Abstract
Afa/Dr diffusely adhering Escherichia coli (Afa/Dr DAEC) strains cause symptomatic urinary tract and intestinal infections. The proinflammatory effects of Afa/Dr DAEC strains in vitro have been not investigated to date. In the present study, we used confluent polarized monolayers of intestinal cell line T84 to evaluate the consequences of epithelial infection by Afa/Dr DAEC strains in terms of proinflammatory response. Polymorphonuclear leukocyte (PMNL) migration across the epithelial barrier was induced after incubation of the T84 monolayers with the wild-type Afa/Dr DAEC strain C1845 harboring the fimbrial F1845 adhesin and strain IH11128 harboring the Dr hemagglutinin, and the E. coli laboratory strain HB101 was transformed with the pSSS1 plasmid, producing Afa/Dr F1845 adhesin. PMNL migrations were correlated with a basolateral secretion of interleukin-8 by T84 cells and were abolished after incubation of epithelial cells with an anti-decay accelerating factor (DAF) antibody that recognized the short consensus repeat 3 domain of DAF (monoclonal antibody 1H4). Moreover, Afa/Dr DAEC strains induced tyrosine phosphorylation of several T84 proteins and activated the mitogen-activated protein kinases (ERK1/2 mitogen-activated protein, P38, and Jun-C kinases). These data demonstrated for the first time that, in vitro, Afa/Dr DAEC strains exert a proinflammatory signal in intestinal epithelial cells.
Among the heterogeneous group of diffusely adhering Escherichia coli (DAEC) organisms, one family of E. coli expresses the related Afa/Dr adhesins (Afa/Dr DAEC) (32). These bacteria adhere to human intestinal epithelial cells by recognizing the brush-border associated decay-accelerating factor (DAF), CD55 (2, 13, 35). The binding of Afa/Dr DAEC strains is a result of the interaction between bacterial adhesin and the short consensus repeat 3 (SCR3) domain of DAF (31). Other possible virulence factors of the Afa/Dr DAEC family, apart from their adhesins, are largely unknown. As well as recognizing the DAF molecule, members of the Afa/Dr family of adhesins also recognize another membrane-associated glycosylphosphatidylinositol-anchored protein, the carcinoembryonic antigen or CD66e (13). The brush border attachment of Afa/Dr DAEC is followed by microvillus injury in fully differentiated Caco-2 cells (2). Brush border lesions result from dramatic rearrangements of apical cytoskeleton proteins such as F-actin, villin, and fimbrin, proteins that play a crucial role in the organization of brush border integrity (35). A change in the distribution of functional brush border-associated proteins controlling the absorption or secretion function has also been observed (35). Cytoskeleton rearrangements have been reported that resulted in a Ca2+-dependent signaling as a result of the activation of a DAF-associated signal transduction cascade (36). Moreover, lesions in tight junctions have been observed and shown to result from rearrangements in at least two tight-junction-associated proteins, ZO-1 protein and occludin (34). Finally, it has been shown recently that a uropathogenic Afa/Dr DAEC strain can enter into epithelial cells by recognizing α5β1 integrin (12). For some authors, the involvement of Afa/Dr DAEC strains in acute diarrhea is controversial (10, 38), but others have shown that these bacteria are significantly detected in some patients with diarrhea (1, 11, 14, 21, 22, 26, 37).
Most cases of bacterial colitis are characterized by the large number of polymorphonuclear leukocyte (PMNL) migrating across the columnar epithelium in response to inflammatory stimuli (9). To date, the proinflammatory responses of the colonic epithelium to Afa/Dr DAEC infection have not been investigated. We used the human intestinal epithelial cell line T84 to explore the inflammatory stimuli induced after the attachment of Afa/Dr DAEC strains to the colonic epithelium. More particularly, we investigated (i) whether Afa/Dr DAEC strains can trigger PMNL migration across polarized monolayers, (ii) whether basolateral secretion of cytokines occurs as a result of the Afa/Dr DAEC-T84 cell interaction, (iii) whether Afa/Dr DAEC strains can activate the tyrosine phosphorylation of T84 proteins and mitogen-activated protein (MAP) kinases, and (iv) whether signal transduction of this type may be involved in the induction of PMNL transmigration.
MATERIALS AND METHODS
Reagents and antibodies.
The phosphatase-conjugated goat anti-interleukin-8 (IL-8) polyclonal antibody was obtained from Sandoz Pharmaceutical (Rueil-Malmaison, France). The polyclonal anti-CD55 antibody and the monoclonal antibody (MAb) anti-CD55 SCR3 (1H4) were from D. M. Lublin (Washington University, St. Louis, Mo.). MAb anti-CD55 (F4-D29) was from Valbiotech (Paris, France), and the polyclonal anti-CD55 antibody (H-319) was from Santa Cruz Biotechnology (Santa Cruz, Calif.). Phospho-Tyr MAb (4G10; diluted 1/6,000) was from Upstate Biotechnologies Incorporation (Paris, France). Phospho-specific p44/p42 MAP kinase (catalog no. 9101; diluted 1/3,000), p38 MAP kinase (catalog no. 9211; diluted 1/1,000), and p54/p46 kinase (catalog no. 9251; diluted 1/2,000) polyclonal antibodies were obtained from New England Biolabs (Beverly, Mass.). Non-phospho-specific MAbs to ERK (K-23), p38 (A-12), and JNK (D-29) were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). MAP kinase inhibitors SB203580 and PD98059 (Calbiochem, La Jolla, Calif.) were dissolved in dimethyl sulfoxide (10 μM; Sigma, Paris, France).
Bacterial strains and growth conditions.
We used the wild-type Afa/Dr DAEC C1845 harboring fimbrial F1845 adhesin (3) and IH11128 harboring Dr hemagglutinin (33) and E. coli laboratory strain HB101 transformed with the pSSS1 plasmid, producing F1845 adhesin (3). E. coli laboratory strain K12-HB101 (gift of Patrice Boquet, INSERM 452, Nice) was used as the negative control. The strains were grown for 18 h at 37°C on complete Freund adjuvant agar containing 1% Casamino Acids (Difco laboratories, Detroit, Mich.), 0.15% yeast extract, 0.005% magnesium sulfate, and 0.0005% manganese chloride in 2% agar. E. coli laboratory strain HB101 was grown at 37°C for 18 h on Luria agar.
Cell culture and electrophysiological studies.
T84 cells (American Type Culture Collection, passages 65 to 90), a human colonic carcinoma cell line, were grown and maintained as confluent monolayers on collagen-coated permeable supports. Monolayers were grown on 0.33-cm2 ring-supported polycarbonate filters (Costar, Cambridge, Mass.) and utilized 6 to 14 days after plating. Confluent monolayers on permeable supports were constructed to permit a basolateral-to-apical migration of PMNL (“inverted inserts”) as previously described (17, 28). We used the following system to assess currents, transepithelial potentials, and resistances: a commercial voltage clamp apparatus (Bioengineering Department, University of Iowa) interfaced with an equilibrated pair of calomel electrodes immersed saturated KCl and with a pair of Ag-AgCl electrodes immersed in Hanks balanced salt solution (HBSS). Agar bridges were then used to interface the electrodes with the solutions on either side of the T84 monolayers (one calomel and one Ag-AgCl electrode in each well) (17, 28). The spontaneous transepithelial electrical potential and the instantaneous potential generated by passing 25 μA of current were measured on inverted monolayers before and after incubating for 4 h with bacteria.
PMNL preparation.
Human PMNL were isolated from whole blood by using a gelatin-sedimentation technique (28). Briefly, whole blood anticoagulated with citrate-dextrose was centrifuged at 300 × g for 20 min (20°C). The plasma and buffy coat were removed and the gelatin-cell mixture was incubated at 37°C for 30 min to remove contaminating red blood cells. Residual red blood cells were then lysed with isotonic ammonium chloride. After a wash in HBSS free from Ca2+ or Mg2+, the cells were counted and resuspended at 5 × 107 PMNL/ml. PMNL (95% pure) with 98% viability by trypan blue exclusion were used within 1 h after isolation.
PMNL transmigration assays.
The physiologically (basolateral-to-apical) directed PMNL transepithelial migration assay has been described elsewhere (25, 28). PMNL transmigration experiments were performed at 37°C on 0.33-cm2 inverts. T84 monolayers were washed three times in warm HBSS, and ca. 5 × 107 CFU of the different strains of E. coli used in the present study in 100 μl were gently placed on the apical surface and incubated for 4 h at 37°C. Nonadherent bacteria were removed from the monolayers by extensive washing and were then transferred back into the 24-well tissues culture tray containing 1.0 ml of HBSS in each lower reservoir (apical membrane was now colonized with E. coli) and 100 μl of HBSS in the upper reservoir (basolateral interface). We then added 106 PMNL to the upper reservoirs. Control transmigration of PMNL was initiated by the addition of formyl-methionine-leucin-phenylalanine (fMLP) (10−7 M) to the lower reservoir and incubation for 15 min to allow a transepithelial chemotactic gradient to form before the addition of PMNL. In some PMNL transmigration experiments, T84 monolayers were incubated with several concentrations of C1845 bacteria (intestinal epithelial monolayers were colonized with 5, 10, 50, or 100 cell-associated C1845 bacteria/epithelial cell) or after incubation with C1845 bacteria for various times (30, 60, or 120 min). Some transmigration experiments were also conducted after incubation of T84 with the MAb 1H4 (dilution, 1/40). Transmigration of PMNL was assayed by measuring the azurophil granule marker myeloperoxidase as described previously (28). As standards we used serial dilutions of the same PMNL used in the experiments. Myeloperoxidase was dissolved in the same way as for the lower reservoirs (in this way, the assay was linear over the range of 0.3 × 104 to 50 × 104 cells/ml). In some experiments the T84 cells were treated before PMNL transmigration by exposure to the specific p38 MAP kinase inhibitor SB203580 (10 μM; Calbiochem, La Jolla, Calif.) for 30 min before being exposed to Afa/Dr DAEC strains and during the incubation period of the experiment. A similar protocol was used for experiments with the ERK1/2 MAP kinase inhibitor PD98059 (25 μM; Calbiochem). Before PMNL transmigration, measurements of transepithelial resistance in T84 cell monolayers after preincubation with Afa/Dr DAEC or after exposure to MAP kinase inhibitors showed no effects during the time course of incubation compared to controls (data not shown).
Electron microscopy study.
Inverted T84 monolayers were rinsed thoroughly in HBSS. Approximately 5 × 107 CFU of the different strains of E. coli strains used in the present study in a volume of 100 μl were gently placed on the apical surface and incubated for 4 h at 37°C and at pH 7.4. In some experiments, the bacterial incubation was performed after preincubation of the T84 monolayers with MAb 1H4 antibody. Nonadherent bacteria were removed from the monolayers by extensive washing, and the cells were then transferred back into the 24-well tissue culture containing HBSS. After being removed from the inserts, the T84 monolayers were fixed with freshly prepared 2% paraformaldehyde in 0.1 M Na cacodylate (pH 7.4) for 1 h at 4°C. Monolayers were rinsed in cacodylate buffer, postfixed in 1% OsO4 for 1 h, dehydrated through graded alcohols, and embedded in epoxy resin. Oriented 1-mm sections were obtained with diamond knives, and multiple areas were thin sectioned. Ultrathin sections were examined on a JEOL 1200 EXII electron microscope. The numbers of adherent bacteria seen per 50 epithelial cells were counted in random sections.
IL-8 production.
In order to characterize the IL-8 secretion in the lower reservoirs after 3 h of epithelial cell infection by the different strains used in the present study, the lower reservoirs (1 ml each) were assayed in triplicate for this cytokine by enzyme-linked immunosorbent assay. The enzyme-linked immunosorbent assay was carried out by using a mouse MAb to IL-8 and a phosphatase-conjugated goat anti-IL-8 polyclonal antibody. The minimum detectable level of IL-8 was typically less than 10 pg/ml.
Preparation of cell lysates for Western blotting.
T84 cells were seeded in 100-mm tissue culture petri dishes At 80 to 90% confluence, the monolayers were washed twice with serum-free Dulbecco modified Eagle medium-F-12 and then grown in fresh culture medium supplemented with 0.1% bovine serum albumin for 12 h. Infection was carried out by adding a late-logarithmic-phase bacteria culture (100 bacteria/cell) alone or after preincubation with a polyclonal antibody. At the times indicated, the infected cells were washed with phosphate-buffered saline and solubilized for 30 min at 4°C in 1 ml of lysis buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1% Nonidet P-40; 2 mM phenylmethylsulfonyl fluoride [PMSF]; 1 mM EDTA; 1 mM aprotinin; 25 mM leupeptin; 1 mM pepstatin; 1 mM AEBF; 10 mM NaF; 5 mM sodium PPi; 10 mM α-glycerophosphate); the lysate was then sonicated and centrifuged at 15,000 rpm for 15 min at 4°C. The protein content of the supernatant was determined by using Bio-Rad DC reagents. Immunoprecipitation and Western blotting were carried out as previously described (4).
Data analysis.
Resistance time courses were compared by two-factor analysis of variance. Myeloperoxidase assays were compared by Student t test. Values are expressed as the means ± the standard errors of the mean of “n” experiments.
RESULTS
Colonization of the T84-apical membrane by wild-type Afa/Dr DAEC strain C1845 harboring the fimbrial F1845 adhesin induces transepithelial migration.
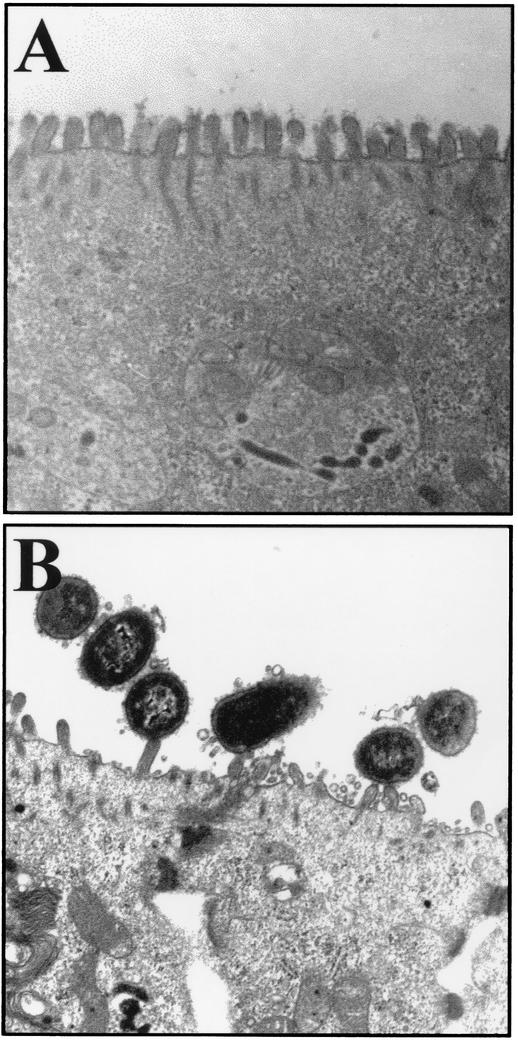
Apical membrane injury induced by wild-type Afa/Dr DAEC strains in Caco-2 cells is morphologically characterized by elongation and nucleation of the microvilli and rearrangement of the brush border-associated cytoskeletal proteins (2, 35). Here, we observed that, after incubation for 2 h, C1845 bacteria interacted with epithelial T84 cells, showing that the bacterial membrane was pressed close to the epithelial cell membrane, and the brush border disappeared at the apical surface (Fig. 1B). Similar results were obtained with IH11128 and HB101/pSSS1 bacteria (not shown), whereas no change in brush border architecture was observed with the nonpathogenic strain K12-HB101 of E. coli (Fig. 1A). Interestingly, C1845 bacteria did not adhere to the brush border after preincubation of T84 cells with MAb anti-DAF 1H4, and the microvilli in the presence of the MAb anti-DAF 1H4 (not shown) looked like the microvilli in Fig. 1A. By counting the number of bacteria adherent to the T84 monolayers (i.e., the number of adherent bacteria observed per 50 epithelial cells), we confirmed that numerous C1845, IH11128, and HB101/pSSS1 bacteria were adherent to the epithelial cells (221 ± 18 versus 245 ± 12 versus 191 ± 23 for C1845 versus IH11128 versus HB101/pSSS1), whereas fewer bacteria were observed on the brush border after preincubation of the epithelial cells with MAb 1H4 (23 ± 8 versus 15 ± 2 versus 20 ± 3 for C1845 versus IH11128 versus HB101/pSSS1, respectively; P < 0.01) than without MAb IH4. Under all of these conditions, T84 cells did not display any nuclear or cytoplasmic apoptotic features such as nuclear condensation or fragmentation or intracytoplasmic vacuoles.
FIG. 1.
Electron microscopy examination of Afa/Dr DAEC interaction with polarized T84 monolayers. (A) T84 cells infected with the nonadherent E. coli HB101 bacteria show a regular brush border. (B) Wild-type Afa/Dr DAEC C1845 bacteria are associated with the brush border and show a disappearance of the regular brush border. Bars, 0.5 μm.
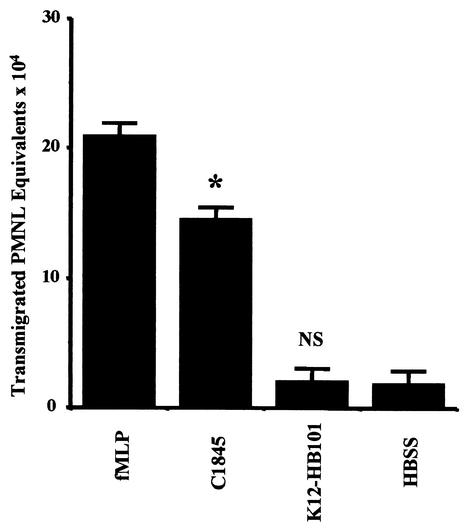
Before starting the transmigration assays, we confirmed that the transepithelial resistance of T84 cells incubated for 4 h with the E. coli strains used in the present study was not affected (data not shown). Apical colonization of T84 monolayers by C1845 bacteria modulates subsequent PMNL-epithelial cell interactions under conditions in which the physiological functions of the monolayers are preserved. As shown in Fig. 2, apical colonization of T84 cells with E. coli C1845 at a density of 100 bacteria/epithelial cell elicited marked the transepithelial migration of PMNL in the physiologic basolateral-to-apical direction, which was 78% of that induced by the PMNL transmigration-inducing peptide fMLP (10−7 M). In contrast, similar exposure to E. coli K12-HB101 failed to stimulate any comparable detectable transepithelial migration of PMNL. The PMNL transepithelial migration was dependent on the density of colonization. Indeed, cell-associated C1845 bacteria at densities of 5, 10, 50, and 100 bacteria/epithelial cell elicited progressive increases in the number of transmigrating PMNL (data not shown). PMNL migration was also dependent on the time course of colonization by the C1845 bacteria (data not shown).
FIG. 2.
Induction of physiologically directed transepithelial migration (basolateral to apical) of PMNL by apical colonization of T84 intestinal cells with wild-type Afa/Dr DAEC strain C1845. PMNL transepithelial migration in control cells was induced by the PMNL transmigrating inducing peptide fMLP (10−7 M). C1845 bacteria elicited >78% of the maximum transmigration response induced by fMLP, in contrast to the control HBSS or to E. coli HB101, a nonpathogen bacterium that induced no transepithelially directed chemotaxis of PMNL (✽, P < 0.005). Data represent the means and standard deviations of at least three experiments, each performed in triplicate.
Transepithelial migration of PMNL is induced similarly by the different Afa/Dr DAEC strains and is blocked by preincubating T84 with MAb anti-DAF (1H4).
As shown in Table 1, attachment of wild-type Afa/Dr DAEC C1845 and IH11128 bacteria to the apical membrane of T84 cells induced a basolateral-to-apical directed migration of PMNL. Attachment of recombinant E. coli pSSS1 harboring the Afa/Dr F1845 adhesin to the apical membrane of T84 cells induced a basolateral-to-apical directed migration of PMNL similar to that observed with the wild-type Afa/Dr DAEC strain C1845 harboring the F1845 adhesin. PMNL transmigrations induced by wild-type Afa/Dr DAEC strains C1845 and IH11128 and by recombinant HB101/pSSS1 E. coli, were, respectively 74, 72, and 56% of that which occurred in response to fMLP (10−7 M). To determine whether the number of PMNL that transmigrate across a monolayer is dependent upon recognition of DAF expressed at the apical membrane of T84 cells, by Afa/Dr DAEC adhesins, similar transmigration assays were conducted with T84 monolayers preincubated with an anti-DAF (IH4) antibody. As shown in Table 1, under these conditions the Afa/Dr DAEC strains used in the present study failed to induce transmigration of PMNL across the monolayers, as did the HBSS or K12-HB101 used as controls.
TABLE 1.
Crucial role of the Afa/Dr DAEC adhesins in induced PMNL transepithelial migration with apical colonization by Afa/Dr DAEC strainsa
| Strain | Mean transmigrated PMNL eq ± SE (104):
|
|
|---|---|---|
| Without MAb anti-CD55 (1H4) | With MAb anti-CD55 (1H4) | |
| Control (HBSS buffer) | 3.1 ± 1 | 2.2 ± 1 |
| fMLP (10−7 M) | 23.2 ± 1.3 | 21.2 ± 0.5 |
| Wild-type C1845 | 19.1 ± 2.1 | 3.5 ± 1.1* |
| Wild-type IH11128 | 16.5 ± 1 | 2.8 ± 1* |
| HB101/pSSS1 (F1845 adhesin) | 14.9 ± 0.5 | 3.4 ± 1.4* |
| HB101 | 2.9 ± 1.2 | 2.4 ± 1 |
A positive control was performed with the PMNL transmigrating inducing peptide fMLP at 10−7 M. All wild-type Afa/Dr DAEC strains and recombinant HB101/pSSS1 harboring Afa/Dr DAEC F1845 adhesin elicited a transepithelial migration of PMNL (not significantly different between PMNL transepithelial migration induced by fMLP versus wild-type Afa/Dr DAEC strains harboring the fimbrial F1845 and Dr hemagglutinin and recombinant E. coli HB101/pSSS1 expressing F1845 adhesin). PMNL transepithelial migrations induced by wild-type Afa/Dr DAEC C1845 and IH11128 strains or HB101/pSSS1 E. coli are inhibited by intestinal epithelial cells preincubated with MAb anti-CD55 (1H4) (∗, <0.01) versus infection without MAb CD55. Data are pooled from 6 to 12 individual monolayers for each condition, and the results are means ± standard errors.
IL-8 secretion. We next showed that wild-type C1845 and IH11128 Afa/Dr DAEC strains and recombinant HB101/pSSS1 E. coli induced T84 cell-polarized monolayers to secrete more IL-8 than control T84 cells (3.5 ± 1 versus 2.67 ± 1.2 versus 3.8 ± 1.5 versus 0.15 ± 0.11 versus 0.1 ± 0.2 ng/ml secreted into the basolateral compartment for strain C1845 versus strain HB101/pSSS1 versus strain IH11128 versus strain K12-HB101 versus control T84 cells, respectively; P < 0.01). IL-8 secretions induced by Afa/Dr DAEC strains were greatly reduced after pretreating the T84 cells with MAb 1H4 (0.25 ± 0.2 versus 0.30 ± 0.2 versus 0.19 ± 0.1 ng/ml secreted into basolateral compartment for strain C1845 versus strain HB101/pSSS1 versus strain IH11128). Moreover, antibodies that neutralize IL-8 (40 μg/ml) did not inhibit the transepithelial migration of PMNL in response to fMLP (10−7 M) but totally inhibited the transepithelial migration of PMNL induced by IL-8 (10 ng/ml) or by apical colonization with wild-type Afa/Dr DAEC strains C1845 and IH11128 (data not shown).
Afa/Dr DAEC strains induce tyrosine phosphorylation of proteins in T84 intestinal epithelial cells.
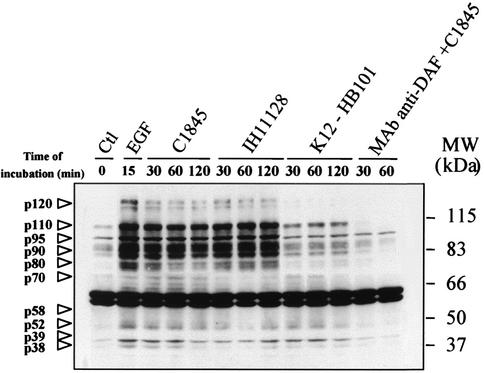
Tyrosine phosphorylation of cellular proteins in T84 cells infected by the wild-type Afa/Dr DAEC strains C1845 and IH11128 at densities of 100 bacteria/epithelial cell was investigated by infecting cells for 30, 60, and 120 min, followed by anti-phosphotyrosine Western blotting analysis of the whole-cell lysates (Fig. 3). Compared to noninfected cells, several tyrosine-phosphorylated proteins were detected in cells after 30 min of infection. Most of the tyrosine-phosphorylated proteins were visualized after the incubation of T84 cells for 120 min with Afa/Dr DAEC strains. Similar results were obtained with the HB101/pSSS1 E. coli (data not shown). Tyrosine-phosphorylated proteins were not detectable in T84 cells infected with K12-HB101 E. coli or after exposure of the T84 cell monolayers to MAb anti-DAF (1H4), followed by incubation with strain C1845. Activation of tyrosine-phosphorylated proteins was detected after the exposure of T84 cells for 15 min to epidermal growth factor (EGF; 10 nM), showing that some EGF-induced phosphorylated proteins are the same as those observed after Afa/Dr DAEC infection.
FIG. 3.
Wild-type Afa/Dr DAEC strains C1845 and IH11128 induce tyrosine phosphorylation in T84 cells. T84 cells were lysed at various times after infection, and samples were then analyzed by immunoblotting with anti-phosphotyrosine antibody as described in Materials and Methods. The putative T84 cell-induced tyrosine-phosphorylated proteins are indicated by arrowheads. Preexposure of the T84 cell monolayers to MAb anti-DAF inhibited tyrosine-phosphorylated proteins induced by C1845. The positive control lane was obtained with cells treated with EGF (10 nM, 15 min). The negative control lane was obtained with cells infected with strain K12-HB101. The micrograph is representative of results obtained at least three times.
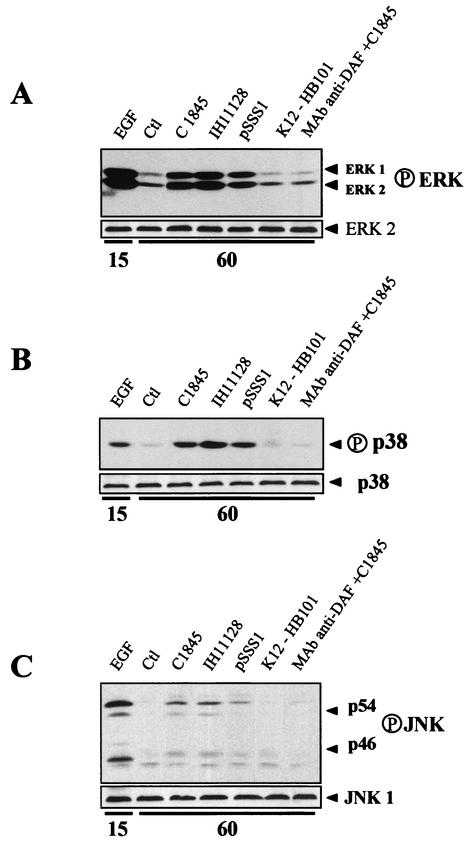
Afa/Dr DAEC strains induce activation of MAP kinases in T84 intestinal epithelial cells. Confluent monolayers of T84 intestinal epithelial cells were infected with wild-type Afa/Dr DAEC strains C1845 and IH11128 or HB101/pSSS1 E. coli at densities of 100 bacteria/epithelial cell. We then investigated the effects of Afa/Dr DAEC infection on the activation of the ERK1/2, p38, and JNK MAP kinase pathways. The kinetics of ERK1/2, p38, and JNK activation after various infection times was assessed by Western blotting with antibodies directed against their phosphorylated forms (Fig. 4). The active forms of ERK1/2 (p42 and p44) and JNK (p46 and p54) appeared after exposure of T84 cells for 30 min to Afa/Dr DAEC strains or HB101/pSSS1 E. coli (not shown), and peaked after 60 min of exposure (Fig. 4A and C), whereas the active form of p38 was detectable after a lag of 60 min after infection (Fig. 4B). A low level of the active form of ERK1/2 was detectable in control cells (Fig. 4A), whereas active forms of p38 or JNK were not (Fig. 4B and C). Activated ERK1/2, JNK, and p38 forms were not detected in lysates extracted from T84 cells infected with strain K12-HB101, whereas activation of these MAP kinases was detected after 15 min of exposure to EGF (10 nM) (Fig. 4). Moreover, adhesion of Afa/Dr DAEC to T84 cells was necessary to activate the ERK1/2, p38, or JNK pathway, since incubating T84 cells with an antibody against the SRC3 domain of DAF (MAb 1H4) failed to activate these kinases (Fig. 4). The total amounts of ERK1/2, JNK, and p38 were evaluated for each lane after a stripping step for 30 min at 50°C in 70 mM Tris (pH 6.7), 2% sodium dodecyl sulfate, and 100 mM 2-mercaptoethanol by Western blotting with anti-ERK2, p38, and JNK1 antibodies (Fig. 4).
FIG. 4.
Activation of MAP kinases in Afa/Dr DAEC-infected epithelial intestinal T84 cells. Cells were lysed 60 min after infection by wild-type Afa/Dr DAEC strains C1845 and IH11128 and recombinant E. coli strain HB101/pSSS1 expressing F1845 adhesin. The positive controls were obtained from cells treated with EGF (10 nM, 15 min). The negative controls were obtained from cells infected with strain K12-HB101 and from uninfected control T84 cells. Samples were analyzed by immunoblotting with antiphosphorylated antibodies as described in Materials and Methods. (A) Activation of ERK1/2 in Afa/Dr DAEC-infected and EGF-stimulated cells. Note that control cells show a constitutive activation of ERK. (B) Activation of p38 MAP kinases in control, Afa/Dr DAEC-infected and EGF-stimulated cells. (C) Activation of JNK in control, Afa/Dr DAEC-infected, and EGF-stimulated cells. MAP kinase activation was not detectable in infected epithelial cells after preexposing T84 monolayers with MAb anti-DAF (1H4). Micrographs are representative of at least three experiments.
Activation of MAP kinases is required for the induction of PMNL transepithelial migration after apical colonization of T84 intestinal cells with Afa/Dr DAEC strains.
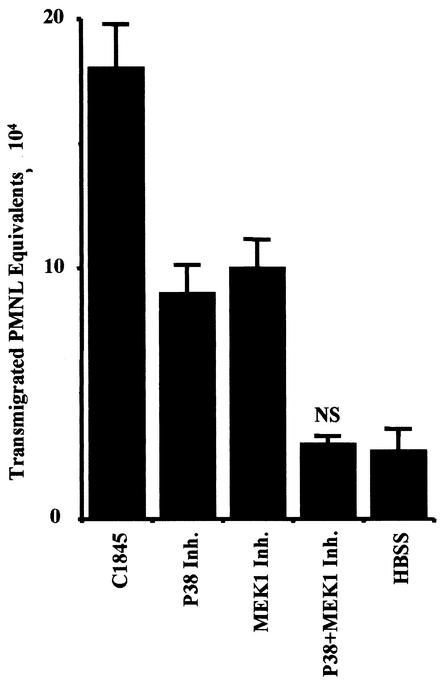
In these experiments, we used the specific MAP kinase inhibitors, SB203580 which blocks p38 kinase activity, and PD98059, which blocks MEK1 kinase activity, thereby preventing ERK1/2 phosphorylation (Fig. 5). In control experiments, the ability of SB203580 (10 μM) to block p38 MAP kinase activity and the ability of PD98059 (25 μM) to block MEK1 kinase activity in wild-type Afa/Dr DAEC C1845-infected T84 cells were confirmed by Western blotting, with the phospho-specific ERK1/2 and p38 antibodies (data not shown). T84 cells were pretreated for 30 min with the MAP kinase inhibitors used singly or in combination. The T84 monolayers were then inoculated with the C1845 bacteria, and transepithelial migration of PMNL was induced as previously described. The p38 inhibitor SB303580 reduced transepithelial migration by 75% (P < 0.001), the MEK1 inhibitor PD98059 reduced transepithelial migration by 72% (P < 0.001), and a combination of the two inhibitors completely eliminated the induction of PMNL transepithelial migration by C1845 (P < 0.0001). Similar results were obtained with wild-type Afa/Dr DAEC strain IH11128 or HB101/pSSS1 E. coli (data not shown).
FIG. 5.
MAP kinase activation is required for the induction of the transepithelial migration of PMNL after apical colonization of T84 intestinal cells with wild-type Afa/Dr DAEC strain C1845. T84 monolayers were cultured alone (HBSS) or were infected with the C1845 bacteria with or without pretreatment with the p38 inhibitor SB203580 (10 μM) and/or the MEK1 inhibitor PD98059 (25 μM). Data represent the mean and standard deviation of at least three experiments, each performed in triplicate. One of three experiments performed. NS, not significantly different from control (HBSS).
DISCUSSION
Our data showed that Afa/Dr DAEC strains can trigger in vitro proinflammatory stimuli through the intestinal epithelial barrier, inducing the recruitment and influx of PMNL. As far as we are aware, this the first model allowing PMNL to migrate across an epithelium after apical colonization by wild-type Afa/Dr DAEC strains, which leads to apical adhesin-receptor-dependent cell signaling. These results are in agreement with some previous hypotheses about the role of Afa/Dr DAEC in colitis and pyelonephritis observed in vivo (11, 33).
Bacterial adherence to host cells is the initial step in infections caused by a uropathogen or enteropathogen such as E. coli. These organisms may carry the determinants for several fimbrial and afimbrial adhesins (19). The members of the Afa/Dr family of adhesins, including Dr, Dr-II, AFA I, AFA III, and F1845, have similar genetic organization (32) and recognize DAF as a receptor (31). DAF is a complement-regulatory protein of 70 to 80 kDa expressed by human carcinoma cells (30), which consists of five domains, i.e., four SCRs (SCR1 to SCR4) followed by a serine-threonine-rich (ST-rich) domain and is attached by a glycosylphosphatidylinositol anchor (6, 20). The SCR3 domain plays a crucial role in the regulatory function of DAF as established by mapping domains in the DAF molecule (7, 27). Interestingly, a previous study had already shown that Afa/Dr DAEC strains bind to the SCR3 domain of DAF (31). In the present study, the findings demonstrate that the recognition of DAF by the Afa/Dr adhesins is required to promote the transepithelial migration of PMNL. More specifically, we have shown that a specific antibody against SCR3 could inhibit the PMNL transepithelial migration induced by epithelial colonization by the wild-type Afa/Dr DAEC strains, demonstrating that the PMNL transmigration response requires bacterial adhesion to the SCR3 domain of DAF.
IL-8 is the main cytokine produced by the epithelial cells known to stimulate PMNL migration. Several studies have shown that T84 cells infected by different bacteria can produce IL-8 (18, 23, 29). Moreover, Clostridium difficile toxin A induces the production of IL-8 from human colonic epithelial cells (5). In this study we showed that IL-8 was secreted by Afa/Dr DAEC-stimulated T84 cells. We next determined that the IL-8 secreted by Afa/Dr DAEC-stimulated T84 cells was responsible for the observed induction of PMNL transepithelial migration by showing that this migration was inhibited by neutralizing antibodies to IL-8. The Afa/Dr DAEC-induced production of IL-8 observed, quite apart from its effect on the induced PMNL transepithelial migration, is of interest in terms of the modulation of intestinal functions. The action of proinflammatory cytokines results in changes in metabolic status. For example, IL-8 increased glucose absorption by an active mechanism (15). It remains to be determined whether the Afa/Dr DAEC-induced production of proinflammatory cytokines has any effect on intestinal functions such as transport and secretion.
Previous reports have shown that the infection of the human embryonic intestinal INT407 cell line by wild-type Afa/Dr DAEC C1845 or HB101/pSSS1 E. coli expressing the Afa/Dr F1845 adhesin promotes F-actin rearrangements and clustering of phosphotyrosines (36). These findings demonstrate that C1845, due to its F1845 adhesin, may induce the recruitment of signal transduction molecules after contact with the epithelial cells. In the present study, we showed that various wild-type strains of Afa/Dr DAEC (C1845 and IH11128) and the recombinant HB101/pSSS1 E. coli induce tyrosine phosphorylation of various proteins in T84 cells. MAP kinases form a group of three serine/threonine kinases, including ERK1 and ERK2 and two stress-activated protein kinases, p38 MAP kinase and JNK. These kinases have been implicated in the host cell response to some bacterial infection (16, 39). Activation of MAP kinases has been found in epithelial cells infected by Salmonella enterica serovar Typhimurium, Helicobacter pylori and enteropathogenic E. coli resulting in the production of the proinflammatory cytokines such as IL-8 (8, 16, 24). In the present study, we investigated the ability of Afa/Dr DAEC strains to activate MAP kinases and correlated these signaling pathways to the transepithelial migration of PMNL induced by Afa/Dr DAEC-infected T84 cells. We showed that both wild-type Afa/Dr DAEC strains (C1845 and IH11128) and recombinant HB101/pSSS1 E. coli induced tyrosine phosphorylation in T84 cells. This tyrosine phosphorylation occurred in T84 cells with the activation of the three MAP kinases ERK1/2, JNK, and p38. Moreover, intimate adhesion of Afa/Dr DAEC to T84 cells was necessary for activation of the ERK1/2, p38, or JNK pathway, since incubating T84 cells with an antibody against the SRC3 domain of DAF (MAb 1H4) failed to activate these signaling pathways. MAP kinase signaling regulates the expression of many proinflammatory cytokines, including chemokines for PMNL, such as IL-8. Both p38 kinase inhibitor and MEK1 inhibitor substantially reduced the PMNL transepithelial migration induced in Afa/Dr DAEC-infected T84 cells. It appears that both the p38 and ERK1/2 pathways have to be activated for a maximal intestinal epithelial cell chemokine response to Afa/Dr DAEC inducing an intense PMNL transepithelial migration.
In conclusion, our study shows for the first time that Afa/Dr DAEC strains have marked proinflammatory effects in vitro on colonic epithelial cells. After adherence of Afa/Dr DAEC through the adhesin interaction with the apical brush border-associated receptor, the DAF, it appears likely that intestinal epithelial cells have evolved mechanisms to participate actively in the signaling loop, which orchestrates colonic inflammation. Finally, we speculate that Afa/Dr DAEC strains may be involved in the pathogenesis of diarrhea observed in some forms of bacterial colitis by activating the transepithelial migration of PMNL.
Acknowledgments
We thank Mireille Mari for excellent technical assistance.
F.B. and P.B. contributed equally to this study.
Editor: A. D. O'Brien
REFERENCES
- 1.Baqui, A. H., R. B. Sack, R. E. Black, K. Haider, A. Hossain, A. R. Alim, M. Yunus, H. R. Chowdhury, and A. K. Siddique. 1992. Enteropathogens associated with acute and persistent diarrhea in Bangladeshi children less than 5 years of age. J. Infect. Dis. 166:792-796. [DOI] [PubMed] [Google Scholar]
- 2.Bernet-Camard, M. F., M. H. Coconnier, S. Hudault, and A. L. Servin. 1996. Pathogenicity of the diffusely adhering strain Escherichia coli C1845: F1845 adhesin-decay accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect. Immun. 64:1918-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilge, S. S., C. R. Clausen, W. Lau, and S. L. Moseley. 1989. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J. Bacteriol. 171:4281-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bocciardi, R., B. Mograbi, B. Pasini, M. G. Borrello, M. A. Pierotti, I. Bourget, S. Fischer, G. Romeo, and B. Rossi. 1997. The multiple endocrine neoplasia type 2B point mutation switches the specificity of the Ret tyrosine kinase towards cellular substrates that are susceptible to interact with Crk and Nck. Oncogene 15:2257-2265. [DOI] [PubMed] [Google Scholar]
- 5.Branka, J. E., G. Vallette, A. Jarry, C. Bou-Hanna, P. Lemarre, P. N. Van, and C. L. Laboisse. 1997. Early functional effects of Clostridium difficile toxin A on human colonocytes. Gastroenterology 112:1887-1894. [DOI] [PubMed] [Google Scholar]
- 6.Brodbeck, W. G., L. Kuttner-Kondo, C. Mold, and M. E. Medof. 2000. Structure/function studies of human decay-accelerating factor. Immunology 101:104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyne, K. E., S. E. Hall, S. Thompson, M. A. Arce, T. Kinoshita, T. Fujita, D. J. Anstee, W. Rosse, and D. M. Lublin. 1992. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J. Immunol. 149:2906-2913. [PubMed] [Google Scholar]
- 8.Czerucka, D., S. Dahan, B. Mograbi, B. Rossi, and P. Rampal. 2001. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect. Immun. 69:1298-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenoglio-Preiser, C. M., P. E. Lantz, M. B. Listrom, M. Davis, and F. O. Rilke. 1989. The non-neoplastic large intestine, p. 427-445. In C. M. Fenoglio (ed.), Gastrointestinal pathology. Raven Press, New York, N.Y.
- 10.Forestier, C., M. Meyer, S. Favre-Bonte, C. Rich, G. Malpuech, C. Le Bouguenec, J. Sirot, B. Joly, and C. De Champs. 1996. Enteroadherent Escherichia coli and diarrhea in children: a prospective case-control study. J. Clin. Microbiol. 34:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giron, J. A., T. Jones, F. Millan-Velasco, E. Castro-Munoz, L. Zarate, J. Fry, G. Frankel, S. L. Moseley, B. Baudry, J. B. Kaper, et al. 1991. Diffuse-adhering Escherichia coli (DAEC) as a putative cause of diarrhea in Mayan children in Mexico. J. Infect. Dis. 163:507-513. [DOI] [PubMed] [Google Scholar]
- 12.Guignot, J., M. F. Bernet-Camard, C. Pous, L. Plancon, C. Le Bouguenec, and A. L. Servin. 2001. Polarized entry of uropathogenic Afa/Dr diffusely adhering Escherichia coli strain IH11128 into human epithelial cells: evidence for alpha5 beta1 integrin recognition and subsequent internalization through a pathway involving caveolae and dynamic unstable microtubules. Infect. Immun. 69:1856-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guignot, J., I. Peiffer, M. F. Bernet-Camard, D. M. Lublin, C. Carnoy, S. L. Moseley, and A. L. Servin. 2000. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells. Infect. Immun. 68:3554-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunzburg, S. T., B. J. Chang, S. J. Elliott, V. Burke, and M. Gracey. 1993. Diffuse and enteroaggregative patterns of adherence of enteric Escherichia coli isolated from aboriginal children from the Kimberley region of Western Australia. J. Infect. Dis. 167:755-758. [DOI] [PubMed] [Google Scholar]
- 15.Hardin, J., K. Kroeker, B. Chung, and D. G. Gall. 2000. Effect of proinflammatory interleukins on jejunal nutrient transport. Gut 47:184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobbie, S., L. M. Chen, R. J. Davis, and J. E. Galan. 1997. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159:5550-5559. [PubMed] [Google Scholar]
- 17.Hofman, P., L. D'Andrea, D. Carnes, S. P. Colgan, and J. L. Madara. 1996. Intestinal epithelial cytoskeleton selectively constrains lumen-to-tissue migration of neutrophils. Am. J. Physiol. 271:C312-C320. [DOI] [PubMed] [Google Scholar]
- 18.Hofman, V., V. Ricci, A. Galmiche, P. Brest, P. Auberger, B. Rossi, P. Boquet, and P. Hofman. 2000. Effect of Helicobacter pylori on polymorphonuclear leukocyte migration across polarized T84 epithelial cell monolayers: role of vacuolating toxin VacA and cag pathogenicity island. Infect. Immun. 68:5225-5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holden, N., C. Cotterill, and D. Gally. 2000. Examination of regulatory cross-talk between the decay accelerating factor-binding fimbrial/afimbrial adhesins and type I fimbriae. Adv. Exp. Med. Biol. 485:143-150. [DOI] [PubMed] [Google Scholar]
- 20.Hourcade, D., M. K. Liszewski, M. Krych-Goldberg, and J. P. Atkinson. 2000. Functional domains, structural variations and pathogen interactions of MCP, DAF, and CR1. Immunopharmacology 49:103-116. [DOI] [PubMed] [Google Scholar]
- 21.Jallat, C., A. Darfeuille-Michaud, C. Rich, and B. Joly. 1994. Survey of clinical isolates of diarrhoeogenic Escherichia coli: diffusely adhering E. coli strains with multiple adhesive factors. Res. Microbiol. 145:621-632. [DOI] [PubMed] [Google Scholar]
- 22.Jallat, C., V. Livrelli, A. Darfeuille-Michaud, C. Rich, and B. Joly. 1993. Escherichia coli strains involved in diarrhea in France: high prevalence and heterogeneity of diffusely adhering strains. J. Clin. Microbiol. 31:2031-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keates, S., A. C. Keates, M. Warny, R. M. Peek, Jr., P. G. Murray, and C. P. Kelly. 1999. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag− Helicobacter pylori. J. Immunol. 163:5552-5559. [PubMed] [Google Scholar]
- 25.Le'Negrate, G., E. Selva, P. Auberger, B. Rossi, and P. Hofman. 2000. Sustained polymorphonuclear leukocyte transmigration induces apoptosis in T84 intestinal epithelial cells. J. Cell Biol. 150:1479-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine, M. M., E. J. Bergquist, D. R. Nalin, D. H. Waterman, R. B. Hornick, C. R. Young, and S. Sotman. 1978. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet i:1119-1222. [DOI] [PubMed] [Google Scholar]
- 27.Lublin, D. M., and K. E. Coyne. 1991. Phospholipid-anchored and transmembrane versions of either decay-accelerating factor or membrane cofactor protein show equal efficiency in protection from complement-mediated cell damage. J. Exp. Med. 174:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madara, J. L., S. Colgan, A. Nusrat, C. Delp, and C. Parkos. 1992. A simple approach to measurement of electrical parameters of cultured epithelial monolayers: use in assessing neutrophil epithelial monolayers transmigration. J. Tissue Culture Methods 14:209-216. [Google Scholar]
- 29.McCormick, B. A., P. M. Hofman, J. Kim, D. K. Carnes, S. I. Miller, and J. L. Madara. 1995. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J. Cell Biol. 131:1599-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niehans, G. A., D. L. Cherwitz, N. A. Staley, D. J. Knapp, and A. P. Dalmasso. 1996. Human carcinomas variably express the complement inhibitory proteins CD46 (membrane cofactor protein), CD55 (decay-accelerating factor), and CD59 (protectin). Am. J. Pathol. 149:129-142. [PMC free article] [PubMed] [Google Scholar]
- 31.Nowicki, B., A. Hart, K. E. Coyne, D. M. Lublin, and S. Nowicki. 1993. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J. Exp. Med. 178:2115-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nowicki, B., R. Selvarangan, and S. Nowicki. 2001. Family of Escherichia coli Dr adhesins: decay-accelerating factor receptor recognition and invasiveness. J. Infect. Dis. 183(Suppl. 1):S24-S27. [DOI] [PubMed] [Google Scholar]
- 33.Nowicki, B., C. Svanborg-Eden, R. Hull, and S. Hull. 1989. Molecular analysis and epidemiology of the Dr hemagglutinin of uropathogenic Escherichia coli. Infect. Immun. 57:446-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peiffer, I., A. B. Blanc-Potard, M. F. Bernet-Camard, J. Guignot, A. Barbat, and A. L. Servin. 2000. Afa/Dr diffusely adhering Escherichia coli C1845 infection promotes selective injuries in the junctional domain of polarized human intestinal Caco-2/TC7 cells. Infect. Immun. 68:3431-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peiffer, I., J. Guignot, A. Barbat, C. Carnoy, S. L. Moseley, B. J. Nowicki, A. L. Servin, and M. F. Bernet-Camard. 2000. Structural and functional lesions in brush border of human polarized intestinal Caco-2/TC7 cells infected by members of the Afa/Dr diffusely adhering family of Escherichia coli. Infect. Immun. 68:5979-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peiffer, I., A. L. Servin, and M. F. Bernet-Camard. 1998. Piracy of decay-accelerating factor (CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F-actin rearrangements in cultured human intestinal INT407 cells. Infect. Immun. 66:4036-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poitrineau, P., C. Forestier, M. Meyer, C. Jallat, C. Rich, G. Malpuech, and C. De Champs. 1995. Retrospective case-control study of diffusely adhering Escherichia coli and clinical features in children with diarrhea. J. Clin. Microbiol. 33:1961-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tacket, C. O., S. L. Moseley, B. Kay, G. Losonsky, and M. M. Levine. 1990. Challenge studies in volunteers using Escherichia coli strains with diffuse adherence to HEp-2 cells. J. Infect. Dis. 162:550-552. [DOI] [PubMed] [Google Scholar]
- 39.Tang, P., C. L. Sutherland, M. R. Gold, and B. B. Finlay. 1998. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect. Immun. 66:1106-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]