest that CNF-1, by acting on T lymphocytes, may increase in an important fashion the virulence of certain strains of E. coli against the intestinal epithelia.
Bacterial colitis is characterized by an acute inflammation with polymorphonuclear leukocyte (PMNL) migration into the digestive lumen in response to inflammatory cytokines (16, 22, 50). This transepithelial migration of PMNL and the consequences of cell-cell interaction on both PMNL and intestinal epithelial cells have been extensively studied using an in vitro model (28, 33, 36). T lymphocytes play an essential role in the immune surveillance at the intestinal epithelium level (46). Dysregulated T-cell responses to enteric bacteria have been implicated in the pathogenesis of some models of colitis (29, 30). Nevertheless, the role of T lymphocytes during the acute phase of bacterial colitis has been poorly investigated.
The recruitment of T lymphocytes from the circulation into the mucosa requires the extravasation of these leukocytes from the microvasculature and their subsequent homing into the lamina propria (6). Leukocyte migration in response to chemoattractants is a crucial phenomenon in both the immune and the inflammatory response. Several members of the Rho family of small GTPases, named Rho, Rac, and Cdc42, act as key regulators of the actin cytoskeleton and different data suggest that Rho proteins are involved in the leukocyte adhesion through integrins activated by chemoattractants (32). Moreover, small GTPases can regulate T-cell chemotaxis in response to stromal cell-derived factor 1α (SDF-1α) (13). Indeed, overexpression of dominant negative Cdc42 and activated mutants of all these Rho GTPases inhibited SDF-1α-induced T-cell chemotaxis (13). Moreover, D'Souza-Schorey et al. have shown that Rac may increase integrin-mediated adherence of T lymphocytes (14).
Certain strains of Escherichia coli produce a toxin named cytotoxic necrotizing factor 1 (CNF-1) (3, 4, 8). These strains can induce urinary or gastrointestinal tract infections (4, 15). CNF-1 mediates its effects via the permanent activation of small Rho GTP-binding proteins, by causing deamidation of p21 Rho Glu63 residue (5, 19-21, 44). Recently, it has been demonstrated that CNF-1 mediates its effect via both a clathrin-independent endocytic mechanisms and an acidic-dependent membrane translocation step in its delivery of the catalytic domain to the cell cytosol (12). The consequences of this activation is mainly characterized by an intense actin cytoskeleton polymerization into the intoxicated cell (3, 7, 18, 27, 47, 48). We have previously shown that CNF-1, when incubated with epithelial cells, decreases the transepithelial migration of PMNL (26). Moreover, we have also demonstrated that CNF-1 can act on PMNL, by inducing a decreased bacteria phagocytosis (27). No information is currently available on the effect of CNF-1 on T lymphocytes and the possible consequences for the epithelial cells of their interaction with such CNF-1-intoxicated T lymphocytes.
In this study, we present evidence that CNF-1 induces T-lymphocyte phenotypic changes by activation of GTP-binding protein Rho. Such CNF-1-treated T lymphocytes cause cytotoxic effects in cultured monolayers of the human intestinal epithelial cell line T84 by increasing their adherence at the basolateral pole of the monolayers. Altogether, these data suggest that during infection by certain E. coli strains producing CNF-1, the adherence and activation status of T lymphocytes may be modified in vivo.
MATERIALS AND METHODS
Reagents.
Highly purified CNF-1 used throughout this work was a generous gift of Gilles Flatau (INSERM U 452, Faculty of Medicine, University of Nice Sophia-Antipolis) and was prepared as described previously (15).
T-cell purification and cell culture.
Heparinized blood samples were separated by Ficoll gradient technique. Peripheral blood mononuclear cells were removed from the gradient, and purified T cells were obtained by positive selection as described previously (40). The cells were washed and maintained in RPMI 1640 medium until utilization. The human leukemic T-cell line Jurkat was cultured in humidified incubators at 37°C, 5% (vol/vol) CO2 in RPMI 1640 medium supplemented with 10% (vol/vol) fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (5 μg/ml). HEp-2 cells were grown in Dulbecco's modified eagle's medium (DMEM) supplemented with 7% calf serum-1% glutamine as previously described (12).
Rho ADP-ribosylation assay.
CNF-1-induced Rho activation in T lymphocytes was determined as previously described (21). Deamidation of Glu63 by CNF-1 results in a significant upshift of the apparent molecular weight of [32P] ADP-ribosylated Rho on SDS-PAGE (21, 44). Molecular effects due to CNF-1 which result in Rho activation can be demonstrated by radiolabeled staining of Rho and deamided Rho by the use of C3 exoenzyme, which add [32P]ADP ribose on Thr37 of Rho. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the deamided protein is detected at a slower migrating band (upshift). Peripheral blood T lymphocytes and Jurkat and HEp-2 cells (107 cells per condition), were incubated with increasing concentrations of CNF-1 (from 1 pM to 3 nM) at 4 and 24 h in supplemented RPMI 1640. Cells were then treated as previously described (9, 21, 44).
Morphological study. (i) Confocal microscopy studies.
F-actin fluorescence staining of control and CNF-1-treated cells (peripheral blood T lymphocytes and Jurkat and HEp-2 cells) was processed as previously described (26). The slides were observed and photographed with a laser scanning fluorescence microscope (Leica, DMIRBE, Lyon, France) equipped for epifluorescence.
(ii) Electron microscopy studies.
Control and CNF-1-treated cells (T lymphocytes and Jurkat cells) were fixed with 2% formaldehyde, in 0.1 M Na cacodylate, pH 7.4 for 1 h at 4°C. Cell pellets were then treated as previously described (26). For immunoelectron microscopy, control or CNF-1-stimulated T-lymphocyte pellets were fixed in 3.7% paraformaldehyde and embedded at low temperature into LR White resin (Hard LR; White, London, United Kingdom). Ultrathin sections were put on 300 mesh nickel grids, washed with phosphate-buffered saline (PBS), then incubated for 1 h at room temperature with anti-CD11a (Immunotech, Luminy, France), anti-CD29 (K20, gift of M. Ticchioni, INSERM 343, Nice, France) or anti-CD103 (β7) (Fib504; American Type Culture Collection [ATCC], Manassas, Va.) antibodies. After being washed with PBS, the grids were incubated for 1 h with 10-nm-thick colloidal gold-conjugated rabbit anti-mouse or goat anti-rat secondary antibodies (dilution 1:20; British Biocell International, Paris, France). Secondary antibody alone and KL1 (anticytokeratine) antibody (Immunotech) were used in each experiments as negative controls. The grids were washed with PBS, then with distilled water and stained with uranyl acetate. Ultrathin sections were examined with a JEOL 1200 XII electron microscope.
In some experiments, Jurkat cells were incubated with 10−9 M CNF-1 for 48 h and then fixed in methanol and stained with May Grunwald-Giemsa (E. Merck, Darmstadt, Germany). To quantify toxic effects in Jurkat cell cultures, 20 microscopic fields (magnification, ×400) for each sample were examined; viable cells (identified by trypan blue exclusion), and cells containing two or more nuclei were counted. Data were expressed as cells per square millimeter.
Flow cytometric analysis. (i) Determination of F-actin.
Control and CNF-1-treated lymphocytes were fixed with 3.7% formaldehyde and incubated for 45 min with PBS containing 500 nM rhodamine-phalloidin. After being washed in PBS, the cellular content in F-actin was measured by flow cytometry.
(ii) Expression of integrins.
Control and CNF-1-treated lymphocytes were fixed in 1% formalin for 30 min at room temperature. The cells were then washed in Hanks balanced salt solution (HBSS) and incubated with goat immunoglobulin for 20 min. The lymphocytes were washed again in HBSS and incubated with either monoclonal antibodies (MAbs) (anti-CD11a; ATCC; diluted 1/1,000), anti-CD29 (K20; INSERM 343; diluted 1/1,000), anti-CD103 (β7) (Fib504; ATCC), or an isotype-matched control for 20 min at room temperature and then washed twice. Cells were then treated as previously described (27).
Migration assays.
Assays for migration of control and CNF-1-treated T lymphocytes were performed in Transwell cell culture chambers with polycarbonate filters (surface, 0. 33 cm2; pore size, 8 μm; Costar). Control T lymphocytes (2 · 106) and CNF-1 pretreated T lymphocytes for 24 h were added to the upper reservoir. One milliliter of HBSS with or without 10 nM SDF-1α (R&D Systems, Lille, France) were added in the lower reservoir. After 6 h of migration, transmigrated cells in the lower reservoir were counted by flow cytometry using a standard of 25 · 103 fluorescent beads (Fluospheres; Beckman Coulter, Paris, France).
Adherence assay to intestinal epithelial cells.
Assays for adherence of control and CNF-1-treated Jurkat cells were performed in Transwell cell culture chambers with polycarbonate filters (surface, 0. 33 cm2; pore size, 8 μm; Costar). The epithelial intestinal cell line (T84) was grown on these filters as previously described (25, 36). Briefly, inverted monolayers were grown as follows: 0.8-mm-thick lexan rings having the same dimension of the base of Costar inserts were attached to the underside of the insert using general Electric RTV silicone glue. After drying overnight, the inserts were sterilized by submersion in 70% ethanol, inverted onto a sterile petri dish in a hood. Collagen (from rat tail tendon) and T84 cells were added to the filter (underside facing up) and cells were allowed to attach overnight before righting the inserts into the 24-well holding plates. Adherence assays were then performed in a mucosa-to-lumen direction (i.e., Jurkat cells or T lymphocytes were added to the upper reservoir in contact with the basal side of T84 monolayers). Jurkat cells were labeled for 16 h with [3H]methyl thymidine (2.5 μCi/ml; ICN) and preincubated or not at 37°C with CNF-1. A total of 2 × 106 cells suspended in 100 μl of RPMI 1640-0.05% bovine serum albumin was added to the upper chamber and 1 ml of the same medium was added to the lower chamber. When indicated, 10 nM SDF-1α was added to the lower chamber. Cells were allowed to adhere for 6 h at 37°C and 5% CO2 atmosphere. Counting of adherent Jurkat cells was evaluated by the measure of incorporated [3H]methyl thymidine by scintillation spectroscopy. Data are pooled from 6 to 12 individual monolayers for each condition, and results are means ± standard errors (SE) of five experiments.
Determination of T-lymphocyte adherence to T84 monolayers was performed with a fluorescence multiwell plate reader (Cytofluor; Perspective Biosystems, Framingham, Mass.). Briefly, 2 · 106 T lymphocytes were added to the upper chamber and 1 ml of HBSS with or without 10 nM SDF-1α was added in the lower reservoir. Cells were allowed to adhere for 6 h at 37°C and 5% CO2 atmosphere. After 1 h of incubation at 37°C and washing with HBSS, cell-associated fluorescence was measured. Assays were realized in triplicate. The results were expressed as relative mean adherence corresponding to the ratio of fluorescence values before and after washing. In some experiments, adherence of CNF1-treated T lymphocytes to T84 monolayers in the presence of SDF-1α was studied in T lymphocytes treated with anti-tumor necrosis factor alpha (anti-TNF-α) MAb (5 μg/ml; Euromedex, Paris, France) or anti-transforming growth factor β1 (anti-TGF-β1) MAb (5 μg/ml; Euromedex) for 1 h.
The morphological consequences of this control T-lymphocyte adherence on T84 monolayers were assessed by electron microscopy. Three different T84 monolayers were examined for each condition after 24 h of contact with lymphocytes treated or not with CNF-1 (16 h, 10−9 M). After removal from the inverts, the monolayers were fixed with 2% freshly prepared formaldehyde, in 0.1 M Na cacodylate, pH 7.4, for 1 h at 4°C. Tissues were rinsed in cacodylate buffer and were then processed as described above. More than 30 T84 cells were examined for each condition.
Analysis of activation of p42-44MAPk and JNK by Western blotting.
Control and CNF-1-treated T lymphocytes were washed in Hanks' balanced salt solution (Sigma, Paris, France), then lysed in cold NP-40 buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 2 mM EDTA, 1% NP-40, 1 mM Na3 VO4, 1 mM phenylmethylsulfonyl fluoride, 25 μM leupeptin, 5 mM benzamidine, 1 μM pepstatin, 25 μM aprotinin, 50 mM sodium β-glycerophosphate, 20 mM sodium pyrophosphate, 0.5 mM dithiothreitol) at a density of 5 · 107 cells/ml. After sonication (two pulses of 8 s), lysates were centrifuged at 15,000 × g for 15 min at 4°C and denatured by boiling in reduced SDS sample buffer. Protein lysates (50 μg per sample) were resolved onto SDS-PAGE and subsequently were electrophoretically transferred to polyvinylidene fluoride membrane (Immobilon-P; Millipore). Membranes were incubated in saturation buffer, and probed with anti-phospho-p42-44MAPK (diluted at 1/2,000) (New England BioLabs, Beverly, Mass.), anti-phospho-JNK (diluted 1/3,000; New England BioLabs), anti-ERK2 (0.1 μg/ml; Santa Cruz Biotechnologies, Santa Cruz, Calif.), anti-JNK1 (0.1 μg/ml; Santa Cruz Biotechnologies) antibodies incubated overnight at 4°C. This labeling was visualized by peroxidase-conjugated anti-rabbit immunoglobulin G (diluted at 1/2,000; New England BioLabs) and by enhanced chemiluminescence (ECL kit; Amersham International, Little Chalfont, Buckinghamshire, United Kingdom). As positive control for T-lymphocyte activation we used phorbol myristate acetate (PMA) (5 ng/ml). In some experiments, T lymphocytes were preincubated 1 h with the MEK inhibitor PD 98059 (Calbiochem, La Jolla, Calif.) at a final concentration of 10 μM before addition of CNF-1. Finally, to assess the possible implication of TNF-α and TGF-β 1 on CNF-1 induced mitogen-activated protein kinase (MAPK) activation, T lymphocytes were first incubated with anti-TNF-α (5 μg/ml; Euromedex, Paris) or TGF-β1 (5 μg/ml; Euromedex) neutralizing antibodies for 1 h before addition of CNF-1.
ELISA and RNase protection assay (RPA) for the detection of cytokines.
To characterize the cytokines produced by CNF-1-treated T lymphocytes, control and treated lymphocytes were assayed in triplicate by enzyme-linked immunosorbent assay (ELISA) for gamma interferon (IFN-γ), interleukin-2 (IL-2), IL-4, IL-8, TGF-β1, TGF-β2, TGF-β3, and TNF-α cytokines. The ELISA was carried out using MAbs to cytokines cited above and phosphatase-conjugated goat anti-cytokine polyclonal antibodies (Sandoz Pharmaceutical, Rueil-Malmaison, France). The minimum detectable dose was determined by adding two standard deviations to the mean optical density value of twenty standard replicates and calculating the corresponding concentration. Cytokine concentrations in each sample were determined by extrapolation from cytokine standard curves. The minimum detectable dose was less than 10 pg/ml for IFN-γ, IL-2, IL-4, or IL-8 cytokines, and less than 5 pg/ml for TGF-β1, TGF-β2, TGF-β3, or TNF-α cytokines.
For RNA extraction and RPA, peripheral T lymphocytes (107 cells/10 ml) were incubated in 10 ml of culture buffer with or without CNF-1 (10−9 M) or PMA (5 ng/ml) at 37°C overnight. Total RNA was isolated by lysing cells with TRIzol reagent (Life Technologies) according to the manufacturer's instructions and analyzed for the level of mRNA expression of IFN-γ, IL-2, IL-4, IL-8, TGF-β1, TGF-β2, TGF-β3, and TNF-α by RiboQuant RPA using the hCK-3 multiprobe template set from Pharmingen (San Diego, Calif.) (24). Briefly, riboprobes were 32P labeled and hybridized overnight in solution with 1 to 2 μg of RNA samples. The hybridized RNA was digested with RNases and the remaining RNase-protected probes were resolved on denaturing polyacrylamide gels and visualized by autoradiography according to the RiboQuant protocol (Pharmingen).
Data analysis.
An unpaired two-tailed Student's t test was used to determine statistical significance for adherence assays and ELISA analyses. Values are expressed as the means ± SE of the means of a number of experiments.
RESULTS
CNF-1 induces T-lymphocyte phenotypic changes by activation of GTP-binding protein Rho.
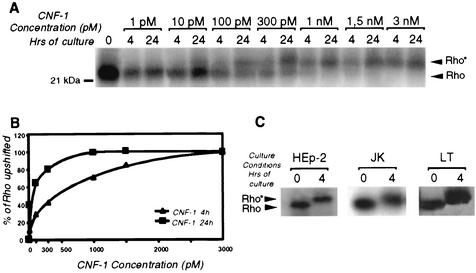
Since the effect of CNF-1 on lymphoid cells was unknown, we assessed whether T lymphocytes were sensitive to CNF-1. We studied the CNF-1 activation of Rho at different times and concentrations using the Jurkat T-cell line. We used HEp-2 as a CNF-1 sensitive cell-line. Rho activation was elicited in a dose-and time-dependent manner (Fig. 1A and B). We then tested this toxin on purified T lymphocytes. We observed the same upshift for T-lymphocyte cells after incubation with CNF-1 (3 · 10−9 M, for 4 h) (Fig. 1C).
FIG. 1.
Mobility shift of Rho in lymphoid cells upon treatment with CNF-1. (A) Jurkat T cells (107 cells/assay) treated or not treated for 4 or 24 h with different concentrations of CNF-1 (1, 10, 100, and 300 pM and 1, 1.5, and 3 nM) were lysed. Rho proteins were [32P]ADP ribosylated for 90 min with C3 exoenzyme. Deamidation of Rho was visualized by SDS-12% PAGE, followed by blotting and autoradiography as described in Materials and Methods. (B) Densitometric scanning of the deamidated Rho protein. (C) Upshift of Rho in T lymphocytes and Jurkat and HEp-2 cells treated or not treated for 4 h with 3 nM of CNF-1 was analyzed by [32P]ADP ribosylation.
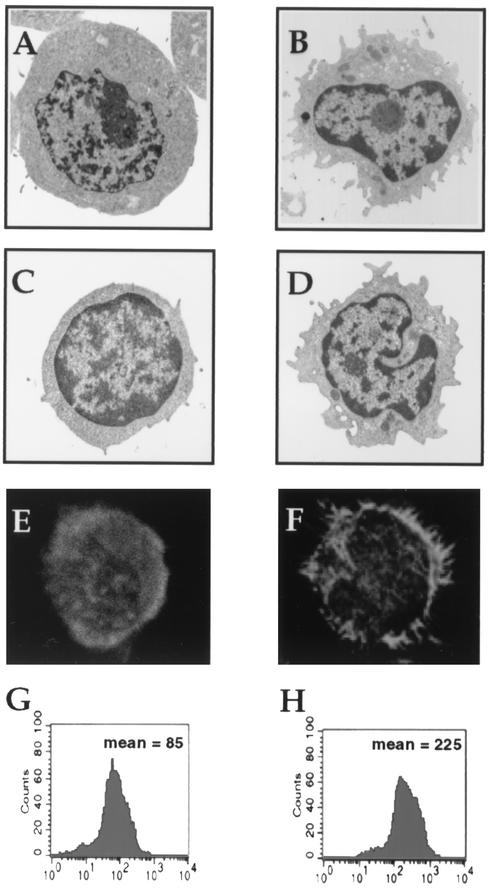
The morphology of control and CNF-1-treated T lymphocytes was assessed by electron microscopy. Changes in the shape of CNF-1 stimulated lymphocytes were detectable after 24 h of treatment. Cells exhibited cytoplasmic projections resembling pseudopodia or filopodia. These effects were detected both in peripheral blood T lymphocytes (Fig. 2A and B) and in Jurkat cells (Fig. 2C and D).
FIG. 2.
Morphological modifications and F-actin reorganization induced by CNF-1 (10−9 M, 24 h) in lymphoid cells. Transmission electron microscopic photographs of control (A) and CNF-1-treated (B) Jurkat T cells and of control (C) and CNF-1-treated (D) peripheral blood T lymphocytes (magnification, ×2,500). F-actin distribution stained with rhodamine-phalloidin (500 nM) in control (E) and CNF-1-treated (F) peripheral blood T lymphocytes were observed by confocal microscopy (magnification, ×2,000). Determination of lymphocyte F-actin by flow cytometry incubated with CNF-1 (H) exhibited a shift in the fluorescence peak, indicating polymerization of actin compared to control cells (G). One of five experiments is shown (each condition performed in triplicate). P < 0.05.
Owing to the fact that CNF-1 induces an important F-actin reorganization in various models, we tested the effect of this toxin (10−9 M for 24 h) on the T-lymphocyte actin cytoskeleton. The F-actin distribution was investigated by conventional fluorescence and by confocal microscopy after rhodamine-phalloidin staining. In most control cells, an evenly distributed subcortical actin band was consistently observed (Fig. 2E). In contrast, CNF-1 treatment caused the concentration of subcortical actin in broad membrane extension (Fig. 2F). As assessed by flow cytometry, the content of F-actin was increased in CNF-1-treated T lymphocytes (10−9 M for 24 h) in comparison to control T lymphocytes (225 ± 11 versus 85 ± 8, means ± SE, for treated cells versus control cells, respectively; P < 0.05) (Fig. 2H and G).
In Jurkat cells incubated 48 h with 10−9M CNF-1, formation of giant multinucleated cells was found. More than 4 nuclei per cell were observed in 80% ± 10% of the treated population whereas only 5% ± 3% of the control population had more than four nuclei per cell (P < 0.001; not shown).
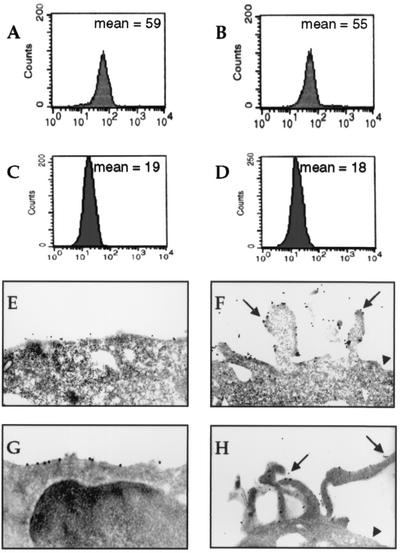
CNF-1 induces clustering of CD29 and CD11a integrins on human T lymphocytes.
Quantification performed by flow cytometry showed that exposure of T lymphocytes to CNF-1 for 24 h failed to modify expression of CD11a (Fig. 3A and B) and CD29 (Fig. 3C and D), as well as CD49d and CD103 (not shown). As shown by electron microscopy CD11a and CD29 preferentially clustered into filopodia in CNF-1-treated cells (Fig. 3F and H) while they were evenly distributed along the plasma membrane in control cells (Fig. 3E and G). Identical results were obtained for the CD49d molecule (data not shown).
FIG. 3.
Effect of CNF-1 on CD29 and CD11a expression. CNF-1 did not cause an increase in CD11a expression (B) or in CD29 expression (D) as assessed by flow cytometry, in comparison with control peripheral blood T lymphocytes (A and C). For CD11a and CD29 staining, numerous beads were regrouped on filopodia in CNF-1-treated cells (anti-CD11a MAb [F]; anti-CD29 MAb [H]) (arrows). No beads were observed in nonfilopodial plasma membrane (arrowheads). Beads were evenly distributed along the plasma membrane in control cells (anti-CD11a MAb [E]; anti-CD29 MAb [G]) (electron microscopy magnification, ×4,500).
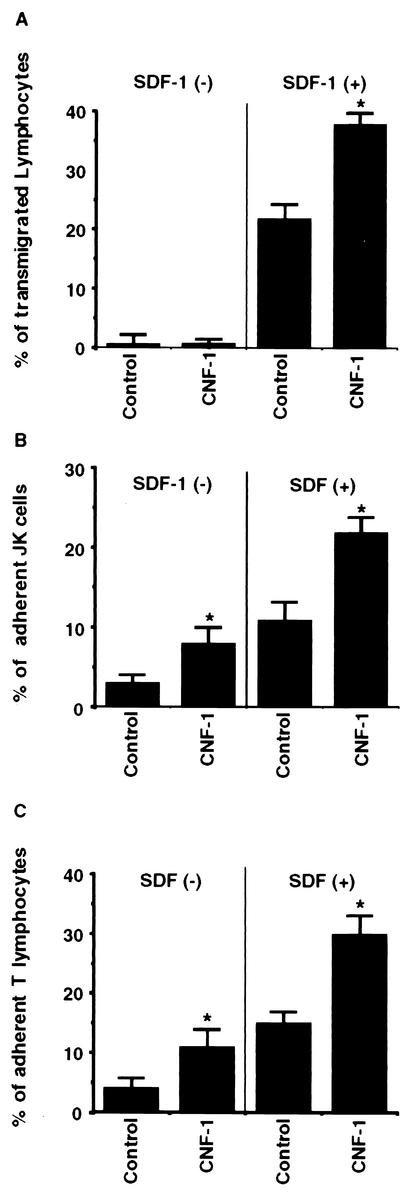
CNF-1 potentiates SDF-1α-induced transmigration of T lymphocytes across acellular filters and enhances the adherence of lymphoid cells to T84 monolayers.
As shown in Fig. 4A, pretreatment of T lymphocytes with CNF-1 enhanced SDF-1α induced migration across acellular filters compared with untreated T lymphocytes.
FIG. 4.
(A) Effect of CNF-1 on T lymphocytes migration across acellular filters. T lymphocytes present in the lower reservoirs were determined after 6 h of transmigration with or without SDF-1α as described in Materials and Methods. Data are pooled from 6 to 12 individual monolayers for each condition and results are means + SE (error bars) of five experiments (*, P < 0.05). Also shown is the effect of CNF-1 on Jurkat T cells (B) or T-lymphocyte (C) adherence to T84 monolayers. Lymphoid cells associated with monolayers were counted after 6 h of transmigration (basolateral-to-apical direction) in the absence or the presence of SDF-1α as described in Materials and Methods; data are pooled from 6 to 12 individual monolayers for each condition, and results are means + SE (error bars) of five different experiments (*, P < 0.05).
Incubation of Jurkat cells with 10−9M CNF-1 for 24 h in the absence of SDF-1α increased four times the number of adherent cells associated with the colonic epithelial cells ([8 ± 1.21] × 104 versus [2 ± 0.9] × 104 cell equivalent, means ± SE, for CNF-1-treated and control cells, respectively; P < 0.05) (Fig. 4B). In parallel, a pretreatment of Jurkat cells with CNF-1 (10−9 M for 24 h) increased twice their adherence to epithelial cells in response to SDF-1α ([32 ± 1.9] × 104 versus [15 ± 1.2] × 104 cell equivalent, means ± SE, for CNF-1-treated versus control cells, respectively; P < 0.05) (Fig. 4B). We found that CNF-1 enhanced the adherence of T lymphocytes to epithelial cells both in the absence or in the presence of SDF-1α (Fig. 4C).
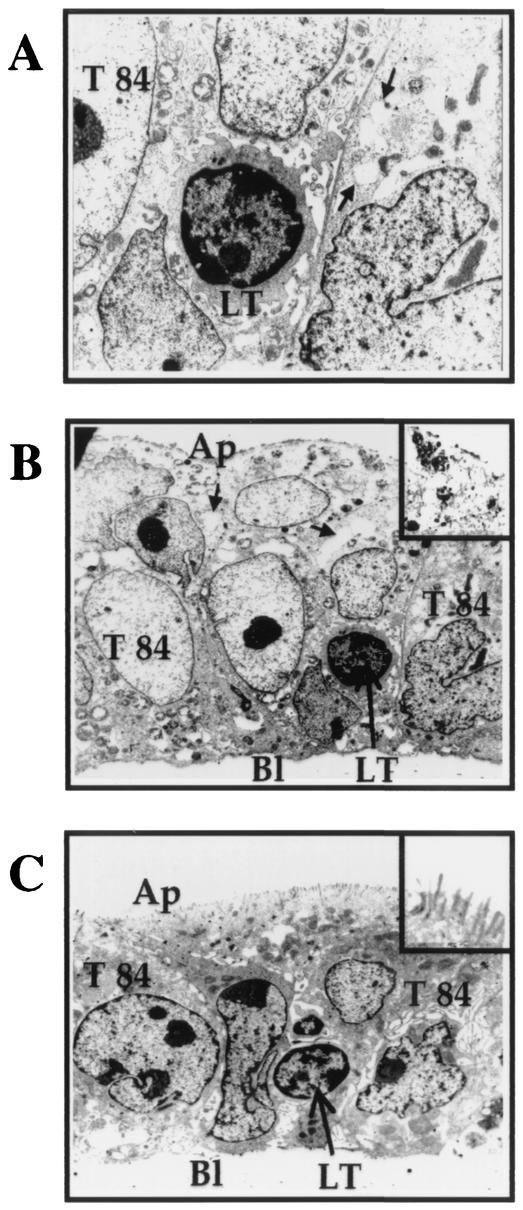
Analysis of epithelial cells following contact (24 h) with CNF-1-treated lymphocytes by electron microscopy revealed a cytoplasmic vacuolation of T84 cells and a profound alteration of the brush border (Fig. 5A and B) and tight junction disruptions (not shown). By contrast, T84 cells cocultured with untreated T lymphocytes showed that the majority of epithelial cells exhibited a regular brush border without disorganization of the monolayers after 24 h (Fig. 5C).
FIG. 5.
Electron micrographs showing CNF-1-induced-epithelial damages in T84 monolayers cocultured for 24 h with CNF1-treated T lymphocytes. CNF1-treated lymphocytes provoked in T84 epithelial cells intracytoplasmic vacuolization (A) (arrows) (magnification, ×2,500), and loss of microvilli (B) (magnification, ×1,800 [magnification for inset, ×2,800]). Contact (24 h) with control T lymphocyte did not affect the morphological features of the T84 monolayers (C) (magnification, ×1,800) and the brush border (inset [magnification, ×2,800]) Abbreviations: Ap, apical side; Bl, basolateral side; LT, lymphocyte.
CNF-1 induces p42-44MAPk activation in T lymphocyte and such activation is required for the increased adherence of T lymphocyte.
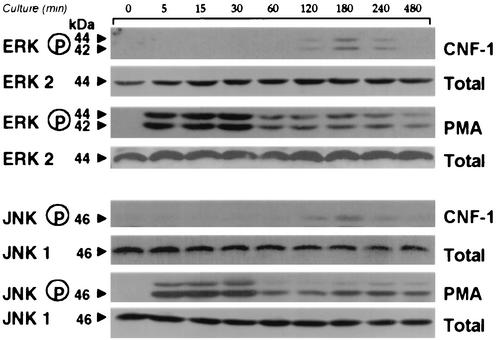
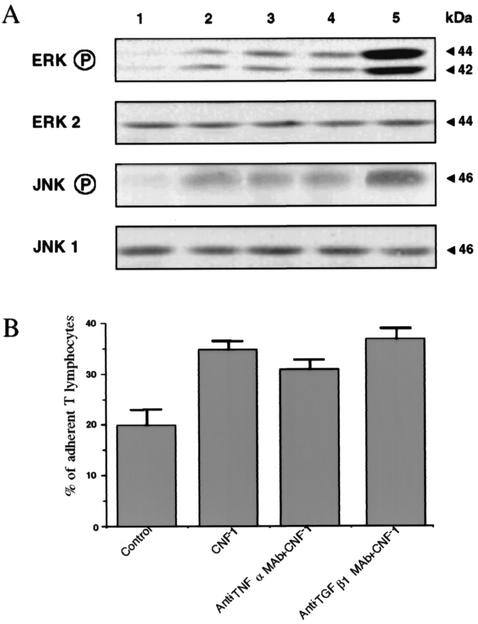
The effect of CNF-1 on the different MAP kinase pathways in T lymphocytes was assessed by the use of phosphospecific MAP kinase antibodies. Phosphorylation of p42-44MAPk started after 2 h of incubation with CNF-1, reached a maximal at 3 h and decreased by 4 h (Fig. 6). CNF-1 also induced JNK activation in a comparable time-course (Fig. 6). PMA-mediated activation of p42-44MAPk and JNK via the PKC pathway was used as a positive control (Fig. 6). The extent of JNK and p42-44MAPk phosphorylation was weaker in CNF-1-treated cells than in PMA-treated cells. No activation was observed in control T lymphocytes (data not shown).
FIG. 6.
Time course of p42-44MAPk and Jun kinase activation in T lymphocytes after incubation with CNF-1. CNF-1 effect was compared to the effect of PMA. Lysates from untreated (lane 1) or CNF-1-treated (1 nM) cells treated for different times or PMA (5 ng/ml)-treated cells were prepared and blotted with phosphospecific MAPK antibodies. Blots were stripped and reprobed for total ERK2 and JNK1.
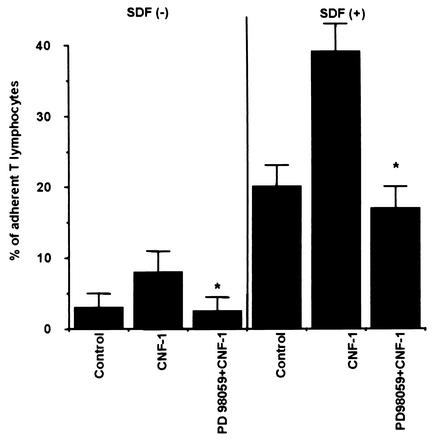
As shown on Fig. 7, pretreatment of T lymphocytes with the MEK inhibitor PD 98059 markedly reduced the increase in adherence of CNF-1-treated T lymphocytes to T84 epithelial cells (Fig. 7). Both spontaneous adherence of CNF-1-treated T lymphocytes to T84 cells and adherence of CNF-1-treated-T lymphocytes to T84 cells induced in response to SDF-1α were decreased following pretreatment of T lymphocytes with PD 98059 (Fig. 7).
FIG. 7.
Effect of the MEK inhibitor PD 98059 on the adherence of CNF-1-treated T lymphocytes to T84 monolayers. T lymphocytes adhering to the T84 monolayers were counted as described in the Material and Methods after 6 h of transmigration (basolateral-to-apical direction) in the absence or the presence of SDF-1α. In some experiments, T lymphocytes were preincubated with the MEK inhibitor PD 98059 and then treated with CNF-1 before transmigration. Data are pooled from 6 to 12 individual monolayers for each condition, and results are means + SE (error bars) of five experiments (*, P < 0.05).
CNF-1 induced production of TNF-α and TGF-β by T lymphocytes.
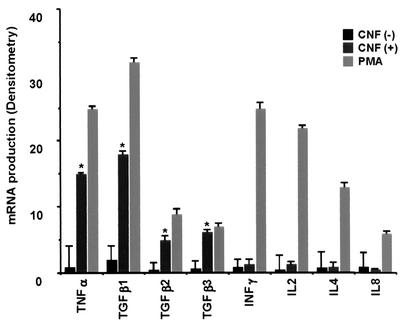
Freshly isolated T lymphocytes constitutively produced low levels of cytokines (Table 1 and Fig. 8). CNF-1 treatment of T lymphocytes induced the production of high levels of TGF-β1, TGF-β2, TGF-β3, and TNF-α proteins (Table 1). TGF-β1, TGF-β2, TGF-β3, and TNF-α mRNA were increased in T lymphocytes following CNF-1 treatment (Fig. 8). Incubation of T lymphocytes with CNF-1 had no effect on IL-2, IL-4, IL-8, and IFN-γ mRNA expression and cytokine production (Table 1 and Fig. 8). The results indicated in Table 1 are means ± SE of three different experiments.
TABLE 1.
Cytokine secretion by T lymphocytes following incubation with CNF-1 (24 h, 1 nM)
| Cytokines | Mean cytokine secretion ± SE (ng/ml)a
|
|
|---|---|---|
| CNF-1 treated | CNF-1 untreated | |
| TNF-α | 2.65* ± 0.41 | 0.67 ± 0.12 |
| TGF-β1 | 3.89* ± 0.33 | 0.5 ± 0.11 |
| TGF-β2 | 1.2* ± 0.30 | 0.1 ± 0.07 |
| TGF-β3 | 1.1* ± 0.27 | 0.2 ± 0.08 |
| IFN-γ | 0.3 ± 0.1 | 0.22 ± 0.1 |
| IL-2 | 0.4 ± 0.1 | 0.5 ± 0.12 |
| IL-4 | 0.2 ± 0.2 | 0.3 ± 0.07 |
| IL-8 | 0.3 ± 0.1 | 0.1 ± 0.03 |
Results are based on three different experiments. *, P < 0.01.
FIG. 8.
Expression of IL-2, IL-4, IL-8, IFN-γ, TNF-α, TGF-β1 TGF-β2, and TGF-β3 mRNA in CNF-1-treated peripheral T lymphocytes (24 h, 1 nM). RNA expression was analyzed by RPA as described in Materials and Methods. *, P < 0.01.
p42-44MAPk activation in CNF-1-treated T lymphocytes (for 3 h, 1 nM) was analyzed by Western blotting after preincubation with TNF-α or TGF-β1 MAbs for 1 h. As shown in Fig. 9A, p42-44MAPk activation in CNF-1-treated T lymphocytes was similar in control cells and in preincubated cells with TNF-α or TGF-β1 MAbs. Similar results were observed for JNK activation in CNF-1-treated T lymphocytes preincubated with TNF-α or TGF-β1 MAbs (Fig. 9A).
FIG. 9.
(A) p42-44MAPk and JNK activation in CNF-1-treated T lymphocytes are linked neither to TNF-α nor to TGF-β1 production. p42-44MAPk and JNK activation in CNF-1-treated T lymphocytes was analyzed by Western blotting in cells preincubated with anti-TNF-α (lane 3) or anti-TGF-β1 (lane 4) MAbs. T lymphocytes were preincubated for 1 h at 37°C with either anti-TNF-α or anti-TGF-β1 antibodies before addition of 1 nM CNF-1 for 3 h. Lane 1, untreated cells; lane 2, CNF-1-treated cells (3 h, 1 nM); lane 5, PMA-treated cells. (B) Increased adherence of CNF-1-treated T lymphocytes to T84 monolayers is not modified in T lymphocytes pretreated with anti-TNF-α or with anti-TGF-β1 MAbs. T lymphocytes associated with monolayers were counted after 6 h of transmigration (basolateral-to-apical direction) induced by SDF-1α as described in Materials and Methods. Data are pooled from 6 to 12 individual monolayers for each condition, and results are means + SE (error bars) of five different experiments.
Increased adherence of CNF-1-treated T lymphocytes to T84 monolayers in the presence of SDF-1α was not modified in CNF-1-treated T lymphocytes preincubated with anti-TNF-α or with anti-TGF-β1 MAbs ([35 ± 1.5] × 104 versus [31 ± 1.8] × 104 versus [37 ± 2] × 104 cell equivalent, means ± SE, for CNF-1-treated T lymphocytes versus CNF-1-treated T lymphocytes preincubated with anti-TNF-α MAb versus CNF-1-treated T lymphocytes preincubated with anti-TGF-β1 MAb, respectively) (Fig. 9B).
DISCUSSION
In this report, we demonstrate that T lymphocytes are sensitive to CNF-1. The toxin has been shown to catalyze deamidation of the GTP-binding Rho protein, thus inducing an hyperpolymerization of the actin cytoskeleton. Following prolonged incubation with CNF-1, a significant number of Jurkat T cells appeared multinucleated. This a likely due to an alteration in cytokinesis consecutive to the actin cytoskeleton remodeling. This is the first report regarding an effect of CNF-1 on lymphoid cells. In fact, most of the above-mentioned effect of the toxin have been observed with other cell types such as epithelial, endothelial, monocytic and granulocytic cells (7, 18, 27, 47).
Furthermore, we showed that SDF-1α induced-migration of CNF-1-treated T lymphocytes across acellular filters was increased compared to untreated T lymphocytes. Laudanna et al. have reported that Rho is involved in the signaling of chemoattractant receptors to trigger adhesion of leukocytes (32). Moreover, Rho GTP-binding proteins play a crucial role in coupling G protein-linked chemoattractant receptors to integrin-mediated adhesion in leukocytes. Here we demonstrate that adherence of CNF-1-treated T lymphocytes (Jurkat cells and peripheral blood T lymphocytes) to the basolateral pole of intestinal epithelial cells was increased via the Rho GTPases activation. Taken together, these data support the hypothesis that, in vivo, CNF-1 could facilitate the migration of T lymphocytes toward the subepithelial space.
The effect of CNF-1 on the increased adherence of T lymphocytes may be due to (i) Rho activation and a subsequent redistribution at the cell surface of adhesion molecules such as CD11a, CD29, and CD49d, and (ii) p42-44MAPk activation. Previous studies have shown that the effects of Rho are mediated by the downstream kinases, Dial and the Rho-dependent kinase ROCK, probably by increasing myosin contractility, leading to stress fiber bundling and focal adhesion formation (46). In parallel, several studies have clearly demonstrated the involvement of p42-44MAPk in adhesion-mediated signaling (41-43). Fincham et al. have recently shown that active p42-44MAPk was present in cellular adhesion sites (17). In the present study, we report that p42-44MAPk was activated in CNF-1-treated T lymphocytes. Moreover, we could demonstrate that this activation was crucial for the enhanced adherence of T lymphocytes to epithelial cells, since the MEK inhibitor PD98059 prevented the toxin-induced increase adherence.
After their recruitment from the circulation, T cells are present into lamina propria or are intimately associated with epithelial surfaces (2, 37, 45). The latter cells, namely, intraepithelial lymphocytes, constitute a distinct population of T cells that may arise from both thymus-dependent and independent pathways (23, 31, 35, 37). The role of T lymphocytes during the onset of bacterial infection has been poorly investigated. However, it has been demonstrated that T lymphocytes can be activated during the first 24 h of bacterial infection (38, 39). During their activation, T lymphocytes can undergo extensive divisions and acquire effector functions (38). Furthermore, when stimulated, intestinal mucosal T lymphocytes produce various inflammatory cytokines which can elicit different effects on intestinal functions (10, 11, 39). Interestingly, these cytokines can act on PMNL during the acute phase of inflammation induced by bacteria. In this line, IFN-γ was shown to modulate the PMNL migration across T84 cells (10). Other cytokines produced by stimulated T lymphocyte such as TGF-β can induce degranulation and oxidant release by adherent human PMNL (1). We provide here evidence that production of TGF-β1, TGF-β2, TGF-β3, and TNF-α were significantly increased upon treatment of T lymphocytes with CNF-1. The toxin-induced increase of TGF-β1 is of particular interest because this cytokine is a key player in inflammatory and immune responses, including PMNL recruitment, adhesion, and increased matrix metalloproteinase secretion and activation (34). The effect on TNF-α production is also noteworthy, because this cytokine acts as a priming agent for PMNL increasing their adherence, their degranulation and oxidative responses (49).
In conclusion, we propose that during acute colitis due to certain E. coli strains, CNF-1 toxin could act on T lymphocytes by increasing their adherence to the intestinal epithelial cells. Moreover, by enhancing the production of TNF-α and TGF-β by T lymphocytes, CNF-1 may in turn amplify the inflammatory consequences of PMNL transepithelial migration in response to the bacterial aggression.
Acknowledgments
We thank Mireille Mari, Dominique Sadoulet, and Bernard Ferrua for their excellent technical assistance.
Editor: J. D. Clements
REFERENCES
- 1.Balazovich, K. J., R. Fernandez, V. Hinkovska-Galcheva, S. J. Suchard, and L. A. Boxer. 1996. Transforming growth factor-beta1 stimulates degranulation and oxidant release by adherent human neutrophils. J. Leukoc. Biol. 60:772-777. [DOI] [PubMed] [Google Scholar]
- 2.Beagley, K. W., and A. J. Husband. 1998. Intraepithelial lymphocytes: origins, distribution, and function. Crit. Rev. Immunol. 18:237-254. [DOI] [PubMed] [Google Scholar]
- 3.Boquet, P. 1998. Cytotoxic necrotizing factor 1 from Escherichia coli: a toxin with a new intracellular activity for eukaryotic cells. Folia Micobiol. 43:285-289. [DOI] [PubMed] [Google Scholar]
- 4.Boquet, P., P. Munro, C. Fiorentini, and I. Just. 1998. Toxins from anaerobic bacteria: specificity and molecular mechanisms of action. Curr. Opin. Microbiol. 1:66-74. [DOI] [PubMed] [Google Scholar]
- 5.Boquet, P., G. Tran Van Nieugh, and P. Sansonetti. 1999. Cell regulation and Rho GTP-binding proteins?, p. 183-199. In P. Jeanteur (ed.). Small Rho GTPases and microbial pathogenicity. Springer-Verlag, Berlin, Germany.
- 6.Campbell, J. J., and E. C. Butcher. 2000. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 12:336-341. [DOI] [PubMed] [Google Scholar]
- 7.Capo, C., S. Meconi, M. V. Sanguedolce, N. Bardin, G. Flatau, P. Boquet, and J. L. Mege. 1998. Effect of cytotoxic necrotizing factor-1 on actin cytoskeleton in human monocytes: role in the regulation of integrin-dependent phagocytosis. J. Immunol. 161:4301-4308. [PubMed] [Google Scholar]
- 8.Caprioli, A., V. Falbo, L. G. Roda, F. M. Ruggeri, and C. Zona. 1983. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological cell alterations. Infect. Immun. 39:1300-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chardin, P., P. Boquet, P. Madaule, M. R. Popoff, E. J. Rubin, and D. M. Gill. 1989. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 8:1087-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colgan, S. P., C. A. Parkos, C. Delp, M. A. Arnaout, and J. L. Madara. 1993. Neutrophil migration across cultured intestinal epithelial monolayers is modulated by epithelial exposure to IFN-gamma in a highly polarized fashion. J. Cell Biol. 120:785-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colgan, S. P., M. B. Resnick, C. A. Parkos, C. Delp-Archer, D. McGuirk, A. E. Bacarra, P. F. Weller, and J. L. Madara. 1994. IL-4 directly modulates function of a model human intestinal epithelium. J. Immunol. 153:2122-2129. [PubMed] [Google Scholar]
- 12.Contamin, S., A. Galmiche, A. Doye, G. Flatau, A. Benmerah, and P. Boquet. 2000. The p21 Rho-activating toxin cytotoxic necrotizing factor 1 is endocytosed by a clathrin-independent mechanism and enters the cytosol by an acidic-dependent membrane translocation step. Mol. Biol. Cell 11:1775-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Pozo, M. A., M. Vicente-Manzanares, R. Tejedor, J. M. Serrador, and F. Sanchez-Madrid. 1999. Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur. J. Immunol. 29:3609-3620. [DOI] [PubMed] [Google Scholar]
- 14.D'Souza-Schorey, C., B. Boettner, and L. Van Aelst. 1998. Rac regulates integrin-mediated spreading and increased adhesion of T lymphocytes. Mol. Cell. Biol. 18:3936-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falzano, L., C. Fiorentini, G. Donelli, E. Michel, C. Kocks, P. Cossart, L. Cabanie, E. Oswald, and P. Boquet. 1993. Induction of phagocytic behaviour in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol. Microbiol. 9:1247-1254. [DOI] [PubMed] [Google Scholar]
- 16.Fenoglio-Preiser, C. M., P. E. Lantz, M. B. Liston, M. Davis, and F. O. Rilke. 1989. The non-neoplastic large intestine, p. 619-725. In C. M. Fenoglio (ed.), Gastrointestinal pathology. Atlas and text. Raven Press, New York, N.Y.
- 17.Fincham, V. J., M. James, M. C. Frame, and S. J. Winder. 2000. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 19:2911-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorentini, C., G. Arancia, A. Caprioli, V. Falbo, F. M. Ruggeri, and G. Donelli. 1988. Cytoskeletal changes induced in HEp-2 cells by the cytotoxic necrotizing factor of Escherichia coli. Toxicon 26:1047-1056. [DOI] [PubMed] [Google Scholar]
- 19.Fiorentini, C., G. Donelli, P. Matarrese, A. Fabbri, S. Paradisi, and P. Boquet. 1995. Escherichia coli cytotoxic necrotizing factor 1: evidence for induction of actin assembly by constitutive activation of the p21 Rho GTPase. Infect. Immun. 63:3936-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorentini, C., A. Fabbri, G. Flatau, G. Donelli, P. Matarrese, E. Lemichez, L. Falzano, and P. Boquet. 1997. Escherichia coli cytotoxic necrotizing factor 1 (CNF1), a toxin that activates the Rho GTPase. J. Biol. Chem. 272:19532-19537. [DOI] [PubMed] [Google Scholar]
- 21.Flatau, G., E. Lemichez, M. Gauthier, P. Chardin, S. Paris, C. Fiorentini, and P. Boquet. 1997. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387:729-733. [DOI] [PubMed] [Google Scholar]
- 22.Galan, J. E., and J. B. Bliska. 1996. Cross-talk between bacterial pathogens and their host cells. Annu. Rev. Cell. Dev. Biol. 12:221-255. [DOI] [PubMed] [Google Scholar]
- 23.Guy-Grand, D., and P. Vassalli. 1993. Gut intraepithelial T lymphocytes. Curr. Opin. Immunol. 5:247-252. [DOI] [PubMed] [Google Scholar]
- 24.Haines, D. S., and D. H. Gillespie. 1992. RNA abundance measured by a lysate RNase protection assay. BioTechniques 12:736-741. [PubMed] [Google Scholar]
- 25.Hofman, P., L. D'Andrea, D. Carnes, S. P. Colgan, and J. L. Madara. 1996. Intestinal epithelial cytoskeleton selectively constrains lumen-to-tissue migration of neutrophils. Am. J. Physiol. 271:C312-C320. [DOI] [PubMed] [Google Scholar]
- 26.Hofman, P., G. Flatau, E. Selva, M. Gauthier, G. Le Negrate, C. Fiorentini, B. Rossi, and P. Boquet. 1998. Escherichia coli cytotoxic necrotizing factor 1 effaces microvilli and decreases transmigration of polymorphonuclear leukocytes in intestinal T84 epithelial cell monolayers. Infect. Immun. 66:2494-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofman, P., G. Le Negrate, B. Mograbi, V. Hofman, P. Brest, A. Alliana-Schmid, G. Flatau, P. Boquet, and B. Rossi. 2000. Escherichia coli cytotoxic necrotizing factor-1 (CNF-1) increases the adherence to epithelia and the oxidative burst of human polymorphonuclear leukocytes but decreases bacteria phagocytosis. J. Leukoc. Biol. 68:522-528. [PubMed] [Google Scholar]
- 28.Hofman, P., M. Piche, D. F. Far, G. Le Negrate, E. Selva, L. Landraud, A. Alliana-Schmid, P. Boquet, and B. Rossi. 2000. Increased Escherichia coli phagocytosis in neutrophils that have transmigrated across a cultured intestinal epithelium. Infect. Immun. 68:449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iqbal, N., J. R. Oliver, F. H. Wagner, A. S. Lazenby, C. O. Elson, and C. T. Weaver. 2002. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J. Exp. Med. 195:71-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito, K., H. Takaishi, Y. Jin, F. Song, T. L. Denning, and P. B. Ernst. 2000. Staphylococcal enterotoxin B stimulates expansion of autoreactive T cells that induce apoptosis in intestinal epithelial cells: regulation of autoreactive responses by IL-10. J. Immunol. 164 :2994-3001. [DOI] [PubMed] [Google Scholar]
- 31.Kagnoff, M. F. 1987. Immunology of the digestive system, p. 1699-1728. In L. R. Johnson (ed.), Physiology of the gastrointestinal tract. Raven Press, New York, N.Y.
- 32.Laudanna, C., J. J. Campbell, and E. C. Butcher. 1996. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science 271:981-983. [DOI] [PubMed] [Google Scholar]
- 33.Le'Negrate, G., E. Selva, P. Auberger, B. Rossi, and P. Hofman. 2000. Sustained polymorphonuclear leukocyte transmigration induces apoptosis in T84 intestinal epithelial cells. J. Cell Biol. 150:1479-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Letterio, J. J., and A. B. Roberts. 1998. Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 16:137-161. [DOI] [PubMed] [Google Scholar]
- 35.Lundqvist, C., V. Baranov, S. Hammarstrom, L. Athlin, and M. L. Hammarstrom. 1995. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int. Immunol. 7:1473-1487. [DOI] [PubMed] [Google Scholar]
- 36.Madara, J. L., S. Colgan, A. Nusrat, C. Delp, and C. Parkos. 1992. A simple approach to measurement of electrical parameters of cultured epithelial monolayers: use in assessing neutrophil epithelial monolayers transmigration. J. Tissue Culture Methods 14:209-216. [Google Scholar]
- 37.Matsuzaki, G., T. Lin, and K. Nomoto. 1994. Differentiation and function of intestinal intraepithelial lymphocytes. Int. Rev. Immunol. 11:47-60. [DOI] [PubMed] [Google Scholar]
- 38.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165:6833-6839. [DOI] [PubMed] [Google Scholar]
- 39.Michetti, P. 2000. Intestinal pathogen or IBD, same T-cell response. Inflamm. Bowel Dis. 6:63-64. [DOI] [PubMed] [Google Scholar]
- 40.Miltenyi, S., W. Muller, W. Weichel, and A. Radbruch. 1990. High gradient magnetic cell separation with MACS. Cytometry 11:231-238. [DOI] [PubMed] [Google Scholar]
- 41.Miyamoto, S., H. Teramoto, J. S. Gutkind, and K. M. Yamada. 1996. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 135:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morino, N., T. Mimura, K. Hamasaki, K. Tobe, K. Ueki, K. Kikuchi, K. Takehara, T. Kadowaki, Y. Yazaki, and Y. Nojima. 1995. Matrix/integrin interaction activates the mitogen-activated protein kinase, p44erk-1 and p42erk-2. J. Biol. Chem. 270:269-273. [DOI] [PubMed] [Google Scholar]
- 43.Renshaw, M. W., D. Toksoz, and M. A. Schwartz. 1996. Involvement of the small GTPase rho in integrin-mediated activation of mitogen-activated protein kinase. J. Biol. Chem. 271:21691-21694. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt, G., P. Sehr, M. Wilm, J. Selzer, M. Mann, and K. Aktories. 1997. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387:725-729. [DOI] [PubMed] [Google Scholar]
- 45.Shibahara, T., M. Si-Tahar, S. K. Shaw, and J. L. Madara. 2000. Adhesion molecules expressed on homing lymphocytes in model intestinal epithelia. Gastroenterology 118:289-298. [DOI] [PubMed] [Google Scholar]
- 46.Singh, B., S. Read, C. Asseman, V. Malmstrom, C. Mottet, L. A. Stephens, R. Stepankova, H. Tlaskavola, and F. Powrie. 2001. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182:190-200. [DOI] [PubMed] [Google Scholar]
- 47.Vouret-Craviari, V., P. Boquet, J. Pouyssegur, and E. Van Obberghen-Schilling. 1998. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol. Biol. Cell. 9:2639-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe, N., T. Kato, A. Fujita, T. Ishizaki, and S. Narumiya. 1999. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell. Biol. 1:136-143. [DOI] [PubMed] [Google Scholar]
- 49.Witko-Sarsat, V., P. Rieu, B. Descamps-Latscha, P. Lesavre, and L. Halbwachs-Mecarelli. 2000. Neutrophils: molecules, functions and pathophysiological aspects. Lab. Investig. 80:617-653. [DOI] [PubMed] [Google Scholar]
- 50.Yardley, J. H. 1986. Pathology of idiopathic inflammatory bowel disease and relevance of specific cell findings: an overview, p. 3-9. In Recent developments in the therapy of inflammatory bowel disease. Proceedings of a Symposium. Myerhoff Center for Digestive Disease at Johns Hopkins, Baltimore, Md.