Abstract
Studies of immunity to Pseudomonas aeruginosa have indicated that a variety of potential immunogens can elicit protection in animal models, utilizing both antibody- and cell-mediated immune effectors for protection. To attempt to optimize delivery of multiple protective antigens and elicit a broad range of immune effectors, we produced an aroA deletion mutant of the P. aeruginosa serogroup O2/O5 strain PAO1, designated PAO1ΔaroA. Previously, we reported that this strain elicits high levels of opsonic antibody directed against many serogroup O2/O5 strains after nasal immunization of mice and rabbits. Here, we assessed the protective efficacy of immunization with PAO1ΔaroA against acute fatal pneumonia in mice. After active immunization, high levels of protection were achieved against an ExoU-expressing cytotoxic variant of the parental strain PAO1 at doses up to 1,000-fold greater than the 50% lethal dose. Significant protection against PAO1 and two of four other serogroup O2/O5 strains was also found, but there was no protection against serogroup-heterologous strains. The serogroup O2/O5 strains not protected against were killed in opsonophagocytic assays as efficiently as the strains with which protection was seen, indicating a lack of correlation of protection and opsonic killing within the serogroup. In passive immunization experiments using challenge with wild-type PAO1 or other noncytotoxic members of the O2/O5 serogroup, there was no protection despite the presence of high levels of opsonic antibody in the mouse sera. However, passive immunization did prevent mortality from pneumonia due to the cytotoxic PAO1 variant at low-challenge doses. These data suggest that a combination of humoral and cellular immunity is required for protection against P. aeruginosa lung infections, that such immunity can be elicited by using aroA deletion mutants, and that a multivalent P. aeruginosa vaccine composed of aroA deletion mutants of multiple serogroups holds significant promise.
Nosocomial infections due to the opportunistic pathogen Pseudomonas aeruginosa are a significant clinical problem (10, 14, 53). These infections, as well as community-acquired corneal infections in wearers of extended-use contact lenses (47, 56), are due to lipopolysaccharide (LPS)-smooth strains of P. aeruginosa, which are distinct from the LPS-rough, mucoid strains found in patients with advanced cystic fibrosis (18). It should be noted, however, that the initial colonizing strains in cystic fibrosis patients are typically LPS smooth (4). The difficulties in treating P. aeruginosa infections are particularly noteworthy. These difficulties stem not only from host factors, such as immunocompromise or the presence of foreign material, but also from bacterial factors such as biofilm formation and intrinsic antibiotic resistance, which have shown a disturbingly rising frequency (16). The intrinsic antibiotic resistance of P. aeruginosa is largely due to the presence of multiple drug efflux pumps (49) and the low permeability of the outer membrane (17). The prevalence of P. aeruginosa infections and the difficulties encountered in treating these infections underlie the need for an effective vaccine.
For LPS-smooth strains, numerous studies have documented the protective efficacy of opsonic antibodies specific for the O-side chain polysaccharide portion of LPS (5, 6, 11, 34, 48). A significant limitation in the development of LPS-specific immunotherapies is the need for multivalent vaccines because of the structural diversity of the O antigens. Based on chemical and serologic variations, the O antigens of P. aeruginosa are currently classified into 20 serogroups, with many serogroups possessing subtype strains having subtle variations in the O antigen (28, 58). The O2/O5 serogroup is probably the most complex and includes the common laboratory strain PAO1, whose genome has been fully sequenced (60). While O antigen-based vaccines have been found to mediate potent protection in animal studies, this protection has, in general, been seen only when the strains used to isolate the vaccine antigen are also used in the challenge studies (6, 7, 45, 46). Broad-based protection is not reliably generated, even against subtype strains within the same serogroup (19, 20). Thus, the serogroup variability of P. aeruginosa coupled with the serogroup-specific nature of protective immune responses have significantly hampered vaccine development.
Recent evidence that P. aeruginosa readily enters lung and corneal epithelial cells during infection (12, 21, 44) raises the possibility that cellular immunity might also be important for the control of P. aeruginosa infections. Indeed, a number of groups have demonstrated T-cell-based immune protection against P. aeruginosa infections (9, 29, 33, 35-38). We hypothesized that a live, attenuated P. aeruginosa vaccine strain could exploit the intracellular phase of P. aeruginosa to elicit a broad immune response encompassing both cell-mediated and antibody-mediated effectors directed at a variety of in vivo-expressed microbial antigens. We previously reported (51) on the construction of an unmarked aroA deletion mutant of PAO1 and found that it was auxotrophic for aromatic amino acids, it had a propensity for intracellular growth, it was highly attenuated in a mouse model of acute pneumonia, and it elicited high levels of opsonic antibody against multiple members of the O2/O5 serogroup. In this report, we present data on the protective efficacy against acute lethal pneumonia in mice after intranasal (i.n.) immunization with the live, attenuated aroA deletion mutant of P. aeruginosa PAO1.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in these experiments, along with their phenotypes and sources, are listed in Table 1.
TABLE 1.
Strains used in this study
| Strain | Description | Source and/or referencea |
|---|---|---|
| P. aeruginosa | ||
| PAO1 | Wild-type strain, LPS-smooth, serogroup O2/O5; subtype epitopes O2a and O2d | M. Vasil |
| PAO1ΔaroA | aroA deletion mutant of PAO1, LPS-smooth, serogroup O2/O5; subtype epitopes O2a and O2d | 51 |
| ExoU+ PAO1 | PAO1(pUCP19exoUspcU), cytotoxic variant of PAO1 carrying the exoU gene and its chaperone gene, spcU, on plasmid pUCP19; carbenicillin resistant | 1 |
| 170003 | Wild-type strain, LPS-smooth, serogroup O2/O5; subtype epitopes O2a and O2b | 20 |
| IATS O16 | Wild-type strain, LPS-smooth, serogroup O2/O5; subtype epitopes O2a, O2b, and O2e | 20 |
| Fisher IT-3 | Wild-type strain, LPS-smooth, serogroup O2/O5; subtype epitopes O2a and O2c | 20 |
| 170006 | Wild-type strain, LPS-smooth, serogroup O2/O5; subtype epitopes O2a, O2d, and O2e | 20 |
| 170007 | Wild-type strain, LPS-smooth, serogroup O2/O5; subtype epitopes O2a, O2d, and O2f | 20 |
| Fisher IT-7 | Wild-type strain, LPS-smooth, serogroup O2/O5; subtype epitope O2a | 20 |
| Fisher IT-4 | Clinical isolate (bacteremia) designated Rhodes, LPS-smooth, serogroup O1 | 50 |
| 6294 | Clinical isolate (corneal infection), LPS-smooth, noncytotoxic, serogroup O6 | BPEI (13, 50) |
| 6206 | Clinical isolate (corneal infection), LPS-smooth, cytotoxic, serogroup O11 | BPEI (13, 50) |
| E. coli HB101 | supE44 hsdS20 (rB− rB−) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 | 3 |
BPEI, Bascom-Palmer Eye Institute, Miami, Fla.
Preparation of bacterial inocula.
Frozen bacterial stocks were plated and grown overnight on tryptic soy agar at 37°C. For immunization, bacteria were suspended in either normal saline or phosphate-buffered saline (PBS). For challenge experiments, bacteria were suspended in PBS containing 1% heat-inactivated fetal calf serum (HyClone). Concentrations were adjusted spectrophotometrically and confirmed by viable counts after serially diluting in PBS containing 1% heat-inactivated fetal calf serum and plating on tryptic soy agar.
Immunization of mice and rabbits.
Six- to eight-week-old female C3H/HeN, C57BL/6, FVB/N, or BALB/c mice (Harlan Sprague-Dawley Farms, Chicago, Ill., or Jackson Laboratory, Bar Harbor, Maine) were housed under virus-free conditions. Before immunization, mice were first anesthetized with 0.2 ml of a mixture of ketamine (6.7 mg/ml) and xylazine (1.3 mg/ml) in 0.9% saline injected intraperitoneally (i.p.). Immunization consisted of placing 10 μl of the live P. aeruginosa ΔaroA vaccine strain, or live Escherichia coli HB101 as a control, on each nostril (total 20 μl per mouse). Escalating doses of 1 × 108 CFU, 5 × 108 CFU, and 1 × 109 CFU were administered at weekly intervals. For studies of i.p. immunization, mice were given similar doses but in 100 μl administered i.p. without prior anesthesia. New Zealand White rabbits (Millbrook Breeding Labs, Amherst, Mass.) were immunized i.n. initially on a similar schedule, followed by repeated i.n. boosting with doses of 109 CFU every 2 to 4 weeks, all with inocula of 100 μl (50 μl per nostril). Rabbits were anesthetized with 2 ml of a mixture of atropine (0.4 mg), ketamine (80 mg), and xylazine (10 mg) injected subcutaneously prior to each i.n. immunization. Rabbits were also immunized with E. coli HB101 as control, with identical schedules and doses. All animal experiments complied with institutional and federal guidelines regarding the use of animals in research.
Challenge studies.
We used our previously described acute fatal pneumonia model after i.n. application of P. aeruginosa in mice sedated with ketamine and xylazine (1). For one experiment, we used an intratracheal challenge route. For challenge experiments after active immunization, infections were performed 3 to 4 weeks after the last dose of vaccine. Mice were followed at least daily for mortality. Moribund animals were sacrificed and considered nonsurvivors. Data are shown as survival to day 5 after challenge. Passive immunization studies utilized immunoglobulin G (IgG) purified with protein G affinity chromatography (Sigma Chemical Co., St. Louis, Mo.) from sera of rabbits immunized i.n. with either E. coli HB101 or PAO1ΔaroA. IgG concentrations were measured by enzyme-linked immunosorbent assay. For passive immunization, IgG was administered i.p., i.n. (after anesthesia with ketamine and xylazine), or both i.p. and i.n., 24 h prior to challenge.
Histological analysis of lungs of immunized mice after challenge.
Adult C3H/HeN mice were immunized and then challenged with ExoU+ PAO1 as described. Mice were sacrificed at 4, 10, and 24 h after challenge. The lungs were immediately instilled with 1 ml of PBS containing 1% paraformaldehyde by means of a tracheal catheter after exposure with a midline neck incision. The lungs were removed, fixed in PBS with 1% paraformaldehyde for 1 h at room temperature, and then placed in 70% ethanol in water at 4°C overnight prior to paraffin embedding. Sections were stained with hematoxylin and eosin.
Opsonophagocytic assays.
Opsonophagocytic assays were conducted as previously described (2, 43), with some modifications. The assay consists of a P. aeruginosa target strain (5 × 106 CFU/ml), dextran-purified human leukocytes (5 × 106 cells/ml), 2.5% infant rabbit serum (Accurate Chemical, Westbury, N.Y.) as the complement source, and test sera. The bacteria were grown in tryptic soy broth containing 1% glycerol as a supplemental carbon source, with the addition of carbenicillin (400 μg/ml) for ExoU+ PAO1. The complement source was not adsorbed with bacteria. Negative controls in all assays included antisera from mice immunized i.n. with E. coli HB101. Tubes with PAO1ΔaroA antisera but without leukocytes served as additional negative controls to help distinguish killing from agglutination. After active immunization, mouse sera were collected and pooled (four mice per immunization group) 3 weeks after the third immunization. For passively immunized mice (n = 5), sera were collected and pooled 22 h after i.p. administration of 1 mg of purified anti-PAO1ΔaroA rabbit IgG.
Statistical analyses.
Survival data were analyzed by Fisher exact test by using Statview (SAS Institute, Cary, N.C.). The 50% lethal doses (LD50s) were determined by probit or logit analysis by using SYSTAT (SYSTAT Software, Inc., Richmond, Calif.). Opsonophagocytic titers, defined as the reciprocal dilution of serum-mediating 50% killing, were calculated by linear regression by using GraphPad Prism (GraphPad Software, San Diego, Calif.). By convention, the concentrations in serum that were used in the calculations were the input rather than the final concentrations, since the initial serum dilution is further diluted fourfold in the assay tube. Under routine conditions, killing of ≥50% is considered biologically significant and therefore serves to classify a serum as positive for opsonic killing activity. Although killing <50% is sometimes statistically significant, this level of killing is not considered biologically significant.
RESULTS
When mice were immunized i.n. with 3 weekly doses of PAO1ΔaroA and then challenged i.n. 3 weeks after the final immunization (Table 2), we observed a high level of protection against lethal pneumonia caused by a cytotoxic variant of PAO1, denoted ExoU+ PAO1. ExoU+ PAO1 expresses the type III secretion system cytotoxin ExoU from a plasmid that includes the ExoU chaperone protein. ExoU is not encoded in the genome of PAO1. We used this strain because we previously found that its i.n. LD50 of 7 × 105 CFU is much lower than that of the parental PAO1 strain (3 × 107 CFU), thereby allowing assessment of protection against very high challenge doses (1). In this acute murine pneumonia model, most mice die within 72 h of challenge with a virulent strain and have evidence of systemic spread of bacteria at the time of death (1). In the present study, if the immunized mice were challenged earlier than 3 weeks after the last immunization, we observed some survival in the E. coli-immunized control groups, likely due to nonspecific activation of immune effectors in the lung after i.n. immunization.
TABLE 2.
Protective efficacy against acute fatal pneumonia due to ExoU+ PAO1 after active immunization with PAO1ΔaroA using different strains of inbred mice and different routes of immunization and challenge
| Mouse strain | Immunization route | Challenge route | Inoculum (CFU) | Multiple of LD50 | No. of surviving mice/no. of mice challenged in vaccine group:
|
|
|---|---|---|---|---|---|---|
| E. coli HB101 | PAO1ΔaroA | |||||
| C3H/HeN | i.n. | i.n. | 5 × 107 | 70 | 0/16 | 16/16b |
| C3H/HeN | i.n. | i.n. | 5 × 108 | 700 | 0/6 | 1/5 |
| BALB/c | i.n. | i.n. | 8 × 108 | 1,100 | 0/15 | 15/15b |
| C57BL/6 | i.n. | i.n. | 5 × 107 | 70 | 0/9 | 9/9c |
| FVB/N | i.n. | i.n. | 5 × 107 | 70 | 0/5 | 5/5c |
| C3H/HeN | i.p. | i.t.a | 5 × 107 | 70 | 0/4 | 4/4c |
| C3H/HeN | i.n. | i.p. | 1 × 108 | 5 | 1/8 | 7/8c |
i.t., intratracheal.
P < 0.001 (Fisher exact test).
P < 0.05 (Fisher exact test).
The resistance to lethal pneumonia after immunization was remarkably robust, as challenge doses in up to 1,100-fold greater than the LD50 were protective after immunization of BALB/c mice with PAO1ΔaroA. C3H/HeN mice were completely protected at doses 70-fold greater than the LD50 but not at 700-fold greater than the LD50 (Table 2). The efficacy of high-dose protection in BALB/c mice but not in C3H/HeN mice indicates murine strain-specific differences in their response to the vaccine and the challenge organism. The complete protection against lethal pneumonia due to ExoU+ PAO1 at 70-fold above its LD50, with 100% of PAO1ΔaroA-immunized mice surviving and 100% of E. coli-immunized control mice dying, was also seen with the C57BL/6 and FVB/N strains of inbred mice (Table 2). Protection was not dependent on the route of immunization, since mice immunized i.p., followed by intratracheal challenge were also fully protected (Table 2). Furthermore, the protection was not dependent on route of challenge since mice immunized i.n., followed by i.p. challenge were also significantly protected (Table 2). When we evaluated the duration of protection in BALB/c mice, we found, remarkably, that 100% (four of four) of PAO1ΔaroA-immunized mice challenged 10 months after the final immunization survived after i.n. challenge with ExoU+ PAO1 at 5 × 107 CFU (70-fold above the LD50), whereas the control E. coli-immunized mice all died (P < 0.05 by Fisher exact test, data not shown).
We next assessed the protective efficacy of i.n. immunization of C3H/HeN mice with PAO1ΔaroA against other P. aeruginosa strains, including the noncytotoxic parental strain PAO1; other serogroup O2/O5 strains, which are considered serogroup-homologous, subtype-heterologous strains (170003, IATS O16, Fisher IT-3, and Fisher IT-7); and serogroup-heterologous strains (Fisher IT-4 [serogroup O1], 6294 [serogroup O6], and 6206 [serogroup O11]) (Table 3). Surprisingly, the protection against lethal pneumonia due to PAO1, the homologous parental strain, was only 70%, even at a dose only 20-fold above the LD50 for this strain, which is nearly 900-fold higher than the LD50 for the ExoU+ PAO1 strain. We also found modest, but significant (P < 0.01), protection against challenge with the subtype-heterologous strains 170003 and Fisher IT-7 at doses from 6- to 29-fold above the LD50s, as well as borderline-significant (P = 0.09) protection against the subtype-heterologous strain IATS O16 at a dose 17-fold above its LD50. There was no protection against the subtype-heterologous, serogroup-homologous strain Fisher IT-3 at a dose only fourfold above its LD50. We were unable to test the other two prototypic serogroup O2/O5 strains (170006 and 170007) due to insufficient virulence of these strains in this infection model. When PAO1ΔaroA-immunized mice were challenged with the serogroup-heterologous P. aeruginosa strains Fisher IT-4, 6294, and 6206, there was no protection (Table 3).
TABLE 3.
Protective efficacy against acute fatal pneumonia due to P. aeruginosa after active nasal immunization of C3H/HeN mice with E. coli HB101 or PAO1ΔaroA
| Challenge strain | Serogroup | LD50(CFU) | Inoculum (CFU) | Multiple of LD50a | No. of surviving mice/no. of mice challenged in vaccine group:
|
|
|---|---|---|---|---|---|---|
| E. coli HB101 | PAO1ΔaroA | |||||
| PAO1 | O2/O5 | 3 × 107 | 6 × 108 | 20 | 0/10 | 7/10b |
| 170003 | O2/O5 | 8 × 107 | 5 × 108 | 6 | 0/10 | 7/12b |
| IATS O16 | O2/O5 | 6 × 107 | 1 × 109 | 17 | 0/10 | 4/10c |
| Fisher IT-3 | O2/O5 | 1 × 108 | 4 × 108 | 4 | 0/6 | 1/7 |
| Fisher IT-7 | O2/O5 | 7 × 106 | 2 × 108 | 29 | 2/10 | 9/10c |
| Fisher IT-4 | O1 | 6 × 108 | 7 × 108 | 1.2 | 0/5 | 0/6 |
| 6294 | O6 | 5 × 107 | 3 × 108 | 6 | 1/6 | 0/6 |
| 6206 | O11 | 4 × 106 | 3 × 108 | 75 | 0/10 | 0/10 |
The LD50 values were determined by probit or logit analysis.
P < 0.01 (Fisher exact test).
P = 0.09 (Fisher exact test).
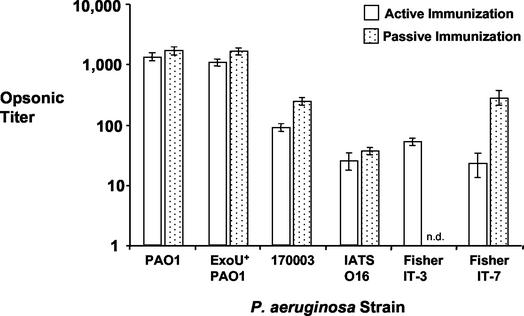
Opsonophagocytic assays were used to test whether the titers of opsonic antibody in the sera from PAO1ΔaroA-immunized mice correlated with the in vivo protection results. We previously reported that a 1:8 dilution of immune mouse antisera mediated high-level killing of five of the seven prototypic serogroup O2/O5 strains, including Fisher IT-3, but was not active against serogroup-heterologous strains (51). Strains 170006 and 170007 were the two serogroup O2/O5 strains not killed well in those experiments. Using immune antisera obtained from the mice after immunization and prior to challenge, we found high levels of opsonic antibody against PAO1, the parental strain, and its cytotoxic variant, ExoU+ PAO1 (Fig. 1). The levels of opsonic killing of the other serogroup O2/O5 strains were markedly less than that of PAO1 and showed no consistent pattern to suggest that there was less activity against Fisher IT-3, the strain not protected against after in vivo challenge. In all experiments, the titers of sera from E. coli-immunized controls were less than 8 (data not shown).
FIG. 1.
Opsonophagocytic titers against P. aeruginosa serogroup O2/O5 strains in pooled sera from actively or passively PAO1ΔaroA-immunized C3H/HeN mice. Titers were determined by linear regression and are expressed as the reciprocal dilution of sera mediating 50% killing. Error bars represent the 95% confidence intervals. n.d., not determined.
To determine the contribution of humoral immunity toward the high-level protection seen against ExoU+ PAO1 (Table 2), we performed passive immunization studies by using IgG purified from antisera of hyper-immunized rabbits given multiple i.n. doses of PAO1ΔaroA or, as a control, E. coli HB101. As shown in Table 4, we found that after i.p. doses of 1.0 mg or after i.n. doses of 0.25 mg of rabbit IgG given 24 h prior to challenge, high-level protection was achieved, but only if challenge doses lower than those used in the active immunization-challenge experiments were used. In these passive transfer studies, there was no protection against a challenge dose of 5 × 107 CFU, but there was 100% protection against a lower challenge dose of 107 CFU after i.p. administration of rabbit IgG. However, improved protection against an inoculum of 5 × 107 CFU of ExoU+ PAO1 was realized when 0.25 mg of the anti-PAO1ΔaroA IgG was administered i.n. in addition to giving 1 mg of IgG i.p. (Table 4), although this was not statistically significant (P = 0.14).
TABLE 4.
Protective efficacy against acute fatal pneumonia due to P. aeruginosa after passive immunization of C3H/HeN mice with rabbit IgG specific for E. coli HB101 or PAO1ΔaroA
| Challenge strain | Inoculum (CFU) | IgG dose (mg) (route) | No. of surviving mice/no. of mice challenged in IgG group:
|
|
|---|---|---|---|---|
| E. coli HB101 | PAO1ΔaroA | |||
| ExoU+ PAO1 | 1 × 107 | 1 (i.p.) | 0/5 | 5/5a |
| 1 × 107 | 0.25 (i.n.) | 0/4 | 4/4a | |
| 5 × 107 | 1 (i.p.) | 0/5 | 0/5 | |
| 5 × 107 | 1 (i.p.), 0.25 (i.n.) | 0/4 | 3/4b | |
| PAO1 | 6 × 108 | 1 (i.p.) | 0/4 | 0/4 |
| 6 × 108 | 1 (i.p.), 0.25 (i.n.) | 0/4 | 0/4 | |
| 6 × 108 | 2 (i.p.), 0.5 (i.n.) | 0/4 | 0/4 | |
| 6 × 108 | 5 (i.p.), 0.5 (i.n.) | 0/2 | 0/2 | |
| 170003 | 5 × 108 | 1 (i.p.) | 0/5 | 0/5 |
| Fisher IT-7 | 2 × 108 | 1 (i.p.) | 0/5 | 0/5 |
P < 0.05 (Fisher exact test).
P = 0.14 (Fisher exact test).
Surprisingly, there was no protection against the parental strain PAO1 after passive immunization with very high doses of IgG (up to 5 mg i.p. and 0.5 mg i.n.) prior to the same challenge inoculum used in active immunization experiments. Furthermore, we found no protection against P. aeruginosa strains 170003, IATS O16, or Fisher IT-7 after passive immunization, again with the same challenge doses as those used in active immunization studies. When we tested the mouse sera for opsonic activity following i.p. administration of the anti-PAO1ΔaroA rabbit IgG, there was high-level killing of all four of the challenge strains (Fig. 1), in many cases higher than the level of killing seen in the active immunization experiments in which significant protection was seen. These results imply that a nonhumoral component of immunity elicited by active immunization significantly contributed to the protection against P. aeruginosa acute lung infection.
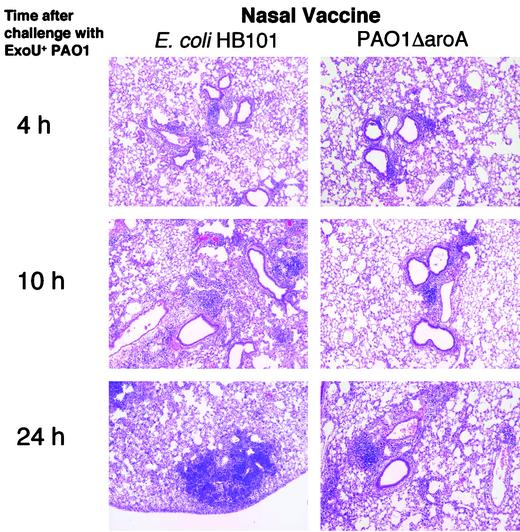
Histological analysis of lungs after i.n. challenge with ExoU+ PAO1 in mice immunized i.n. with either E. coli HB101 (as control) or PAO1ΔaroA showed similar degrees of mild, primarily peri-airway inflammation at 4 h (Fig. 2). However, by 10 h and particularly 24 h after inoculation, the lungs of E. coli HB101-immunized mice had multifocal areas of pneumonia characterized by neutrophil infiltration, alveolar hemorrhage, and the filling of alveoli with proteinaceous debris and bacteria. On the other hand, at 24 h, the lungs of mice that had been immunized with PAO1ΔaroA had only mild to moderate inflammation restricted to the regions near the airways, with overall preservation of normal alveolar architecture. Later time points were not performed since mice in the control group died soon after the 24-h time point.
FIG. 2.
Low-power views (×10) of hematoxylin-and-eosin-stained sections of lungs removed at the indicated time points from C3H/HeN mice infected i.n. with ExoU+ PAO1 after i.n. immunization with E. coli HB101 (left column) or PAO1ΔaroA (right column).
DISCUSSION
In the current study, we evaluated the protective efficacy of nasal immunization with an aroA deletion mutant of the P. aeruginosa serogroup O2/O5 strain PAO1 against acute lethal pneumonia in mice. We previously reported that nasal immunization of mice and rabbits with this strain is well tolerated and generates high levels of opsonic antibody active against serogroup-homologous strains and, in rabbits, even against some serogroup-heterologous strains (51). We found that the opsonic antibodies elicited by the live, attenuated vaccine are primarily directed against the LPS O antigen, since adsorption of the rabbit antisera with O antigen-deficient mutants had no effect on the level of opsonic killing (51). In the present study, we observed high levels of protection (typically with 100% survival in the PAO1ΔaroA-immunized mice and 100% mortality in the E. coli-immunized mice) when immunized inbred mice of several strains were challenged i.n. with ExoU+ PAO1, a cytotoxic variant of the parental strain PAO1 that expresses the type III secretion system cytotoxin ExoU. Immunity did not involve responses to the ExoU protein, given that the vaccine strain does not carry the exoU gene.
The importance of showing that protection extends to multiple strains of inbred mice lies in the observations that during P. aeruginosa infections some strains of inbred mice (e.g., C57BL/6 and C3H/HeN) have been reported to have a propensity toward T helper cell type 1 (TH1) immune responses, characterized by predominant secretion of gamma interferon by T cells, whereas other strains (e.g., BALB/c) show biases toward TH2 immune responses, characterized by predominant secretion of interleukin-4 and interleukin-10 by T cells (22, 29, 30, 41, 42, 59, 61). C57BL/6 mice were found to have increased susceptibility toward chronic bronchopulmonary pneumonia with mucoid P. aeruginosa (29, 59, 61) and corneal infections with LPS-smooth P. aeruginosa (22, 30) compared to BALB/c mice. Other studies, however, have demonstrated that TH1-responding C3H/HeN mice were more susceptible than the TH2-responding BALB/c mice to chronic bronchopulmonary pneumonia with mucoid P. aeruginosa (41, 42). Thus, although the relative importance of TH1 versus TH2 responses in immunity to P. aeruginosa infections is not clear at this time, it is evident that different strains of inbred mice have different immune responses to P. aeruginosa. Despite this, we found high levels of protection against homologous challenge with every strain of inbred mice tested. Our immunized BALB/c mice were somewhat more resistant to P. aeruginosa pneumonia, supporting the results of Stevenson and coworkers (59, 61).
After active immunization, mice challenged with the parental strain PAO1 with an inoculum 20-fold above the LD50 were significantly, but not completely, protected. This finding is in contrast to the complete protection achieved when actively immunized mice were challenged with the more virulent ExoU+ PAO1 strain with an inoculum 70-fold greater than the LD50. This difference was observed despite very high titers of opsonic antibody to PAO1 in the immune mouse sera. Although the reasons for this are not completely clear, it is likely that the large inoculum of PAO1 (6 × 108 CFU per mouse) needed in these studies to obtain high levels of lethality in control mice may have overwhelmed the immune effectors generated by the vaccine. To address this issue, we are currently investigating protective efficacy against lethal pneumonia in mice made neutropenic by cyclophosphamide, which vastly reduces the challenge doses of P. aeruginosa needed for mortality, even with strains poorly virulent in intact mice. Preliminary results suggest a more broadly protective immune response is observed when lower challenge doses can be used and also indicate that the level of neutropenia induced by the cyclophosphamide leaves a sufficient number of functional leukocytes to mediate immune protection. In addition, studies of the efficacy of PAO1ΔaroA vaccination in protecting mice against corneal infections, which require a lower challenge dose to elicit significant pathology, show evidence of high levels of protection against multiple challenge strains, even when they are from heterologous serogroups (T. S. Zaidi, G. P. Priebe, and G. B. Pier, Abstr. 101st Gen. Meet. Am. Soc. Microbiol. 2001, abstr. D-8, 2001).
It is noteworthy that active immunization with the single strain PAO1ΔaroA conferred protection against the subtype-heterologous strains 170003 and Fisher IT-7 and nearly significant protection against the subtype-heterologous strain IATS O16. In general, O-antigen-based vaccine candidates evaluated to date have elicited protection only when the strains used to isolate the vaccine antigen are used in the challenge studies (6, 7, 45). Thus, it is a step forward to observe protection with subtype-heterologous strains even if it is only partial protection. There was no protection against the subtype-heterologous strain Fisher IT-3, a finding which is consistent with the initial studies performed to define the Fisher immunotyping system that showed that IT-3 and IT-7 strains were not cross-protective (11). Although strains 170003, IATS O16, and PAO1 all share a similar carbohydrate backbone in the O-antigen trisaccharide repeat composed of 2-acetamido-3-acetamidino-2,3 dideoxymannuronic acid [Man(2NAc3N)], 2,3-diacetamido-2,3-dideoxymannuronic acid [Man(NAc)2A], and N-acetylfucosamine, the Fisher IT-3 strain has the 5′ epimer of Man(NAc)2A, i.e., 2,3-diacetamido-2,3-dideoxyguluronic acid, in the middle position of its trisaccharide repeat unit (20, 28). Possibly, the Man(NAc)2A residue forms an epitope critical for recognition by protective antibody, but this remains to be determined. This conjecture is further supported by our finding of protection against challenge with the prototype Fisher IT-7 strain after immunization with the PAO1ΔaroA strain. The O antigen of strain Fisher IT-7 differs from PAO1 in that the first sugar in the O-antigen trisaccharide repeat unit, Man(2NAc3N), is replaced by the 5′ epimer, 2-acetamido-3-acetamidino-2,3 dideoxyguluronic acid, indicating that the epitopes formed by this component of the trisaccharide repeat are not involved in binding protective antibody.
An alternative explanation of our protection results is that the O antigen of P. aeruginosa strains of the O2/O5 serogroup undergoes modifications during infection in the host, allowing the challenge strain to generate chemical, and thus serological, changes in the O antigen to avoid preexisting host antibody induced by immunization. Indeed, it was hoped that if this were the case, a live, attenuated vaccine might undergo similar changes and elicit a greater repertoire of antibodies to the O side chains. However, a single vaccine strain may not be able to produce all of the possible variants that can occur in the O side chain of P. aeruginosa strains in a similar serogroup. It has been suggested that in fact all of the related serogroup O2/O5 strains can produce the same, basic trisaccharide repeat unit and that modifications such as O acetylation, monosaccharide epimerization, and variation in linkages that distinguish the subtypes may actually be postsynthetic modifications. A recent study by Raymond et al. (52) has shown that the members of the O2/O5 serogroup, which includes strains serologically designated O16, O18, and O20, have a highly related genetic organization for the genes encoding the O-antigen biosynthetic locus. Strains of different serogroups have highly divergent genes in these places. Structural analysis of the O antigens of several P. aeruginosa O2/O5 strains revealed that the LPS molecular structures from some strains can even be comprised of mixed repeat units representative of different structures within the O2/O5 serogroup (54). If this is the case, then it may be possible that in the presence of protective antibody to one variant structure, strains producing an altered structure that does not bind the antibody will be selected for under immunologic pressure.
Interestingly, within the O2/O5 serogroup, protective efficacy did not correlate with opsonic titer. This might reflect the fact that the P. aeruginosa strains used in vitro did not generate O-antigen variants during the short, 90-min opsonic assay and thus appeared to be highly susceptible to opsonic killing, whereas in vivo O-antigen variants were generated that were not susceptible to the opsonic antibody raised by the attenuated vaccine. Alternately, in addition to opsonic antibody, other immune effectors may be required for protection. A number of studies have implicated that T cells play a significant role in the control of P. aeruginosa infections (9, 29, 33, 35-38, 62), and it is likely that the previously described (51) propensity toward intracellular growth of the aroA deletion mutant would generate some cellular immunity. It might be expected that a cytotoxic strain such as ExoU+ PAO1 would not be susceptible to the typical cellular immune effectors because it would presumably be rapidly released from host cells, as has been suggested by in vitro studies. However, our prior studies with this murine model (1) showed that in vivo a cytotoxic strain was actually better able to survive intracellularly in the lungs than was the noncytotoxic strain PAO1. The contribution of T cells to the protection in this lethal pneumonia model after nasal immunization with PAO1ΔaroA is under active investigation, with current avenues of pursuit including analysis of the protective efficacy in various T cell-knockout mice (G. P. Priebe and G. B. Pier, Abstr. 102nd Gen. Meet. Am. Soc. Microbiol. 2002, abstr. D-74, 2002).
Our finding that protection after active immunization with the live, attenuated strain was most efficacious with the homologous PAO1 strain further supports prior findings (20) that P. aeruginosa vaccines are usually most effective against challenge with the homologous strain from which the vaccine was derived and much less efficacious against challenge even with closely related strains of the same serogroup. This represents a major barrier to developing O-antigen-specific vaccines. Although cross-protection has been achieved by other, non-LPS vaccines, it is notably low in comparison to the level of protection achieved by LPS immunization and homologous-strain challenge (39). We had hoped to overcome this restriction by use of a live, attenuated vaccine. Although there was some success in protection against other strains of the O2/O5 group, the protection was still fairly narrow. However, as opposed to the use of purified LPS O antigens, which interfere with each other's immunogenicity when combined into a multivalent vaccine (20), a multivalent live, attenuated vaccine may be more feasible.
In our passive immunization studies, we found that mice could be protected from challenge with ExoU+ PAO1, but only with relatively high doses of parenteral or nasal IgG and only when relatively low challenge doses were used. There was absolutely no protection in passive immunization studies with PAO1 or other serogroup O2/O5 strains as the challenge strain despite the use of the same inocula as were used in the challenge studies after active immunization (in which significant protection was observed). The inoculum size is likely to be an important factor. It is also possible that the rabbit IgG used in these experiments is not recognized by mouse Fc receptors as effectively as is mouse IgG and is thus able to mediate protection only against the lower challenge doses. However, no such problem with rabbit IgG was encountered in a similar P. aeruginosa pneumonia model after passive immunization with polyclonal rabbit IgG specific for PcrV (55).
The histological studies of lungs after challenge of immunized mice (Fig. 2) show that i.n. immunization did not completely prevent pneumonia but did attenuate its severity. Our previous studies of this acute pneumonia model indicated that death from pneumonia was highly correlated with systemic spread of bacteria (1). It therefore follows that survival of the PAO1ΔaroA-immunized mice is due to prevention of the dissemination of bacteria rather than to the prevention of pneumonia altogether.
A number of P. aeruginosa antigens other than LPS have been investigated as potential vaccines, with most attention being focused on flagella (8, 23, 31), on outer membrane proteins (27, 32, 40) and, more recently, on the PcrV antigen component of the type III secretion system (15, 24, 55, 57). We note that, in these later studies, protection against lethal pneumonia has only been demonstrated with P. aeruginosa strain PA103, a highly virulent, ExoU+ strain that can be given to mice at a relatively low inoculum to generate lethality. Other workers have focused on dendritic-cell-based immunization (25, 26, 62), although the clinical applicability of this approach is questionable. Although protection against heterologous serogroups has sometimes been seen with these vaccines, the protection afforded by non-LPS-based immunotherapeutics has, as a rule, been of only modest potency and is far less than that achieved with LPS-based vaccines and homologous challenge strains.
The present study suggests that nasal immunization with aroA deletion mutants of P. aeruginosa engenders high-level protection against acute lethal pneumonia caused by a homologous challenge strain and a modicum of protection against that caused by subtype strains possessing variations in O antigen structure. These data also indicate that a multivalent vaccine containing aroA deletion mutants of multiple P. aeruginosa serogroups might offer a broadly protective vaccine for P. aeruginosa.
Acknowledgments
This work was supported by NIH grants AI50036 (a Mentored Clinical Scientist Development Award [K08] granted to G.P.P.), AI22535 (G.B.P.), and AI37632 (J.B.G.).
Editor: J. N. Weiser
REFERENCES
- 1.Allewelt, M., F. T. Coleman, M. Grout, G. P. Priebe, and G. B. Pier. 2000. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 68:3998-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ames, P., D. DesJardins, and G. B. Pier. 1985. Opsonophagocytic killing activity of rabbit antibody to Pseudomonas aeruginosa mucoid exopolysaccharide. Infect. Immun. 49:281-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer, H. W., and D. Roulland-Dussoix. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459-472. [DOI] [PubMed] [Google Scholar]
- 4.Burns, J. L., R. L. Gibson, S. McNamara, D. Yim, J. Emerson, M. Rosenfeld, P. Hiatt, K. McCoy, R. Castile, A. L. Smith, and B. W. Ramsey. 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 183:444-452. [DOI] [PubMed] [Google Scholar]
- 5.Cryz, S. J., Jr., E. Furer, and R. Germanier. 1983. Passive protection against Pseudomonas aeruginosa infection in an experimental leukopenic mouse model. Infect. Immun. 40:659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cryz, S. J., Jr., E. Furer, and R. Germanier. 1984. Protection against fatal Pseudomonas aeruginosa burn wound sepsis by immunization with lipopolysaccharide and high-molecular-weight polysaccharide. Infect. Immun. 43:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryz, S. J., Jr., E. Furer, J. C. Sadoff, T. Fredeking, J. U. Que, and A. S. Cross. 1991. Production and characterization of a human hyperimmune intravenous immunoglobulin against Pseudomonas aeruginosa and Klebsiella species. J. Infect. Dis. 163:1055-1061. [DOI] [PubMed] [Google Scholar]
- 8.Doering, G., C. Pfeiffer, U. Weber, A. Mohr-Pennert, and F. Dorner. 1995. Parenteral application of a Pseudomonas aeruginosa flagella vaccine elicits specific anti-flagella antibodies in the airways of healthy individuals. Am. J. Respir. Crit. Care Med. 151:983-985. [DOI] [PubMed] [Google Scholar]
- 9.Dunkley, M. L., R. L. Clancy, and A. W. Cripps. 1994. A role for CD4+ T cells from orally immunized rats in enhanced clearance of Pseudomonas aeruginosa from the lung. Immunology 83:362-369. [PMC free article] [PubMed] [Google Scholar]
- 10.Estahbanati, H. K., P. P. Kashani, and F. Ghanaatpisheh. 2002. Frequency of Pseudomonas aeruginosa serotypes in burn wound infections and their resistance to antibiotics. Burns 28:340-348. [DOI] [PubMed] [Google Scholar]
- 11.Fisher, M. W., H. B. Devlin, and F. Gnabski. 1969. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J. Bacteriol. 98:835-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleiszig, S. M., V. Vallas, C. H. Jun, L. Mok, D. F. Balkovetz, M. G. Roth, and K. E. Mostov. 1998. Susceptibility of epithelial cells to Pseudomonas aeruginosa invasion and cytotoxicity is upregulated by hepatocyte growth factor. Infect. Immun. 66:3443-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleiszig, S. M. J., T. S. Zaidi, M. J. Preston, J. B. Goldberg, M. Grout, D. J. Evans, and G. B. Pier. 1996. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect. Immun. 64:2288-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foca, M., K. Jakob, S. Whittier, P. Della Latta, S. Factor, D. Rubenstein, and L. Saiman. 2000. Endemic Pseudomonas aeruginosa infection in a neonatal intensive care unit. N. Engl. J. Med. 343:695-700. [DOI] [PubMed] [Google Scholar]
- 15.Frank, D. W., A. Vallis, J. P. Wiener-Kronish, A. Roy-Burman, E. G. Spack, B. P. Mullaney, M. Megdoud, J. D. Marks, R. Fritz, and T. Sawa. 2002. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J. Infect. Dis. 186:64-73. [DOI] [PubMed] [Google Scholar]
- 16.Gales, A. C., R. N. Jones, J. Turnidge, R. Rennie, and R. Ramphal. 2001. Characterization of Pseudomonas aeruginosa isolates: occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY antimicrobial surveillance program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S146-S155. [DOI] [PubMed] [Google Scholar]
- 17.Hancock, R. E., and W. A. Woodruff. 1988. Roles of porin and beta-lactamase in beta-lactam resistance of Pseudomonas aeruginosa. Rev. Infect. Dis. 10:770-775. [DOI] [PubMed] [Google Scholar]
- 18.Hancock, R. E. W., L. M. Mutharia, L. Chan, R. P. Darveau, D. P. Speert, and G. B. Pier. 1983. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, non-typable strains deficient in lipopolysaccharide O side chains. Infect. Immun. 42:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatano, K., S. Boisot, D. DesJardins, D. C. Wright, J. Brisker, and G. B. Pier. 1994. Immunogenic and antigenic properties of a heptavalent high-molecular-weight O-polysaccharide vaccine derived from Pseudomonas aeruginosa. Infect. Immun. 62:3608-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatano, K., and G. B. Pier. 1998. Complex serology and immune response of mice to variant high-molecular-weight O polysaccharides isolated from Pseudomonas aeruginosa serogroup O2 strains. Infect. Immun. 66:3719-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauser, A. R., S. Fleiszig, P. J. Kang, K. Mostov, and J. N. Engel. 1998. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect. Immun. 66:1413-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hazlett, L. D., S. McClellan, B. Kwon, and R. Barrett. 2000. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Investig. Ophthalmol. Vis. Sci. 41:805-810. [PubMed] [Google Scholar]
- 23.Holder, I. A., and J. G. Naglich. 1986. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: immunization using divalent flagella preparations. J. Trauma 26:118-122. [DOI] [PubMed] [Google Scholar]
- 24.Holder, I. A., A. N. Neely, and D. W. Frank. 2001. PcrV immunization enhances survival of burned Pseudomonas aeruginosa-infected mice. Infect. Immun. 69:5908-5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kikuchi, T., N. R. Hackett, and R. G. Crystal. 2001. Cross-strain protection against clinical and laboratory strains of Pseudomonas aeruginosa mediated by dendritic cells genetically modified to express CD40 ligand and pulsed with specific strains of Pseudomonas aeruginosa. Hum. Gene Ther. 12:1251-1263. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi, T., S. Worgall, R. Singh, M. A. Moore, and R. G. Crystal. 2000. Dendritic cells genetically modified to express CD40 ligand and pulsed with antigen can initiate antigen-specific humoral immunity independent of CD4+ T cells. Nat. Med. 6:1154-1159. [DOI] [PubMed] [Google Scholar]
- 27.Kim, D. K., J. J. Kim, J. H. Kim, Y. M. Woo, S. Kim, D. W. Yoon, C. S. Choi, I. Kim, W. J. Park, N. Lee, S. B. Jung, B. Y. Ahn, S. W. Nam, S. M. Yoon, and W. J. Choi. 2001. Comparison of two immunization schedules for a Pseudomonas aeruginosa outer membrane proteins vaccine in burn patients. Vaccine 19:1274-1283. [DOI] [PubMed] [Google Scholar]
- 28.Knirel, Y. A. 1990. Polysaccharide antigens of Pseudomonas aeruginosa. CRC Crit. Rev. Microbiol. 17:273-304. [DOI] [PubMed] [Google Scholar]
- 29.Kondratieva, T. K., N. V. Kobets, S. V. Khaidukov, V. V. Yeremeev, I. V. Lyadova, A. S. Apt, M. F. Tam, and M. M. Stevenson. 2000. Characterization of T-cell clones derived from lymph nodes and lungs of Pseudomonas aeruginosa-susceptible and resistant mice following immunization with heat-killed bacteria. Clin. Exp. Immunol. 121:275-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon, B., and L. D. Hazlett. 1997. Association of CD4+ T-cell-dependent keratitis with genetic susceptibility to Pseudomonas aeruginosa ocular infection. J. Immunol. 159:6283-6290. [PubMed] [Google Scholar]
- 31.Landsperger, W. J., K. D. Kelly-Wintenberg, T. C. Montie, L. S. Knight, M. B. Hansen, C. C. Huntenburg, and M. J. Schneidkraut. 1994. Inhibition of bacterial motility with human antiflagellar monoclonal antibodies attenuates Pseudomonas aeruginosa-induced pneumonia in the immunocompetent rat. Infect. Immun. 62:4825-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mansouri, E., J. Gabelsberger, B. Knapp, E. Hundt, U. Lenz, K. D. Hungerer, H. E. Gilleland, Jr., J. Staczek, H. Domdey, and B. U. von Specht. 1999. Safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteers. Infect. Immun. 67:1461-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markham, R. B., J. Goellner, and G. B. Pier. 1984. In vitro T cell-mediated killing of Pseudomonas aeruginosa. I. Evidence that a lymphokine mediates killing. J. Immunol. 133:962-968. [PubMed] [Google Scholar]
- 34.Markham, R. B., and G. B. Pier. 1983. Immunologic basis for mouse protection provided by high-molecular-weight polysaccharide from immunotype 1 Pseudomonas aeruginosa. Rev. Infect. Dis. 5(Suppl. 5):S957-S962. [DOI] [PubMed] [Google Scholar]
- 35.Markham, R. B., G. B. Pier, J. J. Goellner, and S. B. Mizel. 1985. In vitro T cell-mediated killing of Pseudomonas aeruginosa. II. The role of macrophages and T-cell subsets in T-cell killing. J. Immunol. 134:4112-4117. [PubMed] [Google Scholar]
- 36.Markham, R. B., G. B. Pier, and W. G. Powderly. 1988. Suppressor T cells regulating the cell-mediated immune response to Pseudomonas aeruginosa can be generated by immunization with anti-bacterial T cells. J. Immunol. 141:3975-3979. [PubMed] [Google Scholar]
- 37.Markham, R. B., G. B. Pier, and J. R. Schreiber. 1991. The role of cytophilic IgG3 antibody in T cell-mediated resistance to infection with the extracellular bacterium, Pseudomonas aeruginosa. J. Immunol. 146:316-320. [PubMed] [Google Scholar]
- 38.Markham, R. B., and W. G. Powderly. 1988. Exposure of mice to live Pseudomonas aeruginosa generates protective cell-mediated immunity in the absence of an antibody response. J. Immunol. 140:2039-2045. [PubMed] [Google Scholar]
- 39.Matthews-Greer, J. M., and H. E. Gilleland, Jr. 1987. Outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine against heterologous immunotype strains in a burned mouse model. J. Infect. Dis. 155:1282-1291. [DOI] [PubMed] [Google Scholar]
- 40.Matthews-Greer, J. M., D. E. Robertson, G. L. B., and H. E. Gilleland, Jr. 1990. Pseudomonas aeruginosa outer membrane protein F produced in Escherichia coli retains vaccine efficacy. Curr. Microbiol. 20:171-175. [Google Scholar]
- 41.Moser, C., H. P. Hougen, Z. Song, J. Rygaard, A. Kharazmi, and N. Hoiby. 1999. Early immune response in susceptible and resistant mice strains with chronic Pseudomonas aeruginosa lung infection determines the type of T-helper cell response. APMIS 107:1093-1100. [DOI] [PubMed] [Google Scholar]
- 42.Moser, C., H. K. Johansen, Z. Song, H. P. Hougen, J. Rygaard, and N. Hoiby. 1997. Chronic Pseudomonas aeruginosa lung infection is more severe in Th2 responding BALB/c mice compared to Th1 responding C3H/HeN mice. APMIS 105:838-842. [PubMed] [Google Scholar]
- 43.Pier, G. B. 1982. Safety and immunogenicity of high molecular weight polysaccharide vaccine from immunotype 1 Pseudomonas aeruginosa. J. Clin. Investig. 69:303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pier, G. B., M. Grout, T. S. Zaidi, J. C. Olsen, L. G. Johnson, J. R. Yankaskas, and J. B. Goldberg. 1996. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science 271:64-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pier, G. B., and M. Pollack. 1989. Isolation, structure, and immunogenicity of Pseudomonas aeruginosa immunotype 4 high-molecular-weight polysaccharide. Infect. Immun. 57:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pier, G. B., D. Thomas, G. Small, A. Siadak, and H. Zweerink. 1989. In vitro and in vivo activity of polyclonal and monoclonal human immunoglobulins G, M, and A against Pseudomonas aeruginosa lipopolysaccharide. Infect. Immun. 57:174-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poggio, E. C., R. J. Glynn, O. D. Schein, J. M. Seddon, M. J. Shannon, V. A. Scardino, and K. R. Kenyon. 1989. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N. Engl. J. Med. 321:779-783. [DOI] [PubMed] [Google Scholar]
- 48.Pollack, M., and L. S. Young. 1979. Protective activity of antibodies to exotoxin A and lipopolysaccharide at the onset of Pseudomonas aeruginosa septicemia in man. J. Clin. Investig. 63:276-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poole, K., K. Krebes, C. McNally, and S. Neshat. 1993. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J. Bacteriol. 175:7363-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Preston, M. J., S. M. Fleiszig, T. S. Zaidi, J. B. Goldberg, V. D. Shortridge, M. L. Vasil, and G. B. Pier. 1995. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect. Immun. 63:3497-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Priebe, G. P., M. M. Brinig, K. Hatano, M. Grout, F. T. Coleman, G. B. Pier, and J. B. Goldberg. 2002. Construction and characterization of a live, attenuated aroA deletion mutant of Pseudomonas aeruginosa as a candidate intranasal vaccine. Infect. Immun. 70:1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raymond, C. K., E. H. Sims, A. Kas, D. H. Spencer, T. V. Kutyavin, R. G. Ivey, Y. Zhou, R. Kaul, J. B. Clendenning, and M. V. Olson. 2002. Genetic variation at the O-antigen biosynthetic locus in Pseudomonas aeruginosa. J. Bacteriol. 184:3614-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 1999. Nosocomial infections in medical intensive care units in the United States National Nosocomial Infections Surveillance. Syst. Crit. Care Med. 27:887-892. [DOI] [PubMed] [Google Scholar]
- 54.Sadovskaya, I., J. R. Brisson, P. Thibault, J. C. Richards, J. S. Lam, and E. Altman. 2000. Structural characterization of the outer core and the O-chain linkage region of lipopolysaccharide from Pseudomonas aeruginosa serotype O5. Eur. J. Biochem. 267:1640-1650. [DOI] [PubMed] [Google Scholar]
- 55.Sawa, T., T. L. Yahr, M. Ohara, K. Kurahashi, M. A. Gropper, J. P. Wiener-Kronish, and D. W. Frank. 1999. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 5:392-398. [DOI] [PubMed] [Google Scholar]
- 56.Schein, O. D., R. J. Glynn, J. M. Seddon, V. A. Scardino, and K. R. Kenyon. 1989. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N. Engl. J. Med. 321:773-778. [DOI] [PubMed] [Google Scholar]
- 57.Shime, N., T. Sawa, J. Fujimoto, K. Faure, L. R. Allmond, T. Karaca, B. L. Swanson, E. G. Spack, and J. P. Wiener-Kronish. 2001. Therapeutic administration of anti-PcrV F(ab′)2 in sepsis associated with Pseudomonas aeruginosa. J. Immunol. 167:5880-5886. [DOI] [PubMed] [Google Scholar]
- 58.Stanislavsky, E. S., and J. S. Lam. 1997. Pseudomonas aeruginosa antigens as potential vaccines. FEMS Microbiol. Rev. 21:243-277. [DOI] [PubMed] [Google Scholar]
- 59.Stevenson, M. M., T. K. Kondratieva, A. S. Apt, M. F. Tam, and E. Skamene. 1995. In vitro and in vivo T-cell responses in mice during bronchopulmonary infection with mucoid Pseudomonas aeruginosa. Clin. Exp. Immunol. 99:98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 61.Tam, M., G. J. Snipes, and M. M. Stevenson. 1999. Characterization of chronic bronchopulmonary Pseudomonas aeruginosa infection in resistant and susceptible inbred mouse strains. Am. J. Respir. Cell. Mol. Biol. 20:710-719. [DOI] [PubMed] [Google Scholar]
- 62.Worgall, S., T. Kikuchi, R. Singh, K. Martushova, L. Lande, and R. G. Crystal. 2001. Protection against pulmonary infection with Pseudomonas aeruginosa following immunization with P. aeruginosa-pulsed dendritic cells. Infect. Immun. 69:4521-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]